Abstract
A serine/threonine kinase, Melk, was initially cloned in mouse oocytes as a maternal gene, but whose function was unknown. In adult mice, Melk was strongly expressed in the thymus and bone marrow, suggesting a role for Melk in hematopoiesis. We cloned a Melk-like gene from zebra fish (zMelk). zMelk-like gene was expressed in the brain and lateral mesoderm at 12 hours postfertilization (hpf) and in several tissues of adult fish, including the kidney and spleen, both of which are known to be hematopoietic tissues in zebra fish. Abrogation of zMelk-like gene function by zMelk-like gene-specific Morpholino (MO) resulted in abnormal swelling around the tectum region. In addition, the start of blood circulation was severely delayed but, in contrast, the vessel formation seemed normal. Expression of scl, gata-1, and lmo-2 was down regulated at 12 to 14 hpf in the zMelk-like gene MO-injected embryos, and the coexpression of gata-1 rescued the anemic phenotype induced by zMelk-like gene MO. Expression of the zMelk-like gene in embryos enhanced gata-1 promoter-dependent enhanced green fluorescent protein expression, suggesting that the zMelk-like gene affects gata-1 expression at the transcriptional level. Taken together, our data suggest that the zMelk-like gene may play a role in primitive hematopoiesis by affecting the expression of genes critical for hematopoiesis.
By using the PCR-based differential display technique on the eyes and brains of mice of various stages, we cloned a large number of mouse genes and screened them in terms of their primary sequences and expression patterns. The clone described in this work, DD29, is unique, since it encodes a protein kinase having a zinc finger domain. The full-length DD29 turned out to be identical with the previously reported gene Melk (19), which is a member of the sucrose nonfermenting protein kinase (SNF1)/AMPK serine/threonine kinase family (16). Melk was originally identified by differential display to isolate maternal cDNA from unfertilized eggs and preimplantation embryos (19). The expression pattern of Melk during the maturation of oocytes and preimplantation development was typical of that described for maternally expressed genes, including β-actin and E-cadherin (19). Therefore, Melk is surmised to play a role in transducing signals in embryonic cells during preimplantation, but its exact roles in development were not revealed (18). On the other hand, a Melk equivalent gene, Eg3, was cloned from a murine teratocarcinoma cell line (12), and predominant expression of Melk in hematopoietic organs such as the thymus and spleen and several hematopoietic cell lines was reported (12). However, the function of Melk in hematopoiesis has remained unknown. Induction of Melk transcription by T-cell activation was reported (11). So far, five members belonging to the SNF1/AMPK family of mammalian kinases have been reported. SNF1 was originally identified in Saccharomyces cerevisiae and is thought to play a role in the expression of glucose-repressible genes (4). Subsequent studies revealed that the mammalian members of this family also play a role in protecting cells from environmental stress, hypoxia, heat shock, and ischemia (16, 34).
To examine the biological functions of Melk, we took the strategy of using the zebra fish (Danio rerio; z), for this fish is an excellent model vertebrate for the analysis of central nervous system development for many practical reasons, including the high similarity of its genetic and structural organization with that of other vertebrates, including mammals. In addition, another advantage of using the zebra fish is that this animal can be used in Morpholino (MO) antisense oligo-based knockdown experiments (17). In fact, in our present study, we found zebra fish embryos to develop severe anemia when they were injected with MO against the zMelk-like gene. The zebra fish is assumed to be a great tool for understanding normal and disease-related hematopoiesis. Hematopoiesis occurs in sequential waves in vertebrates including zebra fish (38), and striking similarities between zebra fish mutant (mt) phenotypes and human diseases have been reported (1). Furthermore, genes critical for normal mammalian hematopoiesis have been found, and they play a similar role in zebra fish.
In this work, we cloned the zebra fish Melk-like gene. zMelk-like gene was expressed in neural and other tissues, which was similar to that of the mouse Melk (mMelk) expression pattern. We did functional analysis of Melk by using the zebra fish. Unexpectedly, knockdown of the zMelk-like gene by MO oligonucleotides did not affect early embryogenesis, as previously surmised from the expression pattern of mammalian Melk (18). However, we found that disruption of the zMelk-like gene function did affect blood cell development.
MATERIALS AND METHODS
Animals.
Zebra fish (Danio rerio) were purchased from a local pet shop and maintained under a 14-h day, 10-h night cycle at 28.5°C. Embryos were staged according to hours postfertilization (hpf) and morphological criteria (22).
Cloning, construction of DNA and RT-PCR assay.
The zMelk-like gene was cloned by reverse transcription-PCR (RT-PCR) using primers designed based on the sequences of GenBank expressed sequence tag (EST) fragments (BM859334, AW165111), which are homologous to the mMelk sequence. Full-length zMelk-like gene (AB108827) was subcloned from pGEM-T easy vector into pCS2+ vector by using BamHI and EcoRI sites. A mutant zMelk-like gene having alternate codon sequences, zMelk-like 5′mt, was constructed by PCR mutagenesis. A total of 7 nucleotides were substituted as follows: in the original sequence, TTC AGT ATG CCA GTA GAC AGC ACT TCT; in the mutant sequence (mutated nucleotides are underlined), TTC AGC ATG CCT GTC GAT TCT ACT TCT. The initiation codon is expressed in italics. Then, the mt zMelk-like gene was subcloned into pCS2+ vector and subjected to RNA synthesis. zMelk-like genes having a point mutation at threonine 169 (T169A and T169E) were made by PCR mutagenesis. One or two nucleotides were substituted as follows: in the original sequence, ACA (threonine); in mutant sequences (mutated nucleotides are underlined), GCA (alanine, T169A) and GAA (glutamic acid, T169E). Then, the zMelk-likeT169A and zMelk-likeT169E genes were subcloned into pCS2+ vector and subjected to RNA synthesis. Semiquantitative RT-PCR to examine the expression pattern of the zMelk-like gene was done by using mRNA or total RNA extracted from various zebra fish tissues. The amplified transcripts, primer sequences (upstream and downstream), and the length of amplified fragments were as follows: zebra fish Melk-like gene, 5′-TAACAACGTAACCGATTGTAT-3′ and 5′-TACTGTACTACCTTCCTGCA-3′, and 2,130 bp. The conditions for PCR of zebra fish Melk were as follows: 94°C for 1 min, 56°C for 1 min, and 72°C for 3 min for 35 cycles. Full-length zebra fish scl and lmo-2 for injection were cloned by RT-PCR according to the published sequence and subcloned into pCS2+ vector and subjected to RNA synthesis. Full-length zebra fish gata-1 in pCS2+ vector and zebra fish gata-1 promoter enhanced green fluorescent protein (EGFP) were provided by Kobayashi and Yamamoto (Tsukuba University) (23).
RH and genetic mapping.
The genetic map position of the zMelk-like gene was determined by using the LN54 collection of the radiation hybrid (RH) panel (21) which was kindly provided by the Ottawa Health Research Institute. The zMelk-like gene was mapped onto this panel by PCR using the primers 5′-CCTCAAGCACTATGAGGTTTA-3′ (forward) and 5′-ATGTAGATTTTGCTTGTGGTCT-3′ (reverse).
Whole-mount in situ hybridization.
Probes for the scl, gata-1, myoD, sonic hedgehog (shh), fli-1, lmo-2, cdx-4, hoxb6b, c-myb, tie-1, and flk-1 genes were cloned by PCR amplification using the primers designed according to their published sequences. PCR products were subcloned into the pGEM-T easy vector, and digoxigenin (DIG)-labeled sense and antisense RNAs were made by using a DIG-RNA labeling kit (SP6/T7; Roche Diagnostics GmbH, Mannheim, Germany). Whole-mount in situ hybridization was done as described previously except for several minor changes in the protocol (24, 25). Briefly, embryos were fixed in 4% paraformaldehyde (PFA) and stored in methanol. Rehydrated embryos were incubated in 10 μg/ml of proteinase K for 5 min and refixed in 4% PFA for 20 min. Then they were treated with cold acetone for 8 min at −20°C, after which hybridization was done overnight. Embryos were blocked with 2% blocking reagent (Roche Diagnostics GmbH, Mannheim, Germany) consisting of 5% lamb serum, 0.1 M maleic acid (pH 7.5), and 150 mM NaCl and then incubated with anti-DIG-alkaline phosphatase (Roche Diagnostics GmbH, Mannheim, Germany). The enzyme activity was visualized with nitroblue tetrazolium 5-bromo-4-chloro-3-indolylphosphate (BCIP) AP substrate solution (Roche Diagnostics GmbH, Mannheim, Germany). Photographs were taken with a Zeiss Axio Cam attached to a Leica (Vienna, Austria) MZFL III dissecting microscope.
