Abstract
Tumor necrosis factor (TNF) alpha is a cytokine capable of inducing caspase-dependent (apoptotic) cell death in some cells and caspase-independent (necrosis-like) cell death in others. Here, using a mutagenesis screen for genes critical in TNF-induced death in L929 cells, we have found that H-ferritin deficiency is responsible for TNF resistance in a mutant line and that, upon treatment with TNF, this line fails to elevate levels of labile iron pool (LIP), critical for TNF-induced reactive oxygen species (ROS) production and ROS-dependent cell death. Since we found that TNF-induced LIP in L929 cells is primarily furnished by intracellular storage iron, the lesser induction of LIP in H-ferritin-deficient cells results from a reduction of intracellular iron storage caused by less H-ferritin. Different from some other cell lines, the H-ferritin gene in L929 cells is not TNF inducible; however, when H-ferritin is expressed in L929 cells under a TNF-inducible system, the TNF-induced LIP and subsequent ROS production and cell death were all prevented. Thus, LIP is a common denominator of ferritin both in the enhancement of cell death by basal steady-state H-ferritin and in protection against cell death by induced H-ferritin, thereby acting as a key determinant of TNF-induced cell death.
Tumor necrosis factor (TNF) alpha is a cytokine originally identified as a factor that led to the hemorrhagic necrosis of an established tumor (9, 38) and has since been shown to play a crucial role in the pathogenesis of acute and chronic inflammatory diseases (3, 4, 36). TNF can induce apoptotic (caspase-dependent) or necrotic (caspase-independent) cell death in vitro, depending on the nature of the cell line used (5, 16, 52, 53). Although TNF-induced apoptosis may participate in inflammatory responses, TNF-induced necrosis seems to also have a stronge pathophysiological effect, as evidenced by the increased toxicity of TNF in vivo when the pan-caspase inhibitor zVAD, which inhibits apoptosis but enhances necrosis, is applied (10).
Both apoptosis and necrosis are initiated by TNF receptor 1 (TNFR1) clustering and TNFR1-associated death domain (TRADD) recruitment (6, 8, 19, 33, 47). The TNFR1-associated death domain subsequently recruits other effectors, such as the Fas-associated death domain, which is required for caspase-8 autoactivation in the apoptosis pathway. The necrosis pathway may diverge somewhere downstream of these effectors since known proapoptotic caspases, or cytochrome c release, are not involved in this death pathway (16, 17, 51, 56, 57). Furthermore, receptor interacting protein (RIP) in the TNFR1 complex is required for caspase-independent death and has been suggested to be involved in necrosis (23, 35). Despite the differences in TNF-induced apoptosis and necrosis, mitochondria play a role in both types of cell death (7, 18, 48). Reactive oxygen species (ROS) are known to be involved in both TNF-induced apoptosis and necrosis (18).
Iron is utilized as a catalyst at the active site of numerous enzymes involved in oxygen metabolism and electron transport and is thus a key element of redox reactions (26). The largest percentage of intracellular iron is tightly bound to different proteins as a cofactor or for storage. Only a small portion (∼0.2 to 3%) of cellular iron is “iron in transit,” termed the labile iron pool (LIP) (14, 26). LIP constitutes chelatable and redox-active iron and serves as a crossroad of cell iron metabolism. LIP is able to promote the formation of ROS since LIP and ROS levels have been shown to follow similar “rise and fall” patterns (32). Elevated levels of LIP are commonly assumed to compromise cell integrity by ROS. Regulatory mechanisms, such as expression of the iron supplier transferrin receptor and iron-withdrawing protein ferritin, have been implied in maintaining LIP at relatively low levels (1, 46). Ferritin is the major intracellular iron storage protein, consisting of 24 subunits of heavy (H) and light (L) polypeptide chains with varying H/L ratios ranging from H24L0 to H0L24, and controls the level of LIP by accommodating the excess iron (14). Conversely, the ferritin-bound storage iron is released when LIP is low in order to make iron available for cellular functions (26). This concept is supported by experimental evidence showing that the overexpression of H-ferritin reduces steady-state LIP levels and ensuing ROS production (14), whereas the inhibition of H-ferritin expression evokes an increase in the levels and pro-oxidant activity of LIP (27, 28, 44). Iron-regulated ferritin expression through the iron regulatory protein-iron-responsive element system has been well studied (40, 54) and is believed to be a mechanism that controls LIP levels. H-ferritin is also a stress- or inflammation-regulated protein since H-ferritin transcription can be regulated by cytokines, such as TNF, in some cells (12, 37). Alternation of iron metabolism is part of the cellular response to TNF and is involved in TNF-induced cellular events such as NF-κB activation (60). Ferritin expression is generally considered a means of protection against oxidative damage since the induction of ferritin by iron results in a resistance to various types of oxidative stimuli (2, 44, 50). H-ferritin-mediated resistance to TNF-induced apoptosis in HeLa and other cells has also been reported (12, 41).
L929 is murine fibroblast cell line primarily used in TNF bioassay. TNF treatment of L929 cells leads to a caspase-independent cell death with necrotic phenotype (16). In order to further address the less well-defined mechanism of TNF-induced caspase-independent cell death, we have used a random gene disruption approach to generate a series of L929 mutant lines resistant to TNF-induced cell death, a technique that has proven to be an excellent approach for the identification of genes involved in TNF-induced cell death (34, 39, 58). The gene disrupted in one of the TNF-resistant lines was identified as H-ferritin. We observed that in the H-ferritin-deficient (Fermut) cells, the basal levels of total cellular iron and LIP are lower and higher, respectively, than those in wild-type L929 cells. We also found that TNF induces a substantial increase of LIP followed by an extensive increase of ROS generation in wild-type L929 cells. Interestingly, although Fermut cells had higher basal steady-state LIP levels, less LIP induction after TNF stimulation was observed in these cells. This can be explained by our observation that the intracellular iron storage is the major source to furnish the induced LIP. Importantly, ROS production after TNF treatment, which mirrors LIP induction, also exhibits an impaired induction in Fermut cells. Because ROS production is required for TNF-induced L929 cell death (18, 49), the impaired ROS response accounts for, at least in part, the resistance to TNF-induced cell death in Fermut cells. In contrast to some other cell lines (12, 37), L929 cells are not inducible for H-ferritin expression after TNF treatment (59). We show by ectopically expressing H-ferritin under a TNF-inducible promoter that TNF-inducible expression of H-ferritin prevents a TNF-induced increase of LIP and ROS as well as cell death in L929 cells. Thus, LIP can be modulated by multiple mechanisms and LIP level is a central determinant of ROS production and cell death in TNF-treated cells.
MATERIALS AND METHODS
Mutagenesis and identification of mutated gene.
