Abstract
Skeletal muscles are a mosaic of slow and fast twitch myofibers. During embryogenesis, patterns of fiber type composition are initiated that change postnatally to meet physiological demand. To examine the role of the protein phosphatase calcineurin in the initiation and maintenance of muscle fiber types, we used a “Flox-ON” approach to obtain muscle-specific overexpression of the modulatory calcineurin-interacting protein 1 (MCIP1/DSCR1), an inhibitor of calcineurin. Myo-Cre transgenic mice with early skeletal muscle-specific expression of Cre recombinase were used to activate the Flox-MCIP1 transgene. Contractile components unique to type 1 slow fibers were absent from skeletal muscle of adult Myo-Cre/Flox-MCIP1 mice, whereas oxidative capacity, myoglobin content, and mitochondrial abundance were unaltered. The soleus muscles of Myo-Cre/Flox-MCIP1 mice fatigued more rapidly than the wild type as a consequence of the replacement of the slow myosin heavy chain MyHC-1 with a fast isoform, MyHC-2A. MyHC-1 expression in Myo-Cre/Flox-MCIP1 embryos and early neonates was normal. These results demonstrate that developmental patterning of slow fibers is independent of calcineurin, while the maintenance of the slow-fiber phenotype in the adult requires calcineurin activity.
Adult mammalian skeletal muscle is composed of multinucleated myofibers that can be classified based upon expression of one of four adult myosin heavy chain (MyHC) genes that contribute specific contractile properties (23). Muscle fiber types are identified as either type 1 (slow) or type 2 (fast) based upon velocity of contraction and rate of fatigue. Type 1 slow fibers express MyHC-1, are highly oxidative, and are rich in both mitochondria and myoglobin, which gives them their red color. Type 2 fast fibers can be further subdivided as 2A, 2X (also denoted 2D), and 2B based upon expression of MyHC-2A, MyHC-2X, and MyHC-2B, respectively. Type 2A fibers are oxidative and are rich in both mitochondria and myoglobin. In contrast, type 2B fibers are glycolytic and lack myoglobin. Type 2X fibers have an intermediate phenotype. In the mouse embryo, a pattern of fast and slow muscle fibers is established during several waves of myoblast fusion. Primary myofibers are formed during embryonic day 12 (E12) to E14 (18). A second wave of myotube formation occurs during E16 to E18. MyHC-1 is expressed both in the embryo and adult, whereas embryo-specific fast fiber isoforms, MyHC-emb and MyHC-neo, are replaced by adult isoforms after birth.
Postnatally, skeletal fiber-type composition is highly plastic and remodels in response to neuronal input and motor function in order to meet physiological demand (26). For instance, inactivity results in a general shift in MyHC expression and metabolic properties along the progression of 1→2A→2X→2B. Endurance training promotes a shift in the opposite direction, 2B→2X→2A→1 (28). The calcium-activated protein phosphatase calcineurin has been proposed as an important regulator of muscle fiber type that activates the transcription factor nuclear factor of activated T cells (NFAT) to translate activity at the neuromuscular junction into a specific transcriptional response driving fiber type remodeling (4). To test this model, several transgenic mouse lines have been made with skeletal muscle overexpression of constitutively active calcineurin (5, 16, 32). Many of these mice show an increase in type 1 fiber content, although the majority of the muscle is not converted to slow fibers, suggesting that calcineurin activity alone is not sufficient to drive the slow-fiber phenotype.
To identify fiber type characteristics that are dependent on calcineurin activity, loss-of-function experiments have been carried out both in vitro and in vivo. These have relied on drugs that inhibit calcineurin (24) or disruption of genes encoding components of the calcineurin/NFAT signaling pathway (8, 12, 19, 20) and have resulted in a variety of changes in muscle fiber characteristics. These studies demonstrate that MyHC-1 expression is sensitive to calcineurin inhibition. However, whether oxidative type 2A fibers are also dependent on calcineurin is less clear. Studies examining the consequences of calcineurin inhibition on oxidative metabolism, mitochondrial proliferation, and myoglobin content are likewise inconclusive, with results ranging from inhibition to stimulation. Interpretation of drug treatment and gene knockout studies can be complicated, because inhibition of calcineurin signaling in other tissues (such as motor neurons) may contribute to altered muscle fiber phenotypes. To date, no studies have examined whether muscle-autonomous calcineurin signaling is required for fiber type patterning during embryogenesis.
In order to examine the role of calcineurin signaling specifically in muscle fibers we have used a “Flox-ON” approach to generate a transgenic mouse with skeletal muscle-specific overexpression of modulatory calcineurin-interacting protein 1 (MCIP1). MCIP1 binds directly to calcineurin and inhibits phosphatase activity (22). Unlike the calcineurin-inhibitory drugs cyclosporine and FK506, MCIP1 has no apparent toxic side effects. The Flox-MCIP1 mice were crossed with a transgenic mouse line, Myo-Cre, that expresses Cre recombinase specifically in skeletal muscle beginning in myoblasts of developing somites (14). The Myo-Cre/Flox-MCIP1 animals lack type 1 fibers but have neither a change in mitochondrial content nor a shift from oxidative to glycolytic metabolism. Because the MCIP1 transgene is activated in myoblasts prior to primary myotube formation, we have also been able to demonstrate that myocyte-autonomous calcineurin signals are not involved in muscle fiber type patterning during embryogenesis.
MATERIALS AND METHODS
Transgenic mice.
Myo-Cre transgenic mice with skeletal muscle-specific expression of Cre recombinase are described elsewhere (14). Construction of the Flox-MCIP1 transgene was based on a plasmid that contains a 1.6-kb cytomegalovirus (CMV) enhancer/β-actin promoter with loxP sites flanking a chloramphenicol acetyl-transferase (CAT) gene followed by a β-galactosidase gene (lacZ). The lacZ gene and polyadenylation sequence were replaced by a 1-kb fragment encoding a FLAG-tagged MCIP1 with a bovine growth hormone polyadenylation signal.
Fiber type and immunohistochemical analysis of skeletal muscle.
Soleus and gastrocnemius/plantaris (G/P; collected together) were harvested from Myo-Cre/Flox-MCIP1 mice and either flash frozen in embedding medium containing a 3:1 mixture of Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC) and gum tragacanth (Sigma, St. Louis, MO) or fixed in 4% paraformaldehyde and processed for routine paraffin histology. Frozen sections were cut on a cryotome and stained histochemically for ATPase enzyme activity as previously described (30). Paraffin sections were stained immunohistochemically for MyHC-1 and myoglobin.
Silver-stained high-resolution glycerol gels.
Total proteins including myosins were extracted from freshly frozen muscles in extraction buffer (0.3 M KCl, 0.1 M KH2PO4, 50 mM K2HPO4, 10 mM EDTA, pH 6.5) with the addition of Complete protease inhibitor cocktail (Roche, Indianapolis, IN) as described previously (1). The protein extracts were diluted 1:1 (vol/vol) with 60% glycerol, and 0.2 μg of protein was separated on an 8% polyacrylamide gel containing 30% glycerol. The gels were run at 4°C for 40 h at 70 V. Following electrophoresis, the gels were silver stained with Silver Stain Plus (Bio-Rad).
Western blot analysis.
High-resolution glycerol gels or standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels were transferred and analyzed with primary antibodies directed against MyHC-1 (Sigma), Fast MyHC (MY32) (Developmental Studies Hybridoma Bank, Iowa City, IA), calcineurin catalytic subunit A (CnA; Transduction Laboratories), slow fiber-specific troponin I (TnIss) (Santa Cruz), α-tubulin (Sigma), myoglobin (DAKO), cytochrome c (Cyt C) (PharMingen), superoxide dismutase (SOD2) (Stressgen), PGC-1α (Calbiochem), and MCIP1 (3).
