Abstract
The secreted molecule unc-6/netrin is important for guiding axon projections and cell migrations. unc-5 and unc-40/DCC are identified as receptors for unc-6/netrin. The downstream factors of unc-6 receptors are beginning to be elucidated, and some key factors have been identified in various organisms. Here, we showed that SRC-1 interacts with the cytosolic domain of UNC-5 through its SH2 domain. This interaction also requires the intact kinase activity of SRC-1. Downregulation of src-1 by RNA interference decreases the biological processes initiated by the UNC-5 protein and decreases UNC-5 tyrosine phosphorylation. We also generated a chimeric protein consisting of the extracellular domain and transmembrane domain of UNC-5 and an intracellular domain of SRC-1. This fusion protein is able to partially rescue mutant phenotypes caused by unc-5 but not unc-6, unc-40, and unc-34. Our results support a model in which SRC-1 is required for UNC-5-induced axon repulsion and gonad migration signaling pathways and in which localizing SRC-1 activity to UNC-5 is crucial for proper signal transduction in response to unc-6/netrin.
Cell guidance processes are very important biological phenomena and are required for many developmental processes. So far, many axon guidance molecules have been identified. Those can be grouped into four major categories: netrin, semaphorin, slit, and ephrin (12). These molecules guide axons by providing either a chemo-attractive signal, which attracts the growing axon to the targeted area, or a chemo-repellant signal, which keeps the axon away from the improper area. In addition to their role in neuronal function, these molecules play important roles in many other developmental processes.
Netrin is a laminin-like secreted molecule capable of conveying both attractive and repulsive signals depending on the expression of its receptors, UNC-5 and UNC-40/DCC (deleted in colorectal cancer). Axons expressing UNC-40/DCC are attracted toward netrin, and axons expressing both UNC-40/DCC and UNC-5 are repelled by it (9).
Netrin has been studied in many different model systems. Caenorhabditis elegans was the first organism in which unc-6/netrin was identified (13). Later, unc-6/netrin homologues were found in fly (8), zebrafish (17), and mammals (14, 26). These studies confirm that netrin signaling is conserved throughout evolution. In addition to axon guidance, unc-6/netrin homologues participate in various biological processes outside neuronal tissues. In mammals, netrin plays important roles in various types of organ morphogenesis. For example, it controls the shape of branched tubes during lung development (20). It also controls blood vessel branching (21) and stimulates angiogenesis (24). In C. elegans, unc-6/netrin not only directs the formation of neuromuscular junctions, but also controls the migration of distal tip cells (DTCs), which are nonneuronal tissue. A DTC migration defect leads to a misguided gonad shape.
Although many cellular processes have been identified requiring netrin signaling, understanding of netrin-induced intracellular signal transduction is fragmented at best. Also, the fact that netrin has two opposite effects makes netrin attraction/repulsion harder to understand. Both attraction and repulsion should be regulated by unique sets of factors; however, they may share some common effector proteins. For example, unc-34, the Drosophila-enabled homologue in C. elegans, is a common effector for both attraction and repulsion processes (4, 6).
Upon ligand binding, UNC-40/DCC and UNC-5 receptors are tyrosine phosphorylated, and this tyrosine phosphorylation may serve an important role for the downstream events. Mutation of certain tyrosine phosphorylation sites on UNC-5 severely affects axon guidance and DTC migration (15). It has been reported that clr-1, a receptor protein tyrosine phosphatase (RPTP), is able to modulate netrin signaling (3). These data support the importance of tyrosine phosphorylation in netrin signaling. Recent studies also show that DCC and netrin signaling require focal adhesion kinase and SRC. SRC-1 functions immediately downstream of DCC and plays an important role in netrin-induced axon attraction (18, 19, 25). Since both attraction and repulsion processes require DCC, SRC-1 may also be involved in both processes. In this paper, we show that SRC-1 is important for the UNC-5-mediated netrin repulsion signal in C. elegans.
MATERIALS AND METHODS
Cells, transfection procedures, and immunoprecipitation.
Transient transfections of HEK 293 cells were performed using Lipofectamine (Invitrogen). For immunoprecipitation, cells were lysed 36 h posttransfection in NP-40 buffer (20 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1% NP-40, 0.1 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 1 μM dithiothreitol, 100 μM sodium meta-vanadate) and the lysates were subsequently incubated with 1 μg of anti-myc antibody (9E10) at 4°C for 3 hours. Protein G-Sepharose beads (Amersham Biosciences) were added and incubated at 4°C for 2 more hours and then washed three times with NP-40 buffer. The beads were added with 1× sodium dodecyl sulfate sample buffer and run on a 12% sodium dodecyl sulfate-polyacrylamide gel. The samples were then subjected to Western blotting and probed with the appropriate antibodies. In order to inhibit Src family kinase activity, cells were starved for 3 h in the serum-free medium and then the inhibitor PP2 (Calbiochem) was treated at the concentrations indicated in Fig. 1 for 30 min (7).
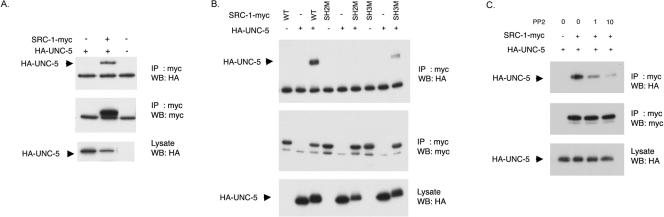
FIG. 1.
The UNC-5 cytosolic domain interacts with SRC-1 in vitro. (A) SRC-1 binds the UNC-5 cytosolic domain. HEK293 cells were transfected with the indicated expression vectors. SRC-1-myc was immunoprecipitated (IP) with anti-myc antibody (9E10) and subjected to Western blot analysis (WB) with the indicated antibodies. (B) The SH2 domain of SRC-1 is important for its interaction with UNC-5. Lysates were subjected to immunoprecipitation with an anti-myc antibody (9E10) and probed for the indicated Western blot analysis. SH2M and SH3M denote SRC-1 mutations in the SH2 domain (R165A) and SH3 domain (W108R W109R), respectively. WT, wild type. (C) PP2 inhibits the interaction between SRC-1 and UNC-5. Transfected HEK293 cells were treated with PP2, an inhibitor of Src family kinases, for 30 min at the indicated concentrations (μM). Lysates were immunoprecipitated with anti-myc antibody (9E10) and probed for the indicated Western blot analysis.
