Abstract
Type 2 diabetes mellitus is a disorder of glucose homeostasis involving complex gene and environmental interactions that are incompletely understood. Mammalian homologs of nematode sex determination genes have recently been implicated in glucose homeostasis and type 2 diabetes mellitus. These are the Hedgehog receptor Patched and Calpain-10, which have homology to the nematode tra-2 and tra-3 sex determination genes, respectively. Here, we have developed Fem1b knockout (Fem1b-KO) mice, with targeted inactivation of Fem1b, a homolog of the nematode fem-1 sex determination gene. We show that the Fem1b-KO mice display abnormal glucose tolerance and that this is due predominantly to defective glucose-stimulated insulin secretion. Arginine-stimulated insulin secretion is also affected. The Fem1b gene is expressed in pancreatic islets, within both β cells and non-β cells, and is highly expressed in INS-1E cells, a pancreatic β-cell line. In conclusion, these data implicate Fem1b in pancreatic islet function and insulin secretion, strengthening evidence that a genetic pathway homologous to nematode sex determination may be involved in glucose homeostasis and suggesting novel genes and processes as potential candidates in the pathogenesis of diabetes mellitus.
The fem-1 gene of Caenorhabditis elegans encodes an ankyrin repeat protein that is part of a signal transduction and transcriptional regulatory pathway controlling cell fate decisions during sex determination in the nematode (10, 17, 31). A mammalian Fem1 gene family, encoding homologs of fem-1, has been characterized and consists of at least three members in the mouse, designated Fem1a, Fem1b, and Fem1c; these have highly conserved homologs in humans, designated FEM1A, FEM1B, and FEM1C, respectively (4, 22, 36-39). A mammalian Fem1 transgene expressed in fem-1 mutants of C. elegans causes partial rescue of masculinization, suggesting evolutionary conservation of ancient biochemical signaling interactions (36). However, the biochemical mechanisms of nematode fem-1 gene action remain unknown, and in vivo physiologic functions of the mammalian Fem1 genes have not yet been defined.
Mammalian homologs of two other nematode sex determination genes, tra-2 and tra-3, have recently been implicated in glucose homeostasis and type 2 diabetes mellitus. The tra-2 gene of C. elegans encodes a transmembrane protein with homology to Patched, the cell surface receptor for Hedgehog (24). The nematode sex determination pathway may be a variant of Hedgehog signaling (27). In addition to the tra-2/Patched homology, the nematode tra-1 gene encodes a transcription factor whose DNA-binding zinc fingers are very similar to those of Drosophila melanogaster Cubitus interruptus and mammalian GLI and GLI-3, which are transcriptional mediators of Hedgehog signaling (40). Recent data have implicated the Patched gene, and Hedgehog signaling, in pancreatic islet β-cell function and glucose homeostasis (16, 34, 35).
The tra-3 gene of C. elegans encodes an atypical calpain protease (2). The initial report identifying the NIDDM1 gene as Calpain-10 recognized that this atypical calpain protease has homology to the nematode tra-3 gene (19). In producing susceptibility to type 2 diabetes mellitus, NIDDM1 is known to interact with a gene, whose identity is unknown, on human chromosome 15 near the CYP19 locus at 15q21.3 (7). This is near 15q22, where FEM1B, the human homolog of mouse Fem1b, localizes (39).
The mammalian Fem1 genes are expressed in tissues of relevance to glucose physiology, including pancreas and skeletal muscle. In the studies described herein, we have used gene targeting by homologous recombination to generate Fem1b knockout (Fem1b-KO) mice with inactivation of the Fem1b gene. We show that these mice display abnormal glucose homeostasis, with abnormal glucose tolerance tests and defective glucose-stimulated insulin secretion. In wild-type mice, the Fem1b gene is expressed in pancreatic islets within both β cells and non-β cells. The Fem1b gene is highly expressed in INS-1E insulinoma cells, a widely used cell culture model of regulated insulin secretion. These findings indicate that Fem1b is involved in pancreatic islet β-cell function and provide further evidence for involvement of a pathway resembling nematode sex determination in mammalian glucose homeostasis.
MATERIALS AND METHODS
Generation of Fem1b-KO mice.
The Fem1b gene was targeted for inactivation by homologous recombination using a well-established general strategy (25). The targeting vector, pZKO(nM), was derived from the vectors pZINI(nM) and pDOUG (which were a generous gift of Chin Chiang, Vanderbilt University) by removal of the LacZ gene from pZINI(nM). A 5.5-kb 5′ genomic BamHI-NotI fragment immediately upstream of Fem1b exon 1 was cloned upstream of PGK-Neo, and a 4-kb 3′ BssHII (plus EcoRI linker)-EcoRI fragment (BssHII site adjacent to the BamHI site which is downstream of Fem1b exon 1 [see reference 36]) was cloned downstream of PGK-Neo, in the targeting vector, to produce a construct, pKO/Fem1b, as shown in Fig. 1. The completed construct was purified by CsCl ultracentrifugation (28). This was used for transfection of mouse 129/SvJ embryonic stem (ES) cells, which were selected with G418 and screened by Southern blot, with positive clones verified with both 5′ and 3′ Southern blot probes. Three Fem1b-targeted ES lines were found and injected into C57BL/6 blastocytes for production of chimeric mice (Genome Systems/Incyte Genomics, St. Louis, MO). Three corresponding chimeric mice were generated which were then bred with C57BL/6 mice, and two of these (mouse 66 and mouse 147 [Fig. 1]) gave germ line transmission. Breeding and experiments with mice were approved by our local Institutional Animal Care and Use Committee.
