Abstract
The PI3K/Akt pathway plays a critical role in the regulation of gene expression induced by numerous stimuli. p300, a transcriptional coactivator, acts in concert with transcription factors to facilitate gene expression. Here, we show that Akt is activated and translocated to the nucleus in response to tumor necrosis factor alpha. Nuclear Akt associates with p300 and phosphorylates its Ser-1834 both in vivo and in vitro. The phosphorylation induces recruitment of p300 to the ICAM-1 promoter, leading to the acetylation of histones in chromatin and association with the basal transcriptional machinery RNA polymerase II. These two events facilitate ICAM-1 gene expression and are abolished by the p300 S1834A mutant, inhibitors of PI3K/Akt, or small interfering RNA of Akt. Histone acetylation is attributed to the Akt-enhanced intrinsic histone acetyltransferase (HAT) activity of p300 and its association with another HAT, p/CAF. Our study provides a new insight into the molecular mechanism by which Akt promotes the transcriptional potential of p300.
p300 and CBP, two transcriptional coactivators, have been demonstrated to play central roles in coordinating and integrating multiple signal-dependent events with the transcriptional apparatus, allowing the appropriate level of gene activity to occur in response to diverse physiological stimuli (14, 39). They have been recognized as key molecules involved in the communication between the transcription factors and the transcriptional machinery, thus appearing to be important in the gene regulation network (9). p300 and CBP exert their transcription-regulating properties through multiple mechanisms. They act as protein bridges, thereby connecting different transcriptional activators via protein-protein interactions to the basal transcriptional machinery, such as TFIIB and TATA-binding protein, as well as the RNA polymerase II complex (14, 39). They also function as a protein scaffold upon which to build a multicomponent transcriptional regulatory complex (28). Another key property is the possession of intrinsic histone acetyltransferase (HAT) activity, raising the possibility that p300 and CBP may cause localized changes in the chromatin structure by acetylation of the N-terminal tails of core nucleosomal histones. This modification neutralizes the positive charge of lysine residues and seems to weaken the interaction between the histone and DNA, allowing DNA to be more accessible to transcription factors (4, 30).
It has long been known that p300 and CBP are phosphoproteins (9, 14). Several studies have reported the regulation of p300 or CBP by protein kinases, such as CaMKIV (18), MAPK (1), or cyclin E-Cdk2 (2), but none of them identified the amino acids targeted by these kinases. Many of these studies showed that p300 and CBP, rather than the transcription factors, are important targets of protein kinases. For instance, MAPK appears to stimulate Elk-1-mediated gene expression by phosphorylating the C-terminal part of CBP rather than Elk-1 itself (19). Only a few studies have correlated phosphorylation of p300/CBP with their functional effects. For example, Ser-89 of p300 was shown to be phosphorylated in vivo, most probably by PKCα (48), PKCδ (49), or AMP-activated protein kinase (45). In these cases, Ser-89 phosphorylation is inhibitory, since the intrinsic HAT activity of p300 is diminished or its interaction with nuclear receptors is blocked (45, 48, 49). The consensus motif RXRXXpS/T, where X is any amino acid, R is arginine, and pS/T is phosphorylated serine or threonine, preferred by Akt has been characterized (3). In vitro and in vivo phosphorylations of transcription factors in this motif have been shown to regulate gene transcriptions. For example, Akt phosphorylated the class O (FOXO) subfamily of Forkhead transcriptional regulators (FKHR, FKHRL1, and AFX), causing inhibition of their transcriptional activities (5, 7, 22). Akt also interacted with and phosphorylated IKKα at Thr-23 to induce nuclear factor κB (NF-κB) activation (31). Database searches indicate the presence of an RXRXXpS/T consensus sequence in the C-terminal part of p300 (1829 to 1834) and CBP (1866 to 1871). It is probable that Akt regulates gene transcription by phosphorylating this motif on these coactivators. In the present study, we present evidence that p300 is phosphorylated at Ser-1834 by Akt both in vivo and in vitro. This Ser-1834 phosphorylation is critical for the transactivation of p300 by stimulating its HAT activity, assembling transcription factors, and recruiting basal transcriptional machinery to the ICAM-1 promoter.
MATERIALS AND METHODS
Plasmids.
The ICAM-1 promoter construct (pIC339) was a gift from P. T. van der Saag (Hubrecht Laboratory, Utrecht, The Netherlands). The NF-κB-, AP-1-, and CRE-luciferase reporters were purchased from Strategene (La Jolla, CA). The constitutively active mutants of p110 (myc-p110*) and Akt (HA-myr-Akt) and the dominant-negative mutant of Akt (Akt KA) were gifts from A. Klippel (Chiron Corporation, Emeryville, CA). Wild-type (wt) p300 was a gift from Y. Nakatani (National Institutes of Health, Bethesda, MD). To generate bacterial expression constructs of glutathione S-transferase (GST)-tagged p300(1714-1883), the CH3 domain of p300 flanked by an EcoRI site and an XhoI site were generated by PCR and subcloned into pGEX5T-3 vector (Amersham Biosciences, Piscataway, N.J.). Mutations of Ser-1834 to alanine in p300 and GST-p300(1714-1883) were generated using a QuikChange site-directed mutagenesis kit (Strategene, La Jolla, CA) according to the manufacturer's instructions. The primers were 5′-CAGGAGGATGGCCGCCATGCAGCGGACTG-3′ and 5′-CAGTCCGCTGCATGGCGGCCATCCTGGTG-3′.
Cell culture.
A549 cells (human alveolar epithelial cell carcinoma cells) were obtained from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin.
U937 cells (human monocytic leukemia cells) were obtained from the Department of Microbiology, College of Medicine, National Taiwan University, and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin.
Quantification of ICAM-1 expression and cell adhesion assay.
The level of cell surface ICAM-1 expression was determined by enzyme-linked immunosorbent assay as described previously (10). Each assay was performed in triplicate. Adhesion of U937 cells to A549 cells was measured as described previously (10). Briefly, U937 cells were labeled with 2′7′-bis(carboxyethyl)-5(6)-carboxyfluorescein (BCECF) and added to the A549 monolayer. Nonadherent cells were removed by gentle washing with phosphate-buffered saline (PBS), and the number of adherent cells was determined by measuring the fluorescence intensity using a CytoFlour 2300 (Millipore, Bedford, MA).
Transfection and luciferase assay.
A549 cells were transfected with reporters and/or plasmids using Tfx-50 (Promega) according to the manufacturer's recommendations. Forty-eight hours after transfection, the transfection efficiency was about 30 to 40%, and cells were treated with inhibitors for 30 min and then tumor necrosis factor alpha (TNF-α) or trichostatin A (TSA) was added for 6 h as indicated. Cell extracts were then prepared, and luciferase and β-galactosidase activities were measured.
RNA interference assay.
To construct small interfering RNA (siRNA) plasmid for targeting Akt1, two DNA oligonucleotides (5′-GATCCGCACCTTCATCATCCGCTGCTTCAAGAGAGCAGCGGATGATGAAGGTGTTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAACACCTTCATCATCCGCTGCTCTCTTGAAGCAGCGGATGATGAAGGTGCG-3′) that encode Akt1 siRNA sequence were designed and cloned into pSilencer 3.1-H1/neo (Ambion, TX) according to the manufacturer's recommendations. The nonsilencing siRNA plasmid used as a control was a gift from W. C. Chang (National Cheng Kung University, Taiwan). Cells were transfected twice (on subsequent days) with these plasmids using Tfx-50 as described above. The effect of Akt knockdown was assessed by the protein levels in cell lysates isolated from 48 h posttransfection.
Preparation of nuclear extracts and DNA affinity protein-binding assay (DAPA).