Plasmid constructs and site-directed mutagenesis.
Standard molecular biology techniques were used. src-1 cDNA (5′ ATGGGTTGCCTGTTTTCA…ACAAATATATATATATAA 3′) and the cytosolic domain of unc-5 cDNAs (5′ AAACGTGGCAATTCAAAA…CAAATTGTGTCCCCATAA 3′) were obtained by PCR from a worm cDNA library and were subcloned into pcDNA3myc or pcDNA3HA (Invitrogen). The pU5HA construct (unc-5::unc-5HA) was a generous gift from J. G. Culotti (University of Toronto). The promoter region of unc-5 (4.6 kb; 5′ CCTGATTGTTTGAGTAAG…ATAATTTCTATTCCAGTA 3′) was obtained by PCR and was used for the transcriptional fusion construct with SRC-1(K290M). For UNC-5-SRC-1 fusion protein, a site-directed mutagenesis kit (Stratagene) was used to generated a SmaI site (5′TGCTGTAAACGTGGCAATTCAAAAAAGTCGGGAATTTCCGGTGGTGGTGGTGGACCCGGGTAATCAGCTTTTCCACAAATTGTGTCCCCATAA 3′). The SmaI site is underlined. Later, either src-1 wild-type or src-1(K290M) cDNA was subcloned into this SmaI site. The various src-1 mutants were generated with a site-directed mutagenesis kit (Stratagene).
C. elegans strain and generation of stable lines.
Nematodes were cultured by standard techniques (2). All C. elegans strains were grown at 20°C. Bristol N2 was a standard strain. The following mutations were used for experiments: for LGI, unc-40(e271), src-1(cj293)/hT2 I, and +/hT2V; for LGIV, unc-5(e53); for LGV, unc-34(e315); and for LGX, unc-6(ev400). Germ line transformation was performed using standard techniques (22). unc-5::src-1(K290M), unc-5::unc-5-src-1, and unc-5::unc-5-src-1(K290M) constructs were injected at 10 ng/μl. sur-5::gfp (100 ng/μl) or plx-2::gfp (100 ng/μl) was used as a coinjection marker. For each clone, at least two independent lines were obtained. The data were obtained from one representative line of each clone. Mating was used for transferring the extrachromosomal array between different genetic backgrounds. Only the worms with a decent green fluorescent protein (GFP) expression pattern were used in our analyses.
RNA interference (RNAi) procedures.
In brief, the HT115(DE3) strain was transformed with L4440 plasmid (Fire Lab vector kit) containing 600 bp of src-1 cDNA (5′ATGGGTTGCCTGTTTTCAAAA…AATTGGGAAATTCCACGCAAT 3′). A construct containing the src-2 cDNA sequence (F49B2.5) was used as a negative control. The transformed HT115(DE3) strains were induced with 1 μM IPTG (isopropyl-β-d-thiogalactopyranoside) for 6 h and used as a food source. Five young adults worms were transferred onto the plates, and their progenies were observed.
Detection of tyrosine phosphorylation in UNC-5.
A worm line integrated with pU5-HA (a hemagglutinin [HA]-tagged version of the UNC-5 construct from J. G. Culotti) was used. The integrated line was grown in liquid culture. After a week, we evenly distributed them into separate liquid cultures with HT115(DE3) E. coli strains producing either src-1 or src-2 double-stranded RNA (dsRNA). After 3 days, we harvested the worms. Subsequently, the worms were sonicated three times in PLC buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 10 mM NaPPi, 10 mM NaF, 100 μM sodium vanadate, and protease inhibitors). The lysates were immunoprecipitated with anti-HA antibody and subjected to Western blotting with anti-phosphotyrosine antibody (4G10; Signal Transduction Laboratory).
Worm tracker system.
L4 worms were collected onto new plates and incubated for 16 h at 20°C. Worms with a complete GFP pattern were selected for further analysis. Each worm was tracked for 5 min under the tracker system (5). The tracker system automatically monitors animal movement by controlling the vision and motion hardware and integrates images of the animal and motion data into behavioral/morphological features such as speed, reversal, and body bending. At least five individual worms for each genotype were tracked, and data were collected.
Gonad migration defects and commissural axon defects.
L4 or young adult animals were mounted on a 2% agarose pad in M9 buffer containing 5 mM sodium azide. Their gonad shapes were observed under a Leica microscope. More than 50 animals were observed for each data point for each strain. Data from three independent countings were subsequently analyzed with SPSS statistical software. Images were taken with a Sony DKC 5000 digital photo camera. Commissural axons were visualized using a GFP marker for motor neurons (plx-2::GFP). More than 15 animals were observed for each data point for each strain.
RESULTS
UNC-5 interacts with SRC-1 in vitro.
To test the possible role of SRC-1 kinase in the unc-5 signaling pathway, we performed in vitro binding experiments between SRC-1 and the cytosolic domain of UNC-5 in HEK 293 cells. SRC-1 is myc epitope tagged at the C terminus, and the UNC-5 cytosolic domain is HA epitope tagged at the N terminus. Cells were transfected with wild-type SRC-1-myc constructs, along with HA-UNC-5. SRC-1-myc was immunoprecipitated, and the presence of HA-UNC-5 in the complex was detected by Western blot analysis. SRC-1 specifically interacts with the cytosolic domain of UNC-5 (Fig. 1A).
Since SRC-1 can bind its interacting proteins through its SH2 and SH3 domains, we mutated either the SH2 or SH3 domain and tested the UNC-5 and SRC-1 interaction. The interaction between UNC-5 and SRC-1 proteins is mediated mainly through the SRC-1 SH2 domain, since mutation of the SH2 domain of SRC-1 severely reduced binding (Fig. 1B). However, the SH3 domain also contributes to binding, because mutation of the SH3 domain partially reduced the overall binding between UNC-5 and SRC-1 (Fig. 1B). We also tested whether SRC-1 kinase activity is important for the interaction. Treatment of SRC-1 kinase inhibitor PP2 (7) dramatically decreased the interaction in a dose-dependent manner (Fig. 1C); therefore, the kinase activity of SRC-1 is important for the binding between SRC-1 and UNC-5. In order to identify the UNC-5 tyrosine phosphorylation sites responsible for the interaction, we generated six UNC-5 mutants in which putative SRC-1 SH2 binding tyrosine residues had been mutated to phenylalanine. Unfortunately, we were unable to detect any difference in the interactions of SRC-1 and UNC-5 mutants from that of the UNC-5 wild type (data not shown). This may imply that multiple tyrosine sites contribute to the interaction or that other tyrosine residues are involved. Together, our data indicate that SRC-1 may phosphorylate UNC-5 and create its own binding sites on UNC-5 molecules.
src-1 knockdown causes gonad migration defects.