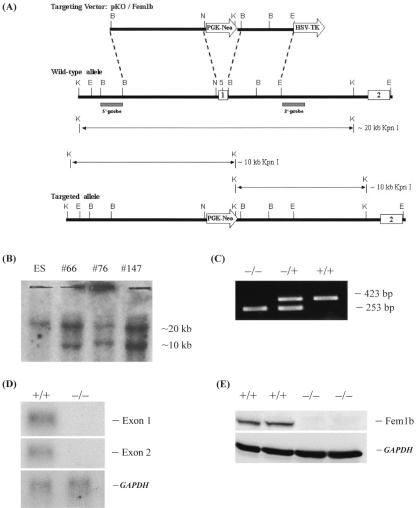
FIG. 1.
Targeted disruption of the mouse Fem1b gene and identification of Fem1b-KO embryonic stem cells and mice. (A) General strategy, with expected KpnI digestion products along with 5′ and 3′ probes for Southern blot. The boxes labeled 1 and 2 represent exons 1 and 2, respectively. Exon 1 is replaced by the PGK-Neo gene (labeled arrow) in the targeted allele. (B) Southern blot analysis with the 5′ probe of KpnI digestion genomic DNA from wild-type ES cells and three Fem1b-targeted clones. (C) Example of PCR genotype analysis showing products from Fem1b-KO homozygotes (−/−), heterozygotes (+/−), and wild-type mice (+/+). (D) Northern blot analysis of Fem1b mRNA expression in testis form wild-type mice (+/+) and Fem1b-KO homozygotes (−/−) using either exon 1 or exon 2 as a probe, with GAPDH used as a control. (E) Western blot analysis of Fem1b protein expression in brain from wild-type mice (+/+) and Fem1b-KO homozygotes (−/−) using an antibody, Li-51, directed against the C terminus of Fem1b, with anti-GAPDH used as a control.
Genotyping.
Genomic DNA was prepared from mouse tail clips using the Puregene DNA Isolation kit (Gentra Systems, Minneapolis, MN) and the manufacturer's instructions. Genotype was determined by PCR of the wild-type (eliminated by knockout) versus Neo alleles. Primer were as follows: mB#1.2F (5′-ACCAAACGGGTGTTGGAGTTG-3′), mB-in/ko#2R, (5′-CAGCAAAGCATAGCTCTGGGA-3′), neo#2F (5′-GGTTCTTTTTGTCAAGACCGAC-3′), and neo#2R (5′-GTAGCCGGATCAAGCGTATG-3′).
PCR conditions were as follows: 94°C for 4 min and then cycles of 94°C for 30 s, 62°C for 30 s, and 72°C for 35 s for 34 cycles, and then 72°C for 8 min.
Glucose and insulin measurements.
Blood glucose from tail vein was measured using a OneTouch FastTake Glucometer (LifeScan, Milpitas, CA). Insulin was measured from plasma, tissue extracts, or cell supernatants using the Rat (Mouse) Sensitive Insulin radioimmunoassay (RIA) kit and the manufacturer's instructions (Linco Research, St. Charles, MO).
In vivo metabolic testing.
For the intraperitoneal glucose tolerance test (iP-GTT), intraperitoneal insulin tolerance test (iP-ITT), and acute-phase glucose-stimulated insulin secretion (A-GSIS) test, there were 12 animals in each group (12 male homozygous Fem1b-KO, 12 male wild-type controls, 12 female homozygous Fem1b-KO, and 12 female wild-type controls), aged 3 to 4 months. The arginine-stimulated insulin secretion test compared eight Fem1b-KO homozygous males with eight wild-type males, aged 6 months. Statistical analyses were performed using SigmaPlot 8.0 (Sigma, St. Louis, MO).
The iP-GTT test was performed after an 18-h overnight fast. d-Glucose (200 mg/ml) was administered at 2 mg/g body weight by intraperitoneal injection. Tail vein blood was sampled for blood glucose determination from nonsedated animals before and at 15, 30, 60, and 120 min after glucose administration. The iP-ITT test was performed on mice in a random-fed state by intraperitoneal administration of human insulin at 0.75 U/kg, and tail vein blood was sampled for blood glucose levels before and at 15, 30, and 60 min. A-GSIS test was performed after an 18-h overnight fast. d-Glucose (200 mg/ml) load was administered at 2 mg/g body weight by intraperitoneal injection, venous blood was sampled before and 2 and 5 min after administration, and serum was separated and stored at −20°C for analysis of the insulin concentration with RIA. Arginine-stimulated insulin secretion test was performed after an overnight fast with l-arginine at 2 mg/g (in phosphate-buffered saline [PBS]) by intraperitoneal injection, with venous blood sampled before and at 2 and 5 min following administration, and serum was separated and stored at −20°C for analysis of insulin concentration by RIA.
Fem1b antibody.
Rabbit polyclonal antibodies directed against a C-terminal epitope of mouse Fem1b (36) with an added cysteine for conjugation (sequence, C-RANDINYQDQIPRTLEEFVGFH) was chemically synthesized (Proteintech Group Inc., Chicago, IL). The peptide was conjugated to keyhole limpet hemocyanin for immunization in two rabbits. The resultant antibody, designated Li-51, was purified from serum using an ImmunoPure(A) immunoglobulin (IgG) purification kit (Pierce Biotechnology, Rockford, IL) and immunoaffinity purified by conjugating the peptide with UltraLink Iodoacetyl gel and then using ImmunoPure Gentle Ag/Ab binding and elution buffers (Pierce Biotechnology, Rockford, IL) to bind and elute the antibodies. The titers were then checked by enzyme-linked immunosorbent assay and were at least 1:10,000 for both rabbits.
Immunohistochemistry.