Microinjection of MO or RNA into zebra fish embryos.
Two MO antisense oligonucleotides (GENE-TOOLS, LLC, Philomath, OR) were designed against 25 bases around the AUG translation initiation codon of the zMelk-like gene. The MO sequences were 5′-TATGCCAGTAGACAGCACTTCTGAA-3′ (start codon is underlined) and 5′-CATCATTGGAGCACAGTTGGTGTAT-3′. The MO was diluted to 0.5 mM with 1× Danieau buffer [58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0 mM HEPES; pH 7.6], and 0.05% phenol red was included as a microinjection control. Fertilized eggs from wild-type zebra fish at the one-cell stage were injected with approximately 1 to 3 nl of DNA solution by using a microinjector (IM-300; Narishige, Tokyo, Japan). Standard control MO, which was available from the same manufacturer, was used as an injection control and had no effect on embryonic development under our experimental conditions. The microinjected embryos were examined under a Leica MZFL III microscope. For RNA injection, capped sense RNAs were synthesized by using an mMessage mMachine in vitro transcription kit (Ambion, Austin, TX) according to the manufacturer's instructions. The synthesized RNAs were diluted to an appropriate concentration with RNase-free water and injected into one- or two-cell-stage embryos.
Light microscopy.
Embryos of appropriate stages were dechorionated and fixed overnight at 4°C in a solution containing 1% glutaraldehyde, 2% formaldehyde, and 0.1 M sodium phosphate (pH 7.4). The samples were washed in 4% sucrose and then fixed in 1% osmium tetroxide in the same buffer for 1 h on ice. They were then dehydrated in a graded series of ethanol and embedded in Epon 812 resin mixture (TAAB Laboratories Equipment Ltd., Berks, United Kingdom). Each block was cut into 0.7-μm-thick sections, which were then stained with toluidine blue and observed under a Zeiss axioplan microscope.
Immunohistochemistry.
Embryos of appropriate stages in frozen OCT were sectioned at a 10-μm thickness and fixed with 4% PFA for 30 min at room temperature. Then the sections were blocked with 2% bovine serum albumin in phosphate-buffered saline for 1 h at room temperature and incubated with histone H3 antibody (Molecular Probes) in blocking solution overnight at 4°C. Antibody was visualized by using a Vectastain Elite ABC mouse immunoglobulin G kit (Vector Laboratories, Inc.) and 3,3′-diaminobenzidine tetrahydrochloride (DAB).
Microangiography, hemoglobin staining, and heart rate.
Microangiography was done as described previously (29). Fluorescein isothiocyanate (FITC)-dextran with a molecular mass of 2,000 kDa (Sigma, St. Louis, MO) was suspended in 1× Danieau buffer at 2 mg/ml. About 3 nl of the prepared solution was injected into the sinus venosa/cardinal vein of the anesthetized embryos at various stages (24, 32, 48, and 54 hpf). Photographs were taken with a MZFL III microscope using a standard FITC filter set. Hemoglobin staining was done as follows. Embryos were treated for 15 min in 40% ethanol containing 0.6 mg/ml o-dianisidine (ICN), 0.01 M sodium acetate (pH 4.5), and 0.65% hydrogen at room temperature in the dark. Then, the reaction was stopped and the embryos were dehydrated by being washed twice with 40% ethanol. The heart rates were counted upon visual inspection of unanesthetized embryos at room temperature (25°C).
RESULTS
Isolation of the zebra fish Melk-like gene with an expression pattern similar to that of mMelk.
By differential display using mouse neural tissues, we cloned a serine/threonine kinase, Melk, as a gene expressed in mouse embryonic neural tissues. mMelk was originally cloned in oocytes as a maternal gene, but whose function was unknown (12, 19). To examine its functions using zebra fish (Danio rerio) as a model system, we then searched an adult zebra fish EST database (GenBank, National Center for Biotechnology Information) for sequences homologous with the mMelk sequence. Two EST sequences, BM859334 and AW165111, which had strong similarities with the respective N and C termini of mMelk, were found. A full-length zebra fish Melk-like clone (AB108827) was obtained from adult zebra fish ovary-derived mRNA by RT-PCR with primers designed based on these EST fragments. RH mapping assigned the zMelk-like gene to linkage group 1, and conserved syntenic genes were not found by comparative analysis with regions of mMelk, which maps to 4B1, and of human Melk (hMelk), which maps to 9p13.1. Thus, we could not conclude from this information whether the zMelk-like gene is a zebra fish orthologue of mammalian Melk or not.
The deduced amino acid sequence of the zMelk-like gene encoded 677 amino acids, and C-terminal and N-terminal regions had more than 70% homology at the nucleotide level with those regions of mMelk and hMelk. In addition, the putative Xenopus egg-specific protein kinase gene Eg3 (31), which was identified by differential screening of genes expressed in the unfertilized eggs, also had significant homology with the zMelk-like gene in both C and N termini (Fig. 1A). Protein sequence alignment between the zMelk-like gene and mMelk showed conserved sequences of the N-terminal kinase domain and C-terminal region at the amino acid level (Fig. 1B). We could not find any significant homology with other genes or motifs in the central region of the zMelk-like gene by database searching. The N-terminal region (residues 13 to 268) contained the putative catalytic domain of protein serine threonine kinases (13, 14). The catalytic domain consisted of 12 subdomains typical of serine/threonine kinase, and this region was highly conserved among mMelk, the zMelk-like gene, Eg3, and hMelk. A leucine zipper motif consisting of a periodic repetition of leucine every seventh residue was found within subdomain VIII of the catalytic domain of mMelk and hMelk, but only an incomplete leucine zipper was found in the zMelk-like gene and in Eg3 (19). In the mMelk and zMelk-like kinases, there was a conserved threonine residue (Thr-167 in mMelk and Thr-169 in the zMelk-like gene) within kinase subdomain VIII. These threonines seem to be equivalent to Thr-197 of protein kinase A, which is known to be an autophosphorylation site (15, 33), suggesting Thr-167 and Thr-169 to be potential autophosphorylation sites of mMelk and zMelk-like genes, respectively. In addition, the periodic repeat of Q (glutamine) every 8 or 9 amino acids was found in the C-terminal region in both the zMelk-like gene and mMelk.
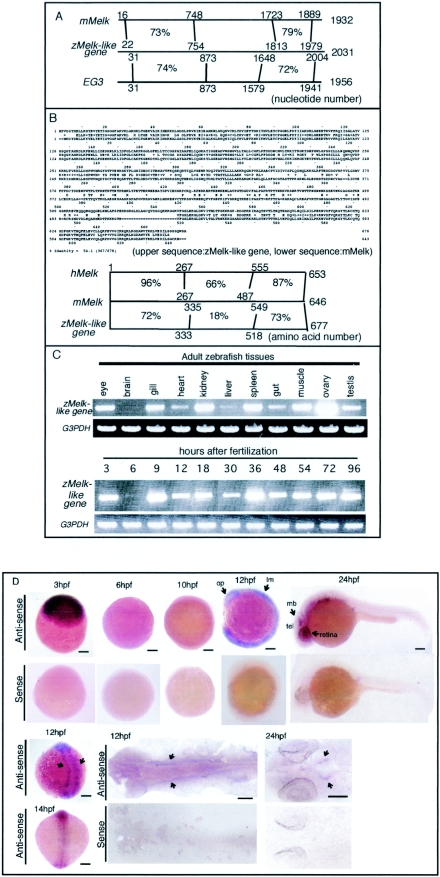
FIG.1.
Expression of zMelk-like gene during zebra fish embryonic development. (A) Schema showing similarity at the nucleotide level among mMelk, zMelk-like gene, and Xenopus EG3. (B) Comparison of zMelk-like gene and mMelk in amino acid sequences. Schema showing similarity at the amino acid among hMelk, mMelk, and zMelk-like gene. (C) Semiquantitative RT-PCR analysis of zMelk-like gene mRNA expression. Total RNA was isolated from various tissues of adult zebra fish (upper panel), and various developmental stages of whole embryos (lower panel). zMelk-like gene transcripts were amplified by using specific primers. G3PDH was used as a control in both panels. (D) Whole-mount in situ hybridization patterns of zMelk-like gene expression during zebra fish embryonic development. Zebra fish embryos at various stages were hybridized with antisense or sense probes. Upper panels are expression of zMelk-like gene at 3, 6 hpf (lateral view, animal pole to top), 12 hpf (lateral view, dorsal to right), and 24 hpf (lateral view, anterior to left): and bottom panels, 12 and 14 hpf (dorsal view, anterior at the top), 12 and 24 hpf (flat-mounted with anterior to left) stages. lm, lateral mesoderm; tel, telencephalon; mb, midbrain; op, optic primordium. Scale bars, 100 μm.
