Abstract
Fas triggers apoptosis via the caspase cascade when bound to its ligand FasL. In type I cells, Fas is concentrated into the plasma membrane lipid rafts, and these domains are required for the apoptotic signal to occur. In contrast, Fas is excluded from the microdomains in type II cells. We report that the coligation with Fas of the membrane receptor CD28 strongly increases Fas-induced apoptosis in type II T lymphocytes, whereas it has no effect in a type I cell line. The effect of CD28 is independent of its intracellular region and requires the recruitment of the microdomains. Indeed, upon CD28 costimulation, Fas is redistributed in the lipid rafts, and their disruption with a cholesterol chelator abrogates the effect of CD28. The microdomain-mediated cell death amplification does not alter death-induced signaling complex formation and is mediated by the enhancement of the mitochondrial apoptotic pathway. These findings indicate that the sensitivity to Fas-induced apoptosis of type II cells can be amplified in vivo by the recruitment of lipid rafts following interactions between nonapoptotic ligand/receptor pairs during cell-to-cell contacts.
Fas (CD95/APO-1) is a transmembrane receptor which belongs to the tumor necrosis factor receptor superfamily. Fas-mediated apoptosis is an important down-regulator of the immune response, as Fas activation has been involved in the deletion of activated T lymphocytes via activation-induced cell death (1, 6, 29) and in the deletion of antigen-presenting cells following contact with activated T lymphocytes (14, 16). Upon FasL or agonistic antibodies stimulation, the Fas intracellular domain called DD (death domain) binds the adaptor protein FADD (Fas-associated protein with death domain), which in turn recruits c-FLIP (FADD-like interleukin-1β [IL-1β]-converting enzyme inhibitory protein) and procaspase 8 and 10. This complex is named the death-inducing signaling complex (DISC). The close vicinity of these caspases allows their activation by autoprocessing, and the active caspase 8 can directly cleave effector caspases, such as caspase 3 (reviewed in reference 11). This pathway is the one used in the so-called type I cells. In other cells called type II cells, the DISC formation is impaired, but enough active caspase 8 is generated to cleave and activate the cytosolic protein BID, which triggers a mitochondrion-dependent apoptotic pathway leading to the activation of caspase 9, which in turn activates caspase 3. This type II pathway can selectively be inhibited upon overexpression of Bcl-2 and Bcl-XL or upon inhibition of caspase 9 (22, 23).
Recently, Muppidi and Siegel reported that Fas is localized in detergent-resistant plasma membrane microdomains termed lipid rafts or microdomains in type I cells and excluded from these domains in type II cells. In type I but not in type II cells, Fas-induced apoptosis depends on the engagement of the microdomains. The microdomains are detergent-resistant cholesterol-rich structures enriched in glycosylphosphatidyl-inositol (GPI)-linked proteins and signal-transducing molecules, e.g., the kinases of the src family (4). Following the recognition of the peptide-major histocompatibility complex, T lymphocyte activation requires the recruitment of microdomains to form the “immunological synapse” at the contact area with the antigen-presenting cell (17, 30, 32). This step is mediated by CD28, which triggers the recruitment of the microdomains at the contact to the T-cell receptor (TCR) molecules.
In a previous work, we reported that an engineered GPI-linked Fas (Fas-GPI), localized into the microdomains, was capable of enhancing Fas-mediated apoptosis in type II cells constitutively expressing functional wild-type Fas (15). By analogy to the TCR activation model, other ligand/receptor interactions are expected to occur concomitantly to the engagement of Fas by the FasL present on the killer cell. We hypothesized that in type II cells, the recruitment of the microdomains could occur via a coreceptor and as a consequence enhance the sensitivity of these cells to Fas-mediated apoptosis. Therefore, we investigated whether type II cells, such as the Jurkat cell line or activated T lymphocytes from peripheral human blood, can be sensitized to Fas engagement through the involvement of the lipid rafts.
MATERIALS AND METHODS
Cell lines and peripheral blood T lymphocytes.
The murine 1A12 cell line expressing human FasL (26) was kindly provided by S. Nagata (Osaka Bioscience Institute, Osaka, Japan). We used two different human T leukemia Jurkat cell lines, one called Jurkat 77 and obtained from P. Anderson (Dana Farber Cancer Institute, Boston, MA) and the other one obtained from J. Thèze (Pasteur Institute, Paris, France). All cell lines were grown in RPMI medium supplemented with 8% heat-inactivated fetal calf serum and 2 mM l-glutamine in a 5% CO2 incubator at 37°C. The Jurkat 77 cell line was 100% positive for CD28 cell membrane expression by flow cytometry, whereas the other Jurkat cell line contained 15% CD28-negative cells, which were isolated by negative sorting using an anti-CD28 monoclonal antibody (MAb) (Immunotech, Marseille, France) adsorbed on goat anti-mouse immunoglobulin-coated magnetic beads (Dynabeads M-450, Dynal, Compiègne, France). Activated CD4+ T lymphocytes from healthy donor blood were obtained as follows. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll centrifugation, washed twice, and mixed at a 1:1 ratio with irradiated stimulatory allogeneic PBMC in the presence of phytohemagglutinin (1 μg/ml; Sigma, L'Isle-d'Abeau-Chesnes, France) and recombinant IL-2 (100 U/ml) in medium supplemented with 10% human serum. After 4 days of culture, cells were washed and resuspended with IL-2 alone. Activated CD4+ T lymphocytes were then enriched by depletion of CD8+ cells using magnetic beads covered with an anti-CD8 MAb (Immunotech).
Antibodies and other reagents.
The anti-human Fas MAb 5D7 and anti-human FasL MAb 14C2 (15) used in flow cytometry and the isotype-matched negative control MAbs 1F10 (immunoglobulin G [IgG]) and 10C9 (IgM) (27) were all generated in the laboratory. For functional studies, the anti-human Fas agonistic MAb 7C11 (IgM) and blocking MAb ZB4 (IgG) and the anti-CD28 MAb (clone CD28.2) were from Immunotech. For the immunoblots, anti-CD28, anti-caspase 8, and anti-BID polyclonal antisera were from R&D Systems (Oxon, United Kingdom), antitransferrin MAb was from Zymed (San Francisco, CA), anti-p56Lck MAb was from Transduction Laboratories (Lexington, KY), anti-caspase 3 and anti-Bcl-2 were from Pharmingen, antiactin was from Sigma, and anti-c-FLIP and anti-Fas antiserum (C-20) were from Santa Cruz Biotechnology (Tebu, Le-Perray-En-Yvelines, France). The J558L hybridoma cell line producing CTLA4-Ig with CTLA4 fragment of mouse origin was obtained from P. Lane (Basel Institute for Immunology, Switzerland). The Fas-Fc chimera was produced in COS cells transfected with a construct generated in the laboratory. As an irrelevant control, we used a homemade COS-derived vIL6-Fc chimeric protein, with vIL6 being the human herpesvirus 8-derived IL-6 homolog. These chimeras were purified on a protein A affinity column. Phorbol myristate acetate, α-cyclodextrin (α-CD), and methyl-β-cyclodextrin (MβCD) were from Sigma. The caspase inhibitors benzyloxycarbonyl-Val-Ala-Asp(Ome)-fluoromethylketone (zVAD-fmk) and benzyloxycarbonyl-Leu-Glu-His-Asp(Ome)-fluoromethylketone (zLEHD-fmk) were from Bachem (Voisins-le-Bretonneux, France).