Following pretreatment with PI3K inhibitor or vehicle, cells were treated with TNF-α for the indicated times, and then nuclear extracts were isolated as described previously (17). Proper isolation of nuclear extract was verified by the detection of the expression of proliferating cell nuclear antigen. Binding of transcription factor to the ICAM-1 promoter was assayed as described previously (12). The biotin-labeled double-stranded oligonucleotides were synthesized by PCR using biotin-labeled 3′ primer, biotin-T10-−24AGTAGCAGAGGAGCTCAGCG−44, and 5′ primers, −346AGACCTTAGCGCGGTGTAGA−326 (−346 to −24) (Scino-Pharm Biotech Ltd., Tainan, Taiwan) according to the human ICAM-1 promoter sequence. The binding assay was performed by mixing 400 μg of nuclear proteins, 2 μg biotin-labeled DNA oligonucleotides, and 20 μl streptavidin-agarose beads (4%) with 70% slurry. The mixture was incubated at room temperature for 1 h with shaking. The beads were pelleted and washed with cold PBS three times. The binding proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blot analysis and probed with specific antibodies.
ChIP assay.
Chromatin immunoprecipitation (ChIP) analysis was performed as described previously (20). DNA immunoprecipitated by anti-p300 (sc-585; Santa Cruz), anti-Flag (F3165; Sigma), anti-RNA polymerase II (sc-5943; Santa Cruz), anti-p300/CBP-associated factor (p/CAF) (sc-13124; Santa Cruz), anti-acetylated histone H3 (06-599; Upstate), or anti-acetylated histone H4 (06-946; Upstate) antibody was purified. PCRs for ChIP were performed using input or pellet DNA serially diluted in fivefold increments to confirm the amplification in a linear range, as described previously (20). The primers 5′-AGACCTTAGCGCGGTGTAGA-3′ and 5′-AGTAGCAGAGGAGCTCAGCG-3′ were utilized to amplify across the ICAM-1 promoter region (−346 to −24). PCR products were analyzed on ethidium bromide-stained agarose gels.
Immunofluorescence staining.
A549 cells, grown on coverslips, were pretreated with Ly294002 or SH-5, followed by TNF-α stimulation for 1 h. The cells were then rapidly washed with PBS and fixed at room temperature for 10 min with 1.5% paraformaldehyde. After being washed with PBS, the cells were blocked for 15 min with 1% bovine serum albumin in TTBS and then incubated with anti-Akt antibody (1:100) for 1 h, washed extensively, and stained for 30 min with fluorescein isothiocyanate-conjugated anti-goat immunoglobulin G (IgG) secondary antibody (1:500). The nucleus was stained with DAPI (4′,6′-diamidino-2-phenylindole). After additional washes, the cells were mounted for analysis with a Leica TCS SP2 confocal spectral microscope.
In vivo phosphorylation.
In vivo labeling with [32P]orthophosphate was performed as described previously (24). A549 cells were cotransfected with Akt or nonsilencing siRNA, HA-myr-Akt or empty vector, and/or Flag-tagged wt p300 or S1834A mutant. After 2 days, the cells were washed twice with Tris-buffered saline and incubated in phosphate-free Dulbecco's modified Eagle's medium (Life Technologies) for 1 h. [32P]orthophosphate (NEN Life Science Products) was then added to achieve a concentration of 0.5 mCi/ml, and the cells were further incubated in appropriate medium for 3 h. The labeled cells were treated with vehicle or TNF-α for 1 h. After being washed twice with cold Tris-buffered saline, the cells were lysed in 1 ml of RIPA buffer. The cells were disrupted completely by scraping and shearing them through a 27-gauge needle. After centrifugation, aliquots of the lysates were precleared with 50 μl of protein A/G PLUS-agarose and then incubated with 2 μg of anti-p300 or anti-Flag antibody. The immune complexes were recovered upon incubation with 40 μl of protein A/G PLUS-agarose at 4°C for 1 h. The pellets were washed twice with cold Tris-buffered saline and twice with RIPA buffer. The immunoprecipitates isolated from the 32P-labeled cells were resuspended in 2× SDS sample buffer, resolved on a 7.5% SDS-polyacrylamide gel, and visualized by autoradiography.
In vitro phosphorylation.
In vitro phosphorylation was assessed as described previously (17). The immunoprecipitates with anti-Akt or antihemagglutinin (anti-HA) antibody were collected, washed, and then incubated at 30°C for 30 min in 20 μl of kinase reaction mixture containing 10 μM [γ-32P]ATP and 1 μg of either bacterially expressed GST-p300(1714-1883) as Akt or immunoprecipitated Flag-p300 as HA-myr-Akt substrate. The reaction was stopped by the addition of an equal volume of Laemmli buffer. Proteins were separated by electrophoresis on 10% SDS-polyacrylamide gels, and phosphorylated GST fusion proteins or Flag-p300 was visualized by autoradiography. The phosphorylation of Flag-p300 by HA-myr-Akt, both immunopurified from cell lysates, was also assessed by Western blotting using anti-phosphoserine antibody (Chemicon, Temecula, CA) after incubation in the kinase reaction mixture without [γ-32P]ATP.
In vitro transcription-translation.
In vitro protein synthesis using an EcoPro T7-coupled transcription-translation system was carried out according to the manufacturer's instructions (Novagen). The in vitro-expressed Flag-p300 was identified by Western blotting using anti-Flag antibody and subjected to a HAT activity assay.
HAT activity assay.
The in vitro HAT activity was assayed by measuring the histone acetylated by p300. The 25-μl reaction mixture containing Flag-tagged wt p300 or S1834E or S1834A mutant, 10 μg histone, and 10 μM acetyl-coenzyme A in HAT assay buffer (50 mM Tris-HCl, pH 8.0, 10% glycerol, 0.1 mM EDTA, and 1 mM dithiothreitol) was incubated at 30°C for 30 min and then subjected to SDS-PAGE for protein separation. The HAT activity of Flag-p300 phosphorylated by HA-myr-Akt, both immunopurified from cell lysates, was also assayed in the presence of histone and acetyl coenzyme A. Acetylated histone was analyzed by Western blotting using anti-acetylated histone H3 antibody.
GST pull-down assay.
Ten microliters of glutathione-agarose beads coupled with GST or GST-p300 was incubated with 1 mg of nuclear protein in 50 μl of kinase buffer (25 mM HEPES, 25 mM β-glycerophosphate, 25 mM MgCl2, 2 mM dithiothreitol, and 0.1 mM NaVO3) overnight at 4°C. Following incubation, the beads were washed twice with lysis buffer. Proteins were eluted with Laemmli SDS sample buffer, separated by 10% SDS-PAGE, and immunoblotted with anti-p/CAF or anti-phosphoserine antibody.
RESULTS
Involvement of the PI3K/Akt pathway in TNF-α-induced ICAM-1 expression.
To examine whether the PI3K/Akt pathway is involved in TNF-α-induced ICAM-1 expression, a PI3K inhibitor, Ly294002, was used. When cells were preincubated with Ly294002 for 30 min, the TNF-α-induced ICAM-1 expression was inhibited by the inhibitor in a dose-dependent manner (Fig. 1A). In parallel with the inhibition of ICAM-1 expression, Fig. 1B shows the blockade of TNF-α-induced U937 cell adhesion to A549 cells by Ly294002. To further investigate the role of the PI3K/Akt pathway in ICAM-1 gene transcription, promoter activity assays were performed using a human ICAM-1 promoter-luciferase construct, pIC339 (−339 to 0). TNF-α-induced ICAM-1 promoter activation was inhibited by Ly294002 in a dose-dependent manner, by SH-5 (a specific Akt inhibitor), or by the overexpression of the dominant-negative kinase-dead Akt mutant (Akt-KA) (Fig. 1C). Ly294002 or SH-5 alone did not affect the basal luciferase activity (data not shown). To further confirm the role of Akt in TNF-α-induced ICAM-1 gene transcription, siRNA-directed gene silencing was used to specifically deplete Akt. As shown in Fig. 1C, loss of Akt blocked TNF-α-induced ICAM-1 promoter activation. Moreover, ectopic expression of the constitutive-active mutant of myc-tagged p110 (myc-p110*), the catalytic subunit of PI3K, or the HA-tagged Akt (HA-myr-Akt) also increased ICAM-1 promoter activity (Fig. 1D). All these results indicated the involvement of the PI3K/Akt pathway in TNF-α-induced ICAM-1 gene transcription.