The pDisrup retroviral vector was used to randomly disrupt genes in L929 cells as described in our previous publications (34, 39, 58). The type of parental L929 cell used in the mutagenesis is a selected subclone which exhibited a spontaneous survival rate (regrowth) of less than 1 in 106 after 48 h of exposure to TNF at 100 ng/ml. The identity of the gene of the viral vector insertion site was determined by 3′ rapid amplification of cDNA ends (RACE) and DNA sequencing of the 3′ RACE product as we described previously (58).
Plasmids and cell transfection.
cDNA of H-ferritin was subcloned into pcDNA6 (Invitrogen) vector or κB151 vector (a gift from V. Kravchenko, The Scripps Research Institute) for reconstitution and inducible expression by TNF, respectively. The calcium phosphate method was used for transfection. Stably transfected clones were selected in normal growth medium with the addition of blasticidin S (10 μg/ml; Invitrogen).
Measurement of cell death.
Cells were trypsinized, collected by centrifugation, washed once with phosphate-buffered saline (PBS), and resuspended in PBS containing 1 μg/ml propidium iodide (PI). The levels of PI incorporation were quantified by flow cytometry on a FACScan flow cytometer (Beckman Coulter EPICS XL).
Measurement of mRNA levels.
Semiquantitative PCR was performed using the Relative reverse transcription (RT)-PCR kit (Ambion, Austin, TX) following the manufacturer's instruction. For real-time PCR analysis, 0.5 μg of total RNA from L929 or A549 cells was used to prepare cDNA by using the Omniscript RT kit (QIAGEN) with oligo(dT)12 as a primer. The SYBR green PCR Master Mix kit (Applied Biosystems) was used in the real-time PCR analysis.
Measurement of total cellular iron.
The total cellular iron was measure as described by Epsztejn et al. (14). Triplicate samples of 5 × 106 cells suspended in 1 ml of an HBS buffer (20 mM HEPES [pH 7.5], 150 mM NaCl) were mixed with an equal volume of an acid mixture (3 N HCl, 10% trichloroacetic acid, 3% thioglycolic acid) and incubated for 2 h at 37°C, cooled, centrifuged at 3,000 rpm for 30 min, and mixed with 0.5 ml batophenan troline sulconate (0.045% in 4.5 N Na acetate-0.2% thioglycolic acid), and the light absorption of the pink solution was read at 535 nm in a UV-VIS photodiode spectrophotometer.
Measurement of relative LIP level.
The LIP levels were measure as described by Epsztejn et al. (15). Because TNF treatment leads to loss of cell viability, we washed away dead cells before the measurement and normalized fluorescence reading with the number of viable cells based on a standard curve of fluorescence intensity cell number. Briefly, the cells were cultured in a 96-well assay plate (white plate with clear bottom, Corning Inc., Corning, NY) and treated with TNF (100 ng/ml) for different periods of time and then loaded with calcein (CA) acetoxymethyl ester (CA-AM; Molecular Probes, Eugene, OR) by changing the medium with or without TNF and with freshly prepared medium containing 0.5 μM CA-AM. The cells were further cultured for 30 min and then washed twice with HBS (20 mM HEPES [pH 7.3], 150 mM NaCl, 20 μM CaCl2, 10 mM glucose) and maintained in HBS. Fluorescence was measured at an excitation of 488 nm and emission of 517 nm using an LS55 luminescence spectrometer (PerkinElmer, Norwalk, CT) equipped with a plate reader accessory. The cells were then treated with 0.1 mM iron chelator salicylaldehyde isonicotinoyl hydrazone (42) for 10 min. The fluorescence of CA in these cells was measured again. The number of cells was then measured by crystal violet assay and used to normalize the fluorescence reading. A standard curve for fluorescence intensity and cell number was drawn by plating different numbers of cells in a 96-well plate and loading them with CA-AM as described above. The ΔF of the measurement normalized two times was obtained, and the relative LIP of each sample was calculated by dividing its ΔF with the ΔF obtained from wild-type cells in resting stage.
Measurements of ROS.
Generation of intracellular superoxides were determined according to the fluorescence of ethidium as a result of the oxidation of hydroethidine (also known as dihydroethidium [HE]; Molecular Probes). HE at 10 μg/μl in dimethyl sulfoxide (DMSO) was stored in nitrogen at −80°C. HE (16 μm) or a DMSO vehicle was added to cells at the same time and incubation took place for 15 min at 37°C. Fluorescence was measured by flow cytometry. Dichlorofluorescin-diacetate (DCFH-DA; Molecular Probes) fluoresces upon oxidation by hydrogen peroxide. DCFH-DA was stored at 4 mm in DMSO at −80°C. Cells were trypsinized, collected by centrifugation, washed once with PBS, and resuspended in PBS. DCFH-DA (20 μm) or a DMSO vehicle was added to cells at the same time. Thirty minutes later, fluorescence was measure by flow cytometry on a FACScan flow cytometer.
Gel shift assay.
Nuclear extracts were prepared from cultured cells, and gel shifts using these nuclear extracts were carried out as previously described (31).
RESULTS
Disruption of the H-ferritin gene in L929 cells confers resistance to TNF-induced cell death.
We used retrovirus insertion-mediated mutagenesis in L929 cells coupled with TNF treatment to select TNF-resistant cell lines generated by the viral insertion. As described in our previous reports (34, 39, 58), the retroviral vector was designed so that the neo gene is fused to the sequence of the exon located at the 3′ end of the viral insertion site. The identities of the disrupted genes in the various TNF-resistant cell lines were determined by 3′ RACE of the fused neo mRNA.
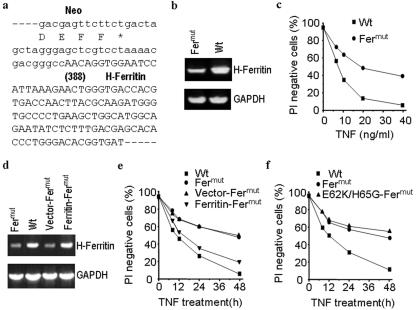
The gene disrupted in one of the TNF-resistant cell lines was identified as H-ferritin. A partial sequence of the fused gene product generated by retroviral insertion in this line (termed Fermut) is shown in Fig. 1a. The neo gene encoded by the viral vector was inserted into the H-ferritin coding region disrupting an H-ferritin gene. Semiquantitative PCR analysis revealed a reduced expression of H-ferritin mRNA (Fig. 1b), confirming that one allele of the H-ferritin gene had been disrupted. Fermut cells showed significant resistance to TNF-induced killing at low or high TNF concentrations (Fig. 1c).
FIG. 1.