Wild-type mice were euthanized, and the pancreas was dissected, fixed with S.T.F. (Streck Laboratories, La Vista, NE), embedded in paraffin, and sectioned at 5 μm. Anti-Fem1b antibody Li-51 was used for immunohistochemistry. We also purchased polyclonal goat antibody (ab801) against Fem1b C terminus (Abcam, Cambridgeshire, United Kingdom). The following other primary antibodies were used: rabbit anti-glucagon, rabbit anti-pancreatic polypeptide, and rabbit anti-somatostatin (Zymed Laboratories, San Francisco, CA); goat anti-glucagon (N-17) and goat anti-somatostatin (D-20) (Santa Cruz Biotechnology, Santa Cruz, CA); and guinea pig anti-human insulin (Linco Research, St. Charles, MO). The following secondary and tertiary antibodies were used: Cy2-conjugated affiniPure donkey anti-guinea pig IgG (heavy plus light chains [H+L]), Cy3-conjugated affiniPure donkey anti-rabbit IgG (H+L), Cy3-conjugated affiniPure donkey anti-goat IgG (H+L), Cy2-conjugated affiniPure donkey anti-rabbit IgG (H+L), biotin-SP-conjugated affiniPure donkey anti-goat IgG (H+L), biotin-SP-conjugated affiniPure donkey anti-rabbit IgG (H+L), and peroxidase-conjugated streptavidin (all from Jackson ImmunoReserach, West Grove, PA). Immunoperoxidase staining utilized the Vectastain Elite ABC-Peroxidase kit and DAB substrate kit (Vector Laboratories, Burlingame, CA). Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling (TUNEL) assay utilized the In Situ Cell Death Detection kit, POD (Roche, Indianapolis, IN). PCNA staining utilized anti-PCNA (FL-261) rabbit primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Whole-pancreas insulin measurement.
Mice were used after an overnight fast, and four Fem1b-KO homozygous mice were compared with four wild-type mice, aged 10 months. After euthanasia, the pancreas was carefully dissected and wet weight was obtained, and the pancreas was gently minced and then homogenized with a hand-held Tissue-Tearor homogenizer in 500 μl per 100 mg tissue of a mixture of acid-ethanol (0.1 N HCl in ethanol). The extract was then incubated at 4°C for 4 h and centrifuged, and the supernatant was stored at −20°C until analysis of insulin with RIA, which was done on 1:5,000 and 1:10,000 dilutions.
Cell culture experiments.
All cell culture reagents were obtained from Sigma (St. Louis, MO), except where otherwise indicated. INS-1E cells (a generous gift from Claes Wollheim and P. Maechler, Geneva, Switzerland) were grown at 37°C in RPMI 1640 medium supplemented with 10 mM HEPES, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 5% fetal calf serum (Invitrogen Corp., Carlsbad, CA), 100 IU/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine in an atmosphere containing 5% (vol/vol) carbon dioxide.
For glucose-stimulated insulin secretion, INS-1E cells were seeded in 24-well tissue culture plates (1 × 105 cells/well). After culture for 4 days, the cells were washed three times in modified Krebs-Ringer bicarbonate HEPES buffer (KRBH) composed of 140 mM NaCl, 3.6 mM KCl, 0.5 mM NaH2PO4, 0.5 mM MgSO4, 1.5 mM CaCl2, 10 mM HEPES, 2 mM NaHCO3, and 0.1% bovine serum albumin (BSA) (Linco) and preincubated in the same medium for another 30 min. The cells were then incubated with KRBH-0.1%BSA containing no glucose or 2.8, 5.6, 11.2, or 16.7 mM glucose for 30 min. For the time course experiment, the cells were stimulated by 11.2 mM glucose for 0, 5, 10, 15, 30, and 60 min. At these time points, cumulative insulin secreted into the medium and cellular insulin content extracted with acid-ethanol were determined by RIA.
For Western and Northern blot analyses of glucose-stimulated insulin secretion, INS-1E cells were seeded on the 100 20-mm polystyrene tissue culture dishes (2 × 106 cells/dish) and grown for 4 days as described above. The cells were washed three times with KRBH-0.1% BSA without glucose and then preincubated in the same medium for another 30 min. The cells were stimulated by 11.2 mM glucose for 0, 5, 10, 15, 20, 30, and 60 min. Protein was prepared from these INS-1E cells by harvesting them in radioimmunoprecipitation assay buffer (50 mM Tris · HCl, pH 7.40, 150 mM NaCl, 2% NP-40, 1% deoxycholate, 0.2% sodium dodecyl sulfate [SDS], 1 mM EDTA) supplemented with protease inhibitor cocktail (Sigma, St. Louis, MO), and then protein concentration was determined by the BCA protein assay kit (Pierce Biotechnology, Rockford, IL), and these samples were used for Western blot (see below). In another experiment, total RNA from INS-1E cells treated in the same way were extracted with TRIzol reagent (Invitrogen) according to the manufacturer's instructions and used for Northern blot analysis (see below).
Northern blot analysis.
Total RNA from INS-1E cells in 60-mm cell culture plates was isolated by the guanidinium-phenol-chloroform extraction method using TRIzol (Invitrogen Corp., Carlsbad, CA) according to manufacturer's directions. RNA concentration was determined spectrophotometrically. RNA samples (10 μg/lane) were analyzed by electrophoresis through a 1.1% agarose-2.2 M formaldehyde gel and transferred to Zeta-Probe membrane (Bio-Rad, Hercules, CA) by capillary flow in 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Transferred RNA was cross-linked to the membrane by UV light illumination and subsequently hybridized overnight at 68°C to radiolabeled probes in PerfectHyb Plus hybridization buffer (Sigma). The final two washes were under high-stringency conditions (0.2× SSC-0.1% SDS at 68°C). cDNA probes for Northern blot were as follows: a 1.8-kb cDNA fragment corresponding to the open reading frame of mouse Fem1b, a 1-kb mouse Patched probe generated by PCR and verified by restriction digest, a 780-bp fragment of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sequence, and a β-actin cDNA (Clontech, Palo Alto, CA).
