Abstract
Purpose
Appropriate antifungal therapy is a major determinant of survival in critically ill patients with invasive fungal disease. We sought to describe whether contemporary dosing of antifungals achieves therapeutic exposures in critically ill patients.
Methods
In a prospective, open-label, multicenter pharmacokinetic study, intensive care unit (ICU) patients prescribed azoles, echinocandins, or polyene antifungals for treatment or prophylaxis of invasive fungal disease were enrolled. Blood samples were collected on two occasions, with three samples taken during a single dosing interval on each occasion. Total concentrations were centrally measured using validated chromatographic methods. Pharmacokinetic parameters were estimated using noncompartmental methods. Antifungal dosing adequacy was assessed using predefined PK/PD targets.
Results
We included 339 patients from 30 ICUs across 12 countries. Median age 62 (interquartile range [IQR], 51–70) years, median APACHE II score 22 (IQR, 17–28), and 61% males. Antifungal therapy was primarily prescribed for treatment (80.8%). Fluconazole was the most frequently prescribed antifungal (40.7%). The most common indication for treatment was intra-abdominal infection (30.7%). Fungi were identified in 45% of patients, of which only 26% had a minimum inhibitory concentration available. Target attainment was higher for patients receiving prophylaxis (> 80% for most drugs). For patients receiving treatment, low target attainment was noted for voriconazole (57.1%), posaconazole (63.2%), micafungin (64.1%) and amphotericin B (41.7%).
Conclusion
This study highlights the varying degrees of target attainment across antifungal agents in critically ill patients. While a significant proportion of patients achieved the predefined PK/PD targets, wide variability and subtherapeutic exposures persist.
Trial registration
ClinicalTrials.gov Identifier: NCT03136926, 2017-04-21.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-025-07793-5.
Keywords: Antifungals, Pharmacokinetics, Critically ill, Intensive care unit, Invasive fungal disease
Take-home message
| This study reveals significant variability in antifungal target attainment among critically ill patients, highlighting the need for individualised dosing strategies. It underscores the importance of therapeutic drug monitoring to optimise antifungal therapy and improve patient outcomes in the intensive care unit. |
Introduction
Invasive fungal diseases are common and potentially devastating nosocomial infections. Incidences of severe fungal infections vary based on geographic location, patient population, and the presence of underlying diseases or comorbidities [1, 2]. For critically ill patients in particular, these infections are associated with substantial morbidity and mortality rates. Recognizing the role of prompt and appropriate antifungal therapy in optimizing patient outcomes [3], adequate dosing should be considered an important quality-of-care intervention.
In critically ill patients, altered pharmacokinetics (PK) reduce the likelihood that standard dosing regimens will achieve maximally effective drug exposures [4, 5]. Factors, such as organ dysfunction, fluid shifts, and concomitant medications, lead to difficult-to-predict drug exposures and potentially sub-optimal therapeutic responses. Achieving target drug concentrations is crucial for ensuring that antifungal therapy is both effective and safe [6]. Importantly, some antifungals carry significant toxicity risks [7], including hepatotoxicity and nephrotoxicity. Additionally, real-time monitoring of patient responses to treatment is challenging due to the non-specific nature of infection symptoms. Therefore, the quality of dosing is measured by achieving target drug exposures that improve patient outcomes while minimizing adverse events.
Existing PK data on antifungals in critically ill patients are mostly derived from small single-center studies with uncertain generalizability and limited capacity to inform the development of robust dosing recommendations. Large multicenter studies such as the Defining Antibiotic Levels in Intensive Care Unit Patients (DALI) study provided valuable insights into antibacterial PK in critically ill patients but had sparse antifungal data across the 68 enrolling intensive care units (ICUs) [8, 9]. The Screening Antifungal Exposure in Intensive Care Units (SAFE-ICU) study was designed after recognizing the need for more comprehensive data on antifungal PK in a multinational cross-section of ICUs.
The overall objective of the SAFE-ICU study was to describe whether in critically ill patients, contemporary dosing of triazole, echinocandin, and polyene antifungals achieve therapeutic exposures that are expected to be associated with optimal outcomes. Secondary objectives were to describe the relationship between observed antifungal exposure and different demographic and clinical characteristics, and identifying whether achieving pre-specified exposures in plasma is associated with improved clinical outcomes.
Methods
Study design and population
The SAFE-ICU study was a prospective, open-labeled, multicenter PK study in adult ICUs, conducted between 2017 and 2018. The protocol for this study has been published previously [10]. Ethical approval was provided by the lead site (Royal Brisbane and Women’s Hospital; HERC/16/QRBW/292), with individual institutional approvals obtained according to local protocols. Written informed consent was obtained for each patient. All procedures followed institutional/national ethical standards and the 1964 Helsinki Declaration and its amendments. This study was registered with ClinicalTrials.gov Identifier: NCT03136926.