H-ferritin mutation leads to a resistance to TNF-induced cell death in L929 cells. (a) The fused mRNA of neo and an endogenous gene in a TNF-resistant L929 clonal cell line was amplified by 3′ RACE. The junction sequence of the fused cDNA is shown, which reveals that the viral insertion occurred at the coding region of the H-ferritin gene. Amino acid sequence at the C terminus of neo is shown beneath the cDNA sequence. The sequence introduced by the viral vector is shown in lowercase. The number in parentheses indicates the position relative to the start codon of ferritin. (b) H-ferritin mRNA is reduced in H-ferritin mutant cells (Fermut). A semiquantitative RT-PCR was performed using the total RNA from wild-type parental (Wt) and Fermut cells, and an ethidium bromide stain is shown. (c) Wild-type and Fermut cells were treated with different concentrations of TNF as indicated. The cells were harvested 48 h after the treatment, and cell viability was assessed by PI exclusion. (d) Stable cell lines were generated from Fermut cells by transfection of H-ferritin expression vector (Ferritin-Fermut) or empty vector (Vector-Fermut). The H-ferritin mRNA levels were examined by semiquantitative PCR. GADPH, glyceraldehyde-3-phosphate dehydrogenase. (e) The cells described for panel d were treated with TNF (40 ng/ml) for different periods of time. Cell viability was assessed by PI exclusion. (f) Stable cell lines were generated from Fermut cells by transfection of an expression vector for an H-ferritin mutant with an inactive ferroxidase center (E62K/H65G-Fermut). The sensitivity to TNF-induced cell death was measured as described for panel e.
To establish whether the TNF resistance of Fermut cells is due to a decrease in H-ferritin expression, we stably transfected the expression vector of H-ferritin under the control of a cytomegalovirus promoter or empty vector into the Fermut cell line (designated Ferritin-Fermut and Vector-Fermut, respectively). The reconstitution of H-ferritin expression was determined by semiquantitative PCR and is shown in Fig. 1d. The expression of H-ferritin in Fermut cells reconstituted sensitivity to TNF-induced cell death, while vector transfection of Fermut cells had no effect on TNF sensitivity (Fig. 1e). Thus, the resistance to TNF-induced cell death observed in the Fermut cell line is due to the reduced level of H-ferritin expression.
In parallel, we also determined whether the ferroxidase activity of H-ferritin is required for the reconstitution of TNF sensitivity in Fermut cells. We expressed in Fermut cells a H-ferritin mutant defective in ferroxidase activity (11) in which E62 and H65 were mutated to K and G, respectively (termed E62K/H65G). Expression of E62K/H65G did not reconstitute the sensitivity to TNF-induced cell death in Fermut cells (Fig. 1f), indicating that the ferroxidase activity of H-ferritin is required for its function in TNF-induced cell death in L929 cells, consistent with its role of converting “free iron” to storage iron.
The resistance of Fermut cells is selective to TNF-induced cell death.
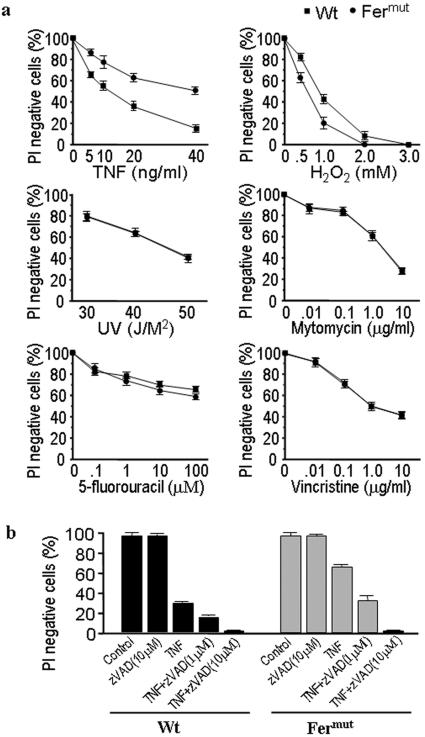
To determine whether the resistance to cell death by H-ferritin deficiency is selective, we examined the sensitivity of Fermut cells to several death stimuli. As shown in Fig. 2, Fermut cells were resistant to TNF-induced cell death but more sensitive to H2O2-induced cell death. The increased sensitivity to H2O2 in Fermut cells is consistent with previous observations that higher H-ferritin levels confer greater resistance to H2O2-induced cell death (14). The sensitivity of Fermut to UV irradiation-, mitomycin-, 5-fluorouracil-, or vincristine-induced cell death was comparable to that observed in the wild-type L929 cells. Thus, H-ferritin-deficiency-mediated TNF resistance is not due to a general promotion of cell survival but is caused by an impairment of the death pathway used by TNF.
FIG. 2.
Fermut cells are selectively resistant to TNF-induced death and have similar sensitivity to zVAD-enhanced cell death in comparison to wild-type L929 cells. (a) Wild-type (Wt) and Fermut cells were treated with different concentrations of TNF, H2O2, mytomycin, 5-fluorouracil, and vincristine for 48, 9, 96, 65, and 96 h, respectively, or with different doses of UV light. Cell viability was measured as described above. (b) Wild-type and Fermut cells were treated with TNF (100 ng/ml) and/or zVAD (1 μM or 10 μM) in different combinations for 24 h. Cell viabilities were measured.
Since inhibition of caspases by the pan-caspase inhibitor zVAD can enhance necrotic cell death in L929 cells (57), we examined whether the mutation of H-ferritin alters zVAD's effect on TNF-induced cell death in L929 cells. As shown in Fig. 2b, caspase inhibition by zVAD equally enhanced TNF-induced cell death in wild-type and Fermut L929 cells.
H-ferritin deficiency alters the levels of total cellular iron and LIP in Fermut cells.
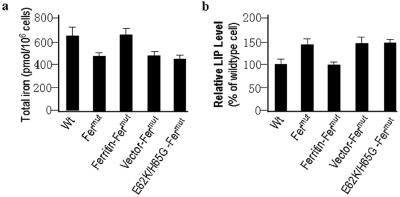
Since H-ferritin plays a central role in regulating cellular iron availability, we measured total iron in Fermut as well as wild-type L929 cells. The total iron was ∼25% lower in Fermut cells than in wild-type L929 cells (Fig. 3a). Expression of H-ferritin, but not the E62K/H65G mutant or empty vector, in Fermut cells increased total iron to a level similar to that in wild-type L929 cells (Fig. 3a). These results indicate that lower expression of H-ferritin in Fermut cells leads to less storage of iron and is consistent with the iron storage function of ferritin.
FIG. 3.
Lower level of H-ferritin in Fermut cells results in low total cellular iron and high basal LIP levels compared with levels in wild-type cells (Wt). (a) Total cellular iron in wild-type, Fermut, and reconstituted lines was measured. H-ferritin deficiency results in reduced total cellular iron. (b) LIP levels in wild-type, Fermut, and reconstituted lines were measured. The results indicate that H-ferritin mutation leads to an increase in the steady level of LIP.
In contrast to the total iron concentration, Fermut cells had ∼30% more LIP than wild-type L929 cells (Fig. 3b). The level of LIP is regulated by H-ferritin expression, since reconstituted Fermut cells (Ferritin-Fermut) had levels of LIP similar to those in wild-type L929 cells (Fig. 3b), and is in accordance with previous reports that cellular levels of H-ferritin are correlated with LIP levels.