Semiquantitative reverse transcription-PCR (RT-PCR) and PCR.
Total RNA was isolated from soleus and tibialis anterior (TA) muscles from wild-type and Myo-Cre/Flox-MCIP1 mice using TriPure Isolation Reagent (Roche). cDNA synthesis of 2 μg of RNA was carried out using a Super Script First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). Annealing temperatures and cycling times for PCR were adjusted for the melting point of each primer set. Several different cycle times and starting cDNA template concentrations were compared to assure that the data presented in the figures represent results in the linear range of detection. Primer sets for PCR can be found in Table 1. To assess relative mitochondrial DNA (mtDNA) abundance, total genomic DNA (nuclear and mtDNA) was isolated from a whole soleus or TA muscle of wild-type and Myo-Cre/Flox-MCIP1 mice. Genomic PCR was carried out with Taq polymerase (Promega) with standard buffer. For each reaction, 250 ng total DNA was used as a template.
TABLE 1.
Sequences of primers used in RT-PCR and cloning of the FLAG-MCIP1 transgene
| Gene product name | Primer pair sequence |
|---|---|
| MyHC-1 | CCTTGGCACCAATGTCCCGGCTC |
| GAAGCGCAATGCAGAGTCGGTG | |
| MyHC-2A | ATGAGCTCCGACGCCGAG |
| TCTGTTAGCATGAACTGGTAGGCG | |
| MyHC-2X | AAGGAGCAGGACACCAGCGCCCA |
| ATCTCTTTGGTCACTTTCCTGCT | |
| MyHC-2B | GTGATTTCTCCTGTCACCTCTC |
| GGAGGACCGCAAGAACGTGCTGA | |
| PGC-1α | CAGACCTGACACAACGCGGACAG |
| CCTGCGCAAGCTTCTCTGAGC | |
| α-actin | GACGGGCAGGTCATCACCAT |
| GCATTTGCGGTGCACAATGG | |
| Myoglobin | CATGGTTGCACCGTGCTCACAG |
| GAGCCCATGGCTCAGCCCTG | |
| MCIP1 | CCTCATGACAGACAGCGAAGC |
| CGTTTGAAGCTCTTAAAATAC | |
| Cyt B | GGAGTCTGCCTAATAGTCCAAATC |
| CAAGGTGGCTTTGTCTACTGAGAAG | |
| 18S RNA | GGACCAGAGCGAAAGCATTT |
| TGCCAGAGTCTCGTTCGTTAT | |
| FLAG-MCIP1 | GAGGAGGTGGATCTGGAGGAC |
| TCAGCTAAGGTGGATCGGTGT |
Transient transfection assays.
Luciferase reporter constructs under the control of a minimal promoter (TATA-Luc) or promoter/enhancer elements from the myoglobin gene (Myb-Luc) (4) were cotransfected into C2C12 cells along with plasmids containing either no insert (control), constitutively active calcineurin (CnA*), or FLAG-tagged MCIP1. Cells were harvested 48 h after transfection. Data are expressed relative to the luciferase activity observed in controls. Results are corrected for variations in transfection efficiency by normalization to expression of a cotransfected pCMV-lacZ plasmid (n = 3 transfections, each assayed in duplicate).
Enzyme assays.
Calcineurin activity was assayed in extracts from gastrocnemius muscles of wild-type and Myo-Cre/Flox-MCIP1 animals using the cellular calcineurin activity assay kit from BIOMOL (Research Laboratories, Plymouth Meeting, PA). Metabolic enzyme activities of citrate synthase (CS), lactate dehydrogenase (LDH), and β-hydroxyacyl coenzyme A (CoA) dehydrogenase (HAD) were assayed on a Beckman DU series 64 spectrometer according to the method of Reed et al. (21). Specific enzyme activities (in micromoles per minute per gram) were calculated from the rate of change of the absorbance (n = 3 for each genotype, assayed in duplicate for each enzyme activity).
Muscle fatigue measurements.
Measurements of muscle fatigue were performed as previously described (30). Briefly, wild-type or transgenic mice were euthanized and soleus and extensor digitorum longus (EDL) muscles were immediately dissected and mounted between a fixed clamp at the base of a water-jacketed organ bath and a Grass FTO3C isometric force transducer. Muscles were equilibrated for 15 min in an oxygenated (95% O2 and 5% CO2) physiological salt solution, containing 120.5 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.5 mM CaCl2, 1.2 mM Na2PO4, 20.4 mM NaHCO3, 10 mM dextrose, and 1 mM pyruvate, at 30°C and pH 7.6. The muscles were adjusted to the optimal length followed by 30 min of resting. Muscles were then stimulated at 100 Hz until the force output dropped to 10% of the initial level. The time taken to reach 10, 30, and 50% of the original force was measured and used as an indication of muscle fatigability.
RESULTS
To inhibit calcineurin signaling specifically in skeletal muscle, we generated Flox-MCIP1 transgenic mice that express MCIP1 upon activation by Cre recombinase and crossed them with Myo-Cre, a transgenic line expressing Cre recombinase in skeletal muscle (14). The Myo-Cre transgene is expressed by embryonic day 9.5 (E9.5) in developing somites. Thus, Cre recombinase is expressed in myoblasts prior to migration of myocytes into the developing limbs. Cardiac and smooth muscle are excluded.
MCIP1 is overexpressed in the skeletal muscle of Myo-Cre/Flox-MCIP1 mice.
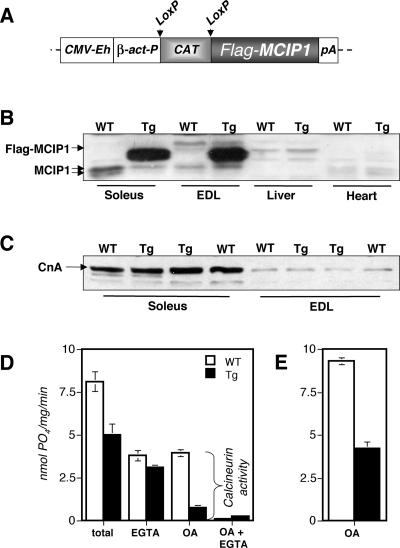
The Flox-MCIP1 transgene consists of a constitutive CMV enhancer/β-actin promoter driving expression of a FLAG epitope-tagged MCIP1 gene. An intervening chloramphenicol acetyl-transferase gene (CAT), flanked by loxP sites, separates the MCIP1 gene from the promoter (Fig. 1A). Following Cre-mediated excision of the CAT gene, the MCIP1 transgene is juxtaposed to the β-actin promoter and continues to be expressed even in the absence of continued Cre recombinase expression. Tissues were harvested from Myo-Cre/Flox-MCIP1 mice to verify skeletal muscle-specific activation of the transgene. The FLAG-tagged MCIP1 transgenic protein was detected in all skeletal muscles tested, including soleus, plantaris, gastrocnemius, tibialis anterior (TA), extensor digitorum longus (EDL), white vastus (WV), diaphragm, and tongue. The transgene was not expressed in heart, liver, kidney, lung, or brain (Fig. 1B and data not shown). There was no apparent fiber type bias in the expression of FLAG-MCIP1, as transgenic protein levels in the soleus, a slow fiber-rich muscle, was equivalent to that found in EDL, a muscle composed exclusively of fast fibers (Fig. 1B). Transgenic protein levels were from 8- to 40-fold higher than endogenous protein depending upon the level of endogenous MCIP1 in that tissue. Endogenous MCIP1 protein levels were reduced in the presence of FLAG-MCIP1 overexpression. The MCIP1 gene is activated in response to calcineurin activity (22), its down regulation in Myo-Cre/Flox-MCIP1 mice suggests that calcineurin activity is inhibited in the muscles of these mice.