FIG. 1.
The PI3K/Akt pathway is required for TNF-α-induced ICAM-1 protein expression and U937 adhesion to A549 cells. (A) Blocking of the PI3K/Akt pathway by Ly294002 reduced ICAM-1 expression induced by TNF-α on A549 cells. Cells were pretreated with 10, 30, or 50 μM Ly294002 for 30 min before incubation with 10 ng/ml of TNF-α for 4.5 h. Surface expression of ICAM-1 was measured by enzyme-linked immunosorbent assay using anti-ICAM-1 antibody as described in Materials and Methods. OD, optical density. (B) Ly294002 inhibited TNF-α-induced U937 cell adhesion to A549 cells. U937 cells, labeled with BCECF, were added to A549 cells pretreated with 10 μM Ly294002 for 30 min before incubation with TNF-α for 4.5 h, and culture was continued at 37°C for 1 h; then, adhesion was measured as described in Materials and Methods. (C) Inhibition of the PI3K/Akt pathway blocked TNF-α-induced ICAM-1 promoter activity. Cells transfected with the pIC339 luciferase expression vector were pretreated with 10, 30, or 50 μM Ly294002 or 20 μM SH-5 or cotransfected with the dominant-negative Akt-KA mutant or Akt siRNA plasmid before incubation with 10 ng/ml of TNF-α for 5 h. Luciferase activity was assayed as described in Materials and Methods. (D) Overexpression of the constitutive-active mutant of p110 or Akt increased ICAM-1 promoter activity. Cells were cotransfected with myc-p110*, HA-myr-Akt, or the empty vector and the pIC339 luciferase expression vector. After 24 h of transfection, luciferase activity was measured. (Insets) Immunoblots of extracts from each sample to detect myc-p110*, Akt, HA-myr-Akt, HA-Akt-KA, or actin level using anti-myc, anti-Akt, anti-HA, or anti-actin antibody, respectively. *, P < 0.05; **, P < 0.01 compared with vehicle or the empty vector. All results are expressed as the mean plus standard error of the mean from three independent experiments.
Inhibition of PI3K/Akt blocked TNF-α-induced recruitment of p300, acetylation of nucleosomal histone, and enhanceosome formation on the ICAM-1 promoter.
The proximal ICAM-1 promoter-enhancer region (−346 to −24) contains several putative recognition sequences for a variety of transcriptional activators, including AP-1, retinoic acid-response element (RARE), C/EBP, NF-κB, Ets-1, interferon-stimulated response element (IRE), Sp1, and AP-2 (Fig. 2A). To investigate the role of the PI3K/Akt pathway in regulating the recruitment of p300 to the promoter, a biotinylated double-stranded DNA fragment containing ICAM-1 promoter from −346 to −24 was generated and used in DAPA. p300 was constitutively expressed in the nucleus (Fig. 2B, lanes 1 to 5), and its recruitment to the ICAM-1 promoter was increased by TNF-α after 30 min of stimulation and sustained to 120 min (Fig. 2B, lanes 3 to 5). The in vivo recruitment of p300 to the ICAM-1 promoter induced by TNF-α was examined by ChIP. Chromatin was immunoprecipitated with anti-p300 antibody, and the ICAM-1 promoter-enhancer region (−346 to −24) containing the essential binding sites for transcriptional activators was amplified by PCR. As shown in Fig. 2C and D, in vivo binding of p300 to the ICAM-1 promoter was increased after treatment with TNF-α in a time-dependent manner. No association of p300 with the β-actin promoter was seen after TNF-α treatment (data not shown). The recruitment of p300 to the ICAM-1 promoter was inhibited by Ly294002 in a dose-dependent manner, as demonstrated by both DAPA and the ChIP assay (Fig. 2B, lanes 8 to 10, and D, lanes 7 to 9). In addition to Akt, PI3K has also been reported to activate the mammalian target of rapamycin (mTOR) kinase, which regulates S6 kinase activation (36). To examine whether the inhibitory effect of Ly294002 could be mediated through mTOR kinase/S6 kinase rather than the PI3K/Akt pathway, Akt inhibitor (SH-5) and mTOR kinase inhibitor (rapamycin) were used. As shown in Fig. 2E, TNF-α-induced p300 recruitment to the ICAM-1 promoter was inhibited by Ly294002 or SH-5, but not rapamycin (lanes 3 to 5). Furthermore, knocking down Akt with siRNA also attenuated the recruitment of p300 to the promoter in a dose-dependent manner (Fig. 2F), indicating that the PI3K/Akt pathway was indeed involved in the TNF-α-induced recruitment of p300 to the ICAM-1 promoter.
FIG. 2.
Inhibition of PI3K/Akt pathway attenuates TNF-α-induced recruitment of p300, acetylation of nucleosomal histone, and enhanceosome formation on the ICAM-1 promoter. (A) Schematic illustration of various transcriptional activator binding sites on the ICAM-1 promoter labeled with biotin. (B) TNF-α-induced p300 recruitment to the ICAM-1 promoter in vitro was inhibited by Ly294002. Cells were stimulated with TNF-α for the indicated time or pretreated with 10, 30, or 50 μM Ly294002 for 30 min before stimulation with TNF-α for 60 min, and then nuclear extracts were prepared and incubated with biotinylated ICAM-1 promoter probes (−346 to −24) or without probe (control) and streptavidin-agarose beads. p300 captured by the beads or in the nuclear extract was detected by Western blotting. (C to F) Inhibition of the PI3K/Akt pathway attenuated TNF-α-induced p300 recruitment, acetylation of nucleosomal histones, and assembly of RNA polymerase II on the ICAM-1 promoter in vivo. Cells were stimulated with TNF-α for the indicated times (C and D) or pretreated with 10, 30, or 50 μM Ly294002 (D) or 30 μM Ly294002, 20 μM SH-5, or 20 ng/ml rapamycin (E) for 30 min or transfected with nonsilencing (control) or Akt siRNA plasmids in a dose-dependent manner (F) before stimulation with TNF-α for 60 min, and then ChIP assays were performed. Chromatin was immunoprecipitated with anti-p300, anti-acetylated histone H3, anti-acetylated histone H4, or anti-RNA ploymerase II (Pol II) antibody or without antibody (control). One percent of the precipitated chromatin was assayed to verify equal loading (Input). Serial dilution in fivefold increments of pellet or input template DNA (C) or fivefold dilutions (D to F) were subjected to PCR using primer to the ICAM-1 promoter region (−24 to −346).
It has been shown that p300, after recruitment to target gene promoters, can acetylate lysine residues within the core histone tails to facilitate the binding of nuclear factors to chromatin by destabilizing the promoter-bound nucleosomes; it then complexes with RNA polymerase II holoenzyme to form enhanceosome, initiating gene transcription (9, 44). As shown in Fig. 2E, the acetylation of histones H3 and H4 and the assembly of RNA polymerase II on the ICAM-1 promoter were increased in response to TNF-α, and these effects were attenuated by Ly294002 and SH-5, but not rapamycin (lanes 3 to 5). These results implied that Akt modulates the promoter recruitment of p300, as well as its HAT activity, leading to the acetylation of core histones and association with basal transcriptional machinery to form enhanceosome.
Nuclear Akt interacts with and phosphorylates p300 in response to TNF-α.
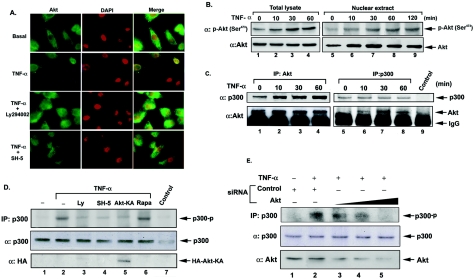
Since Akt mediates the recruitment of p300 to the ICAM-1 promoter, we speculate that Akt should be translocated and activated in the nucleus; it then phosphorylates p300. Immunofluorescence staining demonstrated the nuclear translocation of Akt in response to TNF-α, and this effect was attenuated by Ly294002 or SH-5 (Fig. 3A). Western blotting also showed the phosphorylation and nuclear translocation of Akt after treatment with TNF-α (Fig. 3B). These results confirm that TNF-α induced nuclear translocation and activation of Akt in a PI3K-dependent manner. To examine whether Akt interacts with p300 in vivo, reciprocal immunoprecipitation of nuclear lysates, followed by immunoblot analysis using anti-Akt or anti-p300 antibody, was performed. As shown in Fig. 3C, p300 formed a tight complex with Akt after TNF-α stimulation. It was evident at 10 min and sustained to 60 min.