Establishment of stably transfected cell lines.
The pEGFP-Bcl-2 plasmid (31) was kindly provided by R. Youle (National Institutes of Health, Bethesda, MD). The pEGFP empty vector (Clontech, Palo Alto, CA) was used as a control. The CD28-truncated cDNA lacking CD28 intracellular region (CD28Δ) (25) was kindly provided by A. Weiss (UCSF, San Francisco, CA) in the pAW-neo3 plasmid. Jurkat and H9 cells (5 × 106 cells in 0.8 ml) were transfected with 5 μg of plasmid by electroporation at 280 and 290 V, respectively, and 900 μF using an Easyject+ electroporator (Eurogentec, Seraing, Belgium) and resuspended in culture medium. Selection was performed with G418 (Life Technologies, Cergy-Pontoise, France) at 1.9 mg/ml for Jurkat cells and 1 mg/ml for H9 cells for 15 days. Then the G418-resistant cells were sorted using a Coulter Elite cell sorter (Coultronics, Hialeah, FL) on the basis of green fluorescent protein (GFP) fluorescence and used as polyclonal populations, or cells were cloned by limiting dilutions. The B7-1 cDNA was kindly provided by L. Lanier (UCSF, San Francisco, CA) and subcloned in the puromycin resistance vector pLXSP. The cells were transfected with the pLXSP-B7-1 or the pLXSP plasmids at 290 V and 900 μF, selected with puromycin at 2 μg/ml for 15 days, and then subcloned by limiting dilution.
Flow cytometry staining of cells.
Cells were washed, stained, and analyzed exactly as described previously (15). For the experiments using MβCD, the 1A12 cells were washed in serum-free medium, incubated in serum-free medium for 20 min at 37°C in the presence of the indicated concentration of MβCD, washed again, and finally stained.
Cell cytotoxicity assays.
The 4-h 51Cr release cytotoxicity assays using FasL-expressing cells as effectors were performed as described by Legembre et al. (15). Cytotoxicity assays using beads as effectors were performed as follows. Polystyrene beads with 6-μm diameters (Polybead Polystyrene Microsphere, Polysciences, Eppelheim, Germany) were washed three times with phosphate-buffered saline (PBS) and incubated overnight at room temperature at a ratio of 6 × 105 beads per 50 μl of antibody solution in PBS, with the IgM MAb (anti-Fas or negative control 10C9) at the indicated concentration and the IgG MAb (anti-CD28, anti-CD71, or negative control 1F10). After three washes with PBS, the beads were mixed with the 51Cr-labeled target cells at the indicated ratios, and the chromium released was measured. In experiments with phorbol myristate acetate (25 ng/ml), the cells were first labeled and then incubated for 30 min with the indicated dose of the chemical before adding the beads. In experiments with MβCD, the Jurkat cells and activated CD4+ T lymphocytes were incubated for 20 min in serum-free medium with 2 mM and 5 mM MβCD, respectively, or with α-CD at the same concentration as a control at 37°C and then washed before the addition of the effector cells or beads in medium containing 4% fetal calf serum.
Detergent lysis experiments.
The cells were incubated for the indicated times with MAb-coated beads at a 10:1 (beads/cell) ratio. The cells were lysed in lysis buffer (25 mM HEPES, 1% Triton X-100, 150 mM NaCl, pH 7.4) containing protease inhibitors (1 mM phenylmethylsulfonidefluoride, 5 μg/ml aprotinin, 10 μM leupeptin) for 30 min on ice. After centrifugation (10 min, 4°C, 15,000 rpm), the supernatant was harvested. The microdomains were isolated by ultracentrifugation on a sucrose gradient using the method of Ko et al. (13). To analyze the redistribution of Fas and caspase 8 into the microdomains, Jurkat 77 cells were incubated with 1A12 or 1A12-B7-1 cells for the indicated times and lysed with the lysis buffer. Time zero corresponds to a mix of separately lysed target and killer cells.
Immunoprecipitation of the DISC.
Jurkat 77 cells (7 × 106) were incubated for 25 min at 37°C with magnetic beads (Dynabeads M-450) coated with the anti-Fas MAb at 1 μg/ml and the indicated antibody at a 2:1 (beads/cell) ratio and then lysed for 20 min at 4°C in lysis buffer (25-min activation condition) or were lysed before the beads were added (0-min activation condition). The beads were separated with a magnet and washed three times with 1 ml of lysis buffer. The immunoprecipitate corresponding to 7 × 106 cells was loaded for each condition and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Western blot analysis.
Protein concentration in cellular extracts was determined using the bicinchoninic acid method (Sigma) according to the manufacturer's protocol. Proteins (10 μg per lane unless otherwise stated) were separated by SDS-PAGE on 12% gels in reducing conditions and transferred to a polyvinyldifluoride membrane (Amersham, Buckinghamshire, England), as described previously (15). The peroxidase-labeled secondary antisera used were anti-mouse (Amersham), anti-goat (Vector Laboratories, Burlingame, CA), or anti-rabbit (Zymed).
DNA fragmentation assay.
The target Jurkat cells (3 × 106 per condition) were incubated for 2 h with 30 × 106 coated beads (10:1 ratio). Then the cells were lysed and the DNA was precipitated and analyzed on a 1.5% agarose gel as described previously (28).
Immunofluorescence and imaging.
Jurkat cells were treated with the nonblocking and nonagonistic anti-Fas MAb (5D7) and fluorescein isothiocyanate (FITC)-labeled cholera toxin B for 30 min at 4°C and then mixed with 1A12 or 1A12-B7-1 at a 1:2 ratio. The mixed cells were incubated for 30 min at 37°C and adhered for 5 min at room temperature to poly-l-lysine-coated slides (ESCO, VWR, Strasbourg, France). Cells were then fixed in PBS containing 2% formaldehyde for 15 min, washed twice in PBS-1% bovine serum albumin and stained with the secondary antibody Alexa-594-conjugated goat anti-mouse antibody in PBS-1% bovine serum albumin. Slides were washed with PBS, dried, and mounted with Fluoroprep (Biomerieux, Marcy l'Etoile, France). Images were acquired and processed on a confocal microscope (LSM 510, Carl Zeiss, Jena, Germany) with a 63× objective.
RESULTS
Enhancement of Fas-mediated cell death by coligation of CD28.