FIG. 1.
MCIP1 is overexpressed specifically in skeletal muscle of Myo-Cre/Flox-MCIP1 mice and inhibits calcineurin activity. (A) The Flox-MCIP1 transgene contains a CMV enhancer (CMV-Eh), a β-actin promoter (β-act-P), loxP sites, a CAT gene, a FLAG-tagged MCIP1, and a polyadenylation signal (pA). (B) Western blot demonstrating skeletal muscle-specific expression of the Flox-MCIP1 transgene in Myo-Cre/Flox-MCIP1 mice (Tg) compared to the wild type (WT). Each lane contains 20 μg protein and was probed with anti-MCIP1 antibodies. (C) Western blot analysis of calcineurin protein levels in the Tg animals compared to WT. Each lane contains 20 μg protein and was probed with anti-CnA antibodies. (D) Phosphatase assays of protein extracts from the gastrocnemius of WT and Tg mice. The calcium chelator EGTA was added to inhibit phosphatase activity of calcineurin. Okadaic acid (OA) was added to inhibit the phosphatases PP1 and PP2A. Phosphatase activity is defined as nanomoles of phosphate released per milligram of protein per minute. (E) Calcineurin activity in hind limbs of E17.5 WT and Tg embryos.
Six independent Flox-MCIP1 lines were established. Each showed skeletal-muscle-restricted expression when crossed to Myo-Cre. The line with the highest levels of FLAG-MCIP1 protein was used in our studies, except where noted as Tghigh or Tglow in Fig. 2.
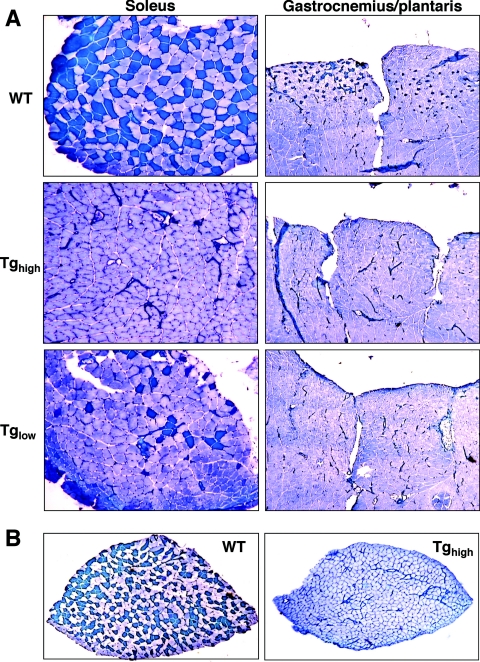
FIG. 2.
The Myo-Cre/Flox-MCIP1 mice lack slow fibers. (A) Metachromatic ATPase fiber type analysis of soleus and gastrocnemius and plantaris from wild type (WT), the high expression Tg (Tghigh), or low expression Tg (Tglow). (B) Metachromatic ATPase stain of a cross-section from an entire soleus muscle from a WT and Tghigh mouse. The average number of fibers in a wild-type cross-section was 845 (±35) compared to 878 (±108) in Myo-Cre/Flox-MCIP1 soleus (n = 4).
The Flox-MCIP1 transgene inhibits calcineurin activity.
To determine whether the Flox-MCIP1 transgene inhibits calcineurin, protein extracts were made from the skeletal muscle of both wild-type and Myo-Cre/Flox-MCIP1 transgenic mice. There was no change in the protein level of the catalytic A subunit of calcineurin (Fig. 1C). Extracts were assayed for calcineurin activity in the presence of excess calcium and calmodulin. First, total cellular phosphatase activity was measured. This activity includes protein phosphatases in addition to calcineurin, primarily PP1 and PP2A. The Myo-Cre/Flox-MCIP1 transgenic animals had 40% lower total phosphatase activity in the gastrocnemius muscle compared to that of wild-type animals (Fig. 1D). Upon addition of the calcium chelator EGTA to inhibit calcineurin activity, the two genotypes had similar phosphatase activities, suggesting that the major difference between wild-type and Myo-Cre/Flox-MCIP1 muscle was due to a lack of calcineurin activity in the transgenic muscle. Indeed, the addition of okadiac acid (OA) to inhibit PP1 and PP2A demonstrated that the transgenic muscle retained approximately 20% of wild-type calcineurin activity. Since extracts from whole muscle include other cell types, in addition to myofibers, the residual calcineurin activity is likely attributable to the calcineurin present in endothelial and smooth muscle cells where the Flox-MCIP1 transgene is not expressed. Calcineurin activity in extracts from the total hind limb of E17.5 Myo-Cre/Flox-MCIP1 embryos was 45% of that from wild-type embryos (Fig. 1E). These results verify that, once activated, the Flox-MCIP1 transgene is a potent inhibitor of calcineurin activity in vivo.
The Myo-Cre/Flox-MCIP1 mice lack slow fibers.
The skeletal muscle fiber type composition of soleus, gastrocnemius, and plantaris muscles from adult wild-type and Myo-Cre/Flox-MCIP1 transgenic male mice was examined using a metachromatic ATPase stain (17). With this technique, type 1 slow fibers stain dark blue, and fast fibers stain various shades of a lighter blue. In wild-type mice, approximately half of the muscle fibers in the soleus were type 1 (Fig. 2A). The remaining fibers were primarily type 2A. The soleus of Myo-Cre/Flox-MCIP1 mice had no reduction in the total number of fibers; however, a dramatic change in fiber type composition was observed. The soleus of Myo-Cre/Flox-MCIP1 mice with the highest FLAG-MCIP1 protein level (Tghigh) contained no type 1 fibers. The soleus of Myo-Cre/Flox-MCIP1 mice from the Flox-MCIP1 line with the lowest level of transgene expression (Tglow) had a dramatic reduction in the number of type 1 fibers, although some type 1 fibers were still present, indicating a dosage effect on slow fiber expression in soleus. The wild-type gastrocnemius and plantaris had focal concentrations of type 1 fibers as expected (Fig. 2A). Type 1 slow fibers were absent in the gastrocnemius and plantaris of the Myo-Cre/Flox-MCIP1 mice regardless of the level of transgene expression. This suggests that a higher level of MCIP1 protein is required to suppress the slow fiber program in soleus than in gastrocnemius and plantaris, reflecting the higher calcineurin protein content in the soleus than in fast fiber-rich muscles of these animals (Fig. 1C).
The cross-sectional area of fibers is reduced in soleus.
Although there was no loss of fiber number in the soleus of the Myo-Cre/Flox-MCIP1 animals compared to the wild type, there was a 30% reduction in cross-sectional area of fibers leading to a reduction in the total cross-sectional area of the soleus muscle (Fig. 2B). This reduction in fiber size was observed primarily in sections of soleus with a total loss of MyHC-1 (Tghigh) but was not apparent in those with some slow fibers remaining (Tglow), suggesting dosage effects of MCIP1 expression. The cross sectional area of type 1 fibers in wild-type soleus was 1,495 ± 447 μm2, and that of type 2A fibers was similar (1,499 ± 347 μm2). The cross-sectional area of fibers in the soleus of the Myo-Cre/Flox-MCIP1 (Tghigh) mice was significantly smaller (1,046 ± 54 μm2) (P < 0.01). Despite the reduction in fiber size, there was no evidence of an increase in the number of centralized nuclei that would indicate muscle damage and regeneration.