FIG. 3.
Nuclear Akt interacts with and phosphorylates p300 in response to TNF-α stimulation. (A) Immunofluorescent staining of Akt in A549 cells was performed using anti-Akt antibody. Cells were stimulated with TNF-α for 60 min or pretreated with Ly294002 or SH-5 for 30 min before incubation with TNF-α and then fixed and stained as described in Materials and Methods. Cell nuclei were stained with DAPI, and Akt was visualized with fluorescein isothiocyanate. DAPI staining was falsely colored red to make visualization of the merge (overlay) easier to distinguish. (B) Total and nuclear extracts from TNF-α-stimulated A549 cells were prepared and then analyzed by Western blotting using anti-phospho-Ser-473 Akt or anti-Akt antibody. (C) Nuclear Akt coimmunoprecipitated with p300 after TNF-α stimulation. Cells were treated with TNF-α for the indicated times. Equal amounts (1 mg) of nuclear extracts were immunoprecipitated (IP) with anti-Akt, anti-p300 antibody, or IgG (control) and then separated by SDS-PAGE on a 7.5% gel and immunoblotted with anti-Akt or anti-p300 antibody as indicated. (D and E) TNF-α-activated Akt phosphorylates p300 in vivo. Cells labeled with [32P]orthophosphate were pretreated with 30 μM Ly294002, 20 μM SH-5, or 20 ng/ml of rapamycin or transfected with the dominant-negative mutant of Akt-KA (D), Akt, or nonsilencing siRNA plasmids (E) before incubation with 10 ng/ml of TNF-α for 1 h. Endogenous p300 was immunoprecipitated with anti-p300 antibody or IgG (control), and phosphorylated p300 was visualized by autoradiography as described in Materials and Methods. The total amounts of p300, HA-Akt-KA, and Akt were detected by Western blotting using anti-p300, anti-HA, and anti-Akt antibodies, respectively.
The in vivo phosphorylation of p300 by Akt was examined by transfection of cells with the dominant-negative Akt-KA mutant or the empty vector for 2 days, and then the cells were labeled with [32P]orthophosphate for 3 h. The radiolabeled cell lysates were immunoprecipitated with anti-p300 antibody, and the phosphorylated p300 was visualized by autoradiography. As shown in Fig. 3D, phosphorylation of p300 by TNF-α was seen (lane 2), and this effect was inhibited by Ly294002, SH-5, and Akt-KA, but not rapamycin (lanes 3 to 6), demonstrating the in vivo phosphorylation of p300 by Akt. To further confirm the effect of Akt, its siRNA was used, and the loss of Akt was observed to attenuate p300 phosphorylation in a dose-dependent manner (Fig. 3E).
Akt phosphorylates p300 at Ser-1834 in vitro and in vivo.
According to a consensus sequence specific for Akt phosphorylation sites (Arg-Xaa-Arg-Xaa-Xaa-Ser/Thr) (3), a putative residue is conserved in both p300 (Ser-1834) and CBP (Ser-1871) (Fig. 4A). We examined whether Ser-1834 of p300 was phosphorylated by Akt. As shown in Fig. 4B, Akt immunoprecipitated from TNF-α-treated cell lysates phosphorylated a GST-tagged p300 peptide (residues 1714 to 1883) [GST-p300(1714-1883)] in a time-dependent manner (Fig. 4B, lanes 1 to 4). When Ser-1834 was mutated to Ala (S1834A), the phosphorylation was attenuated (Fig. 4B, lane 5 to 6). To further verify that Ser-1834 was phosphorylated by Akt, we transfected constitutive-active HA-myr-Akt and dominant-negative HA-Akt-KA into cells and examined their ability to phosphorylate GST-p300(1714-1883) and Flag-p300. As shown in Fig. 4C and D, the constitutive-active Akt mutant (HA-myr-Akt) immunoprecipitated by anti-HA antibody phosphorylated GST-p300(1714-1883) and Flag-p300 immunopurified from cell lysates (Fig. 4C, lane 2, and D, lane 2), but not its S1834A mutant (Fig. 4C, lane 1, and D, lane 4). However, the HA-Akt-KA mutant did not phosphorylate GST-p300(1714-1883) even in the presence of TNF-α stimulation (Fig. 4C, lanes 4).
FIG. 4.
Akt phosphorylates p300 at Ser-1834 in vitro and in vivo. (A) Consensus motif of Akt phosphorylation sites. Sequences of p300/CPB and other known substrates are aligned for comparison. The conserved sequence for phosphorylation by Akt is indicated in boxes. (B, C, and D) Akt phosphorylates p300 at Ser-1834 in vitro. Cells transfected with empty vector (C) or HA-Akt-KA (C) were stimulated with TNF-α for the indicated times (B) or for 60 min (C) or transfected with only HA-myr-Akt (C and D) or Flag-tagged wt or the S1834A mutant of p300 (D). Total cell lysates were prepared and immunoprecipitated with anti-Akt (B) or anti-HA (C and D) antibody. HA-myr-Akt immunoprecipitated by anti-HA antibody was mixed with wt Flag-p300 or its S1834A mutant extracted from unstimulated cells and then subjected to an in vitro phosphorylation assay (D). The amount of GST-p300(1714-1883) was detected by Coomassie brilliant blue staining. (E) Akt phosphorylates p300 at Ser-1834 in vivo. Cells transfected with empty vector, Flag-tagged wt p300, or S1834A mutant or cotransfected with HA-myr-Akt were labeled with [32P]orthophosphate before incubation with 10 ng/ml of TNF-α for 1 h. Flag-p300 was immunoprecipitated by anti-Flag antibody, and phosphorylated Flag-p300 was visualized by autoradiography. The total amount of Flag-p300 and HA-myr-Akt was detected by Western blotting using anti-Flag or anti-HA antibody.
To further confirm the in vivo phosphorylation of Ser-1834 by Akt, A549 cells were cotransfected with Flag-tagged p300 and HA-myr-Akt or their respective empty vectors for 2 days and then subjected to an in vivo phosphorylation assay. As shown in Fig. 4E, wt Flag-p300 was phosphorylated by TNF-α and by the overexpression of HA-myr-Akt (Fig. 4E, lanes 5 and 6). When Ser-1834 was mutated to Ala (Flag-p300 S1834A), these phosphorylations were diminished (Fig. 4E, lanes 8 and 9).
Akt phosphorylation of Ser-1834 enhances the transcriptional activity of p300 by increasing its promoter recruitment, histone acetylation, and enhanceosome formation on the ICAM-1 promoter.
To examine whether Akt-induced Ser-1834 phosphorylation is essential for TNF-α-induced ICAM-1 expression, ICAM-1 promoter activity was examined by overexpression of the Flag-tagged wt p300 or its S1834A mutant. As shown in Fig. 5A, wt p300 increased ICAM-1 promoter activity, and the S1834A mutant also slightly increased promoter activity. However, wt p300 augmented TNF-α-stimulated ICAM-1 promoter activity, and this effect was not seen when the S1834A mutant was overexpressed (Fig. 5A). The balance between the activities of HAT and histone deacetylase (HDAC) controls the steady-state acetylation level of core histone (29). Treatment of mammalian cells with the HDAC inhibitor TSA blocked histone deacetylation and resulted in an increase in gene expression (8). As shown in Fig. 5A, ICAM-1 promoter activity was stimulated by TSA, and wt p300 also augmented its effect. However, mutation of Ser-1834 to Ala abolished this enhancing effect. When wt p300 or S1834A mutant was cotransfected with the constitutively active PI3K (myc-p110*) or Akt (HA-myr-Akt), only wt p300, but not the S1834A mutant, enhanced ICAM-1 promoter activity (Fig. 5B). p300 has been reported to act as a common cofactor for most transcription factors (9, 14). Overexpression of wt p300 in A549 cells also caused an increase in NF-κB-, CREB-, and AP-1-mediated transcriptions, and this effect was enhanced by the mutation of Ser-1834 to Glu, which mimics the phosphorylation status, but attenuated by the mutation of Ser-1834 to Ala (Fig. 5C). These data imply that Akt phosphorylation of p300 at Ser-1834 can regulate the expression of many genes, including ICAM-1.