Western blot analysis.
Whole tissues were homogenized in radioimmunoprecipitation assay buffer containing protease inhibitor cocktail (as noted above with INS-1E cell extracts), and protein concentration was determined with bicinchoninic assay. Whole-tissue protein extracts (80 μg per lane) were separated on a 10% SDS-polyacrylamide gel with stacking gel. For INS-1E cell extracts prepared as described above, proteins were separated on 10% precast SDS-polyacrylamide Mini-gel (20 μg per lane; Bio-Rad). After separation, proteins were transferred to polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). After treatment with 5% blocking reagent (Amersham Biosciences, Piscataway, NJ) in PBST (0.01 M PBS-0.05% Tween 20, pH 7.40), the membrane was incubated with immunoaffinity-purified Li-51 antibody at a 1:400 dilution for 1 h at room temperature. The membrane was washed with PBST three times, followed by incubation with 1:7,000 diluted horseradish peroxidase-labeled donkey anti-rabbit IgG for 1 h at room temperature and then detection with ECL Western blotting reagents (Amersham Biosciences, Piscataway, NJ). The same membrane was then stripped in stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.7) and probed with anti-GAPDH (Chemicon, Temecula, CA) as a control.
RESULTS
Development of Fem1b-KO mice.
We used gene targeting by homologous recombination (25) to generate Fem1b-KO mice with a deletion of Fem1b coding exon 1, which contains the translation initiation codon and the first two ankyrin repeats (36). This approach utilized standard methodology, and the basic elements of the targeting vector and screening strategy by Southern blot and PCR genotyping are shown (Fig. 1A to C). The animals used in the experiments described herein were littermates, and all were on the mixed 129/SvJ × C57BL/6 background. To evaluate the effect of the Fem1b-KO allele on Fem1b mRNA and protein expression, we evaluated tissues that express relatively high levels of Fem1b by Northern blot using probes to both exon 1 and exon 2 and by Western blot with antibody directed against the C terminus of Fem1b. The results (Fig. 1D and E) demonstrate that the Fem1b-KO allele functions as a null allele in the tissues examined. In pancreas, Fem1b expression is confined to islets (see below) which represent only about 1% of pancreas mass. Immunohistochemical analysis demonstrates a loss of specific Fem1b staining in islets of Fem1b-KO homozygotes (see below and Fig. 3C).
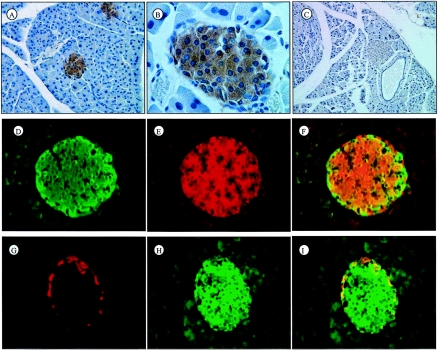
FIG. 3.
Immunolocalization of Fem1b in pancreatic islets and colocalization with pancreatic islet hormones. (A and B) Lower and higher magnification, respectively, of immunoperoxidase staining with antibody Li-51 (against the C terminus of Fem1b) counterstained with hematoxylin. (C) Immunostaining of homozygous Fem1b-KO pancreas with anti-Fem1b C-terminus antibody Li-51, demonstrating the absence of specific staining for Fem1b. (D, E, and F) Immunofluorescence staining of insulin (green), Fem1b (red), and merged image demonstrating that Fem1b is expressed not only in insulin-positive β cells but also in insulin-negative non-β cells. (G, H, and I) Immunofluorescence staining with a combination of antibodies to glucagon and somatostatin (red) and Fem1b (green) and a merged image verifying expression of Fem1b within non-β cells (glucagon-positive α cells and somatostatin-positive δ cells) in addition to β cells.
Glucose homeostasis in Fem1b-KO mice.
As noted above, mammalian homologues of nematode sex determination genes have recently been shown to be involved in glucose homeostasis and type 2 diabetes mellitus. Based on this logic, we evaluated glucose homeostasis in the Fem1b-KO mice by using established experimental methods for mice (3, 6, 15, 23). As a first-line screen, we performed iP-GTT in both male and female Fem1b-KO homozygotes and compared them to wild-type controls. These mice were between 3 and 4 months of age. As shown in Fig. 2A and B, both male and female Fem1b-KO homozygotes display a hyperglycemic response to the iP-GTT compared to wild-type controls.
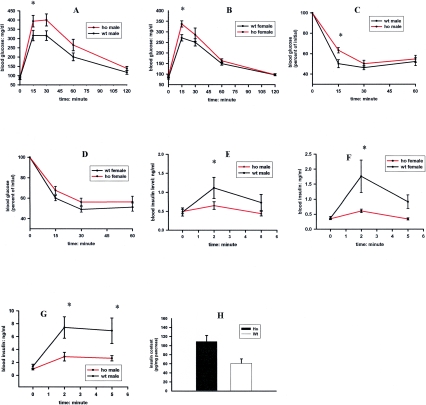
FIG. 2.