LIP induction correlates with ROS production and cell death.
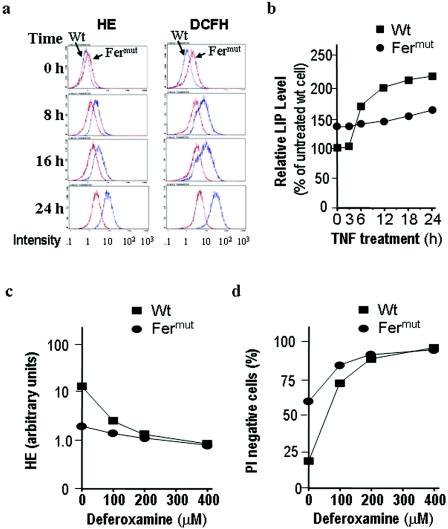
It has been shown that TNF induces ROS production and that ROS induction is required for TNF-induced death in L929 cells. We measured oxidative burst and peroxidase activity with HE and DCFH-DA. Fermut cells have higher basal ROS levels than wild-type L929 cells (Fig. 4a, top panels). This is most likely caused by the higher basal LIP concentrations in Fermut cells (Fig. 3b), further explaining why Fermut cells are more sensitive to H2O2-induced cell death (Fig. 2). Moreover, as reported previously (18), TNF treatment led to an increase in the ROS level in L929 cells (Fig. 4a). Interestingly, although Fermut cells have a higher basal ROS level, these cells showed much less ROS induction by TNF than wild-type cells (Fig. 4a). In other words, ROS levels in TNF-treated Fermut cells were lower than those in TNF-treated wild-type cells. As aforementioned, ROS production is required for TNF-induced cell death in L929 cells and the resistance to TNF-induced cell death in Fermut cells is, at least in part, caused by an impaired ability to produce ROS in response to TNF treatment.
FIG. 4.
TNF-induced ROS production and LIP elevation are impaired in Fermut cells, and the LIP induction is required for TNF-induced ROS production and cell death. (a) Wild-type (Wt) and Fermut cells were treated with TNF (40 ng/ml). ROS production was measured at different times of the treatment with HE and DCFH-DA, respectively. Fermut cells have much less ROS induction by TNF in comparison with wild-type L929 cells. (b) Wild-type and Fermut cells were treated with TNF. LIP levels were measured at different times of the treatment. Fermut cells have much less LIP induction by TNF in comparison with wild-type L929 cells. (c) Wild-type and Fermut cells were pretreated with the indicated concentrations of deferoxamine for 6 h, followed by additional 24-h TNF treatment. The ROS levels were measured by HE staining. (d) Wild-type and Fermut cells were pretreated with the indicated concentrations of deferoxamine for 6 h, followed by 24-h TNF treatment. The survival rates were determined by PI exclusion.
Since LIP is involved in ROS production, we questioned why Fermut cells have higher steady-state levels of LIP before TNF treatment and yet produce less ROS after TNF stimulation. To address this question, we measured LIP during the course of TNF treatment. As shown in Fig. 4b, TNF stimulation significantly increased LIP in the wild-type L929 cells. Although Fermut cells have higher initial LIP levels, the induction of LIP by TNF was modest and the LIP levels in Fermut cells were actually significantly lower than that in wild-type cells after treatment. These results are consistent with the observation by many others that LIP and ROS levels follow similar rise and fall patterns (26, 32) and suggest that reduced ROS induction in TNF-treated Fermut cells is most likely due to impaired LIP induction.
To confirm that TNF-induced ROS production is dependent on LIP induction, we treated the cells with iron chelator deferoxamine. Deferoxamine was able to inhibit TNF-induced ROS production in L929 cells (Fig. 4c). To further determine whether LIP indeed plays a role in TNF-induced ROS-dependent L929 cell death, we measured cell viability in a parallel experiment. Deferoxamine was able to protect against TNF cytotoxicity in L929 cells (Fig. 4d). The survival rates of Fermut and wild-type cells were about the same in the presence of >200 μM of deferoxamine, confirming that H-ferritin deficiency in Fermut alters the chelatable iron pool to influence TNF-induced cell death. These results again demonstrate that LIP induction is required for the ROS generation and cell death of TNF-treated L929 cells.
Low intracellular iron storage in Fermut cells limits LIP induction by TNF.
We next explored why Fermut cells have an impaired LIP response to TNF stimulation. It is known that there exist two major physiological mechanisms that furnish iron to the LIP: iron uptake from outside the cell and iron release from intracellular ferritin (26, 43). Because H-ferritin deficiency in Fermut leads to a reduced storage of iron as assayed for the total iron level (Fig. 3a) and much less LIP induction after TNF treatment in the same cells (Fig. 4b), we hypothesized that the ferritin-bound storage of iron is a major source of iron for furnishing TNF-induced LIP in L929 cells and that reduced iron storage pools result in a corresponding decrease in LIP induction in TNF-treated Fermut cells.
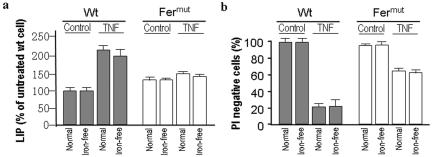
To test this hypothesis, we evaluated whether the uptake of exogenous iron is involved in TNF-induced LIP and cell death. Iron-free medium has been used by other investigators (55) to exclude the involvement of exogenous iron, and the same strategy was employed here to determine the role of exogenous iron. Serum-free medium containing iron [0.1 mg/liter Fe(NO3)3-5 μg/ml iron-saturated transferrin, termed normal] and the same medium without iron and transferrin (iron free) were separately used to determine the effects of iron uptake on TNF-induced L929 cell death. Both the serum-free and iron deprivation conditions did not have significant effects on the viability of L929 cells over a 48-h period of time (data not shown). Wild-type and Fermut cells were cultured in normal and iron-free medium for 12 h and then treated with TNF for an additional 24 h. LIP and cell viability were measured and are shown in Fig. 5a and b. The increase in TNF-induced LIP was only slightly less in wild-type cells under the iron-free condition (Fig. 5a). Similar to wild-type cells, iron deprivation did not have much effect on LIP in Fermut cells (Fig. 5a). These data suggest that extracellular iron is not a major iron source for TNF-induced LIP in L929 cells. Since extracellular iron depletion does not affect the TNF-induced increase of LIP, it is not surprising to see that TNF-induced cell death is similar between cells in normal medium and cells in iron-free medium (Fig. 5b). These data support our hypothesis that the major source of iron to furnish the TNF-induced LIP is of intracellular origin and that reduced iron storage in Fermut cells accounts for the reduced LIP induction by TNF.
FIG. 5.
Endogenous storage iron furnishes TNF-induced LIP in L929 cells. (a) Wild-type (Wt) and Fermut cells were cultured in iron-containing medium (Normal) or iron-free medium (Iron-free) for 8 h, followed by 24-h TNF (40 ng/ml) treatment. LIP levels were measured. (b) Viabilities of the cells treated were determined as described for panel a.