A reduction of unc-6/netrin signaling in C. elegans shows many phenotypes; movement defects (Unc) and gonad migration defects are the two major phenotypes studied so far. Gonad development is guided by DTCs and results in two symmetrical U-shaped gonad arms. DTCs migrate along the body wall and make two 90-degree turns (Fig. 2A). The first turn, which results in ventral-to-dorsal migration, requires UNC-6/netrin secretion from the ventral side and the functions of UNC-5 and UNC-40. Two receptors, UNC-5 and UNC-40, act together in pushing DTCs away from the UNC-6/netrin source at the ventral side. As a result, unc-6, unc-5, and unc-40 mutants show gonad migration defects defined by lack of the first 90-degree turn (Fig. 2B).
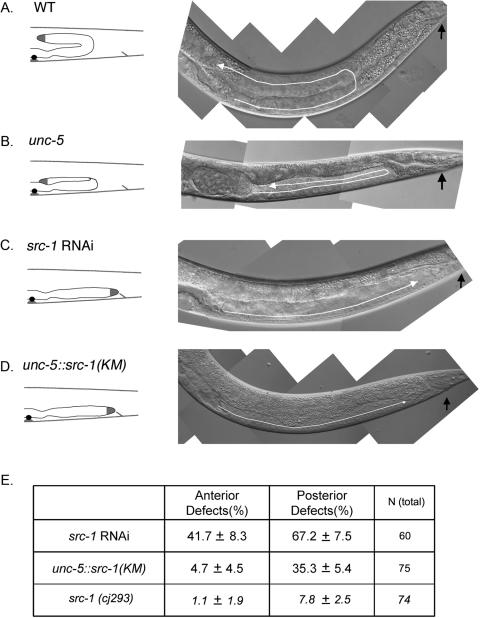
FIG. 2.
src-1 function is important for proper gonad migration. (A to D) The gonad migration defect is caused by low src-1 activity. N2 (A), unc-5 (B), src-1 RNAi (C), and unc-5::src-1(K290M) stable-line (D) gonad phenotypes are shown. Schematic illustrations of gonad morphology are shown on the left. White arrows show the gonad migration paths. Black arrows indicate the position of the anus. Anterior is to the left, and dorsal is on top. (E) Anterior and posterior gonad defects in src-1 RNAi-treated worms and unc-5::src-1(K290M) stable lines. Percentages of defects are shown along with standard deviations. Total numbers of worms observed are also shown. src-1(cj293) represents maternally rescued src-1 mutant worms. Wild-type N2 worms (WT) have no detectable gonad defect.
To test whether src-1 functions downstream of the unc-5 pathway, we first performed src-1 RNAi experiments to phenocopy the unc-5 gonad defects. Healthy worms were fed with Escherichia coli expressing src-1 dsRNA. The majority of their progeny displayed a lethal phenotype consistent with that described in a previous report (1). However, a small percentage of worms escaped the lethal effect of src-1 RNAi and survived to adulthood. These escapees show the Unc (uncoordinated) phenotype. Although neurons are known to be resistant to RNAi treatment, the Unc phenotype may indicate the axon guidance defects caused by src-1 RNAi in neurons. However, we cannot exclude the possibility that the Unc phenotype may be a secondary effect caused by more global defects. We also observed that these escapees tend to have a high percentage of gonadal defects (Fig. 2E). These worms showed a defect in their first gonad turn, implying that the unc-6/netrin signaling pathway has been compromised. However src-1 RNAi also affects the second turn, resulting in a gonad with a straight shape (Fig. 2C). We also used a dominant negative construct of src-1 under the control of the unc-5 promoter [unc-5::src-1(KM)]. The resulting phenotype of the stable line is very similar to that of src-1 RNAi (Fig. 2D). Although src-1(cj293) homozygotes are lethal, they can be maternally rescued when homozygous worms are produced from an src-1(cj293)/hT2 mother. The resulting src-1(cj293) mutants show gonad defects similar to that of src-1 RNAi treatment. The percentage of defects is low compared to that of src-1 RNAi treatment (Fig. 2E), suggesting that the same percentage of gonad defects may be rescued maternally. Therefore, we conclude that the straight gonad shape is a direct result of compromised src-1 activity; also, src-1 is involved in many cellular processes, possibly including the unc-5-mediated repulsion process.
src-1 RNAi antagonizes the rescue of unc-5::unc-5HA constructs on an unc-5 background.
Since the effect of src-1 RNAi is multiple defects in addition to the first gonad turn defect, it was hard to evaluate the importance of src-1 in unc-5 signaling. Because src-1 is involved in a wide variety of cellular processes, one can imagine that the reduction of src-1 function could have pleiotropic effects on various developmental processes. unc-5 worms usually have a high percentage of gonad defects and can be rescued by the injection of the pU5HA (unc-5::unc-5HA) plasmid (15). We observed that injection of pU5HA significantly rescued the gonad migration defects of unc-5 mutants (Fig. 3A). Both the anterior and posterior migration abnormalities observed in unc-5 mutants were rescued. We also detected that the worm's movement was greatly increased to the wild-type level. However, pU5HA did not completely rescue the defects in the unc-5 mutant. The incomplete rescue of unc-5 mutants by pU5HA suggests that the level of unc-5 signaling in animals is lower than that of the wild type. Therefore, the pU5HA-rescued worms may be more sensitive to the downregulation of src-1. The src-1 dsRNA-containing bacteria were diluted fivefold with control bacteria as a feeding food for worms in order to avoid the high percentage of lethality caused by src-1 dsRNA. Under the suboptimal treatment of src-1 RNAi, wild-type N2 worms showed a much milder phenotype; only 16% of the worm displayed the straight-gonad defects (Fig. 3A). We applied this src-1 RNAi condition to the rescued unc-5 worms. After a 56-h feeding, we counted the gonad defects of their progeny. The defects on the rescued unc-5 worms increased about fourfold compared to those without RNAi treatment (Fig. 3A). Although src-1 RNAi treatment also generates the straight-gonad phenotype with the unc-5-rescued worms, the percentage of cells with the straight-gonad phenotype is not much different from the percentage treated on wild-type worms. These observations support an interpretation that the second turn of the gonad is not dependent on unc-5 signaling and should be regulated by yet-unidentified signaling molecules. src-1 may also be involved in this unknown signaling mechanism. Together, our experiments support the notion that src-1 mediates unc-5 signaling.