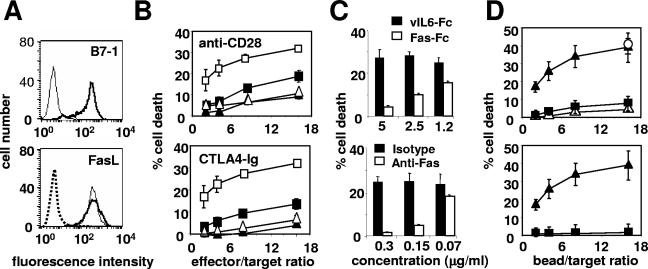
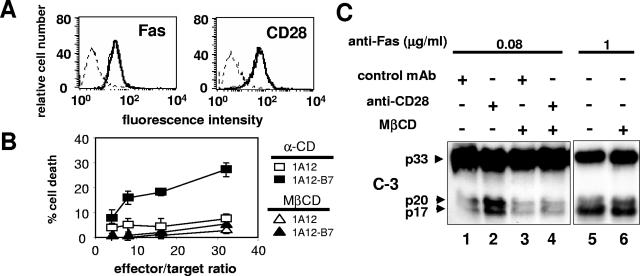
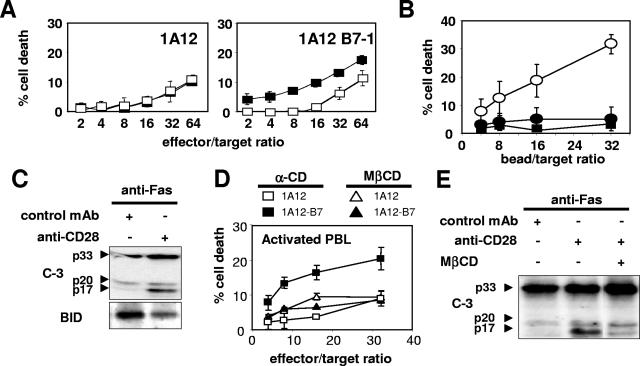
To analyze the effect of the coligation of Fas and CD28 on the apoptosis of the human T-cell line Jurkat 77, we used the human FasL-expressing murine cell line 1A12, which we stably transfected with the cDNA encoding the human CD28 ligand (B7-1 or CD80). A clone (1A12-B7-1), which expressed the same level of membrane FasL as the parent 1A12 cells, was chosen (Fig. 1A). The 1A12-B7-1 cell line was more efficient at killing Jurkat cells than the 1A12 cell line (Fig. 1B). Moreover, blocking the CD28/B7-1 interaction with a neutralizing anti-CD28 MAb or with the soluble Fc chimeric B7-1 receptor CTLA4 (CTLA4-Ig) abrogated the killing triggered by the 1A12-B7-1 clone but had no effect on 1A12-induced cell death (Fig. 1B). Similarly, blocking the FasL/Fas interaction with the soluble chimeric receptor Fas-Fc or with the neutralizing anti-Fas MAb ZB4 completely prevented the killing mediated by the 1A12-B7-1 cells (Fig. 1C). Therefore, CD28 did not trigger any cell death signal on its own, but it was able to efficiently enhance the death signal triggered by the activation of Fas. To analyze the costimulatory signal triggered by CD28 in the absence of the possible interference of other membrane molecules, these experiments were also conducted with polystyrene beads coated with the agonistic anti-Fas IgM MAb 7C11 and an anti-CD28 MAb (Fig. 1D). At a concentration of anti-Fas MAb triggering minimal cell death (0.08 μg/ml), the coadsorption of the anti-CD28 MAb strongly increased cell death (Fig. 1D, upper panel). At high bead/target ratios, the anti-Fas/anti-CD28-coated beads induced as much apoptosis as beads coated with a high concentration (10 μg/ml) of anti-Fas alone (Fig. 1D, empty circle). In contrast, a MAb specific for CD71 (transferrin receptor) had no effect (Fig. 1D), although CD71 is expressed by Jurkat cells at levels comparable to CD28 by flow cytometry (result not shown). Therefore, the effect of CD28 could not be attributed to a mere increase in the avidity of the beads for the cells. In addition, no cell death could be detected when MAb 7C11 was replaced by an irrelevant IgM-negative control MAb (Fig. 1D, lower panel). These results demonstrated that the anti-Fas/anti-CD28 beads behaved like the 1A12-B7-1 effector cells.
FIG. 1.
Fas-mediated cell death is amplified upon CD28 costimulation. (A) The membrane expression of B7-1 (upper panel) and of FasL (lower panel) on the 1A12 cell line (thin line) and on the 1A12-B7-1 cell line (thick line) was analyzed by flow cytometry. The dotted line depicts an isotype control MAb. (B) 1A12 (▴ and ▵) or 1A12-B7-1 (▪ and □) cell lines were incubated with 51Cr-labeled Jurkat 77 target cells at the indicated ratios in the presence of (▴ and ▪) soluble blocking anti-CD28 MAb (upper panel) or CTLA4-Ig (lower panel) or of (▵ and □) the negative control MAb or the control vIL6-Fc. (C) The 1A12-B7-1 cell line was incubated with the 51Cr-labeled Jurkat 77 target cells at an 8:1 ratio in the presence of Fas-Fc or vIL6-Fc (upper panel) or of the blocking anti-Fas MAb ZB4 or the control MAb (lower panel) at the indicated concentrations. (D) Polystyrene beads were coated with antibodies and mixed for 4 h with 51Cr-labeled Jurkat 77 target cells at the indicated ratios before cell death measurement. In the upper panel, anti-Fas MAb 7C11 (0.08 μg/ml) was coated together with the negative IgG control MAb (▪), the anti-CD28 MAb (▴), or the anti-CD71 MAb (▵) at 10 μg/ml. To quantify the maximum value of Fas-mediated apoptosis in this assay, beads were coated with 7C11 at high concentration (10 μg/ml) (○). In the lower panel, beads were coated with either the anti-Fas MAb 7C11 (0.08 μg/ml) and the anti-CD28 MAb (▴) or the negative IgM control MAb (0.08 μg/ml) and the anti-CD28 MAb (▪). Results represent the means and errors of three independently repeated experiments.
CD28 cosignal increases the Fas-mediated apoptotic pathway.
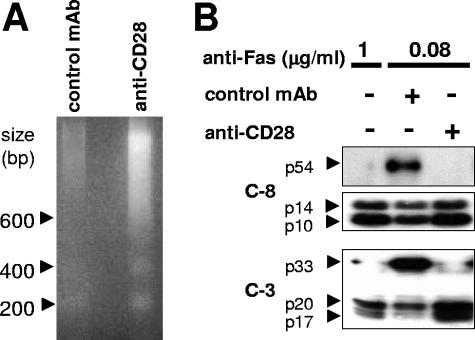
We then analyzed the effect of Fas/CD28 coligation on DNA fragmentation and procaspase 8 and 3 cleavage, which are hallmarks of Fas-induced apoptosis. A suboptimal concentration of anti-Fas MAb (0.08 μg/ml) triggered minimal DNA fragmentation (Fig. 2A) and caspase 8 and 3 activation (Fig. 2B), which were dramatically increased upon coligation of CD28. Therefore, the enhancement of Fas-mediated cell death by CD28 occurred via the canonical Fas apoptotic signaling cascade.
FIG. 2.
CD28 cosignal increases the Fas-mediated apoptotic pathway. Beads were coated with the anti-Fas (7C11) and the indicated antibody and then incubated with target Jurkat 77 cells at a 10:1 (beads/cell) ratio for 2 h before the cells were lysed. (A) DNA fragmentation was analyzed by agarose gel electrophoresis as described in Materials and Methods. (B) Activation of caspase 8 and caspase 3 was analyzed by immunoblotting. The results shown are representative of three independent experiments.
The intracellular region of CD28 is dispensable for enhancing Fas-mediated cell death.