H-ferritin has a role in modulating LIP in TNF-treated cells.
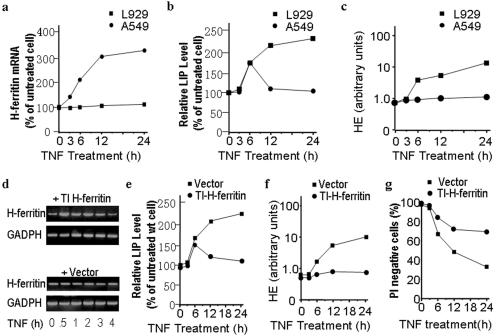
It has been shown that changes in LIP in response to extracellular stimuli are usually transitory and homeostatic in nature (26). The substantial increase of LIP in TNF-treated L929 cells could result from a lack of balance in regulating LIP levels or from the possibility that the TNF-induced change exceeds the cells' homeostatic capacity. The latter seems unlikely because Fermut cells that should have less homeostatic capacity for iron are resistant to TNF-induced LIP and death. Induction of H-ferritin and reduction of the transferrin receptor are commonly used by cells to control cellular iron levels and LIP upon treatment with different stimuli including cytokines. Since the data shown in Fig. 5 indicate that the uptake of iron does not play a major role in TNF-induced LIP in L929 cells, we next addressed the relationship between H-ferritin and LIP levels. Because none of the antibodies available to us could detect H-ferritin in L929 cells, we were unable to directly evaluate the relationship between H-ferritin protein level and LIP (data not shown). As it is reported that TNF can induce H-ferritin expression at the mRNA level (37), we measured H-ferritin mRNA in L929 cells before and after TNF stimulation using real-time PCR. An A549 cell was used for comparison because TNF-induced H-ferritin expression has been reported in this cell line (50). As shown in Fig. 6a, TNF-induced H-ferritin mRNA expression is observed in A549 cells but not in L929 cells. Together with another report showing that no change in H-ferritin at the mRNA or protein levels occurs in TNF-treated L929 cells (59), we conclude that L929 cells do not up-regulate H-ferritin expression in response to TNF stimulation. We measured LIP levels of A549 in parallel with those of L929 cells treated with TNF and found that LIP induction by TNF in A549 cells was as drastic as in L929 cells during the first 6 h of treatment but was transient, declining to the basal levels thereafter. In contrast, LIP levels in TNF-treated L929 cells remained high after 24 h of TNF treatment (Fig. 6b). These data suggest that H-ferritin induction is a mechanism in controlling LIP levels in A549 cells and that this mechanism is absent in L929 cells. ROS induction by TNF is seen much more strongly in L929 cells than in A549 cells, in agreement with the relationship between LIP and ROS (Fig. 6c).
FIG. 6.
Induction of H-ferritin by TNF is absent in L929 cells, and ectopically introducing TNF-inducible H-ferritin inhibits TNF-induced LIP, ROS, and cell death. (a) L929 and A549 cells were treated with TNF (100 ng/ml) for different periods of time. H-ferritin mRNA levels were measured by real-time PCR. (b) LIP levels of the cells treated were as described for panel a. (c) ROS production in the cells treated were as described for panel a. (d) Stable lines of L929 cells transfected with H-ferritin in a TNF-inducible expression vector (TI H-ferritin) or empty vector (Vector) were treated with TNF for different periods of time. H-ferritin mRNA levels were examined by semiquantitative PCR. (e) LIP levels in TI H-ferritin- and vector-transfected L929 cells after TNF treatment of different periods of time. wt, wild type. (f) ROS levels in the cells were as described for panel e. (g) Viability of the cells was as described for panel e.
To further examine whether H-ferritin induction can truly function as a counteracting mechanism to regulate LIP levels, we stably expressed H-ferritin under a TNF-inducible promoter in L929 cells. The induction of H-ferritin in this cell line after TNF treatment was detected by RT-PCR (Fig. 6d). We next examined the effect of TNF-induced expression of H-ferritin on TNF-induced LIP, ROS, and cell death in L929 cells. Indeed, ectopic H-ferritin expression under a TNF-inducible system significantly inhibited TNF-reduced LIP, ROS, and cell death in L929 cells (Fig. 6e through g). These results raise a provocative possibility that it is not the preexisting ferritin but the ferritin induced by a given stimulus that can act as the protective chelator of induced LIP in the prevention of cell death.
TNF-induced NF-κB activation is normal in wild-type and H-ferritin mutated L929 cells.
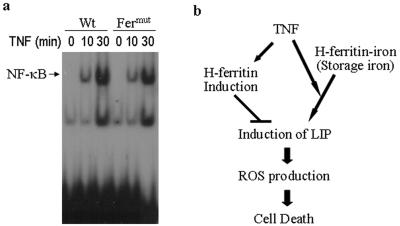
Pham et al. recently reported that H-ferritin is an NF-κB-dependent gene and is primarily responsible for the NF-κB-mediated antiapoptosis effect (41). Since H-ferritin is not inducible in L929 cells, we examined whether L929 cells were defective in TNF-induced NF-κB activation using gel shift assays. As shown in Fig. 7a, TNF induces NF-κB activation in both wild-type and Fermut cells. Thus, the lack of H-ferritin induction in L929 cells is not due to the absence of NF-κB activation upon TNF stimulation. Furthermore, we analyzed the promoter regions of mouse and human H-ferritin and did not find any NF-κB binding sites. Though we cannot exclude the possibility that an NF-κB-like sequence(s) differing from the consensus motif GGGANNYYCC can serve as an NF-κB binding site(s) in the H-ferritin promoter, it is likely that NF-κB-dependent H-ferritin induction is not directly driven by NF-κB. Thus, the lack of TNF-induced H-ferritin expression in L929 cells may not be related to the signaling pathway that controls NF-κB activation.
FIG. 7.
(a) DNA binding activity to NF-κB. Wild-type (Wt) and Fermut L929 cells were treated with TNF for 0, 10, and 30 min. Nuclear extracts were prepared and analyzed by gel shift assay. The autoradiograph is shown. (b) Proposed model of how H-ferritin regulates LIP and the central role of LIP in TNF-induced ROS production and ROS-dependent cell death.