Adult (≥ 18 years) critically ill patients were enrolled according to predefined inclusion and exclusion criteria (electronic supplementary table 1). Fluconazole, voriconazole, posaconazole, isavuconazole, anidulafungin, caspofungin, micafungin and amphotericin B were studied. The choice of antifungal agent and dosing was at the discretion of the treating clinician.
Pharmacokinetic sampling and bioanalysis
PK sampling occurred during a dosing interval on two occasions: first, between the first and third days (occasion 1), and second, between the fourth and seventh days (occasion 2) of the antifungal course in the ICU. On each sampling occasion, three blood samples were drawn from established intravenous access. The first sample (A) was collected 30 min after the completion of the intravenous infusion, or after oral intake. The second sample (B) was taken between 3 and 6 h after the start of drug infusion or administration of enteral dose. The last sample (C) was drawn within 30 min before the next scheduled dose. Immediately after collection, blood samples were placed on ice and centrifuged within 6 h to separate the plasma, which was frozen at − 80 °C and stored locally until shipment to the bioanalytical laboratory. Total concentration of the study antifungals in plasma samples was determined by chromatographic methods at The University of Queensland Centre for Clinical Research. The assays were conducted in compliance with US Food and Drug Administration (FDA) bioanalytical method validation guidance [11].
Clinical data collection
Admission demographic and clinical characteristics were collected prospectively, including age, sex, height, weight, admission details, and severity of illness (APACHE II and III scores or SAPS II score). Additionally, Sequential Organ Failure Assessment (SOFA) score, albumin concentrations, serum creatinine concentrations, aspartate transaminase (AST) and alanine aminotransferase (ALT) concentrations, AST/ALT ratios, and information on whether patients were undergoing continuous renal replacement therapy (CRRT) and receiving vasopressors/inotropes were collected on each sampling occasion. The site of infection (if applicable) was recorded, along with any identified fungi and their susceptibility from collected microbiology samples. Clinical outcomes and 30-day mortality were also documented. Clinical outcomes were assessed by the treating clinician and categorized as either clinical cure, defined as either improvement (a marked or moderate reduction in severity and/or number of signs and symptoms of infection) or resolution (disappearance of all signs and symptoms related to the infection)–, or clinical failure (insufficient lessening of the signs and symptoms of infection to qualify as improvement, including death or indeterminate). Antifungal data, including the antifungal agent, date the study antifungal commenced, date of occasion, dose number, dose administered, frequency, route of administration, infusion duration (when applicable), dosing time, and sampling time points were documented.
Pharmacokinetic analysis
The pharmacokinetic-pharmacodynamic (PK/PD) targets used in this study are stated in electronic supplementary table 2 [6, 12–16]. Where available, the minimum inhibitory concentration (MIC) of the identified fungus was used; otherwise, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) epidemiologic cut-off values (ECOFFs) were applied. Where no fungi were identified, we conservatively used the highest MIC for a pathogen susceptible to the antifungal. The area under the curve from 0 to 24 h (AUC0-24) was estimated using noncompartmental in Phoenix WinNonlin™ version 8.3.5.340. Concentrations from the first (A) and last (C) samples were considered as Cmax and Cmin, respectively.
Statistical analysis
Descriptive statistics summarizing patients’ demographic and clinical characteristics, and PK/PD-related data were reported as number (%) and median (interquartile range, IQR or min–max) as appropriate. The primary endpoint was PK/PD target attainment. Secondary endpoints included clinical outcome and 30-day mortality. Univariate analyses were conducted to explore statistically significant associations (p < 0.05) between endpoints and patients’ characteristics. Comparisons employed the Mann–Whitney U test, Chi-square, or Fisher’s exact test depending on the assumptions of the data. Analyses were conducted separately for patients prescribed treatment and prophylaxis, and by overall and individual antifungal agents. A multivariate logistic regression model was constructed to identify significant characteristics associated with the endpoints. Characteristics with p < 0.10 on univariate analysis were considered for model building. Goodness of fit was assessed by the Hosmer–Lemeshow statistic. Statistical analyses were performed using IBM SPSS Statistics version 28.0.1.0 and Stata/SE 18.0.