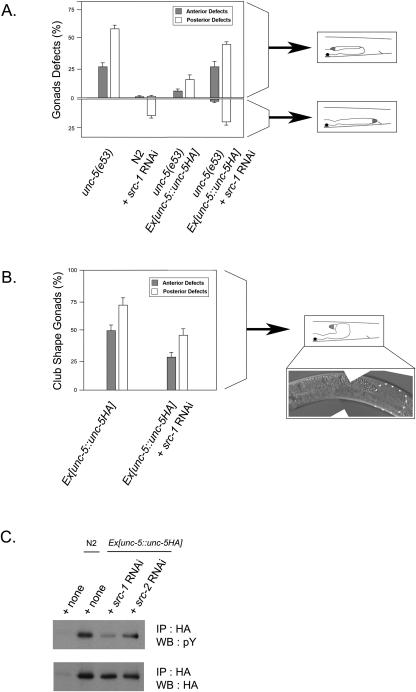
FIG. 3.
Downregulation of src-1 by RNAi affects unc-5 signaling. (A) A rescue of an unc-5 null mutant by Ex[unc-5::unc-5HA] is reversed by src-1 RNAi. Gonad defects were counted for the respective strains. Two distinct gonad defects, a defect on first gonadal turn and a defect on the first and second gonadal turns, were counted separately and subsequently analyzed with SPSS statistical software. The defect on the first gonadal turn was shown on the upper half of the panel, and the defect on first and second gonadal turn was shown in the lower half of the panel. The defects in the anterior and posterior arms of the gonad were counted separately. Schematic illustrations of the gonad morphologies are shown on the right. The total number of worms counted for each strain was more than 50, and error bars represent standard errors (SE). (B) The occurrence of club-shaped gonads by UNC-5 overexpression is reduced by src-1 RNAi. The club-shaped gonads for the respective strains were counted and subsequently analyzed with SPSS statistical software. A schematic illustration of the club-shaped gonad morphology is shown on the right. A club-shaped gonad of the Ex[unc-5::unc-5HA] stable line is also shown. Triangles denote the abnormal gonad shape. The total number of worms counted for each strain was more than 50, and error bars represent SE. (C) The tyrosine phosphorylation in UNC-5 protein is reduced by src-1 RNAi. The indicated worm strains were treated with src-1 or src-2 RNAi. After lysis, the samples were immunoprecipitated (IP) with anti-HA antibody and subjected to Western blotting (WB) with the antiphosphotyrosine antibody (pY).
src-1 RNAi suppresses the defects caused by unc-5 overexpression.
To confirm the src-1 RNAi effect of antagonizing the pU5HA construct, we devised another sensitized experiment. We injected a 10-fold-higher concentration of the pU5HA construct into wild-type worms in order to generate a worm with hyperactive unc-5 signaling. Consistent with a previous report (16), high expression of unc-5 under the wild-type background resulted in a club-shaped gonad phenotype (Fig. 3B). When overexpressed, UNC-5 is thought to be auto-activated independently of the UNC-6 ligand. It has been widely observed that high expression of a transmembrane receptor could result in the formation of a receptor complex on the cell surface without ligand binding. These auto-activated receptors do not have the directional information normally provided by the localized ligand available. Therefore, the club-like gonad shape at the site of first gonad turn is generated and may be a representation of UNC-5 hyperactivity.
We treated these unc-5-overexpressing worms with the suboptimal src-1 RNAi and determined the percentage of club-shaped gonads. After being treated with src-1 RNAi, the unc-5-overexpressing worms tend to have a variety of gonad shapes, as expected from observations of src-1 RNAi in wild-type worms. However, the percentage of club-shaped gonads decreased significantly after the treatments (Fig. 3B). These experiments also support the notion that src-1 mediates unc-5 signaling, and a decrease of src-1 activity is able to suppress hyperactive unc-5 signaling in vivo.
src-1 RNAi reduces UNC-5 tyrosine phosphorylation in vivo.
Upon ligand binding, UNC-5 receptors are tyrosine phosphorylated, and this tyrosine phosphorylation seems to be important for the downstream events (15). In order to test whether src-1 regulates the tyrosine phosphorylation in unc-5 in vivo, we cultured the UNC-5HA-expressing worms with src-1 RNAi. After 3 days, the worms were lysed with sonication and immunoprecipitated with anti-HA antibody. The level of UNC-5 tyrosine phosphorylation was detected by Western blotting. Consistent with our model in which src-1 regulates UNC-5 phosphorylation, the level of UNC-5 tyrosine phosphorylation decreased significantly upon src-1 RNAi treatment (Fig. 3C). It is worth noting that src-1 RNAi did not completely eliminate UNC-5 tyrosine phosphorylation. This could be explained by the possibility that another kinase, such as src-2 (F49B2.5), is also involved in UNC-5 tyrosine phosphorylation. However, we prefer an alternative explanation, namely, that src-1 RNAi is not effective in neurons that also express UNC-5. Consistent with this explanation, src-2 RNAi, which does not produce any noticeable defect in worms (data not shown), had a minor effect on UNC-5 tyrosine phosphorylation. Together, our data provide strong evidence for a role for src-1 in unc-5 tyrosine phosphorylation.
The unc-5 null phenotype is rescued by a UNC-5-SRC-1 fusion construct.