In vivo glucose homeostasis in Fem1b-KO homozygotes (ho) compared to that of wild-type (wt) controls, male and female. For each of these experiments in A to F, there were 12 animals in each group (12 male homozygous Fem1b-KO, 12 male wild-type controls, 12 female homozygous Fem1b-KO, and 12 female wild-type controls). (A and B) Intraperitoneal glucose tolerance test following an 18-h overnight fast (males and females); (C and D) intraperitoneal insulin tolerance test (males and females); (E and F) acute-phase glucose-stimulated insulin secretion (males and females); (G) acute-phase arginine-stimulated insulin secretion in eight Fem1b-KO homozygous males compared to that in eight wild-type males; (H) pancreatic insulin content in fasted mice (four Fem1b-KO homozygous mice and four wild-type mice).
In order to distinguish whether the abnormal iP-GTT was due primarily to insulin resistance, defective insulin secretion, or both, we performed iP-ITT and A-GSIS on these mice. The iP-ITT showed minimally abnormal results (Fig. 2C and D), suggesting that insulin resistance is not the primary defect in homozygotes, although it could be contributing. In contrast, the A-GSIS tests demonstrate that both male and female homozygous Fem1b-KO animals display defective A-GSIS compared to wild-type controls (Fig. 2E and F). In order to test whether the defect is specific for glucose, we tested arginine-induced insulin secretion in a group of male Fem1b-KO homozygotes and compared it to that of wild-type mice (Fig. 2G), and the results show that there is also defective arginine-induced acute-phase insulin secretion.
To evaluate whether the defective acute-phase insulin secretion is related to a defect in secretion per se as opposed to a defect in insulin production, we measured the insulin content of the pancreas in these mice (Fig. 2H), which demonstrates that Fem1b-KO homozygotes have increased insulin content compared to that of wild-type controls. The pancreas and islets of Fem1b-KO mice appear essentially normal by light microscopy and by immunostaining with antibodies against insulin for β cells and antibodies to glucagon, somatostatin, and pancreatic polypeptide for α cells, δ cells, and PP cells, respectively (data not shown). Evaluation of apoptosis by TUNEL assay and cell proliferation by PCNA immunostaining did not reveal any obvious differences between Fem1b-KO homozygotes and wild-type controls (data not shown).
Immunostaining of Fem1b in pancreatic islets.
In humans, FEM1B has been shown to be expressed within whole pancreas (4), but cell type distribution within this organ was unknown. We developed rabbit polyclonal antibodies against Fem1b and used these to characterize expression of Fem1b protein in pancreas. Immunostaining of wild-type pancreas with immunoaffinity-purified antibody shows that Fem1b protein is expressed in pancreatic islets (Fig. 3A and B). As noted above, immunostaining of pancreas from Fem1b-KO homozygotes with rabbit polyclonal anti-Fem1b demonstrates an absence of specific staining (Fig. 3C). Immunostaining of the pancreas with a commercially available goat polyclonal antibody against Fem1b demonstrates the same islet staining pattern, with an absence of specific staining in the Fem1b-KO homozygotes (data not shown). Coimmunostaining with antibodies to insulin, a β-cell marker, demonstrates that Fem1b is expressed in virtually all β cells (Fig. 3D to F). Coimmunostaining with antibodies to glucagon and somatostatin, markers for α cells and δ cells, respectively, demonstrates that the Fem1b protein is also expressed in these non-β cells (Fig. 3G to I).
Fem1b in INS-1E cells.
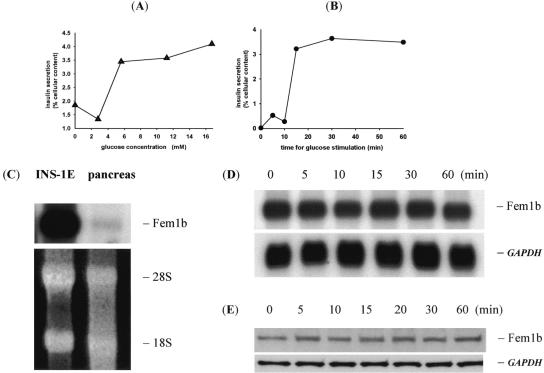
We next evaluated INS-1E cells, a subline of the rat INS-1 insulinoma cell line widely used as a β-cell culture model, and selected for displaying glucose-stimulated insulin secretion within a physiologic range of glucose levels (1, 26). These cells display glucose-induced insulin secretion with a glucose dose response relationship and time course (Fig. 4A and B), essentially as previously described (26). Compared to whole rat pancreas, in which islet β cells represent only about 1% of the total cell mass, Fem1b mRNA is highly expressed in INS-1E cells (Fig. 4C). Fem1b mRNA and protein levels in INS-1E cells are not significantly altered during the time course of an A-GSIS experiment (Fig. 4D and E).
FIG. 4.
Fem1b in INS-1E cells. (A and B) GSIS dose response curve and time course, respectively; (C) Northern blot of Fem1b mRNA expression in INS-1E cells compared to whole rat pancreas. Ethidium bromide stain shows 18S and 28S rRNA to control for loading. (D) Northern blot of GSIS time course with Fem1b cDNA probe and GAPDH probe as a control. (E) Western blot analysis of GSIS time course with anti-Fem1b C-terminus antibody Li-51 and anti-GAPDH as a control.
DISCUSSION
We conclude that Fem1b functions in vivo to regulate insulin secretion and plasma glucose levels. Fem1b-KO mice do not have fasting hyperglycemia but rather have defective acute-phase GSIS. Such defective acute-phase GSIS is the earliest detectable defect in humans destined to develop diabetes and may represent the primary genetic risk factor predisposing to diabetes (13). With aging and superimposed insulin resistance, fasting hyperglycemia and overt diabetes later develop. The early β-cell dysfunction with abnormal postprandial hyperglycemia, in the setting of normal fasting glucose levels, is itself clinically important and associated with increased risk of cardiovascular disease (14). Therefore, the Fem1b-KO mouse model is a key component of the complex pathogenesis of type 2 diabetes mellitus.