DISCUSSION
Using genetic mutagenesis, we systematically searched for genes that play critical roles in TNF-induced L929 cell death, a paradigm for necrotic (caspase-independent) cell death. Our genetic screen has uncovered a number of factors in which full complements of expression are needed for L929 cells to undergo cell death in response to TNF (34, 39, 58). In this study, we characterize a TNF-resistant line that harbors a mutation in the H-ferritin gene. We found that a deficiency of H-ferritin results in resistance to TNF and that reintroduction of H-ferritin restores the sensitivity of L929 Fermut cells to respond to TNF-induced killing. To a first approximation, this finding contradicts a reported protective role of H-ferritin in ROS-mediated cell death; however, further careful studies have revealed the dynamic aspects of ferritin function in controlling the pool of labile iron: H-ferritin can both positively and negatively influence the level of LIP induction by a given stimulus. Low, steady levels of ferritin result in less iron storage, which in turn limits LIP induction if intracellular storage is the major source of iron to supply increased LIP, as is the case in L929 cells; on the other hand, high levels of ferritin newly synthesized by inducible expression mechanisms sequester free iron and thus suppress LIP induction. LIP is therefore a direct limiting factor of ROS production, and ferritin exerts its role on ROS-mediated cell death by controlling cellular levels of LIP. Nevertheless, it is important to note that LIP can also be furnished by extracellular iron in some cell systems and that proteins involved in iron import and export can also affect LIP and thus ROS-mediated cell death (12, 29, 30). For example, apoptotic death of bovine aortic endothelial cells after exposure to doxorubicin (DOX), a widely used anticancer drug that induces ROS, oxidative damage, and apoptosis, is accompanied by a significant increase in the transferrin-receptor-dependent iron uptake (30). Together with data from previous studies, we propose a model shown in Fig. 7 to suggest that cellular ferritin controls LIP levels that in turn play a central role in regulating ROS-mediated cell death.
LIP is needed for the normal metabolism of cells (26). Because of the toxicity of free iron, multiple regulatory mechanisms have been invoked in cells to maintain LIP levels in a safe range (26). Within the safe range, higher, steady levels of LIP in the cell lead to a higher sensitivity to oxidative stress-induced death (2, 14), as in the case of the H-ferritin-deficient cells (Fig. 2). The protective role of higher basal levels of H-ferritin in cell death is best demonstrated in cells that directly encountered oxidative insults (14) and can be interpreted as lower, steady LIP levels in the cell-limited oxidation-mediated damage. However, higher basal levels of H-ferritin cannot prevent LIP induction, most likely due to the possibility that existing ferritin molecules are already occupied by iron. Inducible expression of H-ferritin is an effective replenishing mechanism to suppress LIP induction (Fig. 7).
RIP1 is known to be required for TNF-induced caspase-independent cell death (23, 35). We checked if RIP1 is required for the induction of LIP in TNF-treated cells and found that TNF cannot induce an increase of LIP in RIP1 knockout mouse embryonic fibroblast cells (data not shown), indicating that RIP1 is upstream of LIP induction. Our data suggest that in L929 cells, the iron source used to supply TNF-induced LIP is released from existing ferritin complexes. Degradation of ferritin by the lysosome is known to be a mechanism for the recycling of ferritin-bound iron (43). Thus, lysosomes are likely to have a role in LIP up-regulation in TNF-treated L929 cells. The involvement of lysosomes in TNF-induced cell death has in fact been demonstrated by genetic and biochemical evidence (13, 21, 22, 24, 39, 45). Degradation of ferritin may provide one of the mechanisms of how lysosomes contribute to TNF-induced cell death. Increased LIP in TNF-treated cells may function to increase iron availability for many TNF-induced cellular reactions, including enhancing the respiration chain and other sites that could result in a notable increase of ROS generation. TNF can induce H-ferritin expression in many different types of cells, which may serve to limit LIP induction and therefore control ROS. In contrast, L929 cells cannot be induced by TNF to elevate ferritin expression and hence lack the mechanism to limit LIP formation when exposed to TNF and may be one of the reasons why substantial ROS are produced and cell death occurs in L929 cells (Fig. 7).
A number of studies have used iron loads and iron chelators to address the relationship between iron and cell death (20, 25). While many cell lines are not affected in viability by acute iron loads or iron deprivation, some cell lines can be killed by iron deprivation (25). It is possible that different cells adopt different means to store and reuse iron. Iron deprivation in cells that have low iron storage or are inefficient in the recycling of storage iron may quickly affect cellular metabolisms and then trigger apoptosis. As for the cells with viability unaffected by iron load or deprivation alone, preexposure of iron load in cells can either enhance or inhibit TNF-induced cell death. Different cells may have different abilities to adjust LIP levels, which can be explained by the observations that iron loads can induce ferritin expression in some cells whereas iron treatment has no effect on ferritin expression in other cells. LIP induction by TNF should be suppressed if the expression of ferritin is induced by preexposure of iron, and LIP induction should be enhanced if cells that cannot be induced express ferritin by iron load. While this paper was in preparation, Pham et al. reported that H-ferritin up-regulation by NF-κB inhibits TNF-induced apoptosis through suppressing ROS (41), which is consistent with our finding that induced H-ferritin counteracts ROS induction. Our data further show that H-ferritin, through LIP, controls ROS levels. The work by Pham et al. and us demonstrates that iron metabolism is involved in both apoptotic and necrotic cell death. However, it is unclear at the present time how ROS functions in apoptosis and necrosis. ROS is believed to be upstream of caspases in the apoptotic program (41); however, we also observed that the inhibition of caspases equally enhances TNF-induced necrosis in wild-type and Fermut L929 cells (Fig. 2b), suggesting that LIP and its controlled ROS must function either unrelated or antagonistic to caspase activity in necrosis. Nevertheless, the role of LIP in the control of ROS appears to be the same. Taken together, our current study has provided compelling evidence that it is the inducibility of ferritin rather than the absolute ferritin concentration in the cell that controls levels of stimulus-induced LIP and that LIP is a central controller of TNF-induced ROS and ROS-dependent cell death.
Acknowledgments
We thank P. Ponka for kindly providing us with salicylaldehyde isonicotinoyl hydrazone and V. Kravchenko for the pcDNAκB vector.
This work was supported in part by National Institutes of Health grants AI41637, AI054796, GM37696, and GM67101 and funds from Xiamen University.