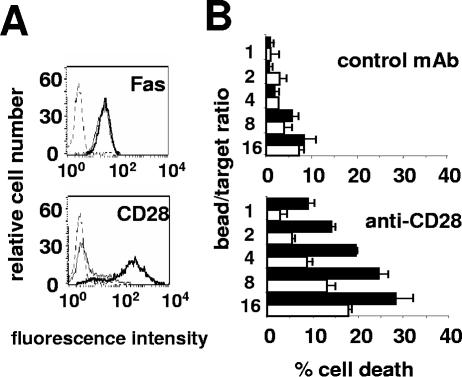
The CD28 intracellular domain has been reported as essential to trigger signaling pathways via phosphatidylinositol 3-kinase (PI3-K) (25). To analyze the role of this region in the enhancement of Fas-mediated cell death, we used a Jurkat cell line almost deficient for CD28 (3; Materials and Methods). We stably overexpressed in these cells an intracellular domain-truncated CD28 construct (CD28Δ) which is unable to transduce any cosignal for T-cell activation (25) or the control empty vector as a control. The cell populations raised expressed identical levels of membrane Fas (Fig. 3A), and a large amount of truncated CD28 was expressed on the cell surface in the Jurkat CD28Δ cell line (Fig. 3A). The control and CD28Δ-transfected cell lines displayed identical sensitivities to Fas stimulation (Fig. 3B). Due to residual expression of wild-type CD28 on around 20% of the control cell line (Fig. 3A), its sensitivity to killing after Fas/CD28 coligation slightly increased. However, the CD28Δ cell line was again much more sensitive to Fas/CD28 cotriggering (Fig. 3B). This result demonstrated that CD28Δ was capable of amplifying Fas-mediated cell death and did not inhibit the endogenous residual wild-type CD28 by acting as a dominant negative decoy receptor as could have been expected. Therefore, the proapoptotic role of CD28 on Fas-induced apoptosis was completely independent of its signal-transducing intracellular region.
FIG. 3.
CD28 intracellular region is not required for enhancing Fas-mediated apoptosis. (A) Fas and CD28 expression were analyzed by flow cytometry analysis on the bulk populations of sorted CD28-negative Jurkat cells stably transfected with the CD28Δ construct (thick line) or the control empty vector (thin line). The dashed line is the negative IgG control MAb. (B) CD28Δ (filled bars) or control (open bars) Jurkat cells were incubated with beads coated with the anti-Fas (0.08 μg/ml) and the negative IgG control MAb (upper panel) or the anti-CD28 MAb (lower panel) at the indicated ratios. Cell death was measured using the 51Cr release assay. Results represent the means and errors of three independently repeated experiments.
CD28 costimulation induces the redistribution of Fas into the microdomains.
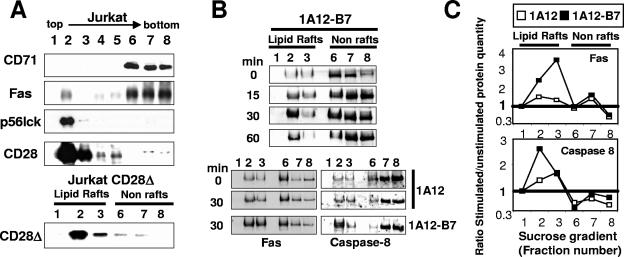
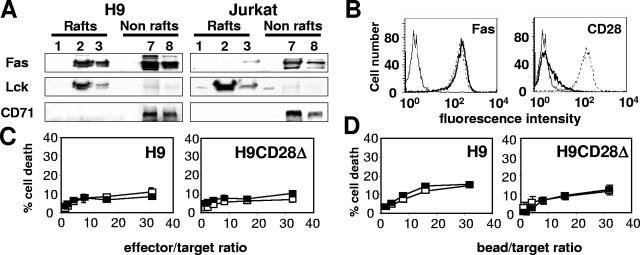
CD28 plays an important role during T-lymphocyte activation via the recruitment of the microdomains (30). By analogy to this function, we asked whether CD28 could increase the Fas-mediated signal via the same mechanism. To address this question, we first analyzed the membrane localization of CD28. The membrane fractions from a Jurkat 77 cell lysate were separated by sucrose gradient ultracentrifugation and analyzed by immunoblotting (Fig. 4A). CD71, which is used as a marker of the nonmicrodomain membranes, was concentrated in the high-density fractions (Fig. 4A, lanes 6 to 8), as was Fas (see also references 13 and 15). Conversely, the tyrosine kinase p56Lck, a resident of the microdomains, was found only in the low-density fractions (Fig. 4A, lanes 2 and 3). Interestingly, both wild-type CD28 and CD28Δ were exclusively localized in the microdomains.
FIG. 4.
Fas is redistributed in the lipid rafts upon CD28 cosignal. (A) Triton X-100 cell lysates prepared from the Jurkat 77 (upper part) or the Jurkat CD28Δ (lower part) cell lines were separated by ultracentrifugation on a sucrose gradient. Ten micrograms of proteins per lane was loaded, separated by SDS-PAGE, and analyzed by immunoblotting for their content in the indicated molecules. (B) Jurkat cells (50 × 106) were incubated at the indicated time with the 1A12 or 1A12-B7-1 cell lines as indicated (ratio, 1:2) and lysed. The lipid rafts were isolated by ultracentrifugation on a sucrose gradient, and Fas or caspase 8 was analyzed by immunoblotting. (Upper panel) Time-dependent relocation of Fas into the microdomains in response to 1A12-B7-1 cells. (Lower panel) Relocation of Fas and caspase 8 in response to 1A12 or 1A12-B7-1 cells after an incubation of 30 min. (C) Densitometry analysis of Fas and caspase 8 distribution. Values correspond to ratios of the intensity of specific bands at 30 min versus 0 min (see Materials and Methods) with 1A12 (□) or 1A12-B7-1 (▪) cells.
We next examined the localization of Fas upon CD28 costimulation. Jurkat 77 cells were incubated at various times with 1A12-B7-1 cells and lysed, and the lipid rafts were separated on sucrose gradient (Fig. 4B, upper panel). A 15-minute-long incubation with 1A12-B7-1 was sufficient to trigger the redistribution of Fas in the lipid rafts, which reached a peak after 30 min. We also analyzed caspase 8, which was also significantly translocated to the lipid rafts (Fig. 4B, lower panel). In comparison, the amount of Fas or caspase 8 redistributed in the lipid rafts was very faint when Fas alone was stimulated with 1A12 cells (Fig. 4B, lower panel). This was confirmed by densitometric analysis of the distribution of both proteins in these membrane fractions (Fig. 4C). To ascertain this, we attempted to visualize the translocation of Fas into the lipid rafts by fluorescence confocal microscopy. The microdomains were identified using the FITC-labeled B subunit of cholera toxin, which displays a high affinity for the ganglioside GM1 which is concentrated into the microdomains. The 1A12 cells stimulated the mobilization of Fas at the contact point between the cells, but the microdomains kept a homogenous distribution on the Jurkat cell surface (Fig. 5A). In contrast, 1A12-B7-1 cells induced the aggregation of the lipid rafts at the interface between the two cells, where the Fas receptors also merged (Fig. 5B). Therefore, Fas was completely colocalized with the lipid rafts upon CD28 coactivation. We can see that the Fas mobilization at the interface seemed to be augmented with the coactivation of CD28 (compare red staining in Fig. 5A and B). This effect could be due to the increased activation of caspase 8, as observed in Fig. 2B, for the Fas/CD28-stimulated Jurkat cells and then to a more efficient formation of the large Fas clusters, which depends on caspase 8 activity (2).
FIG. 5.