Results
A total of 350 patients were recruited from 30 ICUs across 12 countries. Of these, 11 were excluded due to meeting exclusion criteria, withdrawal of consent, incomplete information, or missing samples. Among the remaining 339 patients, 349 antifungal courses were administered (Supplementary Table 3), with 9 patients receiving a second antifungal and one patient receiving a third. Patient characteristics at admission and on occasion 1 are detailed in Table 1, with electronic supplementary table 4 providing stratification by antifungal. Antifungal therapy was primarily prescribed for treatment (274/339, 80.8%). Fluconazole was the most frequently prescribed antifungal, accounting for 142/349 (40.7%) courses. The most common indication for treatment was intra-abdominal infection (84/274, 30.7%). Fungemia was present in 43/274 (15.7%) patients.
Table 1.
Patient characteristics at admission and on occasion 1
| Median (IQR) | |
|---|---|
| Characteristics at admission (n = 339) | |
| Male, n (%) | 207/339 (61.1%) |
| Age (years) | 62 (51–70) |
| Height (m) | 1.70 (1.62–1.75) |
| Weight (kg) | 77.2 (65.0–88.0) |
| BMI (kg/m2) | 26.6 (23.1–30.9) |
| BSA (m2) | 1.9 (1.7–2.0) |
| APACHE II score | 22 (17–28) |
| APACHE III scorea | 75 (57–94) |
| SAPS IIa | 39 (29–50) |
| Clinical characteristics on occasion 1 (n = 346) | |
| SOFA score | 7 (5–11) |
| Albumin (g/dL) | 2.2 (1.9–2.7) |
| Serum creatinine (µmol/L) | 79.6 (55.4–140.4) |
| CrCL—Cockcroft-Gault (mL/min) | 83.9 (50.7–129.5) |
| eGFR—MDRD-4 (mL/min/1.73 m2) | 75.7 (41.9–115.5) |
| eGFR—CKD-EPI (mL/min/1.73 m2) | 87.1 (45.6–107.6) |
| AST | 40 (24–73) |
| ALT | 32 (18–65) |
| AST/ALT ratio | 1.3 (0.8–2.0) |
| CRRT, n (%) | 58/346 (16.8%) |
| Vasopressors/inotropes, n (%) | 184/346 (53.2%) |
| Admitted to ICU from, n (%) | |
| Ward | 145 (42.8%) |
| Operating theater/radiology suite | 75 (22.1%) |
| Emergency department | 70 (20.6%) |
| Other ICU | 36 (10.6%) |
| Home | 1 (0.3%) |
| APACHE III admission diagnosis, n (%) | |
| Non-operative | 198 (59.8%) |
| 503—sepsis with shock, other than urinary | 40 (20.2%) |
| 211—other respiratory diseases | 22 (11.1%) |
| 212—bacterial pneumonia | 21 (10.6%) |
| 501—sepsis, other than urinary | 14 (7.1%) |
| Post-operative | 133 (40.2%) |
| 1412—peritonitis | 27 (20.3%) |
| 1401—GI perforation/rupture (not peritonitis) | 22 (16.5%) |
| 1405—GI neoplasm | 10 (7.5%) |
| 1407—liver transplant | 7 (5.3%) |
| Site of infection (patients receiving treatment = 274) | |
| Intra-abdominal | 84 (30.7%) |
| Blood | 43 (15.7%) |
| Urinary tract | 26 (9.5%) |
| Other | 108 (39.4) |
| Unknown | 13 (4.7%) |
IQR interquartile range; BMI body mass index; BSA body surface area; APACHE acute physiology and chronic health evaluation; SAPS simplified acute physiology score; SOFA sequential organ failure assessment; CrCL creatinine clearance; eGFR estimated glomerular filtration rate; MDRD-4 modification of diet in renal disease 4-variable equation; CKD-EPI chronic kidney disease epidemiology collaboration creatinine equation; AST aspartate aminotransferase; ALT alanine transaminase; CRRT continuous renal replacement therapy; ICU intensive care unit, GI gastrointestinal; other sites of infection include: lung, vascular access-related, intraoral, upper cervical esophageal fistulas, central nervous system abscesses, maxillary sinus, mediastinum, pleural fluid, skin, submandibular abscesses, thoracic abscess, and tissue from neck, among others
aNot performed by all sites
Fungi were identified in 148/274 (54%) treatment patients and 5/65 (7.7%) prophylaxis patients, with Candida albicans (51.7%), Nakaseomyces glabratus (13.6%), and Candida parapsilosis (8.5%) being the most prevalent (Supplementary Table 5). MIC data were available for only 26% of identified fungi. Clinical cure was achieved in 189/274 (69%) patients treated for infection. By day 30 following study enrollment, 133/339 (39.2%) patients receiving antifungal therapy had died, of which 117/133 (88%) received antifungal treatment and 16/133 (12%) prophylaxis.