To examine the tissue distribution of zMelk-like gene transcripts, we then performed RT-PCR using total RNAs isolated from various tissues of the adult zebra fish (Fig. 1C). The zMelk-like gene transcripts were strongly expressed in the eye, gill, kidney, spleen, muscle, ovary, and testis and weakly in the heart, liver, and gut in the adult zebra fish. No signal was detected in the adult brain, but a strong signal was observed in the eye, which was different from the case of mMelk (data not shown). In adult fish, the kidney contains hematopoietic cells and is assumed to be equivalent to mammalian bone marrow in terms of hematopoietic function (35). Thus, the expression of Melk in hematopoietic tissues is a common feature in zebra fish and mice. We next examined the spatial distribution of zMelk-like gene mRNA in developing embryos by whole-mount in situ hybridization using DIG-labeled RNA as probes. zMelk-like gene mRNA was strongly expressed in the entire embryonic region of 3-hpf eggs (Fig. 1D). In contrast, in the shield stage embryos (around 6 hpf), only a very weak signal was found (Fig. 1D). Since zygotic gene expression in zebra fish is known to start around 4 hpf (22), the temporal transition of zMelk-like gene expression was assumed to be typical of the expression of maternal mRNA and its degradation and of newly synthesized zygotic transcription products. RT-PCR analysis of zMelk-like gene mRNA expression from different stages of embryos supported this idea (Fig. 1D). By the six-somite stage (at around 12 hpf), signals appeared in the entire embryo, but slightly stronger expression was observed in the eye primordium and lateral mesoderm of the midtrunk region than elsewhere (Fig. 1D, arrows). A dorsal view and flat-mounted samples of a 12-hpf embryo showed zMelk-like gene expression as bilateral stripes (Fig. 1D, arrows). Later, these stripes spread both rostrally and caudally. By 24 hpf, the signals had disappeared from the caudal half of the embryos and weak expression was observed in the entire anterior half of the embryo except for the brain, where relatively strong signals were found in the ventricular region of the midbrain and telencephalon and in the retina (Fig. 1D).
Ablation of zMelk-like gene function by MO antisense oligonucleotides resulted in severe anemia and slight retardation of eye development.
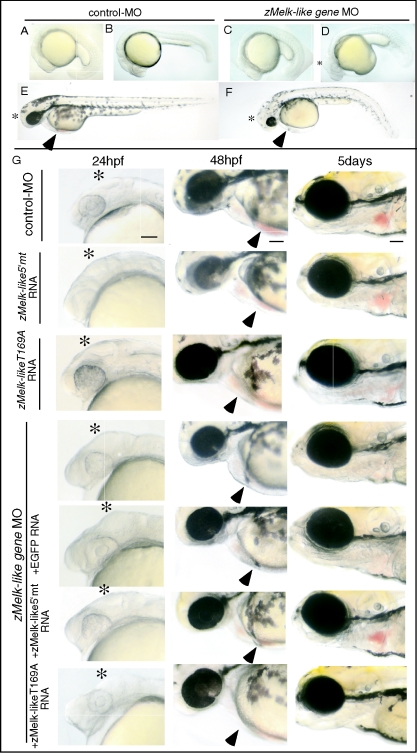
We then analyzed the roles of the zMelk-like gene in the development of these tissues by using MO antisense oligonucleotides. When MO was injected into zebra fish embryos at the one- or two-cell stage, no apparent effect was observed until 12 hpf (data not shown). Although MO is known to suppress the translation of maternal transcripts, it is notable that the ablation of Melk function in early embryogenesis did not show any obvious effects. At 24 hpf, abnormal swelling in the tectum region of the brain and small eyes were found (Fig. 2D, asterisk). These phenotypes were seen in nearly 90% (171/192) of the embryos when 6 ng of zMelk-like gene-first MO was injected (Table 1). In addition, curly and thickened tails and yolk sac extensions were observed (Fig. 2D). However, these tail and yolk sac abnormalities seemed to be nonspecific, since we observed similar effects with other unrelated MO (data not shown). At 48 hpf, the tectum region was still swollen and the eyes were slightly smaller in comparison with those of the control MO-injected embryos (Fig. 2E and F, asterisks).
FIG. 2.
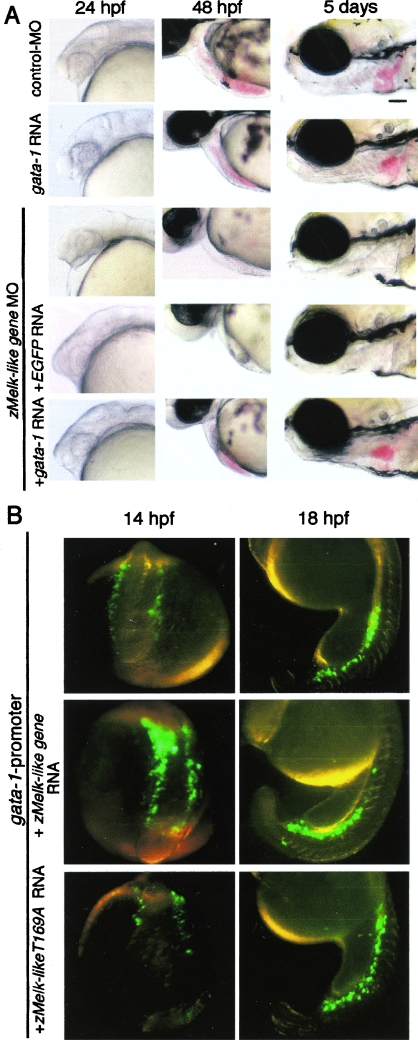
Abrogation of zMelk-like gene function by MO antisense oligonucleotide. Lateral views (anterior to left) of 21 hpf (A and C), 24 hpf (B and D), and 48 hpf (E and F) zebra fish embryos injected with control MO (A, B, and E) or zMelk-like gene MO (C, D, and F) oligonucleotides. (G) Lateral views (anterior to left) of embryos injected with either control MO, zMelk-like 5′mt RNA, zMelk-likeT169A RNA or zMelk-like gene MO are shown. Coinjection of zMelk-like gene MO and either EGFP RNA, zMelk-like 5′mt RNA, zMelk-likeT169A RNA was done and views of these embryos at 24 and 48 hpf and 5 days are shown. Scale bars, 100 μm.
TABLE 1.