CD28 cosignal induces the relocation of Fas into the lipid rafts. Jurkat 77 cells were stained with anti-Fas MAb (5D7) and FITC-labeled cholera toxin B (green) for 30 min at 4°C, washed twice, and then stimulated for 30 min with (A) the 1A12 or (B) the 1A12-B7-1 cell line at 37°C. Anti-Fas was detected with an Alexa-594-conjugated goat anti-mouse antibody (red). For the bottom of panels A and B, the red and green fluorescence intensities of the plasma membrane were measured around the perimeter of the cell and plotted in the corresponding graphs. The y axis represents relative fluorescence intensity of the pixels, and the x axis represents the distance around the periphery of the cell.
Disruption of lipid rafts inhibits the CD28-triggered amplification of Fas-mediated apoptosis.
To confirm the role of the microdomains recruited by CD28 in the amplification of Fas-mediated apoptosis, we examined the consequences of their disruption. This can be achieved by depletion of plasma membrane cholesterol using MβCD, whereas its close homolog α-CD cannot (32). Treatment of the Jurkat cell line with MβCD did not alter the membrane expression of CD28 and of Fas (Fig. 6A). When Jurkat cells were preincubated with MβCD, the CD28-mediated amplification of Fas apoptosis was abrogated (Fig. 6B, compare open and filled triangles), whereas α-CD had no effect (Fig. 6B, compare open and filled squares). To analyze the consequence of microdomain disruption on Fas signaling, the cells were incubated with or without MβCD, washed to remove the excess of chemical, and mixed with the MAb-coated beads for 45 min before cell lysis and analysis of caspase 3 cleavage. As previously described (2, 18), the disruption of lipid rafts did not modify the Fas-mediated apoptosis pathway in our type II cell line (Fig. 6C, compare lanes 5 and 6). In contrast, the pretreatment with MβCD completely abrogated the CD28-mediated amplification of caspase 3 cleavage (Fig. 6C, compare lanes 2 and 4), leading to the conclusion that lipid raft recruitment can enhance Fas-induced cell death in a type II cell line.
FIG. 6.
Disruption of the microdomains inhibits the CD28 cosignal. (A) Stability of membrane receptor expression level following MβCD treatment. Jurkat 77 cells were incubated for 20 min with (thick line) or without (thin line) MβCD (2 mM), and then the surface level of CD28 and Fas was analyzed by flow cytometry. The dashed line is the negative IgG control MAb. (B) Jurkat 77 cells were treated for 20 min with 2 mM MβCD or α-CD and incubated with either 1A12 or 1A12-B7-1 effector cells, and a 51Cr release assay was performed. (C) Jurkat cells were treated as in panel B, incubated for 45 min with beads coated with the indicated antibodies, and then lysed. Caspase 3 activation was analyzed by immunoblotting. The data are representative of three independent experiments.
CD28 costimulation in type I cell line H9 does not modify Fas-triggered apoptosis.
Fas is concentrated in lipid rafts in type I cell lines at a resting state, and microdomains are essential to the induction of the Fas-mediated apoptosis signal in these cells (18). Therefore, we wondered whether the recruitment of CD28-linked microdomains in a type I cell line, such as the H9 cell line (22), could enhance Fas-mediated apoptosis.
As already reported (18), we observed that a large amount of Fas was concentrated in the lipid rafts, in contrast to the type II Jurkat cell line (Fig. 7A). Because CD28 expression in H9 cells was very faint (Fig. 7B), and given the dispensability of CD28 intracellular region for raft recruitment, we overexpressed CD28Δ or the empty control vector in H9 cells. The bulks of stably transfected H9 cells were analyzed by flow cytometry to estimate Fas and CD28 expression (Fig. 7B). H9 CD28Δ and control transfectants displayed comparable levels of Fas (Fig. 7B). To analyze whether the microdomain recruitment could increase Fas signaling in the type I cell line, we performed 51chromium release cytotoxicity assays with the effector cells 1A12 and 1A12-B7-1 (Fig. 7C) and with polystyrene beads coated with a concentration of anti-Fas MAb triggering minimal cell death (1 μg/ml) and either the anti-CD28 MAb or the negative control MAb (Fig. 7D). CD28 recruitment in the H9 CD28Δ cell line did not alter the sensitivity to Fas-induced apoptosis. This observation is consistent with the demonstration that the type I cells already use efficiently the microdomains in response to Fas (18) and suggests that type I cells cannot be modulated by the indirect recruitment of CD28-associated lipid rafts.
FIG. 7.
CD28-mediated microdomain recruitment in a type I cell line does not improve the Fas-dependent apoptotic signal. (A) H9 (type I) and Jurkat 77 (type II) cells were lysed, and lipid rafts were separated from the rest of the membrane by ultracentrifugation on a sucrose gradient. The distribution of the indicated proteins was analyzed by immunoblotting. (B) Fas and CD28 expression in the CD28-deficient H9 cell line transfected with empty vector (thick line) or with the CD28Δ-containing plasmid (dashed line). The thin line represents a negative control MAb. (C) Control H9 cells or CD28Δ-expressing H9 cell lines were incubated with the 1A12 or 1A12-B7-1 cell line, and apoptosis was measured with the 51Cr release assay. (D) H9 and CD28Δ-expressing H9 cells were incubated in the presence of beads coated with the anti-Fas MAb 7C11 (1 μg/ml) and either the anti-CD28 MAb or the negative IgG control MAb (10 μg/ml), and a 51Cr release assay was performed. For all of the 51Cr release assays, results represent the means and errors of three independently repeated experiments.
Microdomain recruitment via CD28 enhances the mitochondrion-dependent apoptotic pathway.
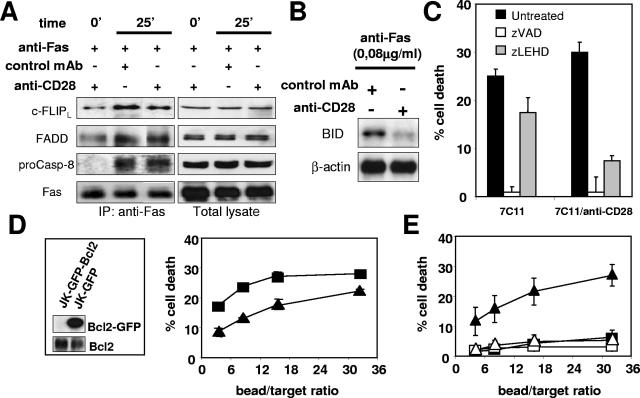
The increase in caspase 8 cleavage (Fig. 2A) prompted us to analyze whether the stimulation of CD28 could enhance the Fas-mediated apoptotic pathway at the step of DISC formation. For this purpose, Jurkat cells were stimulated with magnetic beads coated with MAb 7C11 in combination with the anti-CD28 MAb or the control MAb. Fas was immunoprecipitated, and the DISC components were analyzed by immunoblotting. None was detectable when apoptosis was triggered with the anti-Fas MAb at 0.08 μg/ml, whatever CD28 was coligated or not (result not shown). The DISC components could be immunoprecipitated in detectable amounts only when the anti-Fas MAb concentration reached 1 μg/ml. Such a concentration of MAb 7C11 is required, since the DISC is known to be weakly formed in type II cells (22). However, at a 7C11 concentration of 1 μg/ml, the enhancing effect of CD28 coligation on Fas-induced cell death is still efficient (28% cell death versus 15% in the absence of CD28 ligation, as determined with the 51Cr release assay [result not shown]). Faint amounts of the 54-kDa form of c-FLIP (c-FLIPL) and FADD, but not procaspase 8, were found to be preassociated to Fas prior to activation (Fig. 8A). Following stimulation with the anti-Fas or with the anti-Fas/anti-CD28 beads, procaspase 8 was recruited, and the amount of c-FLIPL and FADD increased, demonstrating the formation of the DISC (Fig. 8A). However, no significant difference could be evidenced for DISC formation between Fas and Fas/CD28 stimulation.