REFERENCES
- 1.Arosio, P., and S. Levi. 2002. Ferritin, iron homeostasis, and oxidative damage. Free Radic. Biol. Med. 33:457-463. [DOI] [PubMed] [Google Scholar]
- 2.Berberat, P. O., M. Katori, E. Kaczmarek, D. Anselmo, C. Lassman, B. Ke, X. Shen, R. W. Busuttil, K. Yamashita, E. Csizmadia, S. Tyagi, L. E. Otterbein, S. Brouard, E. Tobiasch, F. H. Bach, J. W. Kupiec-Weglinski, and M. P. Soares. 2003. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 17:1724-1726. [DOI] [PubMed] [Google Scholar]
- 3.Beutler, B., and A. Cerami. 1986. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature 320:584-588. [DOI] [PubMed] [Google Scholar]
- 4.Beutler, B., and A. Cerami. 1988. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu. Rev. Biochem. 57:505-518. [DOI] [PubMed] [Google Scholar]
- 5.Beyaert, R., and W. Fiers. 1994. Molecular mechanisms of tumor necrosis factor-induced cytotoxicity. What we do understand and what we do not. FEBS Lett. 340:9-16. [DOI] [PubMed] [Google Scholar]
- 6.Boldin, M. P., T. M. Goncharov, Y. V. Goltsev, and D. Wallach. 1996. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1 and TNF receptor-induced cell death. Cell 85:803-815. [DOI] [PubMed] [Google Scholar]
- 7.Brekke, O. L., M. R. Shalaby, A. Sundan, T. Espevik, and K. S. Bjerve. 1992. Butylated hydroxyanisole specifically inhibits tumor necrosis factor-induced cytotoxicity and growth enhancement. Cytokine 4:269-280. [DOI] [PubMed] [Google Scholar]
- 8.Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269-290. [DOI] [PubMed] [Google Scholar]
- 9.Carswell, E. A., L. J. Old, R. L. Kassel, S. Green, N. Fiore, and B. Williamson. 1975. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 72:3666-3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cauwels, A., B. Janssen, A. Waeytens, C. Cuvelier, and P. Brouckaert. 2003. Caspase inhibition causes hyperacute tumor necrosis factor-induced shock via oxidative stress and phospholipase A2. Nat. Immunol. 4:387-393. [DOI] [PubMed] [Google Scholar]
- 11.Cozzi, A., B. Corsi, S. Levi, P. Santambrogio, A. Albertini, and P. Arosio. 2000. Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vivo role of ferritin ferroxidase activity. J. Biol. Chem. 275:25122-25129. [DOI] [PubMed] [Google Scholar]
- 12.Cozzi, A., S. Levi, B. Corsi, P. Santambrogio, A. Campanella, G. Gerardi, and P. Arosio. 2003. Role of iron and ferritin in TNFalpha-induced apoptosis in HeLa cells. FEBS Lett. 537:187-192. [DOI] [PubMed] [Google Scholar]
- 13.Deiss, L. P., H. Galinka, H. Berissi, O. Cohen, and A. Kimchi. 1996. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO J. 15:3861-3870. [PMC free article] [PubMed] [Google Scholar]
- 14.Epsztejn, S., H. Glickstein, V. Picard, I. N. Slotki, W. Breuer, C. Beaumont, and Z. I. Cabantchik. 1999. H-ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties. Blood 94:3593-3603. [PubMed] [Google Scholar]
- 15.Epsztejn, S., O. Kakhlon, H. Glickstein, W. Breuer, and I. Cabantchik. 1997. Fluorescence analysis of the labile iron pool of mammalian cells. Anal. Biochem. 248:31-40. [DOI] [PubMed] [Google Scholar]
- 16.Fiers, W., R. Beyaert, W. Declercq, and P. Vandenabeele. 1999. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene 18:7719-7730. [DOI] [PubMed] [Google Scholar]
- 17.Goossens, V., K. De Vos, D. Vercammen, M. Steemans, K. Vancompernolle, W. Fiers, P. Vandenabeele, and J. Grooten. 1999. Redox regulation of TNF signaling. Biofactors 10:145-156. [DOI] [PubMed] [Google Scholar]
- 18.Goossens, V., J. Grooten, K. De Vos, and W. Fiers. 1995. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc. Natl. Acad. Sci. USA 92:8115-8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 20.Greene, B. T., J. Thorburn, M. C. Willingham, A. Thorburn, R. P. Planalp, M. W. Brechbiel, J. Jennings-Gee, J. Wilkinson, F. M. Torti, and S. V. Torti. 2002. Activation of caspase pathways during iron chelator-mediated apoptosis. J. Biol. Chem. 277:25568-25575. [DOI] [PubMed] [Google Scholar]
- 21.Guicciardi, M. E., J. Deussing, H. Miyoshi, S. F. Bronk, P. A. Svingen, C. Peters, S. H. Kaufmann, and G. J. Gores. 2000. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Investig. 106:1127-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hersh, B. M., E. Hartwieg, and H. R. Horvitz. 2002. The Caenorhabditis elegans mucolipin-like gene cup-5 is essential for viability and regulates lysosomes in multiple cell types. Proc. Natl. Acad. Sci. USA 99:4355-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holler, N., R. Zaru, O. Micheau, M. Thome, A. Attinger, S. Valitutti, J. L. Bodmer, P. Schneider, B. Seed, and J. Tschopp. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1:489-495. [DOI] [PubMed] [Google Scholar]
- 24.Isahara, K., Y. Ohsawa, S. Kanamori, M. Shibata, S. Waguri, N. Sato, T. Gotow, T. Watanabe, T. Momoi, K. Urase, E. Kominami, and Y. Uchiyama. 1999. Regulation of a novel pathway for cell death by lysosomal aspartic and cysteine proteinases. Neuroscience 91:233-249. [DOI] [PubMed] [Google Scholar]
- 25.Jiang, X. P., F. Wang, D. C. Yang, R. L. Elliott, and J. F. Head. 2002. Induction of apoptosis by iron depletion in the human breast cancer MCF-7 cell line and the 13762NF rat mammary adenocarcinoma in vivo. Anticancer Res. 22:2685-2692. [PubMed] [Google Scholar]
- 26.Kakhlon, O., and Z. I. Cabantchik. 2002. The labile iron pool: characterization, measurement, and participation in cellular processes(1). Free Radic. Biol. Med. 33:1037-1046. [DOI] [PubMed] [Google Scholar]
- 27.Kakhlon, O., Y. Gruenbaum, and Z. I. Cabantchik. 2001. Repression of ferritin expression increases the labile iron pool, oxidative stress, and short-term growth of human erythroleukemia cells. Blood 97:2863-2871. [DOI] [PubMed] [Google Scholar]
- 28.Kakhlon, O., Y. Gruenbaum, and Z. I. Cabantchik. 2001. Repression of the heavy ferritin chain increases the labile iron pool of human K562 cells. Biochem. J. 356:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalivendi, S. V., S. Cunningham, S. Kotamraju, J. Joseph, C. J. Hillard, and B. Kalyanaraman. 2004. Alpha-synuclein up-regulation and aggregation during MPP+-induced apoptosis in neuroblastoma cells: intermediacy of transferrin receptor iron and hydrogen peroxide. J. Biol. Chem. 279:15240-15247. [DOI] [PubMed] [Google Scholar]