Abnormal phenotypes caused by zMelk-like gene MO injection and rescue from them by zMelk-like gene mRNA, zMelk-like 5′mt RNA, or zMelk-likeT169A gene mRNA
| Material(s) injected | No. of embryos examineda | No. of embryos with normal or abnormal phenotypes (%)
|
|||
|---|---|---|---|---|---|
| Normal | Abnormalb
|
Lethal | |||
| Tectum swelling | Blood circulation | ||||
| Control MO (6 ng) | 173 | 170 (98%) | 0 (0%) | 0 (0%) | 3 (2%) |
| Control MO (9 ng) | 53 | 52 (98%) | 0 (0%) | 0 (0%) | 1 (2%) |
| zMelk-like gene RNA (300 pg) | 182 | 178 (98%) | 0 (0%) | 0 (0%) | 4 (2%) |
| zMelk-like gene 5′mt RNA (300 pg) | 68 | 67 (99%) | 0 (0%) | 0 (0%) | 1 (1%) |
| zMelk-like geneT169A RNA (300 pg) | 32 | 30 (94%) | 0 (0%) | 0 (0%) | 2 (6%) |
| zMelk-like gene first MO (6 ng) | |||||
| zMelk-like gene first MO (6 ng) | 192 | 14 (7%) | 171 (89%) | 174 (91%) | 4 (2%) |
| zMelk-like gene first MO (6 ng) + EGFP RNA (300 pg) | 120 | 9 (8%) | 107 (89%) | 108 (90%) | 3 (2%) |
| zMelk-like gene first MO (6 ng) + zMelk-like geneRNA (300 pg) | 75 | 53 (71%) | 21 (28%) | 21 (28%) | 1 (1%) |
| zMelk-like gene first MO (6 ng) + zMelk-like gene 5′mtRNA (300 pg) | 88 | 62 (70%) | 24 (27%) | 25 (29%) | 1 (1%) |
| zMelk-like gene first MO (6 ng) + zMelk-like geneT169A RNA (300 pg) | 58 | 4 (7%) | 48 (83%) | 51 (88%) | 3 (5%) |
| zMelk-like gene second MO (6 ng) | |||||
| zMelk-like gene second MO (6 ng) | 167 | 69 (41%) | 66 (40%) | 95 (57%) | 3 (2%) |
| zMelk-like gene second MO (6 ng) + EGFP RNA (300 pg) | 111 | 43 (39%) | 44 (40%) | 66 (59%) | 2 (2%) |
| zMelk-like gene second MO (6 ng) + zMelk-like geneRNA (300 pg) | 70 | 58 (83%) | 11 (16%) | 12 (17%) | 0 (0%) |
| zMelk-like gene second MO (9 ng) | |||||
| zMelk-like gene second MO (9 ng) | 154 | 14 (9%) | 66 (43%) | 137 (89%) | 3 (2%) |
| zMelk-like gene second MO (9 ng) + EGFP RNA (300 pg) | 102 | 10 (10%) | 47 (46%) | 90 (88%) | 2 (2%) |
| zMelk-like gene second MO (9 ng) + zMelk-like gene RNA (300 pg) | 70 | 52 (74%) | 11 (16%) | 18 (26%) | 0 (0%) |
One- or two-cell-stage embryos were injected with the materials indicated.
Number (%) of embryos with abnormal phenotypes, i.e., disintegration of the tectum region due to abnormal swelling or abnormal blood circulation.
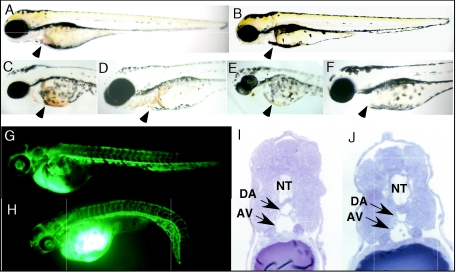
Blood circulation of zebra fish embryo starts to be visible by around 24 to 26 hpf (22, 32). In zMelk-like gene MO-injected embryos, the blood circulation was not apparent at 24 hpf (in 91% of them treated with 6 ng of first MO). It became visible about 29 hpf, but the number of blood cells was far smaller than that for the control MO-injected embryos. Even at 48 (Fig. 2E and F, arrowheads) and 72 (Fig. 3A and B, arrowheads) hpf, only a trace amount of blood cells could be seen.
FIG. 3.
Hematopoiesis and vasculogenesis of MO-injected embryos. (A and B) Lateral views of 72-hpf zebra fish embryos injected with control MO (A) or zMelk-like gene MO (B). (C through F) Hemoglobin staining using o-dianisidine for 58-hpf (C and E) and 72-hpf (D and F) zebra fish embryos injected with the control MO (C and D) or the zMelk-like gene MO (E and F). (G and H) Microangiography by injecting FITC-dextran into control MO (G) or zMelk-like gene MO (H) -injected zebra fish embryos at 48 hpf. (I and J) Plastic, thin, transverse sections of the mid-trunk region of 24-hpf zebra fish embryos injected with control MO (I) or zMelk-like gene MO (J). The sections were stained with toluidine blue. NT, neural tube; DA, dorsal aorta; and AV, axial vein.
To confirm the specificity of these effects, we used a second MO synthesized to be specific against a different region of the zMelk-like gene mRNA. As shown in Table 1, this second MO showed similar effects on zebra fish embryo development, i.e., swelling of the tectum and small eyes. We then examined the dose requirement for the second MO and found that a higher amount (9 ng) of it was required to obtain a similar incidence of the phenotype caused by the first MO (6 ng).
We next examined whether coinjection of the zMelk-like gene RNA could rescue embryos from the MO phenotypes or not. We constructed a mutant zMelk-like gene (zMelk-like 5′mt) having alternate codon sequences at 7 nucleotides and used RNA transcribed from the mutant to reverse the effects of the zMelk-like gene MO. Injection of zMelk-like 5′mt mRNA alone (300 pg) did not cause any morphological defect, at least at 24 or 48 hpf or 5 days (Fig. 2G). Furthermore, we did not observe ectopic or early-onset blood circulation (data not shown). Then, coinjection of 6 ng of first MO together with either the zMelk-like 5′mt mRNA or EGFP mRNA was done (Table 1). Recovery from tectum swelling at 24 hpf (Fig. 2G, left panels, asterisks) and blood circulation at 48 hpf and 5 days (Fig. 2G, middle and right panels, arrowheads) were clearly observed by coinjection with zMelk-like 5′mt RNA but not by that with EGFP RNA. We also examined the effect of coinjection of the wild-type zMelk-like gene mRNA with the MO. Wild-type zMelk-like gene mRNA reduced the incidence of the MO-induced phenotype of tectal swelling and blood circulation from 91% to 28% (Table 1). Coinjection with wild-type zMelk-like gene mRNA also neutralized the effects of the second MO (Table 1). This significant rescue achieved by coinjection with the zMelk-like 5′mt RNA or wild-type zMelk-like gene RNA suggests strongly that the zMelk-like gene MO phenotypes were caused by the specific inhibition of the zMelk-like gene functions.
Since the zMelk-like gene contains a putative serine/threonine kinase domain, we next examined the role of the kinase domain by using a mutant zMelk-like gene having a point mutation within a critical amino acid in the putative ATP binding domain. One of the mutant zMelk-like genes which has substitution of threonine 169 with alanine (T169A) was expected to be destroyed with its kinase activity. We injected zMelk-like geneT169A mRNA with zMelk-like gene MO and found that the phenotype was indistinguishable from that of embryos injected with the zMelk-like gene MO alone (Fig. 2G), suggesting the essential role of the kinase domain in zMelk-like gene function. We also constructed a mutant zMelk-like gene having a substitution of threonine 169 with glutamic acid (T169E) which was expected to make its kinase constitutively active. The T169E mutant rescued the effect of zMelk-like gene MO (data not shown). We tried to confirm whether the T169E is constitutively active or not by different experiments; however, we could not obtain clear evidence suggesting the activity of the T169A (data not shown).
Primitive hematopoiesis was suppressed, but vasculogenesis seemed to occur normally in zMelk-like gene MO-injected embryos.
Although severe anemia was observed until at least 72 h after MO was injected into embryos (Fig. 3A and B), blood circulation and the number of blood cells at about 6 days postfertilization (dpf) were similar in both zMelk-like gene MO-injected and control embryos (data not shown). Since definitive erythropoiesis starts around 4 dpf (1), there are two possibilities, i.e., that definitive hematopoiesis as seen in control embryos had occurred in zMelk-like gene MO-injected embryos at around 6 dpf or that the effects of MO disappeared around this time. In addition to the defect in blood cells, the beating of the heart tube was slower than that of the control MO-injected embryos. At 32 hpf, the heart rate of control MO-injected embryos was 100 ± 3 beats per min, which is comparable to previously reported values (2, 20), whereas the rate for the zMelk-like gene MO-injected embryos was 81 ± 4 beats per min. At 48 hpf, the heart rate of the former was 150 ± 3 beats per min and that of the latter was 121 ± 4 beats per min.
The zMelk-like gene was expressed relatively strongly in the lateral mesoderm. Since this mesoderm is a place where the differentiation of primitive erythroid progenitors is initiated, we next analyzed the effect of zMelk-like gene MO on hematopoiesis directly by hemoglobin staining with o-dianisidine (7). Blood cell pools could be seen in the duct of Cuvier of control MO-injected embryos at 36 hpf, but they were absent in the zMelk-like gene MO-injected embryos (data not shown). Even at 48 hpf, stain deposits in the zMelk-like gene MO-injected embryos were not detectable (Fig. 3C and E, arrowheads). By 72 hpf, blood cells circulated through the heart and branchial vessels in control embryos, but zMelk-like gene MO-injected embryos did not express detectable levels of hemoglobin at that time (Fig. 3D and F, arrowheads). There was no evidence of blood pooling or hemorrhage. To examine the possibility that the apoptosis occurs with blood cells, we examined apoptotic cells by staining embryos with acridine orange at 48 and 72 hpf, but no difference in the staining pattern between control- and zMelk-like gene-MO injected embryos was observed (data not shown).