FIG. 8.
CD28 cosignal does not alter DISC formation but enhances the mitochondrion-dependent apoptotic pathway. (A) Magnetic beads were coated with the anti-Fas MAb (1 μg/ml) and the indicated antibody (10 μg/ml) and then incubated with 7 × 106 Jurkat 77 cells for 25 min at a 2:1 (beads/cell) ratio before the cells were lysed. As a control, cells were lysed before the beads were added (time zero). The DISC was immunoprecipitated, and its components c-FLIPL, FADD, procaspase 8, and Fas were analyzed by immunoblotting. A total cell lysate was also analyzed as a control. (B) Beads coated with anti-Fas and the indicated MAb were incubated with target Jurkat cells for 2 h at a 10:1 (beads/cell) ratio. The cells were lysed, and the uncleaved p22 BID was detected by immunoblotting. The cleaved fragments are not detected with the antibody used. Detection of β-actin is used as a control for protein loading. (C) 51Cr-labeled Jurkat 77 cells were incubated for 30 min at 37°C with the pan-caspase inhibitor zVAD-fmk or the specific caspase 9 inhibitor zLEHD-fmk (10 and 50 μM, respectively) and then mixed with beads coated with a high concentration of anti-Fas MAb alone (1 μg/ml, white bars) or anti-Fas MAb (0.08 μg/ml) with the anti-CD28 MAb (black bars) at a 10:1 (beads/cell) ratio for 4 h. Apoptosis was measured by 51Cr release assay. (D) Jurkat 77 cells were transfected with GFP-Bcl-2 or GFP, and protein expression was analyzed in cell lysates by performing a Bcl-2 immunoblot. The sensitivity of Jurkat GFP (▪) and Jurkat GFP-Bcl-2 (▴) cells to Fas-mediated apoptosis was analyzed in a 51Cr release assay using beads coated with a high concentration of 7C11 (1 μg/ml). (E) Jurkat GFP (filled symbols) or Jurkat GFP-Bcl-2 (open symbols) cells were incubated with beads coated with anti-Fas (0.08 μg/ml) and anti-CD28 (triangles) or the negative IgG control (squares). For all of the 51Cr release assays, results represent the means and errors of three independently repeated experiments, and graphs are representative of three independent experiments.
As CD28 did not enhance apoptosis in the H9 cell line and DISC formation, a feature of type I cells, in the Jurkat cell line, we then hypothesized that CD28-mediated microdomain recruitment could enhance the mitochondrion-dependent type II apoptotic pathway. We first analyzed the activation of the mitochondrion-targeting molecule BID. This was examined via the disappearance of its precursor form p22, which occurs after its activation by cleavage. When compared to beads coated with the anti-Fas MAb alone, the anti-Fas/anti-CD28 beads led to a more pronounced activation and loss of p22 BID (Fig. 8B).
Second, we analyzed the caspase 9 activity, which is induced via the mitochondrial release of cytochrome c. Caspase 9 activity can be specifically blocked with the peptidic inhibitor zLEHD-fmk, whereas the inhibitor zVAD-fmk blocks all caspases. As such, zVAD-fmk completely inhibited the cell death triggered by beads covered with a high anti-Fas concentration (1 μg/ml of 7C11) or with the anti-Fas/anti-CD28 beads (Fig. 8C, 7C11/anti-CD28), showing that apoptosis triggered by Fas/CD28 coligation is totally dependent on caspase activation. In contrast, zLEHD-fmk strongly impaired the death signal triggered by the Fas/CD28 coligation while having a weaker effect on the signal mediated by Fas alone (75% versus 34% inhibition, respectively), indicating that the effect of CD28 preferentially involved caspase 9.
Bcl-2 inhibits the mitochondrial cell death pathway. We generated Jurkat cells overexpressing a GFP-Bcl-2 chimera or GFP alone as a control (Fig. 8D, insert) and displaying comparable amounts of surface CD28 and Fas as detected by flow cytometry (result not shown). The Jurkat GFP-Bcl-2 cells were slightly less sensitive than the control cells to killing via Fas, as shown with beads coated with anti-Fas MAb at a high concentration (1 μg/ml) (Fig. 8D). In contrast, Bcl-2 overexpression totally abrogated the enhancing effect of CD28 (Fig. 8E, compare filled and empty triangles). Taken together, these experiments demonstrated that in a type II cell line the microdomain recruitment selectively acted through the mitochondrion-dependent pathway of Fas-mediated apoptosis.
CD28-mediated microdomain recruitment enhances Fas-mediated apoptosis of primary activated CD4+ T lymphocytes from peripheral blood.
Primary activated CD4+ T cells are refractory to bivalent Fas stimulus but become sensitive after TCR restimulation (18). Fas is excluded from the microdomains in these primary activated T cells and is redistributed in these structures after TCR restimulation. Therefore, primary activated T cells behaved as type II cells (18). To extend our findings to primary immune cells, we analyzed whether the primary activated CD4+ T lymphocytes could be sensitized to Fas-mediated apoptosis pathway via the recruitment of CD28-associated microdomains. CD4+ T lymphocytes were enriched by negative selection from PBMC of healthy donors and polyclonally activated with phytohemagglutinin and IL-2. As observed with the Jurkat cells, the 1A12-B7-1 cell line killed more efficiently the activated CD4+ lymphocytes than the 1A12 cell line, and blocking the CD28/B7-1 interaction with the neutralizing anti-CD28 MAb inhibited the killing triggered by the 1A12-B7-1 cell line but not by the 1A12 cell line (Fig. 9A). When using MAb-coated beads as effectors with a concentration of anti-Fas MAb triggering minimal apoptosis, the coligation of CD28 also enhanced Fas-mediated cell death, whereas CD28 did not trigger cell death by itself (Fig. 9B). Fas/CD28 coligation via antibody-coated polystyrene beads efficiently increased the cleavage of caspase 3 and BID compared to the cleavage triggered by the anti-Fas MAb (Fig. 9C). Moreover, pretreatment of the CD4+ T lymphocytes with MβCD abrogated the enhancing effect of CD28 on Fas-induced cell death (Fig. 9D), as well as on caspase 3 cleavage (Fig. 9E). Therefore, as observed with Jurkat cell line, the Fas-mediated apoptosis in the primary activated T lymphocytes can be efficiently increased by microdomain recruitment.
FIG. 9.