Myo-Cre/Flox-MCIP1 mice lack type 1 slow fiber-specific contractile proteins.
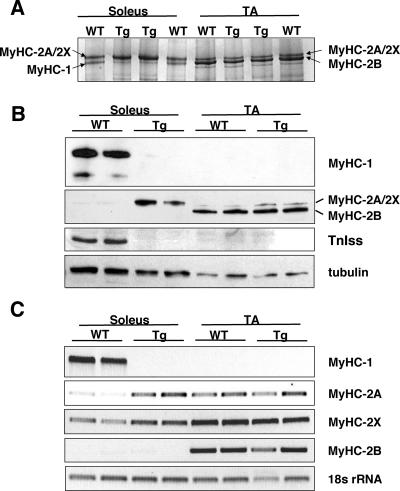
High-resolution glycerol gels were used to resolve the myosin heavy chain isoforms from soleus and TA of Myo-Cre/Flox-MCIP1 and wild-type mice. Soleus muscle extracts from wild-type mice showed two bands of relatively equal intensity (Fig. 3A). The faster migrating lower band is MyHC-1, whereas the upper band contains the fast myosin heavy chains MyHC-2A and MyHC-2X. The lower MyHC-1 band was absent in the Myo-Cre/Flox-MCIP1 muscle, whereas the upper myosin band doubled in intensity, suggesting that expression of either MyHC-2A or MyHC-2X compensated for the loss of MyHC-1. The pattern of myosin heavy chain isoforms in the TA muscle of the Myo-Cre/Flox-MCIP1 mice was not noticeably changed compared to wild-type mice (Fig. 3A). In TA, the predominant myosin heavy chain isoform is MyHC-2B, with the balance of fibers being type 2X and type 2A. Western blot analyses confirmed the glycerol gel findings (Fig. 3B). MyHC-1 protein was present in the soleus of wild-type animals but absent from the soleus of Myo-Cre/Flox-MCIP1 mice. A similar pattern was seen for the slow fiber-specific troponin I (TnIss) protein, indicating that other slow fiber-specific contractile components, in addition to MyHC-1, are under the control of calcineurin (Fig. 3B). An antibody specific for fast MyHC isoforms showed a robust increase in the level of MyHC-2A/2X in the soleus of Myo-Cre/Flox-MCIP1 mice compared to wild-type mice. In contrast, the change in MyHC-2A/2X protein in the TA of Myo-Cre/Flox-MCIP1 mice was negligible (Fig. 3B).
FIG. 3.
MyHC-1 is replaced by MyHC-2A in Myo-Cre/Flox-MCIP1 muscles. (A) Silver-stained, high-resolution glycerol gel resolving MyHC isoforms in extracts from the soleus and TA of wild-type (WT) and Tg mice. Each lane contains 0.2 μg of protein. (B) Western blot analysis of the same extracts. Total protein (2 μg), separated on high-resolution glycerol gels, was transferred to nitrocellulose and probed with antibody specific for MyHC-1 or the My32 antibody, which detects fast MyHC isoforms. Protein extracts (20 μg) were probed with anti-TnIss or anti-tubulin antibodies. (C) RT-PCR analysis of adult MyHC isoform expression in soleus and TA of the collateral leg of WT and Tg animals. 18S rRNA was used as a control.
To confirm that MyHC-2A was the primary myosin isoform compensating for the loss of MyHC-1, the upper band from the soleus of Myo-Cre/Flox-MCIP1 mice was excised from the glycerol gel and subjected to analysis by mass spectroscopy. Peptides from three different myosin heavy chains were identified. The most prominent species was MyHC-2A, with minor contributions from MyHC-2B and MyHC-1. No peptides specific for adult MyHC-2X, embryonic (MyHC-emb), or neonatal (MyHC-neo) species were detected. This suggests that an increase in the adult MyHC-2A compensated primarily for the loss of MyHC-1 in the soleus of these mice.
Semiquantitative RT-PCR was performed to assess changes in transcript levels of the four adult myosin heavy chains. MyHC-1 transcripts were undetectable in the muscles of Myo-Cre/Flox-MCIP1 mice (Fig. 3C). There was a dramatic increase in the transcript levels of MyHC-2A in the soleus of Myo-Cre/Flox-MCIP1 mice compared to wild-type mice. RT-PCR showed a consistent, but slight, increase in MyHC-2X transcripts in Myo-Cre/Flox-MCIP1 soleus compared to the wild type. In TA, MyHC-2A, MyHC-2X, and MyHC-2B, transcript levels were unaltered in Myo-Cre/Flox-MCIP1 mice compared to the wild-type mice. Transcription of MyHC-2B was not altered. Neither MyHC-emb nor MyHC-neo transcripts were detected in any of the adult tissues (data not shown).
In summary, in the presence of exogenous MCIP1, the soleus showed a dramatic shift in MyHC isoform expression from MyHC-1 to MyHC-2A and MyHC-2X, whereas there was very little change in MyHC-2A, MyHC-2X, and MyHC-2B expression or protein levels in the TA. Thus, the only muscles altered by MCIP1 inhibition of calcineurin appear to be those containing type 1 slow fibers.
MCIP1 overexpression does not alter myoglobin levels.
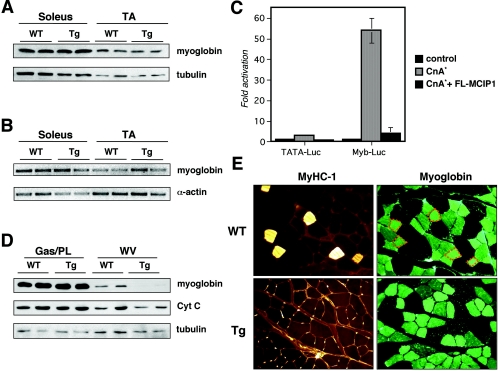
Upon examination of the hind limb of Myo-Cre/Flox-MCIP1 transgenic mice, the distribution and abundance of red versus white muscle appeared to be similar to the wild type. Consistent with this, Western blot analysis showed that there was no difference in the overall level of myoglobin protein in the soleus and TA of the Myo-Cre/Flox-MCIP1 mice compared to the wild type (Fig. 4A). Furthermore, there was also no change in the level of myoglobin transcript abundance in the muscles of Myo-Cre/Flox-MCIP1 animals compared to the wild type (Fig. 4B). This result was surprising based on our previous data showing that the myoglobin promoter is activated by calcineurin (4) and that an activated calcineurin transgene can increase myoglobin in vivo (32). To verify that our FLAG-tagged MCIP construct would inhibit calcineurin activation of the myoglobin promoter, we tested it in transient transfection assays. Expression of FLAG-MCIP1 did indeed inhibit activation of the myoglobin reporter plasmid (Fig. 4C). Thus, although the myoglobin promoter can be activated by calcineurin in tissue culture, within the context of the soleus and TA muscle, myoglobin expression is not solely dependent on calcineurin.
FIG. 4.
Myoglobin levels are not reduced in the Myo-Cre/Flox-MCIP1 mice. (A) Western blot analysis of myoglobin protein. Each lane contains 5 μg of protein. (B) RT-PCR analysis of myoglobin transcription in the collateral leg of the same animals. Skeletal α-actin was used as a control. (C) Transient transfection assays of C2C12 myoblasts with a minimal TATA-luciferase (TATA-Luc) or a myoblobin-luciferase (Myb-Luc) cotransfected with an empty expression vector, or ones expressing a constitutively active form of calcineurin (CnA*) and FLAG-MCIP1. (D) Western blot analysis of 20 μg of protein from gastrocnemius and plantaris (Gas/PL) and WV of wild-type (WT) and Tg mice. (E) Serial sections of plantaris muscle from WT and Tg animals reacted with antibodies specific for MyHC-1 or myoglobin. The outline of MyHC-1-positive fibers in the WT are noted in orange in the upper left myoglobin-stained section.