FIG. 5.
Akt phosphorylation of Ser-1834 enhances the transcriptional activity of p300 by increasing its promoter recruitment, histone acetylation, and enhanceosome formation on the ICAM-1 promoter. (A) The p300 S1834A mutant inhibited TNF-α- or TSA-induced ICAM-1 promoter activity. A549 cells were cotransfected with pIC339 and the Flag-tagged wt p300 or p300 S1834A mutant, or the empty vector, and then treated with 10 ng/ml of TNF-α or 1 μM TSA for 6 h. Luciferase activity was measured. (Inset) Immunoblots of extracts from each sample to detect the level of Flag-tagged wt p300 or S1834A mutant using anti-Flag antibody. (B) The p300 S1834A mutant inhibited ICAM-1 promoter activity induced by the constitutive-active mutant of p110 or Akt. Cells were cotransfected with myc-p110*, HA-myr-Akt, or empty vector and the Flag-tagged wt or S1834A mutant of p300. After 24 h of transfection, luciferase activity was measured. (Inset) Immunoblots of extracts from each sample to detect the level of myc-p110*, HA-myr-Akt, or Flag-p300 using anti-myc, anti-HA, or anti-Flag antibody, respectively. (C) Phosphorylation of p300 at Ser-1834 is critical for NF-κB-, CREB-, and AP-1-mediated transcriptions. Cells were cotransfected with the Flag-tagged wt or the S1834A or S1834E mutant of p300 and NF-κB-, CRE-, or AP-1-luciferase reporters, and then luciferase activity was measured. (D) Mutation of Ser-1834 to Ala abolished the recruitment of p300, acetylation of nucleosomal histones, and RNA polymerase II to the ICAM-1 promoter. ChIP assays were performed in cells transfected with the Flag-tagged wt or S1834A mutant of p300 or the empty vector. Cells were pretreated with 50 μM Ly294002 for 30 min, followed by TNF-α stimulation for 60 min, and then chromatin was immunoprecipitated with anti-Flag, anti-acetylated histone H3, or anti-RNA ploymerase II (Pol II) antibody or without antibody (control). The precipitated ICAM-1 promoter region (−24 to −346) was amplified by PCR; 1% of the chromatin was assayed to verify equal loading (Input). All results were expressed as the mean plus standard error of the mean from three independent experiments.
To further explore why Akt phosphorylation of Ser-1834 increased the transcriptional activity of p300, Flag-tagged wt p300 and its S1834A mutant were transfected into cells and ChIP assays were performed. As shown in Fig. 5D, recruitment of Flag-tagged wt p300 to the ICAM-1 promoter after TNF-α stimulation was seen (lane 5), and this effect was abolished by Ly294002 and the mutation of Ser-1834 to Ala (Fig. 5D, lanes 4 to 6 and 7 to 8, respectively). Moreover, acetylation of histone H3 and assembly of RNA polymerase II on the ICAM-1 promoter induced by TNF-α were enhanced by wt p300 but attenuated by the S1834A mutant (Fig. 5D, compare lanes 1 and 2, 4 and 5, and 7 and 8). The inhibitory effect of Ly294002 on these two events was also seen (Fig. 5D, compare lanes 2 and 3, and 5 and 6, respectively). These data indicated that Akt-mediated phosphorylation of Ser-1834 enhanced the transcriptional activity of p300 by increasing its recruitment to the ICAM-1 promoter and HAT activity and the subsequent association with RNA polymerase II to form enhanceosome.
Akt phosphorylation of p300 at Ser-1834 increases its intrinsic HAT activity and its association with p/CAF.
To examine whether phosphorylation of Ser-1834 increased the intrinsic HAT activity of p300, wt Flag-tagged p300 and its S1834A mutant cotransfected with HA-myr-Akt or HA-Akt KA mutant were immunoprecipitated by anti-Flag antibody. The HAT activity was assayed by measuring the acetylation of histone H3. As shown in Fig. 6A, wt p300, but not the S1834A mutant, acetylated histone H3 in response to TNF-α stimulation (Fig. 6A, lanes 1 to 4), and this effect was inhibited by the Akt-KA mutant (Fig. 6A, lanes 5 and 6). HA-myr-Akt enhanced the acetylation of histone H3 induced by wt p300 (compare lanes 7 and 1), but not the S1834A mutant (Fig. 6A, compare lanes 8 and 3). These results showed that phosphorylation of p300 at Ser-1834 increased its intrinsic HAT activity. To further demonstrate that phosphorylation of Ser-1834 by Akt increased the HAT activity, wt p300 or its S1834A mutant extracted by Flag tag was mixed with myr-Akt extracted by HA tag from unstimulated cell lysates and then subjected to in vitro phosphorylation and HAT activity assays. As shown in Fig. 6B, increases of phosphorylation and HAT activity were seen only in wt p300, but not the S1834A mutant (Fig. 6B, lanes 2 and 4). To confirm this finding, p300 was expressed in vitro and subjected to a HAT activity assay. As shown in Fig. 6C, histone H3 was acetylated by wt p300, and this effect was enhanced by the S1834E mutant but attenuated by the S1834A mutant (Fig. 6C, lanes 2 to 4). All the data clearly demonstrated that Akt phosphorylation of p300 at Ser-1834 is critical for the regulation of intrinsic HAT activity.
FIG. 6.
Phosphorylation of p300 at Ser-1834 increases its intrinsic HAT activity. After cotransfection with Flag-tagged wt p300 or S1834A mutant and HA-Akt KA or HA-myr-Akt for 48 h, cells were treated with TNF-α for 1 h. Then, the wt and S1834A mutants of Flag-p300 were immunopurified by anti-Flag antibody or by IgG (control). (A) HA-myr-Akt immunoprecipitated from unstimulated cell lysates was mixed with wt Flag-p300 or its S1834A mutant and incubated in kinase assay buffer for 30 min. (B) Flag-tagged wt p300 or S1834E or S1834A mutant protein was expressed in vitro and immunopurified by anti-Flag antibody. (C) HAT activity was then assayed as described in Materials and Methods. Levels of acetylated histone H3, histone H3, immunoprecipitated Flag-p300, and HA-myr-Akt were estimated by Western blotting using anti-acetylated histone H3, anti-histone H3, anti-Flag, and anti-HA antibodies, respectively.
Ser-1834 is close to the C/H3 domain of p300, which is responsible for the recruitment of numerous transcriptional activators, as well as other HAT-possessing proteins, such as p/CAF and SRC-1 (46, 47). The associated HAT activity from p/CAF has also been reported to synergize the transcriptional activation of p300 (13, 44). Thus, we further examined if Ser-1834 phosphorylation affects the protein-protein interaction between p300 and p/CAF. In a GST pull-down assay, the phosphorylation of GST-p300(1714-1883) and its interaction with p/CAF were increased after TNF-α stimulation in the presence of ATP (Fig. 7A, lanes 3 to 4), and these effects were attenuated by Ly294002 (Fig. 7A, compare lanes 4 and 6) or in the absence of ATP (Fig. 7A, compare lanes 4 and 8). GST-300(1714-1883) S1834A mutant or GST alone did not bind p/CAF (Fig. 7A, lanes 9 and 10, and 1 and 2). Moreover, the recruitment of p/CAF to the ICAM-1 promoter was enhanced by TNF-α stimulation (Fig. 7B, lane 2), and this effect was attenuated by Ly294002 (Fig. 7B, lane 3). Wild-type p300 enhanced, but the S1834A mutant inhibited, TNF-α-induced recruitment of p/CAF to the ICAM-1 promoter (Fig. 7C, lanes 3 and 4, and 5 and 6). These data implied that Akt phosphorylation of Ser1834 enhanced the recruitment of p300 together with p/CAF to the ICAM-1 promoter by increasing their protein-protein interaction.