Both male and female homozygotes display abnormal glucose tolerance. This is associated with a defect in insulin secretion in response to both glucose and arginine, suggesting a generalized defect in insulin secretion from pancreatic islet β cells in the Fem1b-KO mice. The increased pancreatic insulin content in these mice argues that the defect is due to insulin secretion rather than insulin production. Since Fem1b is also expressed in skeletal muscle, the major site of insulin-dependent glucose disposal, we cannot exclude from our studies that there may also be a defect in this process in the Fem1b-KO mice. In addition, the studies of glucose homeostasis reported herein were done in homozygous Fem1b-KO mice and wild-type mice. Preliminary data did not demonstrate an obvious difference in glucose tolerance tests between Fem1b-KO heterozygotes and wild-type mice (data not shown). Future experiments will quantitatively evaluate the effect of Fem1b-KO allele dosage in the setting of more overt insulin resistance and will evaluate more quantitatively, by euglycemic-hyperinsulinemic clamp methodology, whether some degree of skeletal muscle insulin resistance may also be present in the Fem1b-KO mice. In addition, whether there may be developmental compensation for Fem1b loss of function from the other family members Fem1a and Fem1c remains to be explored.
The role of Fem1b in pancreatic islet insulin secretion strengthens evidence that a genetic pathway homologous to nematode sex determination may be involved in mammalian glucose homeostasis (Fig. 5). This novel pathway could be involved in the β-cell dysfunction seen in type 2 diabetes mellitus. Calpain proteases, including the tra-3 homolog Calpain-10/NIDDM1, have been shown to be involved in β-cell function (21, 32, 41). Since Calpain-10/NIDDM1 is known to interact with a gene that is near where human FEM1B localizes, to increase susceptibility to type 2 diabetes (7, 39), whether FEM1B could be the responsible interacting gene becomes a pertinent question.
FIG. 5.
(A) Simplified representation of sex determination pathway of C. elegans downstream of X-chromosome dosage compensation genes (adapted from reference 1). Genes at one level negatively regulate (◂) the activity of genes at the next downstream level (for example, fem-1 + fem-2 + fem-3 negatively regulate the activity of tra-1). Highlighted in red are those genes that have been shown to have homologs in mammals that are involved in glucose homeostasis and diabetes. (B) List of the highlighted C. elegans sex determination genes and their corresponding mammalian homologs with phenotypes related to glucose homeostasis, with representative references or source.
Likewise, whether Fem1b actually functions in a signaling pathway along with Calpain-10/NIDDM1 becomes an important question but will not be simple to address. In the nematode, the ordering of genes within the sex determination pathway was based on extensive genetic epistasis analysis (17), and although biochemical interactions among some of the encoded proteins are now known, none involving fem-1 have been defined. Therefore, it is unclear how fem-1 interacts with tra-3, or any other gene, in the regulatory cascade. There is no evidence that fem-1 is itself a proteolytic substrate of tra-3. Rather, the target of nematode tra-3 calpain protease activity appears to be tra-2 (2, 30), which functions upstream of fem-1 (Fig. 5).
Fem1b is expressed in both β cells and non-β cells of pancreatic islets, making it possible that Fem1b-KO alleles cause a functional defect in insulin secretion either directly within β cells, indirectly via interactions with non-β cells and humoral factors, or both. Whether Fem1b gene function in islets, like fem-1 gene function in nematodes, is related to transcriptional regulation and cell fate determination, rather than other mechanisms, cannot be answered until the precise functional defect is further clarified. Fem1b mRNA and protein levels in INS-1E cells are not acutely regulated during acute glucose-stimulated insulin secretion (Fig. 4D and E), although subcellular distribution and posttranslational modifications of Fem1b protein have yet to be characterized under these conditions. In this context, it is noteworthy that in C. elegans sex determination, fem-1 mRNA and protein is expressed in both male and female somatic tissues, suggesting that regulation of fem-1 activity is posttranscriptional and likely posttranslational (12). Whether there may be conservation of regulatory mechanisms governing mammalian Fem1 function is unknown. INS-1E cells offer a physiologically relevant cell culture model in which to address mechanisms of Fem1b action and regulation.
In addition, the human FEM1B gene has been implicated in apoptosis via an interaction with the death receptor Fas (4). Apoptosis may be involved both in the autoimmune destruction of β cells in type 1 diabetes mellitus and in graft rejection of transplanted β cells (11). Calpain-10/NIDDM1 has recently been shown to be involved in apoptosis of β cells (21). As noted above, using the TUNEL assay, we noted no overt differences between Fem1b-KO homozygotes and wild-type mice. However, these experiments were not done in the setting of specific apoptotic stimuli or in the setting of genetic predisposition to autoimmune destruction of β cells such as in the nonobese diabetic mouse (5, 8, 29, 33). In the nematode, although fem-1 is not part of the general pathway of programmed cell death, it interacts with the egl-41 gene to control apoptosis of the hermaphrodite-specific neurons within the context of sex determination (9, 18). The egl-41 gene has recently been shown to be the same as sel-10, which encodes an F-box protein that may target the fem-1 protein for proteasomal degradation in hermaphrodites (20).
In summary, the Fem1b gene is expressed in pancreatic islets and functions in vivo to regulate insulin secretion. Although the mechanism of this regulation by Fem1b remains to be established, this finding strengthens evidence that a genetic pathway homologous to nematode sex determination may be involved in mammalian glucose homeostasis and promises to offer insight into novel genes and processes as potential candidates in the pathogenesis of diabetes mellitus.