To determine whether myoglobin expression was independent of calcineurin in all muscles, we assessed myoglobin levels in a variety of other muscle groups, including gastrocnemius, plantaris, EDL, and WV. The only muscle group with a detectable decrease in myoglobin protein was WV (Fig. 4D). Myoglobin protein levels vary between muscle fiber types and correlate with the oxidative capacity of the muscle. Thus, it is abundant in oxidative type 1 slow fibers and in oxidative type 2A fast fibers. Type 2B fibers, the most glycolytic, lack myoglobin entirely. WV is the most glycolytic, fast fiber-rich muscle in the mouse hind limb and normally contains very low levels of myoglobin compared to other muscle groups. The decrease in myoglobin in the WV of the Myo-Cre/Flox-MCIP1 mice compared to the wild type suggests that calcineurin activity can promote expression of myoglobin in vivo under specific circumstances. However, our results also suggest that the majority of myoglobin expression in skeletal muscle of young adult mice is not dependent on calcineurin activity and therefore must be expressed under the control of other regulatory factors.
We used immunohistochemistry to determine whether the normal wild-type distribution of myoglobin among muscle fiber types was maintained in the Myo-Cre/Flox-MCIP1 mice. A region of the gastrocnemius proximal to the plantaris that usually contains all four adult muscle fiber types is shown in Fig. 4E. Serial sections were stained for either MyHC-1 or myoglobin. MyHC-1 was absent in Myo-Cre/Flox-MCIP1 muscle. However, three different intensities of myoglobin reactivity were seen in both wild-type and Myo-Cre/Flox-MCIP1 muscle, indicating that there was no change in the distribution or pattern of myoglobin staining despite the lack of type 1 fibers.
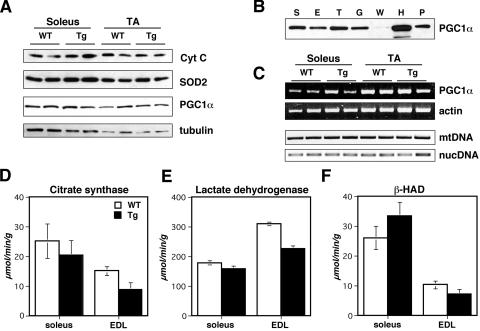
MCIP1 overexpression does not change mitochondrial abundance.
There was no apparent change in the abundance of mitochondria in soleus and TA muscles based on Western analysis of two different mitochondrial proteins, cytochrome c (Cyt C) and mitochondrial superoxide dismutase (SOD2) (Fig. 5A). Semiquantitative PCR to assess mitochondrial DNA (mtDNA) content also did not indicate a change in the abundance of mtDNA in Myo-Cre/Flox-MCIP1 muscle compared to wild-type muscle (Fig. 5C). The coactivator PGC-1α has been hypothesized to function as an important regulator of mitochondrial biogenesis in muscle (15). As expected, PGC-1α protein was highest in oxidative muscle such as heart, soleus, plantaris, and TA while lower in more glycolytic muscles such as gastrocnemius, EDL, and WV of wild-type animals (Fig. 5B). No change was observed in the level of either PGC-1α protein (Fig. 5A) or transcripts (Fig. 5C) in the soleus and TA muscles of Myo-Cre/Flox-MCIP1 mice compared to the same muscle in wild-type mice. Interestingly, as with myoglobin, there appeared to be a slight reduction in Cyt C protein in the WV of Myo-Cre/Flox-MCIP1 mice compared to wild-type mice (Fig. 4D).
FIG. 5.
Metabolism is not altered in the Myo-Cre/Flox-MCIP1 mice. (A) Western blot analysis of 20 μg of protein from wild-type (WT) and Tg animals. (B) Western blot analysis of PGC-1α protein in 20 μg of protein from WT S (soleus), E (EDL), T (TA), G (gastrocnemius), H (heart), and P (plantaris). (C) RT-PCR of PGC-1α and skeletal α-actin of the collateral legs from panel A and a comparison of relative mtDNA copy number using semiquantitative PCR of the mtDNA gene for cytochrome B (Cyt B) and the endogenous MCIP1 nuclear gene (nucDNA). (D) Citrate synthase, (E) lactate dehydrogenase, and (F) β-hydroxyacyl-CoA dehydrogenase activities in the soleus and EDL muscles of WT and Tg mice.
MCIP1 overexpression does not shift metabolic activity.
To examine the influence of the Myo-Cre/Flox-MCIP1 transgene on metabolism, three different enzyme activities were measured in muscle extracts. Citrate synthase (CS) and lactate dehydrogenase (LDH) were used to assess oxidative and glycolytic metabolism, respectively. β-Hydroxyacyl-CoA dehydrogenase (HAD) activity was used as a measure of fatty acid oxidation (FAO). As expected, CS and HAD activity were higher in wild-type soleus than in wild-type EDL (Fig. 5D and F). Conversely, LDH levels were higher in EDL than in soleus (Fig. 5E). There was no statistically significant change in any of the three enzyme activities in the soleus of the Myo-Cre/Flox-MCIP1 mice compared to the wild type (P > 0.1). This was true whether the activities were normalized to protein content (as shown) or to muscle wet weight. In EDL of Myo-Cre/Flox-MCIP1 mice, the activities of all three enzymes were slightly depressed, with CS and LDH reaching statistical significance (P < 0.01). These results indicate that inhibition of calcineurin did not cause a major shift in the enzyme activity from oxidative metabolism to glycolytic, although there may be a mild suppression of total metabolic activity in some muscles of the animals carrying the Myo-Cre/Flox-MCIP1 transgene.
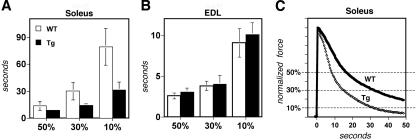
Soleus muscles from the Myo-Cre/Flox-MCIP1 mice fatigue more rapidly than wild-type soleus.
The fatigue rates of soleus and EDL muscles from the Myo-Cre/Flox-MCIP1 mice were compared to that of wild-type muscles. The time required to lose 50%, 70%, or 90% of contractile force was calculated for each muscle group and genotype. The soleus muscle of the Myo-Cre/Flox-MCIP1 mice fatigued more rapidly than soleus from wild-type mice (Fig. 6A and C). There was no difference in the rate of fatigue of EDL from Myo-Cre/Flox-MCIP1 mice compared to EDL from wild-type mice (Fig. 6B).
FIG. 6.
Myo-Cre/Flox-MCIP1 soleus muscles fatigue more rapidly. Muscle fatigue measurements for (A) soleus and (B) EDL comparing wild-type (WT) muscles (n = 6) with Tg muscles (n = 6). The time taken to reach 50%, 30%, or 10% of the original force was measured. (C) Overlay of representative traces from a Tg and WT soleus muscle.
Expression of MyHC-1 and fiber type patterning are normal during embryogenesis of Myo-CRE/Flox-MCIP1 mice.