CD28 corecruitment increases Fas-mediated apoptosis in primary activated CD4+ T lymphocytes from peripheral blood. (A) 51Cr-labeled activated CD4+ T lymphocytes were incubated with 1A12 or 1A12-B7-1 cells in the absence (▪) or in the presence (□) of the blocking anti-CD28 MAb. (B) 51Cr-labeled activated CD4+ T lymphocytes were incubated with beads coated with the anti-Fas MAb (2 μg/ml) and either the negative IgG control MAb (•) or the anti-CD28 MAb (○). As a control, beads were coated with the anti-CD28 MAb and the negative IgM control MAb (▪). (C) Primary activated CD4+ T lymphocytes were incubated for 2 h with beads coated with the anti-Fas MAb at 2 μg/ml and either the anti-CD28 MAb or an irrelevant IgG MAb. The cells were lysed, and total proteins (10 μg/lane) were separated by SDS-PAGE before immunoblotting for caspase 3 and BID. (D) Primary activated CD4+ T lymphocytes were treated for 20 min with 5 mM MβCD or α-CD and incubated with either 1A12 effector cells or 1A12-B7-1 cells, and a 51Cr release assay was performed. (E) Primary activated CD4+ T lymphocytes were treated for 20 min with 5 mM MβCD or left untreated. After washing, the cells were incubated for 45 min with beads coated with the indicated antibodies and then lysed, and total proteins (10 μg/lane) were separated by SDS-PAGE before immunoblotting for caspase 3. For all of the 51Cr-release assays, results represent the means and errors of three independently repeated experiments.
DISCUSSION
In this report, we demonstrated that CD28 and Fas coengagement amplifies the Fas apoptotic pathway in type II cells. This effect was independent of the CD28 intracellular region and required the recruitment of the lipid rafts. Several groups have reported that Fas was localized in the microdomains in type I but not in type II cells (8, 18) and that microdomains are involved in the Fas-mediated apoptosis pathway in type I but not in type II cells (18). We give evidence that in a type I cell line, the recruitment of CD28-containing microdomains is unable to improve the Fas-mediated apoptotic pathway. We also demonstrate that when type II cells become more sensitive to the forced redistribution of Fas into these microdomains, they don't do so by shifting to type I. Indeed, CD28 costimulation did not improve the formation of the DISC but rather strongly enhanced the mitochondrion-dependent apoptotic pathway. This discrepancy could indicate that in type I cells, the Fas-containing microdomains are different from those recruited by CD28 in type II cells. Alternatively, these microdomains may be identical in both types of cells but behave only as nonspecific “cofactors” enhancing the Fas-induced signaling pathway, which is known to be different between type I and type II cells. In the latter case, the contribution of the microdomains to the apoptotic signal in type I cells could not be increased further, as a plateau is already reached (18). We also observed a dramatic increase in caspase 8 cleavage when Fas and CD28 were cotriggered. This may at first glance seem contradictory to the steady level of DISC formation, since procaspase 8 recruitment was not modified. However, it has been demonstrated that caspase 8 activation can also occur downstream of the mitochondrial cascade (9), since it is a potential substrate of caspase 9 and/or caspase 3 (24), which are both strongly involved in the CD28 cosignal.
Upon CD28 engagement, its intracellular region recruits and activates the PI3-K signaling pathway, which is involved in the protection of T lymphocytes from Fas-mediated apoptosis (12). Although our conclusions seem at first glance contradictory to these findings, we actually do not challenge these results in the present study for the following two reasons. First, we focused on the effect on Fas stimulation of CD28-mediated microdomain recruitment and not on the consequences on Fas sensitivity of the costimulation of CD28 with TCR. Second, we demonstrated that CD28 amplifies the Fas-mediated cell death pathway independently of its intracellular region, which is known to be absolutely required for activating the PI3-K pathway (25). It is noteworthy that in the same way as for caspase 8, which plays a major role in both T-cell activation and T-cell elimination (5, 20), the costimulatory molecule CD28 could be used in the two opposite processes. Therefore, the T-lymphocyte activation process would require for its completion that pathways involved in the delayed elimination of the T cells are functional, which may decrease the risk of uncontrolled T-cell proliferation.
We demonstrate that the recruitment of microdomains can improve Fas-mediated apoptosis in so-called type II cells. As activated lymphocytes are described as type II cells, with Fas being excluded from microdomains (18), our findings suggest that target cells could use this mechanism to eliminate the surrounding T lymphocytes for evading the immune system, e.g., in the case of transformed or virus-infected cells. Indeed, it has been reported that Hodgkin's lymphoma cells express FasL (7) and also overexpress the CD28 ligands CD80 and CD86 (21). Similarly, cytomegalovirus-infected dendritic cells coexpress FasL and CD28 ligands. These antigen-presenting cells can eliminate activated T lymphocytes via a Fas-dependent mechanism, while these T cells were resistant to FasL alone. Therefore, the infected dendritic cells could play the role of trojan horses which, instead of stimulating T-cell activation via antigen presentation, can enhance T-cell deletion, allowing the cytomegalovirus to evade the immune response (19).
We recently demonstrated that a microdomain-concentrated GPI-linked chimeric Fas protein was able to amplify the Fas signaling pathway. Therefore, the costimulation of Fas-mediated apoptosis through microdomain recruitment may involve membrane coreceptors distinct from CD28. It has been reported that cells from squamous head-and-neck carcinoma, one of the most immunosuppressive human cancers, express high levels of membrane FasL. Strikingly, these cells selectively enhanced the mitochondrial pathway of apoptosis in Jurkat target cells compared to the stimulation of Fas alone (10). Although this mechanism remains unsolved to date, we suggest the possible involvement of a microdomain-recruiting coreceptor in this immune evasion phenomenon. More generally, we propose that the recruitment of the microdomains to Fas through nonapoptotic ligand/receptor pairs during cell-to-cell contacts represents an as-yet-unraveled general pathway for modulating Fas-induced cell death.
Acknowledgments
We thank J. Déchanet-Merville for insightful discussions. We thank M. E. Peter (Ben May institute for cancer research, Chicago, IL) for providing anti-c-FLIP (clone NF-6) and F. Belloc (laboratoire d'hématologie, Hôpital du Haut-Lévèque, CHU de Bordeaux) for the cell sorting experiments.
P.L. is supported by a grant from the Association pour la Recherche sur le Cancer. This work was supported by grants from the Association pour la Recherche sur le Cancer and from the Ligue Contre le Cancer des Landes, de la Charente, de la Dordogne, de la Gironde, des Pyrénées-Atlantiques.