So far, we reduced src-1 activity and tested its effect in unc-5 signaling. In an effort to test an effect of increased src-1 activity, we tried to generate stable worm lines containing unc-5::src-1 transcriptional fusion constructs. Despite various concentrations of the unc-5::src-1 tested, we were unable to obtain a stable line. We conclude that expression of this construct may interfere with essential developmental processes and result in a lethal phenotype.
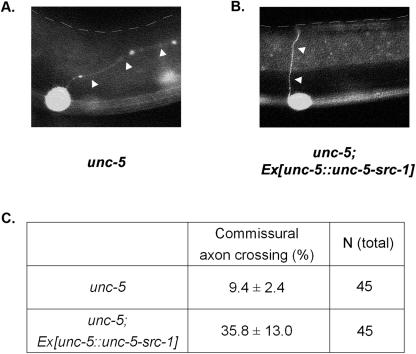
In another effort to have an src-1 construct that can be localized at the unc-5 signaling sites, we generated unc-5 and src-1 fusion constructs under the control of the unc-5 promoter (Fig. 4A). This construct is composed of the unc-5 extracellular domain, the transmembrane domain, and the UNC-5 intracellular domain replaced by SRC-1. A polyglycine linker is added between the UNC-5 portion and SRC-1, giving src-1 kinase free access to its target substrates. Expression of this UNC-5-SRC-1 fusion protein produced little effect in the wild-type N2 worms. However, it rescued a variety of defects in unc-5 null worms. For Fig. 4B, two pictures were taken separately at a 4-second interval and then combined using image-processing software. The unc-5 null mutant was almost paralyzed compared to the wild-type worm. However, unc-5 null mutant worms containing the unc-5-src-1 fusion construct became as mobile as the wild type. We also examined the commissural axon structure for each genotype. A commissural axon projection from the ventral to dorsal side is visualized with a GFP marker for motor neurons. Axon structure is defective in unc-5 (Fig. 5A), and its defects can be rescued by the UNC-5-SRC-1 fusion construct (Fig. 5B).
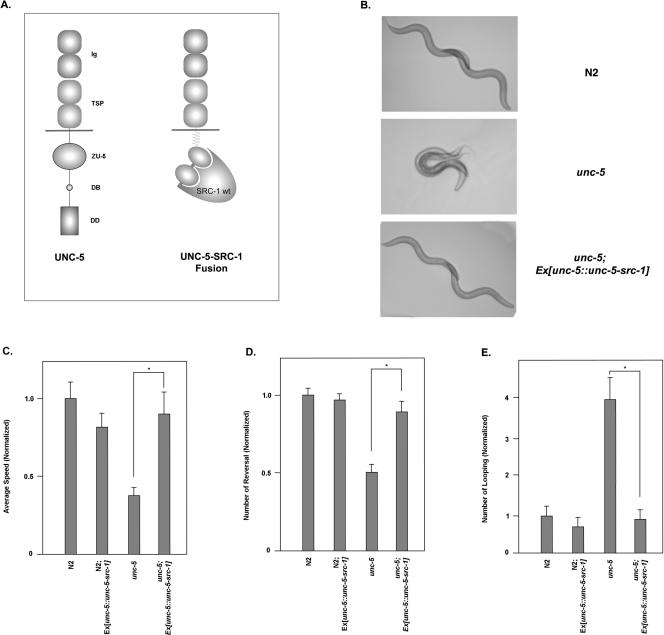
FIG. 4.
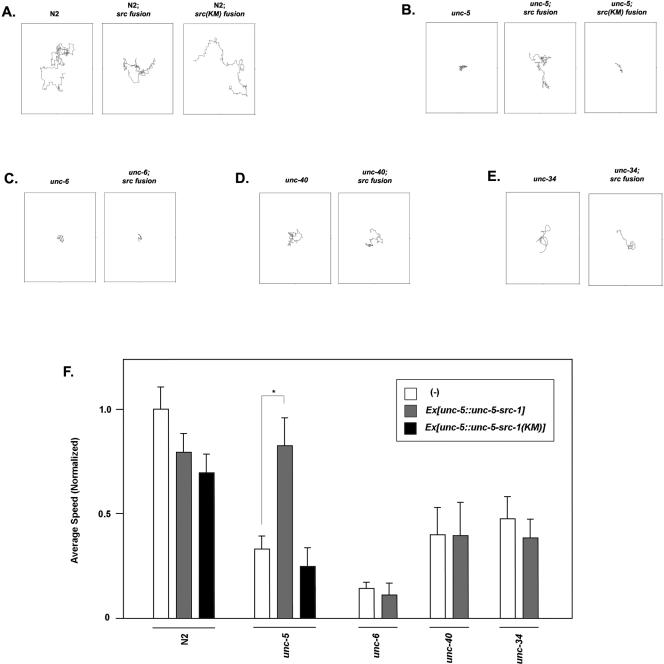
Rescue of the unc-5 null phenotype by the UNC-5-SRC-1 fusion construct. (A) Schematic diagram of UNC-5 and the UNC-5-SRC-1 fusion protein. UNC-5 is composed of two immunoglobulin domains (Ig), two thrombospondin domains (TSP), a transmembrane domain, a ZO-1/Unc-5 domain (ZU-5), a DCC binding domain (DB), and the death domain (DD). UNC-5-SRC-1 fusion protein contains the extracellular and transmembrane domain of UNC-5, a glycine linker region, and SRC-1 protein. (B) The UNC-5-SRC-1 fusion construct is able to rescue the unc-5 null mutant. For each of the indicated genetic backgrounds, two pictures were taken at the 0- and 4-s time points. Subsequently, the Adobe Photoshop program was used to combine two images. (C to E) The unc-5 null defect is rescued by the UNC-5-SRC-1 fusion construct. Average speeds (C), numbers of reversals (D), and numbers of looping (E) of the respective strains were monitored for 5 min. An individual worm was tracked with Tracker System, and the collected data were subsequently processed with SPSS statistical software. For the indicated strains, at least five individual worms are analyzed under the tracker system. The total number of worms counted for each strain was more than 5, and error bars represent SE. Asterisks on each panel indicate that two data points have a significant difference based on the Student t test.
FIG. 5.