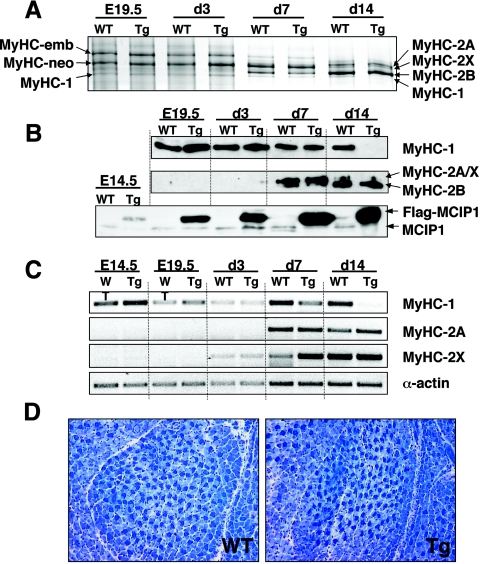
MyHC-1 is expressed both during embryogenesis and in the adult. To determine whether MyHC-1 expression is under the control of calcineurin during embryogenesis, we used RT-PCR and Western blot analysis to examine changes in myosin heavy chain expression. Whole embryos, with the heart removed, were harvested at E14.5, after the completion of the primary round of myotube fusion but prior to the initiation of secondary fusion. Lower limbs were harvested at E19.5 after the completion of the secondary round of myotube fusion but before birth. Lower limbs were harvested postnatally at days 3, 7, and 14.
High-resolution glycerol gels showed that there was no change in the level of MyHC-1, MyHC-emb, and MyHC-neo in the Myo-Cre/Flox-MCIP1 embryos compared to wild-type embryos (Fig. 7A). The distribution of adult My HC-1, -2A, -2X, and -2B appeared to be normal in the Myo-Cre/Flox-MCIP1 neonates at day 7. MyHC-1 was present in the Myo-Cre/Flox-MCIP1 tissues through day 7 but was absent in the hind limb at day 14 (Fig. 7A). These results were confirmed by Western blot analysis showing MyHC-1 protein in the Myo-Cre/Flox-MCIP1 mice was comparable to wild-type mice through day 7 and undetectable at day 14 (Fig. 7B). MyHC-2A and MyHC-2B proteins were not present until day 7. Changes in transcript levels preceded those observed in protein levels. Expression of MyHC-1 in the Myo-Cre/Flox-MCIP1 animals was similar to that of the wild type throughout embryogenesis and the early postnatal period (Fig. 7C). By postnatal day 7, expression of MyHC-1 had begun to diminish and was absent entirely in the hind limb of 14-day-old Myo-Cre/Flox-MCIP1 transgenic pups. Expression of the adult fast MyHC isoforms was not detected until after birth (Fig. 7A).
FIG. 7.
Slow fiber formation and patterning is not altered in the Myo-Cre/Flox-MCIP1 embryos. (A) Silver-stained, high-resolution glycerol gel of 0.2 μg of total protein extracted from E19.5 embryo hind limbs and neonatal pup's hind legs at days 3, 7, and 14. (B) Western blot analysis of the same protein extracts. High-resolution glycerol gels loaded with 2 μg of protein were transferred and probed with antibodies against MyHC-1 or Fast MyHC. A total of 20 μg of protein was run for detection of the Flag-MCIP1 transgene product. (C) Semiquantitative RT-PCR of RNA extracted from E14.5 whole embryos minus the heart, and hind limbs of E19.5 and days 3, 7, and 14. (D) Metachromatic ATPase stain of EDL muscle of E19.5 wild-type (WT) and Tg embryos.
MCIP1 transgenic protein was visible by E14.5, and robust levels were present in the adult (Fig. 7B and 1B). Metachromatic ATPase fiber type analysis of the lower hind limb of E19.5 embryos confirmed the presence of abundant type 1 fibers in both wild-type and Myo-Cre/Flox-MCIP1 mice (Fig. 7D). Furthermore, the pattern and distribution of dark-blue-stained type 1 fibers was indistinguishable between Myo-Cre/Flox-MCIP1 and wild-type embryos, further suggesting that fiber type patterning is independent of calcineurin during embryogenesis. Taken together, these results suggest that, during embryogenesis, slow fiber MyHC-1 expression is independent of calcineurin signaling and that within the first week of life there is a switch in the regulation of the MyHC-1 gene to an absolute requirement for calcineurin activity.
DISCUSSION
Expression of slow fiber contractile proteins is dependent on calcineurin activity.
The findings in this study shed new light on the role of calcineurin in the development and maintenance of skeletal muscle characteristics. Our results demonstrate that, in the skeletal muscle of young adult mice, the contractile components of type 1 slow fibers are under the control of calcineurin, whereas other characteristics, such as oxidative capacity and myoglobin content, are primarily independent of calcineurin activity. In contrast, during embryogenesis and early postnatal growth, slow fiber expression is under the control of a developmental program that is independent of calcineurin.
In the hind limb, soleus muscle contains the highest percentage of type 1 fibers and accordingly displayed the greatest change in fiber type content. In a wild-type soleus, roughly half of the fibers are type 1, yet there was no loss of total fiber number in the Myo-Cre/Flox-MCIP1 animals. Instead, there was a compensatory increase in type 2A fibers. This shift in MyHC isoform content is similar to that seen in models of spinal cord injury, where neuronal activation of the muscle is minimized but the neuromuscular junction remains intact (28). This contrasts with the changes in MyHC isoforms that occur after denervation. In denervated soleus both MyHC-1 and MyHC-2A are lost, while MyHC-2B expression increases (25). Taken together, these results suggest that neuronal activity may maintain expression of slow fiber contractile components through activation of the calcineurin pathway, whereas oxidative type 2A fibers respond to signals from the neuromuscular junction other than calcineurin.
Muscle fiber number is not dependent on a myocyte-autonomous calcineurin signal.
Previous studies have shown that adult mice lacking either NFATC3 (NFATC3−/−) or the beta isoform of the catalytic domain of calcineurin (CnAβ−/−) have fewer muscle fibers compared to wild-type mice (12, 19). This reduction in myofiber number is a consequence of a reduction in the number of primary myofibers formed during embryogenesis. The Myo-Cre transgene activates expression of the Flox-MCIP1 transgene in muscle progenitors by E9.5, prior to this first round of myoblast fusion (14), yet there is no reduction in the total number of muscle fibers in adult Myo-Cre/Flox-MCIP1 mice. This suggests that the dependence of fiber number on calcineurin signaling seen in NFATC3−/− and CnAβ−/− mice either is not myocyte autonomous or occurs very early in embryogenesis, prior to when the Flox-MCIP1 transgene was activated by Myo-Cre.
Calcineurin is expressed in a wide variety of cell types, and its loss in tissues other than muscle of the NFATC3−/− and CnAβ−/− mice could influence primary myotube fusion. It is relevant to note that in the soleus of CnAβ−/− mice, MyHC-2B levels are elevated, reminiscent of the shift in MyHC isoforms seen in denervated muscle. Could loss or reduction of calcineurin activity in the motor neurons of NFATC3−/− and CnAβ−/− mice lead to the reduction in muscle fibers observed in these lines? The Flox-MCIP1 mouse will provide an ideal tool for testing such a hypothesis by providing cell type-specific inhibition of calcineurin signaling in any cell type for which a tissue-specific CRE-expressing line can be generated.
Contribution of calcineurin to myofiber size is context dependent.