FIG. 7.
Akt phosphorylation of p300 at Ser-1834 increases its association with p/CAF. (A) Akt phosphorylation of p300 at Ser-1834 binds p/CAF in vitro. A549 cells were pretreated with 50 μM Ly294002 for 30 min before incubation with 10 ng/ml of TNF-α for 60 min. Nuclear extracts were prepared and pulled down from the glutathione-agarose beads coupled with wt GST-p300(1714-1883) or S1834A mutant as described in Materials and Methods. Levels of phospho-GST-p300(1714-1883) and pulled-down or input p/CAF were detected by Western blotting using anti-phosphoserine or anti-p/CAF antibody, respectively. The amount of GST or GST-p300(1714-1883) was detected by Coomassie brilliant blue staining. (B and C) Ly294002 and the p300 S1834A mutant inhibited the recruitment of p/CAF to the ICAM-1 promoter induced by TNF-α. Cells were pretreated with Ly294002 for 30 min or transfected with Flag-tagged wt p300 or the S1834A mutant or the empty vector before stimulation with TNF-α for 60 min, and then ChIP assays were performed using anti-p/CAF antibody or without antibody (control). Precipitated ICAM-1 promoter region (−24 to −346) was amplified by PCR; 1% of the chromatin was assayed to verify equal loading (Input). (D) HAT activity of p/CAF contributed to TNF-α-induced ICAM-1 promoter activity. A549 cells transfected with pIC339 and wt p/CAF, p/CAF HAT− mutant, or the empty vector were pretreated with Ly294002 for 30 min, followed by stimulation with TNF-α for 60 min. Luciferase activity was then measured. (E) Enhancement of Akt-induced ICAM-1 promoter activity by p/CAF was inhibited by the p300 S1834A mutant. Cells were cotransfected with wt p/CAF, p/CAF HAT− mutant, or the empty vector and the HA-myr-Akt, Flag-tagged wt, or the S1834A mutant of p300, and then luciferase activity was measured. The results were normalized to the β-galactosidase activity and expressed as the mean plus standard error of the mean of three independent experiments performed in triplicate.
To examine whether the HAT activity of p/CAF is essential for TNF-α-induced ICAM-1 expression, cells were overexpressed with wt p/CAF, or a p/CAF HAT deletion (HAT−) mutant, and the ICAM-1 promoter luciferase reporter. Wild-type p/CAF, but not the p/CAF HAT− mutant, enhanced the TNF-α-induced ICAM-1 promoter activity, and this effect was blocked by Ly294002 (Fig. 7D). Moreover, wt p/CAF augmented the constitutive-active Akt- or wt p300-induced, but not S1834A mutant-induced, ICAM-1 promoter activity (Fig. 7E). This synergism was not seen when the p/CAF HAT− mutant was overexpressed (Fig. 7E). All these data provide evidence that the synergic effect of HAT activity induced by p300 and p/CAF is Akt dependent and is essential for ICAM-1 gene transcription.
DISCUSSION
PI3K/Akt pathway activation by TNF-α has been well documented. Its roles in TNF-α-induced ICAM-1 expression on A549 alveolar epithelial cells and in causing monocytes to adhere to these cells are explored in the present study. NF-κB was demonstrated to be critical for TNF-α-induced ICAM-1 expression on A549 cells (10). It has been reported that PI3K/Akt induced NF-κB activation through targeting IKKα or increasing p65 transactivation via phosphorylation of its Ser-529 or Ser-536 (31, 35, 40). Our data showed that Ly294002 attenuated both Akt activation and ICAM-1 expression. However, inhibition of the PI3K/Akt pathway by Ly294002 or wortmannin did not affect TNF-α-induced IKK activation, IκBα degradation, NF-κB-specific DNA-protein binding, or phosphorylation of p65 at Ser-529 or Ser-536 (see Fig. S1 in the supplemental material). Therefore, PI3K/Akt pathway regulation of gene transcription in A549 cells was not through NF-κB or p65 transcriptional activity. In agreement with these findings, several studies have also shown that inhibition of the PI3K/Akt pathway did not prevent TNF-α- or interleukin-1-induced NF-κB activation and IκBα degradation in hepatocytes and A549 and human umbilical vein endothelial cells (25, 32, 41). Therefore, the PI3K/Akt pathway seems not to induce NF-κB activation in every cell type (42). Instead of targeting the IKK/NF-κB activation pathway, we showed a new mechanism by which Akt regulated TNF-α-induced ICAM-1 expression by phosphorylating p300. Akt is activated in a PI3K-dependent manner and translocates to the nucleus. Nuclear Akt binds to p300 and phosphorylates its Ser-1834, leading to an increase in HAT activity, which induces acetylation of the core histone tails. This may promote access of transcription factors to gene promoters (43) and lead to recruitment of basal transcriptional machinery RNA polymerase II to promoters. All these events facilitate the formation of enhanceosomes and enhance gene transcriptions.
Akt phosphorylation of p300 at Ser-1834 regulates TNF-α-induced ICAM-1 expression. This effect is attributed to the increase in recruitment of p300 to the ICAM-1 promoter and the increase in HAT activity, which results in the acetylation of histone H3 or H4 on the ICAM-1 promoter. Several lines of evidence support these notions. First, TNF-α-activated Akt translocates to the nucleus, where p300 is predominantly located, and the association between them has also been observed. Second, in vivo and in vitro phosphorylation of p300 by Akt was demonstrated by the results in which PI3K/Akt inhibitors, dominant-negative Akt-KA mutant, Akt siRNA, or p300 S1834A mutant inhibited the phosphorylation of p300. The specific inhibition of the expression of Akt, but not actin and p300, by Akt siRNA (Fig. 1C and 3E, respectively) might exclude the possibility of its targeting other genes. Third, Akt siRNA and mutation of Ser-1834 to Ala diminished the recruitment of p300 to the ICAM-1 promoter. Fourth, the S1834A mutant attenuated ICAM-1 promoter activity induced by TNF-α or by the constitutively active PI3K (myc-p110*) or Akt (HA-myr-Akt). Finally, Akt phosphorylation of Ser-1834 leads to an increase in the HAT activity of p300. This was demonstrated by the findings that the constitutive-active Akt mutant induced the phosphorylation and HAT activity of wt p300, but not the S1834A mutant (Fig. 6A and B). Furthermore, in vitro intrinsic HAT activity was increased when Ser-1834 was mutated to Glu, while it was decreased when Ser was mutated to Ala (Fig. 6C). The in vivo HAT activity demonstrated that the increase in acetylated histone H3 or H4 on the ICAM-1 promoter was inhibited by the PI3K/Akt inhibitors or the S1834A mutant (Fig. 2E and 5D). These Akt-mediated enhancements in HAT activity may be attributed to two mechanisms. One is through enhanced intrinsic HAT activity, probably due to a conformational change after phosphorylation of Ser-1834, as suggested by Ait-Si-Ali et al. (1), who found an increase in HAT activity of CBP after its C-terminal part was phosphorylated by ERK1. Phosphorylation-induced conformational change that exposes the catalytic domain of ATF-2, thereby activating its HAT, has also been suggested by Kawasaki et al. (21). In addition to possessing intrinsic HAT activity, p300 could also associate with other HATs, such as p/CAF and SRC-1, through its CH3 domain (46, 47). It has been reported that the HAT activity of p/CAF is essential for the enhancement of the transcriptional activity of p300 (13). Since Ser-1834 is close to the CH3 domain, the interaction between p300 and p/CAF was examined and found to increase upon Ser-1834 phosphorylation (Fig. 7A). Therefore, the p300-associated p/CAF may also contribute to the increased HAT activity of p300. MAPK/ERK kinase kinase 1, p42/p44 MAPK, and Akt have also been demonstrated to phosphorylate p300/CBP within their C-terminal parts and to increase their HAT and/or transcriptional activities (1, 26, 38). Phosphorylations in the C-terminal region thus seem to be a common mechanism for up-regulating the transcriptional activity of p300/CBP. In contrast, phosphorylation at the N terminus, e.g., Ser89 by PKCα, was found to inhibit the HAT activity of p300 (48).