The UNC-5-SRC-1 fusion protein is able to rescue the motor neuron defect caused by the unc-5 mutation. (A and B) Commissural axon structure for unc-5(e53) (A) and the unc-5(e53) line with the fusion construct (B) are shown. The axon structure is visualized with the plx-2::GFP construct. The plx-2 promoter expresses GFP protein on B-type motor neurons. White arrows denote commissural axons for motor neurons. The anterior side is to the left, and the dorsal side is on top. The dotted line indicates the dorsal edge of worms. (C) Percentages of proper commissural axon crossing for the respective genotypes were shown along with standard deviations. Total numbers of worms observed are also shown. wt, wild type.
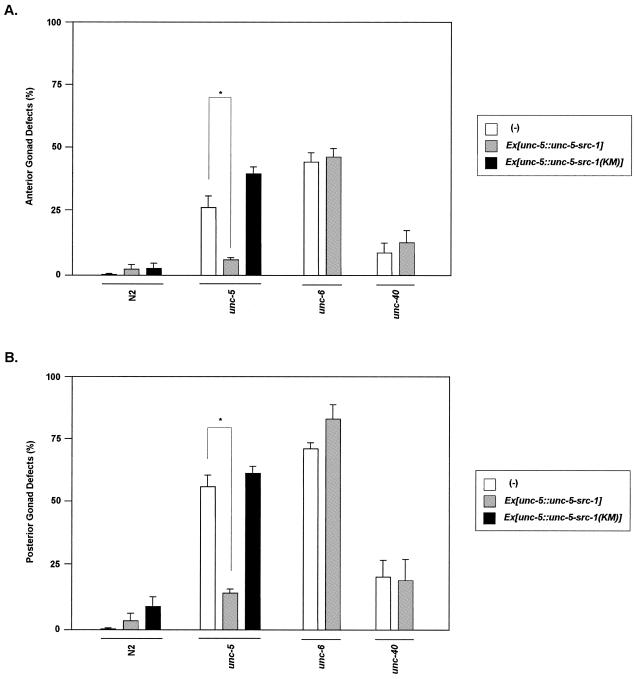
We used a behavior analysis system (tracker system [5]) trying to quantify the worm movement in a more precise way. After being collected with the tracker system, data were processed with statistical software (Fig. 4C to E). The average speed, frequency of reversal movement, and frequency of looping were recovered almost to the wild-type level with the UNC-5-SRC-1 fusion protein. Student t tests were used to confirm that differences between gonads with and without the fusion proteins are significant. We also looked at gonad rescue in unc-5. The unc-5 defects in both the anterior and posterior gonad decreased dramatically in the presence of the UNC-5-SRC-1 fusion protein (Fig. 7).
FIG. 7.
The UNC-5-SRC-1 fusion construct rescues the gonad migration defect caused by unc-5 but not unc-6 or unc-40 mutants. Anterior (A) and posterior (B) gonad defects of the respective strains are counted. The total number of worms counted for each strain was more than 150, and error bars represent SE. The asterisk indicates that two data points have a significant difference based on the Student t test.
To determine whether the kinase activity of src-1 is required for its ability to rescue the unc-5 mutant, we constructed an unc-5-src-1(KM) mutant fusion construct. This mutant fusion construct was unable to rescue the unc-5 mutant defect (Fig. 6). Therefore, src-1 kinase activity is important for the proper signaling downstream of unc-5. This result supports the idea that the UNC-5-SRC-1 fusion proteins can replace UNC-5 in vivo. It also indicates that one of the in vivo roles of UNC-5 is to recruit SRC-1 to the site of UNC-6 ligand action.
FIG. 6.
The UNC-5-SRC-1 fusion construct rescues the movement defect caused by unc-5 but not unc-6, unc-40, or unc-34 mutants. (A to E) The movement path of each individual worm was followed for 5 min. The respective genetic backgrounds were denoted at the top of each panel. Worms were tracked with the tracker system and subsequently analyzed with SPSS statistical software. (F) Statistical representation of the effects of the UNC-5-SRC-1 fusion or UNC-5-SRC-1(K290M) fusion on different Unc mutants. At least five individual worms from the respective genetic backgrounds were tracked and subsequently analyzed with SPSS statistical software. The total number of worms counted for each strain was more than 5, and error bars represent SE. The asterisk indicates that two data points have a significant difference based on the Student t test.
The UNC-5-SRC-1 fusion protein behaves differently in different genetic backgrounds.
To further investigate the mechanism of UNC-5-SRC-1 fusion protein, we investigated how it behaves under different genetic backgrounds, including the unc-6, unc-40, and unc-34 backgrounds. In order to have the same extrachromosomal array on different genetic backgrounds, we used mating to generate the necessary strains. First, a wild-type transgenic worm line was established with the fusion construct. The transgenic worms were mated with different genetic backgrounds. Subsequently, the desired genotypes with the fusion construct were selected. Finally, two major categories were observed: movement defects (Fig. 6) and gonad defects (Fig. 7).
We found that unc-6 could not be rescued by the UNC-5-SRC-1 fusion construct. UNC-6 is the ligand for UNC-5 and provides the directionality to UNC-5. The lack of UNC-6 would have a significant effect on targeting the UNC-5-SRC-1 fusion protein at the site of repulsion. The fusion proteins would be evenly distributed in the unc-6 mutant worms because of the lack of signaling molecules. Although SRC-1 activity is intact, the even distribution of the fusion proteins could not execute its directional information, which is lacking in the unc-6 mutant.
Mutation of unc-40, which forms a complex with unc-5 as the functional repulsive netrin receptor, was not rescued by the UNC-5-SRC-1 fusion protein. This is consistent with observations made in mammalian netrin signaling. Src family kinases are involved in UNC-40/DCC tyrosine phosphorylation. Therefore, it is likely that tyrosine phosphorylation of UNC-40 is involved in unc-6/netrin signaling. Without UNC-40 protein, the unc-6 signaling pathway cannot be rescued even if UNC-5-SRC-1 fusion proteins were recruited to the right place, because one of the key downstream targets of SRC-1, UNC-40, is absent. Furthermore, UNC-40 itself may recruit other proteins, and this function cannot be replaced by UNC-5-SRC-1 fusion proteins.
unc-34 is an Enabled homologue in worms (27) and thought to be a link between the receptors and actin filaments. The UNC-5-SRC-1 fusion construct did not rescue the defects in unc-34. This is consistent with the model that unc-34 is a further downstream component from the receptor in unc-5 axon repulsion signaling. Activation of src-1 cannot rescue the defects caused by mutation of downstream genes. These data support the idea that src-1 is a receptor-proximal component of unc-5 signaling, and localization of src-1 activity is a key event in controlling signal transduction.