A 30% reduction in fiber size was observed in the soleus of the Myo-Cre/Flox-MCIP1 mice; however, no reduction in fiber size was seen in any other muscle group. Measurements of fiber size can be easily influenced by the location of the cross-section, the angle of sectioning, and stretch of the muscle upon mounting. Therefore, caution needs to be used in the interpretation of this observation. However, a similar reduction in the fiber cross-sectional area has been reported in the soleus of CnAβ−/− mice (20) and all muscle groups of NFATC2−/− mice (9). In adult skeletal muscle, existing multinucleate myofibers can grow in size through fusion with additional mononucleated myoblasts recruited from a reserve myogenic satellite stem cell population. Recent data suggest that NFATC2 controls expression of some of the recruitment factors involved in this process (10). Thus, inhibition of calcineurin in the Myo-Cre/Flox-MCIP1 mice may adversely influence the recruitment and fusion of new myoblasts, resulting in smaller myofiber size. Slow fiber-rich muscles contain more satellite cells than fast muscles (7). It has been suggested that muscles such as soleus, which are used continuously, have a higher turnover rate during normal physiological maintenance of the muscle and thus recruit more satellite cells. This may explain the decrease in fiber size in the soleus of the Myo-Cre/Flox-MCIP1 animals but not in the gastrocnemius or plantaris despite the loss of type 1 fibers in these muscles. Studies are under way to determine whether the skeletal muscle regenerative capacity of these animals is impaired.
Oxidative capacity in young adult animals is largely independent of calcineurin activity.
We have shown previously that forced expression of constitutively active calcineurin in skeletal muscle will increase both type 1 fiber abundance and myoglobin content (16, 31). Furthermore, the promoters from the myoglobin, MyHC-1, and MyHC-2A genes each respond to activation by calcineurin in transient transfections (2, 4, 27). Skeletal muscle-specific overexpression of a PGC-1α transgene is also sufficient to increase type 1 slow fiber content and the abundance of both myoglobin and mitochondria (15). It was therefore surprising to find that neither transcription nor protein levels of myoglobin, MyHC-2A, and PGC-1α were reduced in the soleus and TA of Myo-Cre/Flox-MCIP1 mice. Transcription of MyHC-2A actually increased to compensate for the loss of MyHC-1 despite robust inhibition of calcineurin by MCIP1. Our results demonstrate that oxidative metabolism, mitochondrial proliferation, and expression of MyHC-2A, PGC-1α, and myoglobin are primarily independent of calcineurin activity in the skeletal muscle of healthy, young adult mice. Thus, while calcineurin may be essential for the expression of MyHC-1, it is not necessary to maintain many of the other characteristic properties of slow fibers. There may, however, be circumstances under which these characteristics can be influenced by calcineurin activity. The WV of the Myo-Cre/Flox-MCIP1 mice illustrates this and may explain some of the conflicting results from various laboratories that have fueled a controversy over the precise function of calcineurin in regulating fiber type and oxidative capacity of skeletal muscle.
Calcineurin promotes fatigue resistance.
The rate at which a contracting muscle fatigues is a function of both its capacity to generate ATP and the rate at which it consumes ATP. Type 1 fibers are the most fatigue resistant because of their high oxidative capacity and a lower rate of ATP hydrolysis by MyHC-1. Despite similar oxidative capacities, the soleus muscle of the Myo-Cre/Flox-MCIP1 mice fatigued much more rapidly than those of wild-type mice. This suggests that the fatigue-resistant properties of type 1 fibers, compared to type 2A fibers, are primarily dependent on the rate of ATP hydrolysis by MyHC-1 rather than their ability to generate ATP. Consistent with this, there was no change in either MyHC isoform content or the fatigue properties of EDL muscles. Therefore, although inhibition of calcineurin may not reduce the ability of muscles to generate ATP, it can increase the rate of ATP consumption in muscle groups normally containing type 1 fibers and thus adversely affect overall energy balance.
Developmental patterning of type 1 slow fibers is independent of calcineurin.
Despite a complete lack of type 1 fibers in the adult Myo-Cre/Flox-MCIP1 mice, the patterning and expression of slow fibers during embryogenesis and early postnatal development appeared unaltered. This suggests that there is a developmental switch in the regulation of MyHC-1 expression from calcineurin independent to calcineurin dependent. The Hedgehog signaling pathway has been linked to specification of the slow fiber phenotype during vertebrate development (11). Our results suggest that inhibition of calcineurin in the myocyte does not reverse commitment to a slow fiber fate established by this patterning. Furthermore, slow fiber determination remains independent of calcineurin into the second week after birth. Newborn pups are capable of moving their limbs, indicating functional neuromuscular junctions and motor neuron activity. Thus, the switch to calcineurin dependence is unlikely to be a direct response to muscle activity. Changes in MyHC transcription preceded changes in MyHC protein content probably owing to the long half-life of these proteins. There was no evidence of fiber atrophy or fiber loss during this process; rather, we envision a gradual change through a mixed fiber type phenotype similar to that observed during remodeling of adult muscle. Determining the mechanisms that regulate this developmental switch in the control of slow muscle fiber expression may yield important therapeutic tools for maintaining appropriate muscle fiber patterning in individuals with neuromuscular damage or during extended bed rest.
Is inhibition of calcineurin different than losing calcineurin protein?
Our results contrast with those obtained using a “Flox-OUT” approach to delete the regulatory subunit of calcineurin (CnB) (19). In the MLC-cre/CnB(fl/fl) mice, type 1 and type 2A fibers were lost with an increase in glycolytic type 2B fibers. It is not obvious why the oxidative fiber content of the MLC-cre/CnB(fl/fl) mice differs from the Myo-Cre/Flox-MCIP1 mice. One possibility is that inhibition of calcineurin by MCIP1 is selective, affecting activity against some targets while sparing others. Deletion of the MCIP1 gene from mouse (29) and its homologue from yeast (13) suggests that, while high levels of MCIP1 proteins inhibit calcineurin, very low levels may actually facilitate calcineurin signaling. In the case of the Myo-Cre/Flox-MCIP1 mice, FLAG-MCIP1 levels are in vast excess of the endogenous MCIP1 protein. Thus, we think it unlikely that the FLAG-MCIP1 protein is acting to facilitate calcineurin signaling. Furthermore, the loss of MyHC-1 from the Myo-Cre/Flox-MCIP1 mice is complete, whereas MLC-cre/CnB(fl/fl) mice maintain a portion of their type I fibers. This suggests that calcineurin activity is more fully inhibited in the Myo-Cre/Flox-MCIP1 animals.
A more intriguing possibility is that more than phosphatase activity has been lost from the MLC-cre/CnB(fl/fl) mice. The muscles of MLC-cre/CnB(fl/fl) mice were depleted for the catalytic CnA subunit in addition to CnB (19), whereas CnA protein levels were normal in the Myo-CRE/Flox-MCIP1 mice (Fig. 2C). In skeletal muscle calcineurin is localized to the sarcomere (6). Could loss of the CnA/CnB complex have altered sarcomeric structure or function? More studies will be required to determine exactly why these two different approaches to calcineurin inhibition have different impacts on overall oxidative fiber content.
Summary.
Contractile components unique to type 1 fibers, such as MyHC-1 and TnIss, appear absolutely dependent upon calcineurin activity, whereas oxidative capacity, myoglobin content, and mitochondrial abundance are not. Fatigue resistance is also dependent on calcineurin as a consequence of MyHC-1 loss. The Myo-Cre/Flox-MCIP1 animals demonstrate that fiber type patterning during embryogenesis is independent of calcineurin, which allows expression of slow fiber contractile components prior to the establishment of a functional neuromuscular junction. The mechanisms that control the switch from calcineurin-independent to calcineurin-dependent slow fiber expression and whether calcineurin is necessary during regeneration of damaged muscle remain to be determined. Further studies will need to focus on understanding how calcineurin signals integrate with other signaling pathways to coordinate muscle plasticity in response to physical activity, innervation, and hormonal signals.