Next, we examined whether the ablation of zMelk-like gene functions would affect vascular-network formation. FITC-dextran was used to visualize vasculogenesis in zebra fish embryos. FITC-dextran was injected into the sinus venosus/cardinal vein at various stages (30, 48, 56, and 72 hpf) of development, and blood vessels were examined 10 min after the injection. At 48 hpf, there was no significant difference in the pattern of FITC signals between the zMelk-like gene MO-injected embryos and the control MO-injected ones (Fig. 3G and H). Similarly, in other stages (30, 56, and 72 hpf), no significant abnormality of the vascular network in the zMelk-like gene MO-injected embryos was observed (date not shown). Furthermore, observation of transverse sections of the midtrunk of 48-hpf embryos revealed that the dorsal aorta and axial vein formation occurred at the appropriate region (Fig. 3I and J, arrows), supporting the idea that the zMelk-like gene is not necessary for the proper formation of blood vessels.
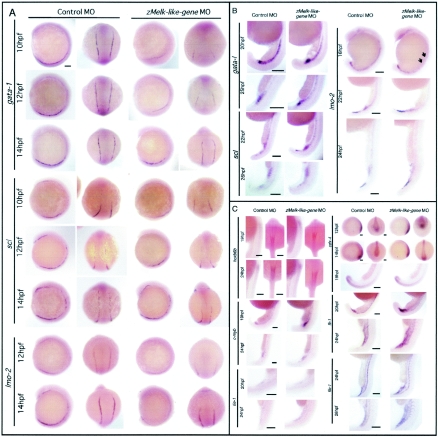
scl, gata-1, and lmo-2 are critical transcription factors for vertebrate embryonic hematopoiesis (10, 38). So next we analyzed the expression pattern of these genes in zMelk-like gene MO- or control MO-injected embryos by whole-mount in situ hybridization. During zebra fish embryogenesis, gata-1 mRNA is reported to be initially detected at the two-somite stage in putative progenitors that reside in two stripes flanking the paraxial mesoderm (7). As shown in Fig. 4A, at 10 hpf, two gata-1-positive stripes were observed in both control MO-injected embryos and zMelk-like gene MO-injected embryos, but the length of the stripes in the latter was far shorter and the signals significantly weaker than those in the former. When we looked at 12-hpf embryos, a similar tendency was observed (Fig. 4A). The length of the area with gata-1-positive signals was about half of that of the control MO-injected embryos, and the intensity was far weaker in the zMelk-like gene MO-injected embryos. Once the embryos had reached 14 hpf, signals in the zMelk-like gene MO-injected embryos were still weaker than those in the control ones, but the difference in the expression pattern had become more subtle. We next examined the expression of scl by in situ analysis. In normal development, scl is known to be expressed at a slightly earlier stage than gata-1, as two stripes situated in the lateral margins of the mesoderm (9), and at 10 to 14 hpf, we detected scl transcripts in the anterior and posterior lateral plate mesoderms. However, in the 10- and 12-hpf zMelk-like gene MO-injected embryos, expression of scl in the anterior lateral plate was hardly detected. The length of the plate expressing scl and the intensity of scl signals in the posterior lateral plate were shorter and weaker, respectively, in zMelk-like gene MO-injected embryos than in the control MO-injected ones (Fig. 4A). However, at 14 hpf, the pattern of scl expression between the two groups was similar. Therefore, in later stages, the expression pattern of both gata-1 and scl became indistinguishable between zMelk-like gene MO-injected embryos and control MO-injected ones.
FIG. 4.
Whole-mount in situ analysis of various hematopoietic and vascular markers. Expression of various hematopoietic genes in control and in zMelk-like gene MO-injected embryos. (A) Whole-mount in situ hybridization of gata-1, scl, and lmo-2. Lateral view with anterior at the top (first and third columns from the left) and dorsal view with anterior at the top (second and fourth column from the left) of probe-hybridized zebra fish embryos injected with either control MO or zMelk-like gene MO are shown. Scale bars, 100 μm. (B) Lateral views of whole-mount control MO- or zMelk-like gene MO-injected embryos at 16 to 26 hpf in situ hybridized for gata-1, scl, and lmo-2 mRNA. Scale bars, 150 μm. (C) Embryos injected with control MO or zMelk-like gene MO were harvested at the indicated stages, and whole-mount in situ hybridization was done by using hoxb6b, c-myb, tie-1, cdx-4, fli-1, and flk-1 probes. Expression patterns of hoxb6b at 19 and 24 hpf (lateral view with dorsal to right and dorsal view with anterior at the top), c-myb at 19 and 24 hpf (lateral view of ICM region), tie-1 at 20 and 24 hpf (lateral view of ICM region), cdx-4 at 12 hpf, 14 hpf (lateral view with anterior to left and posterior view with dorsal at the top), cdx-4 at 19 hpf (lateral view of ICM region), fli-1 at 20 and 24 hpf (lateral views of ICM region) and flk-1 at 24 and 28 hpf (lateral view of ICM region) are shown. Results of antisense probe hybridization are shown in the figure. Sense probe control experiments were done for all of the probes, and no significant signals were detected. Scale bars, 100 μm.
The LIM domain transcription factor lmo-2 interacts with scl and is essential for primitive and definitive hematopoiesis (37). Like scl, lmo-2 is expressed in endothelial and hematopoietic progenitors and in cells of the erythroid and megakaryocytic lineage (10). In zebra fish embryos, lmo-2 is expressed in the two stripes of the ventral mesoderm, as well as in the two anterior stripes, by 12 hpf (35). The signals of lmo-2 in zMelk-like gene MO-injected embryos were faint in both the anterior and posterior at 12 hpf and at 14 hpf, and intensity of the signals was still weaker than that observed with control MO-injected embryos (Fig. 4A). Then, by 16 hpf, expression of lmo-2 in the lateral mesoderm of zMelk-like gene MO-injected embryos was clearly observed in the most posterior region but not in the midtrunk (Fig. 4B, arrows). In zebra fish, by 18 hpf, the ventral mesodermal cells converge to form the intermediate cell mass (ICM) (1). Around 22 to 24 hpf, lmo-2 was strongly expressed in the posterior ICM in both control and zMelk-like gene MO-injected embryos with a similar pattern (Fig. 4B). Similarly, although scl and gata-1 expression was suppressed in the lateral mesoderm around 12 to 14 hpf (Fig. 4A), their expression was recovered around 20 to 22 hpf in the ICM in zMelk-like gene MO-injected embryos, and the patterns and intensity of the signals were indistinguishable from those of the control MO-injected embryos (Fig. 4B). We then analyzed yet other hematopoietic markers by whole-mount in situ analysis of control MO- and zMelk-like gene MO-injected embryos (Fig. 4C). Cdx-4, a homeobox transcription factor, was recently identified as the locus mutated in kugelig (kgg), which is a zebra fish mutant with an early defect in hematopoiesis (6). cdx-4 was expressed similarly in terms of both pattern and intensity in zMelk-like gene MO- and control MO-injected embryos (Fig. 4C). It is notable that the cdx-4 expression in early embryos such as at 12 hpf and 14 hpf was also not perturbed by injection of the zMelk-like gene MO. Hoxb6b is one of the hox genes downstream of cdx-4 (6). We found that the hoxb6b mRNA was expressed similarly in zMelk-like gene MO-injected embryos at both 19 hpf and 24 hpf (Fig. 4C). As also shown in Fig. 4C, other hematopoietic genes such as fli-1, flk-1, c-myb, and tie-1 showed no apparent difference in their expression pattern or the strength of signals between the two groups. Since the zMelk-like gene was not expressed in the ICM, its effects on hematopoiesis in this region may not be due to the direct regulation of ICM function. These results indicate that suppression of the zMelk-like gene affected the expression of gata-1 and scl in the early stage but was not involved in subsequent stages.
We also examined the expression pattern of en-2 (18 hpf, 24 hpf, 28hpf), islet-1 (22 hpf, 24 hpf, 28 hpf), dlx-2 (22 hpf, 24 hpf), wnt-1 (20 hpf, 24 hpf), hlx-1 (20 hpf, 24 hpf, 28 hpf), shh (20 hpf, 24 hpf), and myoD (20 hpf, 24 hpf), but again, no significant difference in the expression patterns between the two groups of embryos was observed (data not shown).