Acknowledgments
We thank Joe Files for obtaining financial assistance from the University of Mississippi Medical Center for development of the Fem1b-KO mice.
This work was supported by a Department of Veterans Affairs Merit Award to J.F.M.
REFERENCES
- 1.Asfari, M., D. Janjic, P. Meda, G. Li, P. A. Halban, and C. B. Wollheim. 1992. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130:167-178. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, T. M., and J. Hodgkin. 1996. The tra-3 sex determination gene of Caenorhabditis elegans encodes a member of the calpain regulatory protease family. EMBO J. 15:4477-4484. [PMC free article] [PubMed] [Google Scholar]
- 3.Brissova, M., M. Shiota, W. E. Nicholson, M. Gannon, S. M. Knobel, D. W. Piston, C. V. Wright, and A. C. Powers. 2002. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J. Biol. Chem. 277:11225-11232. [DOI] [PubMed] [Google Scholar]
- 4.Chan, S. L., K. O. Tan, L. Zhang, K. S. Yee, F. Ronca, M. Y. Chan, and V. C. Yu. 1999. F1Aα, a death receptor-binding protein homologous to the Caenorhabditis elegans sex-determining protein, FEM-1, is a caspase substrate that mediates apoptosis. J. Biol. Chem. 274:32461-32468. [DOI] [PubMed] [Google Scholar]
- 5.Chervonsky, A. V., Y. Wang, F. S. Wong, I. Visintin, R. A. Flavell, C. A. Janeway, Jr., and L. A. Matis. 1997. The role of Fas in autoimmune diabetes. Cell 89:17-24. [DOI] [PubMed] [Google Scholar]
- 6.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 7.Cox, N. J., M. Frigge, D. L. Nicolae, P. Concannon, C. L. Hanis, G. I. Bell, and A. Kong. 1999. Loci on chromosomes 2 (NIDDM1) and 15 interact to increase susceptibility to diabetes in Mexican Americans. Nat. Genet. 21:213-215. [DOI] [PubMed] [Google Scholar]
- 8.Darwiche, R., M. M. Chong, P. Santamaria, H. E. Thomas, and T. W. Kay 2003. Fas is detectable on beta cells in accelerated, but not spontaneous, diabetes in nonobese diabetic mice. J. Immunol. 170:6292-6297. [DOI] [PubMed] [Google Scholar]
- 9.Desai, C., and H. R. Horvitz. 1989. Caenorhabditis elegans mutants defective in the functioning of the motor neurons responsible for egg laying. Genetics 121:703-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doniach, T., and J. Hodgkin. 1984. A sex-determining gene, fem-1, required for both male and hermaphrodite development in Caenorhabditis elegans. Dev. Biol. 106:223-235. [DOI] [PubMed] [Google Scholar]
- 11.Efrat, S., and N. Fleischer. 2000. Engineering the pancreatic β-cell, p. 539. In D. LeRoith, S. I. Taylor, and J. M. Olefsky (ed.), Diabetes mellitus—a fundamental and clinical text, 2nd ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 12.Gaudet, J., I. VanderElst, and A. M. Spence. 1996. Post-transcriptional regulation of sex determination in Caenorhabditis elegans: widespread expression of the sex-determining gene fem-1 in both sexes. Mol. Biol. Cell 7:1107-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerich, J. E. 2002. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes 51(Suppl. 1):S117-S121. [DOI] [PubMed] [Google Scholar]
- 14.Gerich, J. E. 2003. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch. Intern. Med. 163:1306-1316. [DOI] [PubMed] [Google Scholar]
- 15.Gillies, M. C., and T. E. Mandel. 1990. The evolution of function and response to arginine challenge and pregnancy of portally and systemically placed islet cell grafts in streptozotocin diabetic mice. Metabolism 39:1253-1258. [DOI] [PubMed] [Google Scholar]
- 16.Hebrok, M., S. K. Kim, B. St. Jacques, A. P. McMahon, and D. A. Melton. 2000. Regulation of pancreas development by hedgehog signaling. Development 127:4905-4913. [DOI] [PubMed] [Google Scholar]
- 17.Hodgkin, J. 1987. Sex determination and dosage compensation in Caenorhabditis elegans. Annu. Rev. Genet. 21:133-154. [DOI] [PubMed] [Google Scholar]
- 18.Hodgkin, J., T. Doniach, and M. Shen. 1985. The sex determination pathway in the nematode Caenorhabditis elegans: variations on a theme. Cold Spring Harbor Symp. Quant. Biol. 50:585-593. [DOI] [PubMed] [Google Scholar]
- 19.Horikawa, Y., N. Oda, N. J. Cox, X. Li, M. Orho-Melander, M. Hara, Y. Hinokio, T. H. Lindner, H. Mashima, P. E. Schwarz, L. del Bosque-Plata, Y. Horikawa, Y. Oda, I. Yoshiuchi, S. Colilla, K. S. Polonsky, S. Wei, P. Concannon, N. Iwasaki, J. Schulze, L. J. Baier, C. Bogardus, L. Groop, E. Boerwinkle, C. L. Hanis, and G. I. Bell. 2000. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat. Genet. 26:163-175. [DOI] [PubMed] [Google Scholar]