REFERENCES
- 1.Alderson, M. R., T. W. Tough, T. Davis-Smith, S. Braddy, B. Falk, K. A. Schooley, R. G. Goodwin, C. A. Smith, F. Ramsdell, and D. H. Lynch. 1995. Fas ligand mediates activation-induced cell death in human T lymphocytes. J. Exp. Med. 181:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algeciras-Schimnich, A., L. Shen, B. C. Barnhart, A. E. Murmann, J. K. Burkhardt, and M. E. Peter. 2002. Molecular ordering of the initial signaling events of CD95. Mol. Cell. Biol. 22:207-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bani, L., V. Pasquier, M. Kryworuchko, J. Salamero, and J. Theze. 2001. Unstimulated human CD4 lymphocytes express a cytoplasmic immature form of the common cytokine receptor gamma-chain. J. Immunol. 167:344-349. [DOI] [PubMed] [Google Scholar]
- 4.Cerny, J., H. Stockinger, and V. Horejsi. 1996. Noncovalent associations of T lymphocyte surface proteins. Eur. J. Immunol. 26:2335-2343. [DOI] [PubMed] [Google Scholar]
- 5.Chun, H. J., L. Zheng, M. Ahmad, J. Wang, C. K. Speirs, R. M. Siegel, J. K. Dale, J. Puck, J. Davis, C. G. Hall, S. Skoda-Smith, T. P. Atkinson, S. E. Straus, and M. J. Lenardo. 2002. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419:395-399. [DOI] [PubMed] [Google Scholar]
- 6.Dhein, J., H. Walczak, C. Baumler, K. M. Debatin, and P. H. Krammer. 1995. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 373:438-441. [DOI] [PubMed] [Google Scholar]
- 7.Dutton, A., J. D. O'Neil, A. E. Milner, G. M. Reynolds, J. Starczynski, J. Crocker, L. S. Young, and P. G. Murray. 2004. Expression of the cellular FLICE-inhibitory protein (c-FLIP) protects Hodgkin's lymphoma cells from autonomous Fas-mediated death. Proc. Natl. Acad. Sci. USA 101:6611-6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eramo, A., M. Sargiacomo, L. Ricci-Vitiani, M. Todaro, G. Stassi, C. G. Messina, I. Parolini, F. Lotti, G. Sette, C. Peschle, and R. De Maria. 2004. CD95 death-inducing signaling complex formation and internalization occur in lipid rafts of type I and type II cells. Eur. J. Immunol. 34:1930-1940. [DOI] [PubMed] [Google Scholar]
- 9.Fulda, S., S. A. Susin, G. Kroemer, and K. M. Debatin. 1998. Molecular ordering of apoptosis induced by anticancer drugs in neuroblastoma cells. Cancer Res. 58:4453-4460. [PubMed] [Google Scholar]
- 10.Gastman, B. R., X. M. Yin, D. E. Johnson, E. Wieckowski, G. Q. Wang, S. C. Watkins, and H. Rabinowich. 2000. Tumor-induced apoptosis of T cells: amplification by a mitochondrial cascade. Cancer Res. 60:6811-6817. [PubMed] [Google Scholar]
- 11.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 12.Jones, R. G., A. R. Elford, M. J. Parsons, L. Wu, C. M. Krawczyk, W. C. Yeh, R. Hakem, R. Rottapel, J. R. Woodgett, and P. S. Ohashi. 2002. CD28-dependent activation of protein kinase B/Akt blocks Fas-mediated apoptosis by preventing death-inducing signaling complex assembly. J. Exp. Med. 196:335-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko, Y. G., J. S. Lee, Y. S. Kang, J. H. Ahn, and J. S. Seo. 1999. TNF-alpha-mediated apoptosis is initiated in caveolae-like domains. J. Immunol. 162:7217-7223. [PubMed] [Google Scholar]
- 14.Koppi, T. A., T. Tough-Bement, D. M. Lewinsohn, D. H. Lynch, and M. R. Alderson. 1997. CD40 ligand inhibits Fas/CD95-mediated apoptosis of human blood-derived dendritic cells. Eur. J. Immunol. 27:3161-3165. [DOI] [PubMed] [Google Scholar]
- 15.Legembre, P., P. Moreau, S. Daburon, J. F. Moreau, and J. L. Taupin. 2002. Potentiation of Fas-mediated apoptosis by an engineered glycosylphosphatidylinositol-linked Fas. Cell Death Differ. 9:329-339. [DOI] [PubMed] [Google Scholar]
- 16.Matsue, H., D. Edelbaum, A. C. Hartmann, A. Morita, P. R. Bergstresser, H. Yagita, K. Okumura, and A. Takashima. 1999. Dendritic cells undergo rapid apoptosis in vitro during antigen-specific interaction with CD4+ T cells. J. Immunol. 162:5287-5298. [PubMed] [Google Scholar]
- 17.Montixi, C., C. Langlet, A. M. Bernard, J. Thimonier, C. Dubois, M. A. Wurbel, J. P. Chauvin, M. Pierres, and H. T. He. 1998. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 17:5334-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muppidi, J. R., and R. M. Siegel. 2004. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat. Immunol. 5:182-189. [DOI] [PubMed] [Google Scholar]
- 19.Raftery, M. J., M. Schwab, S. M. Eibert, Y. Samstag, H. Walczak, and G. Schonrich. 2001. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15:997-1009. [DOI] [PubMed] [Google Scholar]
- 20.Salmena, L., B. Lemmers, A. Hakem, E. Matysiak-Zablocki, K. Murakami, P. Y. Au, D. M. Berry, L. Tamblyn, A. Shehabeldin, E. Migon, A. Wakeham, D. Bouchard, W. C. Yeh, J. C. McGlade, P. S. Ohashi, and R. Hakem. 2003. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 17:883-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savage, K. J., S. Monti, J. L. Kutok, G. Cattoretti, D. Neuberg, L. De Leval, P. Kurtin, P. Dal Cin, C. Ladd, F. Feuerhake, R. C. Aguiar, S. Li, G. Salles, F. Berger, W. Jing, G. S. Pinkus, T. Habermann, R. Dalla-Favera, N. L. Harris, J. C. Aster, T. R. Golub, and M. A. Shipp. 2003. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood 102:3871-3879. [DOI] [PubMed] [Google Scholar]
- 22.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scaffidi, C., I. Schmitz, J. Zha, S. J. Korsmeyer, P. H. Krammer, and M. E. Peter. 1999. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J. Biol. Chem. 274:22532-22538. [DOI] [PubMed] [Google Scholar]
- 24.Slee, E. A., M. T. Harte, R. M. Kluck, B. B. Wolf, C. A. Casiano, D. D. Newmeyer, H. G. Wang, J. C. Reed, D. W. Nicholson, E. S. Alnemri, D. R. Green, and S. J. Martin. 1999. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 144:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein, P. H., J. D. Fraser, and A. Weiss. 1994. The cytoplasmic domain of CD28 is both necessary and sufficient for costimulation of interleukin-2 secretion and association with phosphatidylinositol 3′-kinase. Mol. Cell. Biol. 14:3392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka, M., T. Suda, K. Haze, N. Nakamura, K. Sato, F. Kimura, K. Motoyoshi, M. Mizuki, S. Tagawa, S. Ohga, K. Hatake, A. H. Drummond, and S. Nagata. 1996. Fas ligand in human serum. Nat. Med. 2:317-322. [DOI] [PubMed] [Google Scholar]
- 27.Taupin, J. L., B. Acres, K. Dott, D. Schmitt, M. P. Kieny, N. Gualde, and J. F. Moreau. 1993. Immunogenicity of HILDA/LIF either in a soluble or in a membrane anchored form expressed in vivo by recombinant vaccinia viruses. Scand. J. Immunol. 38:293-301. [DOI] [PubMed] [Google Scholar]
- 28.Tian, Q., J. Taupin, S. Elledge, M. Robertson, and P. Anderson. 1995. Fas-activated serine/threonine kinase (FAST) phosphorylates TIA-1 during Fas-mediated apoptosis. J. Exp. Med. 182:865-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vignaux, F., and P. Golstein. 1994. Fas-based lymphocyte-mediated cytotoxicity against syngeneic activated lymphocytes: a regulatory pathway? Eur. J. Immunol. 24:923-927. [DOI] [PubMed] [Google Scholar]
- 30.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 283:680-682. [DOI] [PubMed] [Google Scholar]
- 31.Wolter, K. G., Y. T. Hsu, C. L. Smith, A. Nechushtan, X. G. Xi, and R. J. Youle. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139:1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xavier, R., T. Brennan, Q. Li, C. McCormack, and B. Seed. 1998. Membrane compartmentation is required for efficient T cell activation. Immunity 8:723-732. [DOI] [PubMed] [Google Scholar]