Akt-mediated phosphorylation of Ser-1834 also increases the interaction of p300 with C/EBPβ (data not shown), which has been demonstrated to recruit p300 by interacting with residues 1752 to 1859 (containing a CH3 domain) (27). It has been reported that docking p300 to C/EBPβ induces phosphorylation of several sites within the C-terminal domain of p300. Mutation of these sites substantially impairs the activity of p300 as a coactivator of C/EBPβ (37). This property may be shared by other C/EBP family members, as well as other transcription factors, such as Sp1, Elk1, Msx3, and Hox (23), raising the possibility that p300 links the specific transcription factors to the basal transcriptional machinery through protein-protein interaction (27).
Akt plays an important role in prosurvival and antiapoptotic signaling (6). For instance, Akt-induced phosphorylation and nuclear translocation of Mdm2 leads to p53 degradation, thus promoting cell survival (50). However, p300/CBP has been reported to increase the transcriptional activities of NF-κB and p53 through acetylation (12, 15), resulting in the respective prosurvival and proapoptotic effects (14). The phosphorylation and regulation of p300 by Akt probably provides a novel mechanism determining cell survival and proliferation. The interaction between Akt and p300 was found to maintain the steady-state level of p300 and to enter the G1 phase of the cell cycle (11). HDAC inhibitors induce Akt phosphorylation of p300, recruitment of p300 to the chromatin, and modulation of its transactivation potential, leading to the modulation of NF-κB transcription in non-small-cell lung cancer cell lines. This Akt-dependent stimulation of NF-κB-mediated gene expression blunted the proapoptotic action of HDAC inhibitors (26). In the present study, we also showed that Akt-dependent recruitment of p300 and enhancement of HAT activity augmented NF-κB-mediated ICAM-1 transcription (Fig. 5A and 5B; 7D and 7E).
The PI3K/Akt pathway implicated in the regulation of cell-cell adhesion via expression of ICAM-1 has also been reported. For instance, a glucose analog of blood group H antigen (H-2g) and ligation of PAR-1 induces ICAM-1 expression and cell adhesion via PI3K-dependent pathways (34, 51). Our previous studies have demonstrated that ICAM-1 overexpression induces tumor cell invasion across the Matrigel (16). This Akt-mediated ICAM-1 expression might be critical for tumor cell invasion and metastasis. Involvement of the PI3K/Akt pathway in vascular endothelial growth factor-induced ICAM-1 expression and migration has also been reported in brain microvascular endothelial cells (33).
In summary, phosphorylation of p300 at Ser-1834 by Akt mediates two effects. One is increasing the recruitment of p300 to the ICAM-1 promoter. The other is increasing the acetylation of histone H3 or H4 on the ICAM-1 promoter. This acetylation process is derived from the intrinsic HAT activity of p300 and its association with another HAT, p/CAF. These two events promote the formation of a transcriptional enhanceosome complex with RNA polymerase II holoenzyme, leading to an increase in ICAM-1 gene transcription. This regulatory mechanism may have important implications in the gene transcription related to inflammation and tumorigenesis.
Supplementary Material
Acknowledgments
This work was supported by a research grant from the National Science Council of Taiwan (NSC 93-2320-B-002-103).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ait-Si-Ali, S., D. Carlisi, S. Ramirez, L. C. Upegui-Gonzalez, A. Duquet, P. Robin, B. Rudkin, A. Harel-Bellan, and D. Trouche. 1999. Phosphorylation by p44 MAP kinase/ERK1 stimulates CBP histone acetyl transferase activity in vitro. Biochem. Biophys. Res. Commun. 262:157-162. [DOI] [PubMed] [Google Scholar]
- 2.Ait-Si-Ali, S., S. Ramirez, F. X. Barre, F. Dkhissi, L. Magnaghi-Jaulin, J. A. Girault, P. Robin, M. Knibiehler, L. L. Pritchard, B. Ducommun, D. Trouche, and A. Harel-Bellan. 1998. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature 396:184-186. [DOI] [PubMed] [Google Scholar]
- 3.Alessi, D. R., F. B. Caudwell, M. Andjelkovic, B. A. Hemmings, and P. Cohen. 1996. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399:333-338. [DOI] [PubMed] [Google Scholar]
- 4.Bannister, A. J., and T. Kouzarides. 1996. The CBP co-activator is a histone acetyltransferase. Nature 384:641-643. [DOI] [PubMed] [Google Scholar]
- 5.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96:7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657-664. [DOI] [PubMed] [Google Scholar]
- 7.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 8.Camarero, N., A. Nadal, M. J. Barrero, D. Haro, and P. F. Marrero. 2003. Histone deacetylase inhibitors stimulate mitochondrial HMG-CoA synthase gene expression via a promoter proximal Sp1 site. Nucleic Acids Res. 31:1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan, H. M., and N. B. La Thangue. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114:2363-2373. [DOI] [PubMed] [Google Scholar]
- 10.Chen, C., C. Chou, Y. Sun, and W. Huang. 2001. Tumor necrosis factor alpha-induced activation of downstream NF-κB site of the promoter mediates epithelial ICAM-1 expression and monocyte adhesion. Involvement of PKCα, tyrosine kinase, and IKK2, but not MAPKs, pathway. Cell Signal 13:543-553. [DOI] [PubMed] [Google Scholar]
- 11.Chen, J., S. S. Halappanavar, J. R. St-Germain, B. K. Tsang, and Q. Li. 2004. Role of Akt/protein kinase B in the activity of transcriptional coactivator p300. Cell Mol. Life Sci. 61:1675-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng, W. G., Y. Zhu, and K. K. Wu. 2003. Up-regulation of p300 binding and p50 acetylation in tumor necrosis factor-alpha-induced cyclooxygenase-2 promoter activation. J. Biol. Chem. 278:4770-4777. [DOI] [PubMed] [Google Scholar]
- 13.Deng, W. G., Y. Zhu, and K. K. Wu. 2004. Role of p300 and PCAF in regulating cyclooxygenase-2 promoter activation by inflammatory mediators. Blood 103:2135-2142. [DOI] [PubMed] [Google Scholar]
- 14.Goodman, R. H., and S. Smolik. 2000. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14:1553-1577. [PubMed] [Google Scholar]
- 15.Gu, W., and R. G. Roeder. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595-606. [DOI] [PubMed] [Google Scholar]
- 16.Huang, W. C., S. T. Chan, T. L. Yang, C. C. Tzeng, and C. C. Chen. 2004. Inhibition of ICAM-1 gene expression, monocyte adhesion and cancer cell invasion by targeting IKK complex: molecular and functional study of novel α-methylene-γ-butyrolactone derivatives. Carcinogenesis 25:1925-1934. [DOI] [PubMed] [Google Scholar]
- 17.Huang, W. C., J. J. Chen, and C. C. Chen. 2003. c-Src-dependent tyrosine phosphorylation of IKKβ is involved in tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 expression. J. Biol. Chem. 278:9944-9952. [DOI] [PubMed] [Google Scholar]
- 18.Impey, S., A. L. Fong, Y. Wang, J. R. Cardinaux, D. M. Fass, K. Obrietan, G. A. Wayman, D. R. Storm, T. R. Soderling, and R. H. Goodman. 2002. Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron 34:235-244. [DOI] [PubMed] [Google Scholar]