Acknowledgments
This work was supported by National Institutes of Health grant RO1 HL072016-01 and American Heart Association Texas Affiliate grant 0160099Y.
We thank Shane Kanatous for his advice and excellent discussions during the preparation of the manuscript.
REFERENCES
- 1.Agbulut, O., P. Noirez, F. Beaumont, and G. Butler-Browne. 2003. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol. Cell. 95:399-406. [DOI] [PubMed] [Google Scholar]
- 2.Allen, D. L., and L. A. Leinwand. 2002. Intracellular calcium and myosin isoform transitions. Calcineurin and calcium-calmodulin kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter. J. Biol. Chem. 277:45323-45330. [DOI] [PubMed] [Google Scholar]
- 3.Bush, E., J. Fielitz, L. Melvin, M. Martinez-Arnold, T. A. McKinsey, R. Plichta, and E. N. Olson. 2004. A small molecular activator of cardiac hypertrophy uncovered in a chemical screen for modifiers of the calcineurin signaling pathway. Proc. Natl. Acad. Sci. USA 101:2870-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin, E. R., E. N. Olson, J. A. Richardson, Q. Yang, C. Humphries, J. M. Shelton, H. Wu, W. Zhu, R. Bassel-Duby, and R. S. Williams. 1998. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 12:2499-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn, S. E., E. R. Chin, and R. N. Michel. 2000. Matching of calcineurin activity to upstream effectors is critical for skeletal muscle fiber growth. J. Cell Biol. 151:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey, N., J. A. Richardson, and E. N. Olson. 2000. Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc. Natl. Acad. Sci. USA 97:14632-14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson, M. C., and E. Schultz. 1982. The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat. Rec. 202:329-337. [DOI] [PubMed] [Google Scholar]
- 8.Horsley, V., B. B. Friday, S. Matteson, K. M. Kegley, J. Gephart, and G. K. Pavlath. 2001. Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J. Cell Biol. 153:329-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horsley, V., and G. K. Pavlath. 2004. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs 176:67-78. [DOI] [PubMed] [Google Scholar]
- 10.Horsley, V., and G. K. Pavlath. 2003. Prostaglandin F2(alpha) stimulates growth of skeletal muscle cells via an NFATC2-dependent pathway. J. Cell Biol. 161:111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes, S. M. 2004. Muscle differentiation: a gene for slow muscle? Curr. Biol. 14:R156-R157. [PubMed] [Google Scholar]
- 12.Kegley, K. M., J. Gephart, G. L. Warren, and G. K. Pavlath. 2001. Altered primary myogenesis in NFATC3(-/-) mice leads to decreased muscle size in the adult. Dev. Biol. 232:115-126. [DOI] [PubMed] [Google Scholar]
- 13.Kingsbury, T. J., and K. W. Cunningham. 2000. A conserved family of calcineurin regulators. Genes Dev. 14:1595-1604. [PMC free article] [PubMed] [Google Scholar]
- 14.Li, C., M. Czubryt, J. McAnally, R. Bassel-Duby, J. A. Richardson, F. F. Wiebel, A. Nordheim, and E. N. Olson. 2005. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc. Natl. Acad. Sci. USA 102:1082-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin, J., H. Wu, P. T. Tarr, C. Y. Zhang, Z. Wu, O. Boss, L. F. Michael, P. Puigserver, E. Isotani, E. N. Olson, B. B. Lowell, R. Bassel-Duby, and B. M. Spiegelman. 2002. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797-801. [DOI] [PubMed] [Google Scholar]
- 16.Naya, F. J., B. Mercer, J. Shelton, J. A. Richardson, R. S. Williams, and E. N. Olson. 2000. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem. 275:4545-4548. [DOI] [PubMed] [Google Scholar]
- 17.Ogilvie, R. W., and D. L. Feeback. 1990. A metachromatic dye-ATPase method for the simultaneous identification of skeletal muscle fiber types I, IIA, IIB and IIC. Stain Technol. 65:231-241. [DOI] [PubMed] [Google Scholar]
- 18.Ontell, M., and K. Kozeka. 1984. The organogenesis of murine striated muscle: a cytoarchitectural study. Am. J. Anat. 171:133-148. [DOI] [PubMed] [Google Scholar]
- 19.Parsons, S. A., D. P. Millay, B. J. Wilkins, O. F. Bueno, G. L. Tsika, J. R. Neilson, C. M. Liberatore, K. E. Yutzey, G. R. Crabtree, R. W. Tsika, and J. D. Molkentin. 2004. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J. Biol. Chem. 279:26192-26200. [DOI] [PubMed] [Google Scholar]
- 20.Parsons, S. A., B. J. Wilkins, O. F. Bueno, and J. D. Molkentin. 2003. Altered skeletal muscle phenotypes in calcineurin Aα and Aβ gene-targeted mice. Mol. Cell. Biol. 23:4331-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed, J. Z., P. J. Butler, and M. A. Fedak. 1994. The metabolic characteristics of the locomotory muscles of grey seals (Halichoerus grypus), harbour seals (Phoca vitulina) and Antarctic fur seals (Arctocephalus gazella). J. Exp. Biol. 194:33-46. [DOI] [PubMed] [Google Scholar]
- 22.Rothermel, B. A., R. B. Vega, and R. S. Williams. 2003. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc. Med. 13:15-21. [DOI] [PubMed] [Google Scholar]
- 23.Schiaffino, S., and C. Reggiani. 1996. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol. Rev. 76:371-423. [DOI] [PubMed] [Google Scholar]
- 24.Schiaffino, S., and A. Serrano. 2002. Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends Pharmacol. Sci. 23:569-575. [DOI] [PubMed] [Google Scholar]
- 25.Serrano, A. L., M. Murgia, G. Pallafacchina, E. Calabria, P. Coniglio, T. Lomo, and S. Schiaffino. 2001. Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc. Natl. Acad. Sci. USA 98:13108-13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieck, G. C., and M. Regnier. 2001. Invited review: plasticity and energetic demands of contraction in skeletal and cardiac muscle. J. Appl. Physiol. 90:1158-1164. [DOI] [PubMed] [Google Scholar]
- 27.Swoap, S. J., R. B. Hunter, E. J. Stevenson, H. M. Felton, N. V. Kansagra, J. M. Lang, K. A. Esser, and S. C. Kandarian. 2000. The calcineurin-NFAT pathway and muscle fiber-type gene expression. Am. J. Physiol. Cell Physiol. 279:C915-C924. [DOI] [PubMed] [Google Scholar]
- 28.Talmadge, R. J. 2000. Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve 23:661-679. [DOI] [PubMed] [Google Scholar]
- 29.Vega, R. B., B. A. Rothermel, C. J. Weinheimer, A. Kovacs, R. H. Naseem, R. Bassel-Duby, R. S. Williams, and E. N. Olson. 2003. Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 100:669-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu, H., S. B. Kanatous, F. A. Thurmond, T. Gallardo, E. Isotani, R. Bassel-Duby, and R. S. Williams. 2002. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296:349-352. [DOI] [PubMed] [Google Scholar]
- 31.Wu, H., F. J. Naya, T. A. McKinsey, B. Mercer, J. M. Shelton, E. R. Chin, A. R. Simard, R. N. Michel, R. Bassel-Duby, E. N. Olson, and R. S. Williams. 2000. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 19:1963-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu, H., B. Rothermel, S. Kanatous, P. Rosenberg, F. J. Naya, J. M. Shelton, K. A. Hutcheson, J. M. DiMaio, E. N. Olson, R. Bassel-Duby, and R. S. Williams. 2001. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 20:6414-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]