The expression of gata-1 rescued the effects of the zMelk-like gene MO in embryos.
Since suppression of the expression of gata-1, scl, and lmo-2 was observed by the injection of zMelk-like gene MO, we next examined whether the coexpression of these genes with zMelk-like gene MO rescued the MO-induced phenotype or not. We first examined effects of the expression of gata-1 in embryos. Various doses of mRNA of full-length gata-1 were injected into zebra fish embryos, and embryos were examined at 24 hpf, 48 hpf, and 5 days after injection. As shown in Fig. 5A, injection of 50 pg of gata-1 mRNA did not show apparent abnormal morphological defects and the onset of blood circulation was normal (data not shown). However, 100 pg of gata-1 mRNA resulted in the appearance of significant population of the lethal phenotype (Table 2). Using the mRNA doses which did not induce abnormal embryos, we next examined whether coexpression of the gata-1 rescued the phenotype induced by zMelk-like gene MO. Embryos injected with zMelk-like gene MO and gata-1 mRNA (50 pg) were examined at 24 hpf, 48 hpf, and 5 days after injection (Fig. 5A). The expression of gata-1 reversed the phenotype induced by zMelk-like gene MO. We had done exactly the same set of experiments using full-length mRNA for scl and lmo-2; however, we could not observe rescue of the phenotype induced by zMelk-like gene MO by the injection of scl or lmo-2 mRNA.
FIG. 5.
Effects of gata-1 gene expressions for phenotype induced by the injection of zMelk-like gene MO and regulation of gata-1 promoter by a Melk-like gene. (A) Lateral views (anterior to left) of embryos injected with either control MO, gata-1 RNA or zMelk-like gene MO are shown. Coinjection of zMelk-like gene MO and either EGFP RNA, or gata-1 RNA was done and views of these embryos at 24 and 48 hpf and 5 days (lateral view with anterior to left) are shown. Scale bars, 100 μm. (B) Effects of zMelk-like gene for activation of zebra fish gata-1 promoter in the embryos. Zebra fish gata-1 promoter EGFP and mRNA for either control, zMelk-like gene, or zMlke-likeT169A were coinjected into fertilized eggs and the expression of EGFP was examined under fluorescence dissection microscope. Dorsal views of embryos at 14 hpf and lateral views with anterior to left embryos at 18 hpf are shown.
TABLE 2.
Abnormal phenotypes caused by zMelk-like gene MO injection and its rescue by gata-1 RNAa
| Material(s) injected | No. of embryos examineda | No. of embryos with normal or abnormal phenotypes (%)
|
|||
|---|---|---|---|---|---|
| Normal | Abnormalb
|
Lethal | |||
| Tectum swelling | Blood circulation | ||||
| Control MO (6 ng) | 61 | 59 (97%) | 0 (0%) | 0 (0%) | 2 (3%) |
| zMelk-like gene first MO (6 pg) | 97 | 3 (3%) | 85 (87%) | 91 (94%) | 3 (3%) |
| zMelk-like gene first MO (6 ng) + EGFP RNA (50 pg) | 96 | 4 (4%) | 83 (86%) | 89 (92%) | 3 (3%) |
| zMelk-like gene first MO + Gata-1 RNA | |||||
| zMelk-like gene first MO (6 ng) + Gata-1 RNA (10 pg) | 83 | 33 (40%) | 42 (50%) | 48 (58%) | 2 (2%) |
| zMelk-like gene first MO (6 ng) + Gata-1 RNA (50 pg) | 80 | 56 (70%) | 16 (20%) | 22 (27%) | 2 (3%) |
| zMelk-like gene first MO (6 ng) + Gata-1 RNA (100 pg) | 80 | 20 (25%) | 4 (5%) | 28 (35%) | 32 (40%) |
| Gata-1 RNA | |||||
| Gata-1 RNA (10 pg) | 41 | 40 (98%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Gata-1 RNA (50 pg) | 38 | 36 (95%) | 0 (0%) | 0 (0%) | 2 (5%) |
| Gata-1 RNA (100 pg) | 42 | 21 (50%) | 0 (0%) | 4 (10%) | 17 (40%) |
One- or two-cell-stage embryos were injected with the materials indicated.
Number (%) of embryos with abnormal phenotypes, i.e., disintegration of the abnormal swelling (tectum region) or abnormal blood circulation.
Gata-1 promoter activity was modulated by the expression of the zMelk-like gene.
Since the reversed phenotype of zMelk-like gene MO by gata-1 expression was observed, we next examined the mechanism of the effect of zMelk-like gene in gata-1 expression by using gata-1 promoter EGFP. The gata-1 8.1-kb 5′ promoter region contains a sufficient genomic region to direct proper expression of the gata-1 gene (23). We first confirmed the expression of gata-1 promoter EGFP in zebra fish embryos by our hand. As shown in Fig. 5B, gata-1 promoter-directed EGFP was expressed in the lateral mesoderm at 14 hpf and ICM at 18 hpf, both of which were proper expression regions of endogenous gata-1. We then examined the effect of coexpressed zMelk-like gene mRNA with gata-1 promoter EGFP (Fig. 5B; Table 3). Coinjection of wild-type zMelk-like gene mRNA enhanced the expression of gata-1 promoter EGFP expression at 14 hpf, suggesting that the zMelk-like gene positively affects gata-1 promoter activity (Fig. 5B). However, the zMelk-likeT169A gene did not affect the expression, indicating the essential role of the kinase domain of the zMelk-like gene. At 18 hpf, we did not observe the clear enhancement of EGFP expression in the presence of the wild-type zMelk-like gene. We used full-length gata-1 mRNA as a positive control in the experiments, and the expression of gata-1 enhanced gata-1 promoter EGFP (Table 3), as expected from previous paper (23). Ectopic expression of EGFP was observed with gata-1 expression; however, ectopic expression was never observed with zMelk-like gene expression, suggesting that expression of the zMelk-like gene alone was not sufficient to promote gata-1 promoter activation. We also examined the coinjection of gata-1 mRNA and zMelk-like gene mRNA in addition to gata-1 promoter EGFP, but we could not observe any apparent enhancement of the expression level of EGFP in comparison with that of the embryos injected with gata-1 mRNA and gata-1 promoter EGFP.
TABLE 3.
Zebra fish gata-1 promoter-dependent EGFP expression in the presence of zMelk-like gene or gata-1
| Material(s) injected | No. of embryos examineda | No. of EGFP-positive embryos (%)
|
||||
|---|---|---|---|---|---|---|
| No expression | Strong level | Weak level | Ectopic | Lethal | ||
| gata-1 promoter EGFP(50 pg) | 65 | 3 (4%) | 5 (7%) | 53 (83%) | 2 (3%) | 2 (3%) |
| gata-1 promoter EGFP(100 pg) + zMelk-like gene (300 pg) | 72 | 4 (6%) | 33 (45%) | 28 (39%) | 3 (4%) | 4 (6%) |
| gata-1 promoter EGFP(100 pg) + gata-1 (200 pg) | 56 | 5 (9%) | 33 (60%) | 7 (12%) | 36 (65%) | 8 (14%) |
Expression levels were arbitrary examined under a fluorescence dissection microscope. Typical examples of strong and weak levels are shown in Fig. 5B.
DISCUSSION
In this report, we identified the zMelk-like gene, which showed an expression similar to that of mMelk, by taking advantage of the strong sequence similarity with mMelk at the C- and N-terminal regions. Knockdown of the zMelk-like gene product indicated that this product may play a role in primitive hematopoiesis in the zebra fish.
Although the zMelk-like gene does not have similarity with mMelk at the middle part of the molecule, several features of expression patterns were similar between the zMelk-like gene and mMelk. For example, both genes were expressed in embryonic brain and retina, especially in the ventricular zone. Furthermore, both were expressed in hematopoietic tissues in adult animals. We tried to find mouse gene(s) having similarity with the middle region of the zMelk-like gene by database searching, but no such gene or fragment was found. Vice versa, our attempt to find sequences in the zebra fish database that were homologous with the middle region of mMelk was not successful. Taken together, our data suggest that the zMelk-like gene may be a zebra fish orthologue of mMelk.