- 30.Kotamraju, S., C. R. Chitambar, S. V. Kalivendi, J. Joseph, and B. Kalyanaraman. 2002. Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: role of oxidant-induced iron signaling in apoptosis. J. Biol. Chem. 277:17179-17187. [DOI] [PubMed] [Google Scholar]
- 31.Kravchenko, V. V., Z. Pan, J. Han, J.-M. Herbert, R. J. Ulevitch, and R. D. Ye. 1995. Platelet-activating factor induces NF-kB activation through a G protein-coupled pathway. J. Biol. Chem. 270:14928-14934. [DOI] [PubMed] [Google Scholar]
- 32.Kruszewski, M. 2003. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat. Res. 531:81-92. [DOI] [PubMed] [Google Scholar]
- 33.Li, H., and J. Yuan. 1999. Deciphering the pathways of life and death. Curr. Opin. Cell Biol. 11:261-266. [DOI] [PubMed] [Google Scholar]
- 34.Li, J., Q. Li, C. Xie, H. Zhou, Y. Wang, N. Zhang, H. Shao, S. C. Chan, X. Peng, S. C. Lin, and J. Han. 2004. β-actin is required for mitochondria clustering and ROS generation in TNF-induced, caspase-independent cell death. J. Cell Sci. 117:4673-4680. [DOI] [PubMed] [Google Scholar]
- 35.Lin, Y., S. Choksi, H. M. Shen, Q. F. Yang, G. M. Hur, Y. S. Kim, J. H. Tran, S. A. Nedospasov, and Z. G. Liu. 2004. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J. Biol. Chem. 279:10822-10828. [DOI] [PubMed] [Google Scholar]
- 36.Liu, Z. G., and J. Han. 2001. Cellular responses to tumor necrosis factor. Curr. Issues Mol. Biol. 3:79-90. [PubMed] [Google Scholar]
- 37.Miller, L. L., S. C. Miller, S. V. Torti, Y. Tsuji, and F. M. Torti. 1991. Iron-independent induction of ferritin H chain by tumor necrosis factor. Proc. Natl. Acad. Sci. USA 88:4946-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Old, L. J. 1985. Tumor necrosis factor (TNF). Science 230:630-632. [DOI] [PubMed] [Google Scholar]
- 39.Ono, K., X. Wang, and J. Han. 2001. Resistance to tumor necrosis factor-induced cell death mediated by PMCA4 deficiency. Mol. Cell. Biol. 21:8276-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pantopoulos, K. 2004. Iron metabolism and the IRE/IRP regulatory system: an update. Ann. N. Y. Acad. Sci. 1012:1-13. [DOI] [PubMed] [Google Scholar]
- 41.Pham, C. G., C. Bubici, F. Zazzeroni, S. Papa, J. Jones, K. Alvarez, S. Jayawardena, S. E. De, R. Cong, C. Beaumont, F. M. Torti, S. V. Torti, and G. Franzoso. 2004. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell 119:529-542. [DOI] [PubMed] [Google Scholar]
- 42.Ponka, P., J. Borova, J. Neuwirt, O. Fuchs, and E. Necas. 1979. A study of intracellular iron metabolism using pyridoxal isonicotinoyl hydrazone and other synthetic chelating agents. Biochim. Biophys. Acta 586:278-297. [DOI] [PubMed] [Google Scholar]
- 43.Radisky, D. C., and J. Kaplan. 1998. Iron in cytosolic ferritin can be recycled through lysosomal degradation in human fibroblasts. Biochem. J. 336:201-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Regan, R. F., N. Kumar, F. Gao, and Y. Guo. 2002. Ferritin induction protects cortical astrocytes from heme-mediated oxidative injury. Neuroscience 113:985-994. [DOI] [PubMed] [Google Scholar]
- 45.Roberg, K., and K. Ollinger. 1998. Oxidative stress causes relocation of the lysosomal enzyme cathepsin D with ensuing apoptosis in neonatal rat cardiomyocytes. Am. J. Pathol. 152:1151-1156. [PMC free article] [PubMed] [Google Scholar]
- 46.Rouault, T. A. 2003. How mammals acquire and distribute iron needed for oxygen-based metabolism. PLoS Biol. 1:E79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salvesen, G. S., and V. M. Dixit. 1999. Caspase activation: the induced-proximity model. Proc. Natl. Acad. Sci. USA 96:10964-10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schulze-Osthoff, K., A. C. Bakker, B. Vanhaesebroeck, R. Beyaert, W. A. Jacob, and W. Fiers. 1992. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J. Biol. Chem. 267:5317-5323. [PubMed] [Google Scholar]
- 49.Shoji, Y., Y. Uedono, H. Ishikura, N. Takeyama, and T. Tanaka. 1995. DNA damage induced by tumour necrosis factor-alpha in L929 cells is mediated by mitochondrial oxygen radical formation. Immunology 84:543-548. [PMC free article] [PubMed] [Google Scholar]
- 50.Smirnov, I. M., K. Bailey, C. H. Flowers, N. W. Garrigues, and L. J. Wesselius. 1999. Effects of TNF-alpha and IL-1beta on iron metabolism by A549 cells and influence on cytotoxicity. Am. J. Physiol. 277:L257-L263. [DOI] [PubMed] [Google Scholar]
- 51.Tartaglia, L. A., T. M. Ayres, G. H. Wong, and D. V. Goeddel. 1993. A novel domain within the 55 kd TNF receptor signals cell death. Cell 74:845-853. [DOI] [PubMed] [Google Scholar]
- 52.Tewari, M., and V. M. Dixit. 1996. Recent advances in tumor necrosis factor and CD40 signaling. Curr. Opin. Genet. Dev. 6:39-44. [DOI] [PubMed] [Google Scholar]
- 53.Tewari, M., and V. M. Dixit. 1995. Fas- and tumor necrosis factor-induced apoptosis in inhibited by the poxvirus crmA gene product. J. Biol. Chem. 270:3255-3260. [DOI] [PubMed] [Google Scholar]
- 54.Torti, F. M., and S. V. Torti. 2002. Regulation of ferritin genes and protein. Blood 99:3505-3516. [DOI] [PubMed] [Google Scholar]
- 55.Truksa, J., J. Kovar, T. Valenta, M. Ehrlichova, J. Polak, and P. W. Naumann. 2003. Iron deprivation induces apoptosis independently of p53 in human and murine tumour cells. Cell Prolif. 36:199-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vandevoorde, V., G. Haegeman, and W. Fiers. 1997. Induced expression of trimerized intracellular domains of the human tumor necrosis factor (TNF) p55 receptor elicits TNF effects. J. Cell Biol. 137:1627-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vercammen, D., R. Beyaert, G. Denecker, V. Goossens, G. Van Loo, W. Declercq, J. Grooten, W. Fiers, and P. Vandenabeele. 1998. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187:1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, X., K. Ono, S. O. Kim, V. Kravchenko, S. C. Lin, and J. Han. 2001. Metaxin is required for tumor necrosis factor-induced cell death. EMBO Rep. 2:628-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warren, S., S. V. Torti, and F. M. Torti. 1993. The role of iron in the cytotoxicity of tumor necrosis factor. Lymphokine Cytokine Res. 12:75-80. [PubMed] [Google Scholar]
- 60.Xiong, S., H. She, H. Takeuchi, B. Han, J. F. Engelhardt, C. H. Barton, E. Zandi, C. Giulivi, and H. Tsukamoto. 2003. Signaling role of intracellular iron in NF-κB activation. J. Biol. Chem. 278:17646-17654. [DOI] [PubMed] [Google Scholar]