DISCUSSION
Netrin is a well-characterized axon guidance molecule. A growth cone can be either attracted toward or pushed away from the netrin source depending on the expression of receptors (9). This process must be reversible and well balanced, because a mild change of cyclic AMP or Ca2+ concentration at the growth cone can reverse the effect of netrin (10, 23). Existing evidence suggests that netrin is also involved in nonneuronal tissue development. Extensive efforts have been made to identify components of the netrin signaling pathway and also to comprehend how these components achieve proper signal transduction. Some of the downstream effectors for attraction (6, 18, 19, 25) and repulsion (4) have been recently elucidated. However, the whole picture is far from complete. Here, we provide evidence that the unc-5-mediated axon repulsion process requires SRC-1 activity.
We have shown that SRC-1 and the cytosolic domain of UNC-5 physically interact. This interaction requires SRC-1 kinase activity, the intact SRC-1 SH2 domain, and UNC-5 tyrosine phosphorylations. We have also shown that tyrosine phosphorylations on UNC-5 are mediated by SRC-1 kinase both in vivo and in vitro.
Not only do SRC-1 and UNC-5 interact physically, they also interact functionally. An UNC-5-SRC-1 fusion protein is able to rescue an unc-5 mutation in C. elegans. In wild-type animals, UNC-5 is activated asymmetrically along the UNC-6 gradient on the cell surface. This asymmetric activation of UNC-5 ensures the growth cone turning or directionality of DTC migration. Since the proper targeting of SRC-1 activity is enough to rescue unc-5 mutant animals, it is tempting to speculate that a major role of UNC-5 could be to concentrate SRC-1 at the growth cone turning site. The UNC-5-SRC-1 fusion protein may require UNC-6 for proper directionality in vivo. The inability of the UNC-5-SRC-1 fusion protein to rescue unc-6 is also consistent with this idea. These results suggest that the asymmetric activation of the UNC-5 receptor is very important for the proper guidance process, and evenly distributed SRC-1 activity cannot direct the proper turning response.
It has been reported recently that DCC, the mammalian homolog of UNC-40, interacts with FAK and SRC, and this interaction is crucial to the netrin-mediated attraction response (18, 19, 25). Therefore, SRC-1 is not only important for repulsion but also important for attraction. Activation of SRC-1 may result in the opposite effect on growth cone turning, depending on what downstream factors/substrates are available. UNC-5 and UNC-40 receptors may recruit other downstream molecules in addition to SRC-1. Even though SRC-1 is the common activator, the differences in recruited molecules may decide the final effect of repulsion or attraction.
Currently, there is no direct evidence to speculate what might be the targets of SRC-1 kinase other than the netrin receptors. However, the importance of tyrosine phosphorylation in netrin signaling is further supported by a recent report that clr-1, a receptor protein tyrosine phosphatase, is involved in netrin signaling (3). A loss-of-function mutation in clr-1 enhances netrin-dependent attraction. These studies further support the importance of tyrosine phosphorylation in netrin signaling. UNC-34 is reported to be a downstream of CLR-1 and also involved in both repulsion and attraction (4, 6).
UNC-34 may be regulated by phosphorylation, possibly phosphorylated by SRC-1, and dephosphorylated by CLR-1. This hypothesis is consistent with the idea that UNC-5-SRC-1 fusion protein cannot rescue the unc-34 mutant. UNC-40 may also facilitate SRC-1 recruitment to the UNC-5/UNC-40 receptor complex and enhance the increased tyrosine phosphorylations on UNC-5. These phosphorylation sites generate the binding sites for SRC-1 itself. Hence, the initial interaction of SRC-1 with the UNC-5/UNC-40 complex can increase the SRC-1 concentration near the receptor complex and may result in signal amplification. Subsequently, SRC-1 can phosphorylate the other proteins recruited by the receptors, possibly including UNC-34.
SRC-1 is involved in many other signaling pathways; therefore, it is interesting to consider the possible cross talk between signaling pathways. Among many signaling pathways, integrin signaling is particularly intriguing. In one recent study, laminin-1, a major component of basement membrane assembly, is shown to convert netrin-mediated attraction into repulsion (11). Those authors demonstrated that extracellular matrix molecules can modify the growth cone response toward diffusible guidance cues. Our data showed that localized activation of SRC-1 can replace the need for UNC-5 on axon repulsion. Therefore, it is tempting to speculate that the effect of laminin-1 on axon repulsion may be mediated through the integrin receptor and the subsequent activation of SRC-1. The integrin receptor can activate the SRC-1 subpopulation locally, and activated SRC-1 may convert UNC-40 into a repulsive receptor. Since netrin associates with the laminin network (29), the two ligands may work together in vivo. Studies of Drosophila melanogaster also indicated that integrin regulates the responsiveness of axons to another guidance molecule, Slit (28). The shared downstream effectors of integrin and UNC-5 signaling and potential regulation of netrin signaling by integrin suggest that integrin and UNC-5 signaling may be convergent and cooperative.
Acknowledgments
We thank Min Han (University of Colorado at Boulder) for critical reading of the manuscript and valuable suggestions. We also thank John Feng and Shawn Xu (University of Michigan) for their help and expertise on the worm tracking system. We thank Theresa Stiernagle (Caenorhabditis Genetics Center) for providing C. elegans strains and Joseph G. Culotti (University of Toronto) for the generous gift of the unc-5 construct. We also appreciate the help and kindness of W.L. Yen.
This work was supported by grants from the National Institutes of Health (K.L.G.).