Primitive hematopoiesis in the zebra fish embryo occurs in the region located between the notochord and endoderm of the trunk called the ICM (1). The earliest expression of hematopoietic genes gata-1, scl, and lmo-2 commences in bilateral stripes within the lateral plate mesoderm at approximately the five-somite stage and 11 hpf, respectively (10, 26). We found that in the zMelk-like gene MO-injected embryos, the expression of gata-1, scl, and lmo-2 around 12 to 14 hpf was severely suppressed but that it seemed relatively normal in the later stage, suggesting that the zMelk-like gene may be involved in the regulation of these genes' expression only in the early stage. Recently, cdx-4, a homeobox transcription factor, was identified as the locus mutated in kugelig (kgg), which is a zebra fish mutant with a defect in the early stage of hematopoiesis (6). In kgg mutants, the number of scl-positive hematopoietic precursor cells and gata-1-positive erythroid precursor cells are decreased (6). Since the expression of cdx-4 was not altered by the injection of zMelk-like gene MO, we surmise that the zMelk-like gene plays a role downstream of cdx-4. hox genes are candidate targets of cdx-4, and in fact, rescue from the kgg phenotype by the overexpression of some hox genes has been reported (6). The fact that we did not observe a reduction in the expression of hoxb6b, one of the hox genes whose expression is suppressed in kgg (10), suggests that the target of the zMelk-like gene lies in between hox and scl. Although redundant activities of multiple hox genes were suggested (6), hoxb6b alone is not sufficient to reverse the kgg phenotype completely. Thus, there is another possibility, that the zMelk-like gene regulates the expression or function of hox members other than hoxb6b. The zMelk-like gene expression pattern at about 12 to 14 hpf did not overlap with that of gata-1, scl, and lmo-2, but weak expression of the zMelk-like gene was observed within most of the lateral mesoderm area. Thus, we could not rule out the possibility that the zMelk-like gene directly regulates gata-1 or scl expression in this region. Enhancement of the gata-1 promoter by expression of the zMelk-like gene supported this idea. Positive feedback of the gata-1 promoter by gata-1 itself was reported (23). Gata-1 is known to be phosphorylated at their serine residues (5), and the involvement of mitogen-activation protein kinase in the phosphorylation of gata-1 was recently suggested (36). Requirement of the kinase domain of the zMelk-like gene for enhancement of the gata-1 promoter was evident; however, we need further experiments to conclude whether direct the phosphorylation of gata-1 by the zMelk-like gene occurs or zMelk-like kinase is in the upstream kinase cascade of gata-1 regulation. On the other hand, since the zMelk-like gene was not expressed in the anterior or posterior ICM, and because gata-1 and scl expression in this region was not affected in zMelk-like gene MO-injected embryos, the suppression of blood circulation by abrogation of products of the zMelk-like gene may not occur through the direct regulation of gata-1 and scl expression in the ICM. Currently, we do not have evidence suggesting mechanisms of the anemia phenotype; however, we surmised several possibilities. One is that the underdetection level of zMelk-like gene in ICM affected the proliferation and/or differentiation of hematopoietic cells through regulating the expression of the gene(s), which we did not examine in this work. Alternatively, these unknown genes may be downstream of the gene cascade of gata-1/scl expressed in the lateral plate mesoderm. Or, more speculatively, zMelk-like gene expressed in the anterior half of embryos regulating the deriver of soluble factor(s), which is essential for blood circulation, into ICM can be surmised.
We could observe rescue of the phenotype induced by zMelk-like gene MO only by the expression of the gata-1 gene, suggesting the importance of gata-1 expression in the early phase of embryogenesis for hematopoiesis in zebra fish. scl and lmo-2 are expressed earlier than gata-1 in the hematopoietic program in vertebrates (1); however, we could not observe recovery of the zMelk-like gene MO-induced phenotype by expression of these genes. Our results may suggest that these genes were not sufficient to promote gata-1 expression. However, we could not rule out that the expression level of scl and lmo-2 was not sufficient for the activity.
There are at least five “bloodless” zebra fish mutants including vlad tepes (28), cloche (clo), spadetail (spt) (35), and bloodless (bls) (26). bls is a dominant mutant with phenotypes ranging from complete absence of circulating blood cells to severe anemia (26). Its phenotype is detected with the onset of circulation. In the bls mutant, the expression of gata-1 and scl is severely impaired in the lateral mesoderm as well as in the ICM around 24 hpf (26). Because of the intact expression of gata-1 and scl in the ICM in the zMelk-like gene MO-injected embryos, different mechanisms for the regulation of hematopoiesis between the zMelk-like gene and bls are suggested. Both clo and spt have defects in primitive and definitive hematopoiesis (35). gata-1 expression in these mutants at 24 hpf is severely impaired. Furthermore, the clo mutants also have significantly decreased expression of vascular genes (35). The phenotype of zMelk-like gene MO-injected embryos seems to be hematopoietic specific, suggesting that this gene may play a role after the commitment of cells to hematopoietic and vascular endothelial cell lineages.
As part of a large-scale chemical mutagenesis screening of the zebra fish genome, more than 50 mutants that affect hematopoiesis, comprising 25 complementation groups, were isolated (30, 32). The majority of these mutations could be classified into five phenotypic classes based on their hematopoietic phenotype (32). Since hemoglobin staining showed no or an extremely reduced amount of heme in the zMelk-like gene MO-injected embryos, impairment of the heme synthesis pathway appears to be another defect in the zMelk-like gene MO-injected embryo. There are several mutants grouped as hypochromic anemia, such as sauternes (sau) (3) or chardonnay (8), and they have mutations in the heme synthesis pathway. However, in these cases, a relatively normal number of circulating erythrocytes was observed up to 2 or 3 days after the onset of circulation, thus suggesting that the zMelk-like gene affects more than just the heme synthesis pathway.
Onset of definitive hematopoiesis in the zebra fish starts around 4 dpf (3, 27). Blood circulation was first observed around 6 dpf in zMelk-like gene MO-injected embryos, and so, although primitive hematopoiesis was defective in the zMelk-like gene product-deleted zebra fish, the definitive hematopoiesis may not have been perturbed.
Since mMelk and zMelk-like genes are expressed strongly in hematopoietic tissues in adult animals, there is a possibility that Melk also plays a role in adult hematopoiesis and functions in hematopoietic cells. Mouse Melk (MPK38) was reported to be expressed in the cells of the T lineage and macrophage/monocyte lineage (12), implicating a potential function of Melk in early T-cell activation and macrophage functions. In fact, elevation of the expression level of Melk (MPK38) in T-cell activation was reported, but its exact function was not revealed (11). zMelk-like gene transcripts were strongly detectable in the thymus of the adult zebra fish, suggesting conserved function of it in T-cell immunology.
Acknowledgments
We thank Y. Aoki for technical support, C. Nakata and R. Kurita for general discussions, and Y. Matsumura for secretarial help. We thank Kobayashi and Yamamoto (Tsukuba University) for providing the pCS2-gata-1 and gata-1 promoter EGFP plasmids.
This work was supported by the RIKEN Center for Developmental Biology.