- 19.Janknecht, R., and A. Nordheim. 1996. MAP kinase-dependent transcriptional coactivation by Elk-1 and its cofactor CBP. Biochem. Biophys. Res. Commun. 228:831-837. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, K., C. Angelin-Duclos, S. Park, and K. L. Calame. 2003. Changes in histone acetylation are associated with differences in accessibility of V(H) gene segments to V-DJ recombination during B-cell ontogeny and development. Mol. Cell. Biol. 23:2438-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawasaki, H., L. Schiltz, R. Chiu, K. Itakura, K. Taira, Y. Nakatani, and K. K. Yokoyama. 2000. ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature 405:195-200. [DOI] [PubMed] [Google Scholar]
- 22.Kops, G. J., N. D. de Ruiter, A. M. De Vries-Smits, D. R. Powell, J. L. Bos, and B. M. Burgering. 1999. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398:630-634. [DOI] [PubMed] [Google Scholar]
- 23.Legube, G., and D. Trouche. 2003. Regulating histone acetyltransferases and deacetylases. EMBO Rep. 4:944-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, J., E. Gao, and C. R. Mendelson. 1998. Cyclic AMP-responsive expression of the surfactant protein-A gene is mediated by increased DNA binding and transcriptional activity of thyroid transcription factor-1. J. Biol. Chem. 273:4592-4600. [DOI] [PubMed] [Google Scholar]
- 25.Madge, L. A., and J. S. Pober. 2000. A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NFκB in human endothelial cells. J. Biol. Chem. 275:15458-15465. [DOI] [PubMed] [Google Scholar]
- 26.Mayo, M. W., C. E. Denlinger, R. M. Broad, F. Yeung, E. T. Reilly, Y. Shi, and D. R. Jones. 2003. Ineffectiveness of histone deacetylase inhibitors to induce apoptosis involves the transcriptional activation of NF-kappa B through the Akt pathway. J. Biol. Chem. 278:18980-18989. [DOI] [PubMed] [Google Scholar]
- 27.Mink, S., B. Haenig, and K. H. Klempnauer. 1997. Interaction and functional collaboration of p300 and C/EBPβ. Mol. Cell. Biol. 17:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munshi, N., M. Merika, J. Yie, K. Senger, G. Chen, and D. Thanos. 1998. Acetylation of HMG I(Y) by CBP turns off IFN beta expression by disrupting the enhanceosome. Mol. Cell 2:457-467. [DOI] [PubMed] [Google Scholar]
- 29.Narlikar, G. J., H. Y. Fan, and R. E. Kingston. 2002. Cooperation between complexes that regulate chromatin structure and transcription. Cell 108:475-487. [DOI] [PubMed] [Google Scholar]
- 30.Ogryzko, V. V., R. L. Schiltz, V. Russanova, B. H. Howard, and Y. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953-959. [DOI] [PubMed] [Google Scholar]
- 31.Ozes, O. N., L. D. Mayo, J. A. Gustin, S. R. Pfeffer, L. M. Pfeffer, and D. B. Donner. 1999. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82-85. [DOI] [PubMed] [Google Scholar]
- 32.Pan, Z. K., S. C. Christiansen, A. Ptasznik, and B. L. Zuraw. 1999. Requirement of phosphatidylinositol 3-kinase activity for bradykinin stimulation of NF-κB activation in cultured human epithelial cells. J. Biol. Chem. 274:9918-9922. [DOI] [PubMed] [Google Scholar]
- 33.Radisavljevic, Z., H. Avraham, and S. Avraham. 2000. Vascular endothelial growth factor up-regulates ICAM-1 expression via the phosphatidylinositol 3 OH-kinase/AKT/nitric oxide pathway and modulates migration of brain microvascular endothelial cells. J. Biol. Chem. 275:20770-20774. [DOI] [PubMed] [Google Scholar]
- 34.Rahman, A., A. L. True, K. N. Anwar, R. D. Ye, T. A. Voyno-Yasenetskaya, and A. B. Malik. 2002. Gαq and Gβγ regulate PAR-1 signaling of thrombin-induced NF-κB activation and ICAM-1 transcription in endothelial cells. Circ. Res. 91:398-405. [DOI] [PubMed] [Google Scholar]
- 35.Reddy, S. A., J. H. Huang, and W. S. Liao. 2000. Phosphatidylinositol 3-kinase as a mediator of TNF-induced NF-kappa B activation. J. Immunol. 164:1355-1363. [DOI] [PubMed] [Google Scholar]
- 36.Schmelzle, T., and M. N. Hall. 2000. TOR, a central controller of cell growth. Cell 103:253-262. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz, C., K. Beck, S. Mink, M. Schmolke, B. Budde, D. Wenning, and K. H. Klempnauer. 2003. Recruitment of p300 by C/EBPβ triggers phosphorylation of p300 and modulates coactivator activity. EMBO J. 22:882-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.See, R. H., D. Calvo, Y. Shi, H. Kawa, M. P. Luke, Z. Yuan, and Y. Shi. 2001. Stimulation of p300-mediated transcription by the kinase MEKK1. J. Biol. Chem. 276:16310-16317. [DOI] [PubMed] [Google Scholar]
- 39.Shikama, N., J. Lyon, and N. B. La Thangue. 1997. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell. Biol. 7:230-236. [DOI] [PubMed] [Google Scholar]
- 40.Sizemore, N., S. Leung, and G. R. Stark. 1999. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol. Cell. Biol. 19:4798-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teshima, S., H. Nakanishi, M. Nishizawa, K. Kitagawa, M. Kaibori, M. Yamada, K. Habara, A. H. Kwon, Y. Kamiyama, S. Ito, and T. Okumura. 2004. Up-regulation of IL-1 receptor through PI3K/Akt is essential for the induction of iNOS gene expression in hepatocytes. J. Hepatol. 40:616-623. [DOI] [PubMed] [Google Scholar]
- 42.Vermeulen, L., W. G. De, S. Notebaert, B. W. Vanden, and G. Haegeman. 2002. Regulation of the transcriptional activity of the nuclear factor-κB p65 subunit. Biochem. Pharmacol. 64:963-970. [DOI] [PubMed] [Google Scholar]
- 43.Vettese-Dadey, M., P. A. Grant, T. R. Hebbes, C. Robinson, C. D. Allis, and J. L. Workman. 1996. Acetylation of histone H4 plays a primary role in enhancing transcription factor binding to nucleosomal DNA in vitro. EMBO J. 15:2508-2518. [PMC free article] [PubMed] [Google Scholar]
- 44.Wade, P. A., D. Pruss, and A. P. Wolffe. 1997. Histone acetylation: chromatin in action. Trends Biochem. Sci. 22:128-132. [DOI] [PubMed] [Google Scholar]
- 45.Yang, W., Y. H. Hong, X. Q. Shen, C. Frankowski, H. S. Camp, and T. Leff. 2001. Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J. Biol. Chem. 276:38341-38344. [DOI] [PubMed] [Google Scholar]
- 46.Yang, X. J., V. V. Ogryzko, J. Nishikawa, B. H. Howard, and Y. Nakatani. 1996. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382:319-324. [DOI] [PubMed] [Google Scholar]
- 47.Yao, T. P., G. Ku, N. Zhou, R. Scully, and D. M. Livingston. 1996. The nuclear hormone receptor coactivator SRC-1 is a specific target of p300. Proc. Natl. Acad. Sci. USA 93:10626-10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan, L. W., and J. E. Gambee. 2000. Phosphorylation of p300 at serine 89 by protein kinase C. J. Biol. Chem. 275:40946-40951. [DOI] [PubMed] [Google Scholar]
- 49.Yuan, L. W., J. W. Soh, and I. B. Weinstein. 2002. Inhibition of histone acetyltransferase function of p300 by PKCδ. Biochim. Biophys. Acta 1592:205-211. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, B. P., Y. Liao, W. Xia, Y. Zou, B. Spohn, and M. C. Hung. 2001. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 3:973-982. [DOI] [PubMed] [Google Scholar]
- 51.Zhu, K., M. A. Amin, M. J. Kim, K. J. Katschke, Jr., C. C. Park, and A. E. Koch. 2003. A novel function for a glucose analog of blood group H antigen as a mediator of leukocyte-endothelial adhesion via intracellular adhesion molecule 1. J. Biol. Chem. 278:21869-21877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.