REFERENCES
- 1.Bei, Y., J. Hogan, L. A. Berkowitz, M. Soto, C. E. Rocheleau, K. M. Pang, J. Collins, and C. C. Mello. 2002. SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev. Cell 3:113-125. [DOI] [PubMed] [Google Scholar]
- 2.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, C., T. W. Yu, C. I. Bargmann, and M. Tessier-Lavigne. 2004. Inhibition of netrin-mediated axon attraction by a receptor protein tyrosine phosphatase. Science 305:103-106. [DOI] [PubMed] [Google Scholar]
- 4.Colavita, A., and J. G. Culotti. 1998. Suppressors of ectopic UNC-5 growth cone steering identify eight genes involved in axon guidance in Caenorhabditis elegans. Dev. Biol. 194:72-85. [DOI] [PubMed] [Google Scholar]
- 5.Feng, Z., C. J. Cronin, J. H. Wittig, Jr., P. W. Sternberg, and W. R. Schafer. 2004. An imaging system for standardized quantitative analysis of C. elegans behavior. BMC Bioinformatics 5:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gitai, Z., T. W. Yu, E. A. Lundquist, M. Tessier-Lavigne, and C. I. Bargmann. 2003. The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron 37:53-65. [DOI] [PubMed] [Google Scholar]
- 7.Hanke, J. H., J. P. Gardner, R. L. Dow, P. S. Changelian, W. H. Brissette, E. J. Weringer, B. A. Pollok, and P. A. Connelly. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271:695-701. [DOI] [PubMed] [Google Scholar]
- 8.Harris, R., L. M. Sabatelli, and M. A. Seeger. 1996. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron 17:217-228. [DOI] [PubMed] [Google Scholar]
- 9.Hong, K., L. Hinck, M. Nishiyama, M. M. Poo, M. Tessier-Lavigne, and E. Stein. 1999. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97:927-941. [DOI] [PubMed] [Google Scholar]
- 10.Hong, K., M. Nishiyama, J. Henley, M. Tessier-Lavigne, and M. Poo. 2000. Calcium signalling in the guidance of nerve growth by netrin-1. Nature 403:93-98. [DOI] [PubMed] [Google Scholar]
- 11.Hopker, V. H., D. Shewan, M. Tessier-Lavigne, M. Poo, and C. Holt. 1999. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature 401:69-73. [DOI] [PubMed] [Google Scholar]
- 12.Huber, A. B., A. L. Kolodkin, D. D. Ginty, and J. F. Cloutier. 2003. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 26:509-563. [DOI] [PubMed] [Google Scholar]
- 13.Ishii, N., W. G. Wadsworth, B. D. Stern, J. G. Culotti, and E. M. Hedgecock. 1992. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron 9:873-881. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy, T. E., T. Serafini, J. R. de la Torre, and M. Tessier-Lavigne. 1994. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78:425-435. [DOI] [PubMed] [Google Scholar]
- 15.Killeen, M., J. Tong, A. Krizus, R. Steven, I. Scott, T. Pawson, and J. Culotti. 2002. UNC-5 function requires phosphorylation of cytoplasmic tyrosine 482, but its UNC-40-independent functions also require a region between the ZU-5 and death domains. Dev. Biol. 251:348-366. [DOI] [PubMed] [Google Scholar]
- 16.Kruger, R. P., J. Lee, W. Li, and K. L. Guan. 2004. Mapping netrin receptor binding reveals domains of Unc5 regulating its tyrosine phosphorylation. J. Neurosci. 24:10826-10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauderdale, J. D., N. M. Davis, and J. Y. Kuwada. 1997. Axon tracts correlate with netrin-1a expression in the zebrafish embryo. Mol. Cell. Neurosci. 9:293-313. [DOI] [PubMed] [Google Scholar]
- 18.Li, W., J. Lee, H. G. Vikis, S. H. Lee, G. Liu, J. Aurandt, T. L. Shen, E. R. Fearon, J. L. Guan, M. Han, Y. Rao, K. Hong, and K. L. Guan. 2004. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat. Neurosci. 7:1213-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, G., H. Beggs, C. Jurgensen, H. T. Park, H. Tang, J. Gorski, K. R. Jones, L. F. Reichardt, J. Wu, and Y. Rao. 2004. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat. Neurosci. 7:1222-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, Y., E. Stein, T. Oliver, Y. Li, W. J. Brunken, M. Koch, M. Tessier-Lavigne, and B. L. Hogan. 2004. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr. Biol. 14:897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu, X., F. Le Noble, L. Yuan, Q. Jiang, B. De Lafarge, D. Sugiyama, C. Breant, F. Claes, F. De Smet, J. L. Thomas, M. Autiero, P. Carmeliet, M. Tessier-Lavigne, and A. Eichmann. 2004. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature 432:179-186. [DOI] [PubMed] [Google Scholar]
- 22.Mello, C., and A. Fire. 1995. DNA transformation. Methods Cell Biol. 48:451-482. [PubMed] [Google Scholar]
- 23.Ming, G. L., H. J. Song, B. Berninger, C. E. Holt, M. Tessier-Lavigne, and M. M. Poo. 1997. cAMP-dependent growth cone guidance by netrin-1. Neuron 19:1225-1235. [DOI] [PubMed] [Google Scholar]
- 24.Park, K. W., D. Crouse, M. Lee, S. K. Karnik, L. K. Sorensen, K. J. Murphy, C. J. Kuo, and D. Y. Li. 2004. The axonal attractant Netrin-1 is an angiogenic factor. Proc. Natl. Acad. Sci. USA 101:16210-16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren, X. R., G. L. Ming, Y. Xie, Y. Hong, D. M. Sun, Z. Q. Zhao, Z. Feng, Q. Wang, S. Shim, Z. F. Chen, H. J. Song, L. Mei, and W. C. Xiong. 2004. Focal adhesion kinase in netrin-1 signaling. Nat. Neurosci. 7:1204-1212. [DOI] [PubMed] [Google Scholar]
- 26.Serafini, T., T. E. Kennedy, M. J. Galko, C. Mirzayan, T. M. Jessell, and M. Tessier-Lavigne. 1994. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78:409-424. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui, S. S., and J. G. Culotti. 1991. Examination of neurons in wild type and mutants of Caenorhabditis elegans using antibodies to horseradish peroxidase. J. Neurogenet. 7:193-211. [DOI] [PubMed] [Google Scholar]
- 28.Stevens, A., and J. R. Jacobs. 2002. Integrins regulate responsiveness to slit repellent signals. J. Neurosci. 22:4448-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yurchenco, P. D., and W. G. Wadsworth. 2004. Assembly and tissue functions of early embryonic laminins and netrins. Curr. Opin. Cell Biol. 16:572-579. [DOI] [PubMed] [Google Scholar]