REFERENCES
- 1.Amatruda, J., and L. I. Zon. 1999. Dissecting hematopoiesis and disease using the zebrafish. Dev. Biol. 216:1-15. [DOI] [PubMed] [Google Scholar]
- 2.Baker, K., K. S. Warren, G. Yellen, and M. C. Fishman. 1997. Defective “pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc. Natl. Acad. Sci. USA 94:4554-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlie, A., A. Donovan, S. J. Pratt, B. H. Paw, A. C. Oates, C. Brugnara, H. E. Witkowska, S. Sassa, and L. I. Zon. 1998. Positional cloning of the zebrafish sauternes gene: a model for congenital sideroblastic anaemia. Nat. Genet. 20:244-250. [DOI] [PubMed] [Google Scholar]
- 4.Celenza, J. L., and M. Carlson. 1986. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science 233:1175-1180. [DOI] [PubMed] [Google Scholar]
- 5.Crossley, M., and S. H. Orkin. 1994. Phosphorylation of the erythroid transcription factor GATA-1. J. Biol. Chem. 269:16589-16596. [PubMed] [Google Scholar]
- 6.Davidson, A. J., P. Ernst, Y. Wang, M. P. S. Dekens, P. D. Kingsley, J. Palls, S. Korsmeyer, G. Q. Daley, and L. I. Zon. 2003. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature 425:300-306. [DOI] [PubMed] [Google Scholar]
- 7.Detrich, H. W., III, M. W. Kieran, F. Y. Chan, L. M. Barone, K. Yee, J. A. Rundstadler, S. Pratt, D. Ransom, and L. I. Zon. 1995. Intraembryonic hematopoietic cell migration during vertebrate development. Proc. Natl. Acad. Sci. USA 92:10713-10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donovan, A., A. Brownlie, M. O. Dorschner, Y. Zhou, S. J. Pratt, B. Paw, R. B. Phillips, C. Thisse, B. Thisse, and L. I. Zon. 2002. The zebrafish mutant gene chardonnay (cdy) encodes divalent metal transporter 1 (DMT1). Blood 100:4655-4659. [DOI] [PubMed] [Google Scholar]
- 9.Gering, M., A. R. F. Rodaway, B. Gottgens, R. K. Patient, and A. R. Green. 1998. The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 17:4029-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gering, M., Y. Yamada, T. H. Rabbitts, and R. K. Patient. 2003. Lmo2 and Scl/Tal1 convert non-axial mesoderm into haemangioblasts which differentiate into endothelial cells in the absence of Gata1. Development 130:6187-6199. [DOI] [PubMed] [Google Scholar]
- 11.Gil, M., Y. Yang, and H. Ha. 1998. MPK38 expression is upregulated in immature T cells activated by concanavalin A. Immunol. Lett. 64:79-83. [DOI] [PubMed] [Google Scholar]
- 12.Gil, M., Y. Yang, Y. Lee, I. Choi, and H. Ha. 1997. Cloning and expression of a cDNA encoding a novel protein serine/threonine kinase predominantly expressed in hematopoietic cells. Gene 195:295-301. [DOI] [PubMed] [Google Scholar]
- 13.Hanks, S. K., and T. Hunter. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. [PubMed] [Google Scholar]
- 14.Hanks, S. K., and A. M. Quinn. 1991. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 200:38-62. [DOI] [PubMed] [Google Scholar]
- 15.Hanks, S. K., A. M. Quinn, and T. Hunter. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42-52. [DOI] [PubMed] [Google Scholar]
- 16.Hardie, G. D., D. Carling, and M. Carlson. 1998. The AMP-activated/snf1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67:821-855. [DOI] [PubMed] [Google Scholar]
- 17.Heasman, J. 2002. Morpholino oligos: making sense of antisense? Dev. Biol. 243:209-214. [DOI] [PubMed] [Google Scholar]
- 18.Heyer, B. S., H. Kochanowski, and D. Solter. 1999. Expression of Melk, a new protein kinase, during early mouse development. Dev. Dyn. 215:344-351. [DOI] [PubMed] [Google Scholar]
- 19.Heyer, B. S., J. Warsowe, D. Solter, B. B. Knowles, and S. L. Ackerman. 1997. New member of the Snf1/AMPK kinase family, Melk, is expressed in the mouse egg and preimplantation embryo. Mol. Reprod. Dev. 47:148-156. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh, D. J.-Y., and C.-F. Liao. 2002. Zebrafish M2 muscarinic acetylcholine receptor: cloning, pharmacological characterization, expression patterns and roles in embryonic bradycardia. Br. J. Pharmacol. 137:782-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hukriede, N. A., L. Joly, M. Tsang, J. Miles, P. Tellis, J. A. Epstein, W. B. Barbazuk, F. N. Li, B. Paw, J. H. Postlethwait, T. J. Hudson, L. I. Zon, J. D. McPherson, M. Chevrette, I. B. Dawid, S. L. Johnson, and S. C. Ekker. 1999. Radiation hybrid mapping of the zebrafish genome. Proc. Natl. Acad. Sci. USA 96:9745-9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimmel, C., W. W. Ballard, S. R. Kimmel, B. Ullmann, and T. Schilling. 1995. Stages of embryonic development of the zebrafish. Dev. Dyn. 203:253-310. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, M., K. Nishikawa, and M. Yamamoto. 2001. Hematopoietic regulatory domain of gata1 gene is positively regulated by GATA1 protein in zebrafish embryos. Development 128:2341-2350. [DOI] [PubMed] [Google Scholar]
- 24.Kurita, R., H. Sagara, Y. Aoki, B. A. Link, K. Arai, and S. Watanabe. 2003. Suppression of lens growth by alphaA-crystallin promoter-driven expression of diphtheria toxin results in disruption of retinal cell organization in zebrafish. Dev. Biol. 255:113-127. [DOI] [PubMed] [Google Scholar]
- 25.Kurita, R., Y. Tabata, H. Sagara, K. Arai, and S. Watanabe. 2004. A novel smoothelin-like, actin-binding protein required for choroidal fissure closure in zebrafish. Biochem. Biophys. Res. Commun. 313:1092-1100. [DOI] [PubMed] [Google Scholar]
- 26.Liao, E. C., N. S. Trede, D. Ransom, A. Zapata, M. Kieran, and L. I. Zon. 2002. Non-cell autonomous requirement for the bloodless gene inprimitive hematopoiesis of zebrafish. Development 129:649-659. [DOI] [PubMed] [Google Scholar]
- 27.Liao, E. C., and L. I. Zon. 1999. Conservation of themes in vertebrate blood development, p. 569-582. In S. Moody (ed.), Cell lineage and fate determination. Academic Press, San Diego, Calif.
- 28.Lyons, S. E., N. D. Lawson, L. Lei, P. E. Bennett, B. M. Weinstein, and P. P. Liu. 2002. A nonsense mutation in zebrafish gata1 causes the bloodless phenotype in vlad tepes. Proc. Natl. Acad. Sci. USA 99:5454-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasevicius, A., and S. C. Ekker. 2000. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 26:216-220. [DOI] [PubMed] [Google Scholar]
- 30.North, T. E., and L. I. Zon. 2003. Modeling human hematopoietic and cardiovascular diseases in zebrafish. Dev. Dyn. 228:568-583. [DOI] [PubMed] [Google Scholar]
- 31.Paris, J., and M. Phillippe. 1990. Poly(A) metabolism and polysomal recruitment of maternal mRNAs during early Xenopus development. Dev. Biol. 140:221-224. [DOI] [PubMed] [Google Scholar]
- 32.Ransom, D. G., P. Haffter, J. Odenthal, A. Brownlie, E. Vogelsang, R. N. Kelsh, M. Brand, F. J. M. van Eeden, M. Furutani-Seiki, M. Granato, M. Hammerschmidt, C.-P. Heisenberg, Y.-J. Jiang, D. A. Kane, M. C. Mullins, and C. Nusslein-Volhard. 1996. Characterization of zebrafish mutants with defects in embryonic hematopoiesis. Development 123:311-319. [DOI] [PubMed] [Google Scholar]
- 33.Steinberg, R. A., R. D. Cauthron, M. M. Symcox, and H. Shuntoh. 1993. Autoactivation of catalytic (C alpha) subunit of cyclic AMP-dependent protein kinase by phosphorylation of threonine 197. Mol. Cell. Biol. 13:2332-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki, A., G.-I. Kusakai, A. Kishimoto, J. Lu, T. Ogura, M. F. Lavin, and H. Esumi. 2003. Identification of a novel protein kinase mediating Akt survival signaling to the ATM protein. J. Biol. Chem. 278:48-53. [DOI] [PubMed] [Google Scholar]
- 35.Thompson, M. A., D. G. Ransom, S. J. Pratt, H. MacLennan, M. W. Kieran, H. W. Detrich III, B. Vail, T. L. Huber, B. Paw, A. J. Brownlie, A. C. Oates, A. Fritz, M. A. Gates, A. Amores, N. Bahary, W. S. Talbot, H. Her, D. R. Beier, J. H. Postlethwait, and L. I. Zon. 1998. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev. Biol. 197:248-269. [DOI] [PubMed] [Google Scholar]
- 36.Towatari, M., M. Ciro, S. Ottolenghi, S. Tsuzuki, and T. Enver. 2004. Involvement of mitogen-activated protein kinase in the cytokine-regulated phosphorylation of transcription factor GATA-1. Hematol. J. 5:262-272. [DOI] [PubMed] [Google Scholar]
- 37.Wadman, I., J. X. Li, R. O. Bash, A. Forster, H. Osada, T. H. Rabbitts, and R. Baer. 1994. Specific in vivo association between the bHLH and LIM proteins implicated in human T cell leukemia. EMBO J. 13:4831-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zon, L. I. 1994. Developmental biology of hematopoiesis. Blood 86:2876-2891. [PubMed] [Google Scholar]