- 20.Jager, S., H. T. Schwartz, H. R. Horvitz, and B. Conradt. 2004. The Caenorhabditis elegans F-box protein SEL-10 promotes female development and may target FEM-1 and FEM-3 for degradation by the proteasome. Proc. Natl. Acad. Sci. USA 101:12549-12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, J. D., Z. Han, K. Otani, H. Ye, Y. Zhang, H. Wu, Y. Horikawa, S. Misler, G. I. Bell, and K. S. Polonsky. 2004. RyR2 and calpain-10 delineate a novel apoptosis pathway in pancreatic islets. J. Biol. Chem. 279:24794-24802. [DOI] [PubMed] [Google Scholar]
- 22.Krakow, D., E. Sebald, L. M. King, and D. H. Cohn. 2001. Identification of human FEM1A, the ortholog of a C. elegans sex-differentiation gene. Gene 279:213-219. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni, R. N., M. Holzenberger, D. Q. Shih, U. Ozcan, M. Stoffel, M. A. Magnuson, and C. R. Kahn. 2002. Beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat. Genet. 31:111-115. [DOI] [PubMed] [Google Scholar]
- 24.Kuwabara, P. E., P. G. Okkema, and J. Kimble. 1992. tra-2 encodes a membrane protein and may mediate cell communication in the Caenorhabditis elegans sex determination pathway. Mol. Biol. Cell 3:461-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansour, S. L., K. R. Thomas, and M. R. Capecchi. 1988. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336:348-352. [DOI] [PubMed] [Google Scholar]
- 26.Merglen, A., S. Theander, B. Rubi, G. Chaffard, C. B. Wollheim, and P. Maechler. 2004. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 145:667-678. [DOI] [PubMed] [Google Scholar]
- 27.Puoti, A., M. Gallegos, B. Zhang, M. P. Wickens, and J. Kimble. 1997. Controls of cell fate and pattern by 3′ untranslated regions: the Caenorhabditis elegans sperm/oocyte decision. Cold Spring Harbor Symp. Quant. 62:19-24. [PubMed] [Google Scholar]
- 28.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Savinov, A. Y., A. Tcherepanov, E. A. Green, R. A. Flavell, and A. V. Chervonsky. 2003. Contribution of Fas to diabetes development. Proc. Natl. Acad. Sci. USA 100:628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokol, S. B., and P. E. Kuwabara. 2000. Proteolysis in Caenorhabditis elegans sex determination: cleavage of TRA-2A by TRA-3. Genes Dev. 14:901-906. [PMC free article] [PubMed] [Google Scholar]
- 31.Spence, A. M., A. Coulson, and J. Hodgkin. 1990. The product of fem-1, a nematode sex-determinating gene, contains a motif found in cell cycle control proteins and receptors for cell-cell interactions. Cell 60:981-990. [DOI] [PubMed] [Google Scholar]
- 32.Sreenan, S. K., Y. P. Zhou, K. Otani, P. A. Hansen, K. P. Currie, C. Y. Pan, J. P. Lee, D. M. Ostrega, W. Pugh, Y. Horikawa, N. J. Cox, C. L. Hanis, C. F. Burant, A. P. Fox, G. I. Bell, and K. S. Polonsky. 2001. Calpains play a role in insulin secretion and action. Diabetes 50:2013-2020. [DOI] [PubMed] [Google Scholar]
- 33.Thomas, H. E., and T. W. Kay. 2000. Beta cell destruction in the development of autoimmune diabetes in the non-obese diabetic (NOD) mouse. Diabetes Metab. Res. Rev. 16:251-261. [DOI] [PubMed] [Google Scholar]
- 34.Thomas, M. K., N. Rastalsky, J. H. Lee, and J. F. Habener. 2000. Hedgehog signaling regulation of insulin production by pancreatic β-cells. Diabetes 49:2039-2047. [DOI] [PubMed] [Google Scholar]
- 35.Thomas, M. K., J. H. Lee, N. Rastalsky, and J. F. Habener. 2001. Hedgehog signaling regulation of homeodomain protein islet duodenum homeobox-1 expression in pancreatic beta-cells. Endocrinology 142:1033-1040. [DOI] [PubMed] [Google Scholar]
- 36.Ventura-Holman, T., M. F. Seldin, W. Li, and J. F. Maher. 1998. The murine Fem1 gene family: homologs of the Caenorhabditis elegans sex-determination protein FEM-1. Genomics 54:221-230. [DOI] [PubMed] [Google Scholar]
- 37.Ventura-Holman, T., and J. F. Maher. 2000. Sequence, organization, and expression of the human FEM1B gene. Biochem. Biophys. Res. Commun. 267:317-320. [DOI] [PubMed] [Google Scholar]
- 38.Ventura-Holman, T., D. Lu, X. Si, E. B. Izevbigie, and J. F. Maher. 2003. The Fem1c genes: conserved members of the Fem1 gene family in vertebrates. Gene 314:133-139. [DOI] [PubMed] [Google Scholar]
- 39.Ventura-Holman, T., N. B. Hander, and J. F. Maher. 2000. The human FEM1B gene maps to chromosome 15q22 and is excluded as the gene for Bardet-Biedl syndrome, type 4. Am. J. Med. Sci. 319:268-270. [DOI] [PubMed] [Google Scholar]
- 40.Zarkower, D., and J. Hodgkin. 1992. Molecular analysis of the C. elegans sex-determining gene tra-1: a gene encoding two zinc finger proteins. Cell 70:237-249. [DOI] [PubMed] [Google Scholar]
- 41.Zhou, Y. P., S. Sreenan, C. Y. Pan, K. P. Currie, V. P. Bindokas, Y. Horikawa, J. P. Lee, D. Ostrega, N. Ahmed, A. C. Baldwin, N. J. Cox, A. P. Fox, R. J. Miller, G. I. Bell, and K. S. Polonsky. 2003. A 48-hour exposure of pancreatic islets to calpain inhibitors impairs mitochondrial fuel metabolism and the exocytosis of insulin. Metabolism 52:528-534. [DOI] [PubMed] [Google Scholar]