Pharmacokinetics
Of the 349 antifungal courses, 346/349 (99.1%) were sampled on occasion 1, with 104 (29.8%) commenced in the 24 h before the first sample collection. On occasion 2, 233 (66.8%) courses were sampled.
Table 2 shows the median daily doses and total weight-based daily doses of the study antifungals. Significant differences were found between the weight-based daily doses for treatment and prophylaxis with fluconazole, voriconazole and micafungin (p < 0.05). PK exposures per occasion for each antifungal prescribed for treatment are presented in Fig. 1, with for prophylaxis data shown in electronic supplementary figure 1.
Table 2.
Antifungal data for PK/PD target attainment in critically ill patients
| Antifungal | PK/PD target | Antifungal courses studied | Daily dose Median (min–max) |
Target attainment | |
|---|---|---|---|---|---|
| Days of antifungal course in the ICU | |||||
| 1–3 days (n = 327) | 4–7 days (n = 206) | ||||
|
Fluconazole 213 courses |
Candidemia treatment [6] AUC0-24/MIC ≥ 100 |
177 |
400 mg (100–1200) 5.0 mg/kg (0.8–17.5) |
80.7% (88/109) | 89.7% (61/68) |
|
Prophylaxis AUC0-24/MIC ≥ 55 |
36 |
400 mg (200–400) 3.8 mg/kg (1.9–7.4) |
91.3% (21/23) | 92.3% (12/13) | |
|
Voriconazole 40 courses |
Invasive aspergillosis treatment [6] Cmin ≥ 2–6 mg/L |
33 |
280 mg (100–410)a 3.9 mg/kg (1.8–6.0)a |
52.9% (9/17) | 37.5% (6/16) |
|
Prophylaxis Cmin ≥ 1–6 mg/L |
7 |
200 mg (150–300)a 2.4 mg/kg (2.4–4.7)a |
75% (3/4) | 100% (3/3) | |
|
Posaconazole 22 courses |
Invasive aspergillosis treatment [6] Cmin > 1 mg/L |
9 |
300 (300–600) 4.7 mg/kg (2.5–9.6) |
37.5% (3/8) | 0% (0/1) |
|
Prophylaxis [6] Cmin > 0.5 mg/L |
13 |
300 (300–600) 4.8 mg/kg (2.6–11.7) |
81.8% (9/11) | 100% (2/2) | |
|
Isavuconazole 10 courses |
Invasive aspergillosis treatment [12, 13] Cmin between 1 and 5.13 mg/L |
8 |
200 mg (200–1116) 2.8 mg/kg (1.8–15) |
60% (3/5) | 100% (3/3) |
|
Prophylaxisb Cmin between 1 and 5.13 mg/L |
2 |
400 mg (200–600) 4.9 mg/kg (2.5–7.4) |
0% (0/1) | 100% (1/1) | |
|
Anidulafungin 76 courses |
Treatment: against Candida albicans [14] fAUC0-24/MIC > 20.6 against Nakaseomyces glabratus [14] fAUC0-24/MIC > 7.0 against Candida parapsilosis [14] fAUC0-24/MIC > 7.6 |
66 |
100 mg (100–200) 1.4 mg/kg (0.6–3.1) |
78.4% (29/37) | 79.3% (23/29) |
|
Prophylaxisb fAUC0-24/MIC > 7.0 |
10 |
100 mg (100–200) 1.5 mg/kg (1.2–3.6) |
83.3% (5/6) | 75% (3/4) | |
|
Caspofungin 65 courses |
Treatment: against Candida albicans [14] AUC0-24/MIC > 865 against Nakaseomyces glabratus [14] AUC0-24/MIC > 450 against Candida parapsilosis [14] AUC0-24/MIC > 1185 |
51 |
50 mg (50–70) 0.7 mg/kg (0.4–1.0) |
90.9% (30/33) | 83.3% (15/18) |
|
Prophylaxisb AUC0-24/MIC > 865 |
14 |
70 mg (35–70) 0.7 mg/kg (0.6–0.9) |
88.9% (8/9) | 80% (4/5) | |
|
Micafungin 64 courses |
Treatment [6]: against Candida sp. [6] AUC0-24/MIC > 3000 against Candida parapsilosis [6] AUC0-24/MIC > 285 |
49 |
100 mg (100–100) 1.4 mg/kg (0.9–2.4) |
67.7% (21/31) | 72.2% (13/18) |
|
Prophylaxisb AUC0-24/MIC > 3000 |
15 |
100 mg (100–100) 1.1 mg/kg (0.8–1.8) |
50% (4/8) | 42.9% (3/7) | |
|
L-Amphotericin B 41 courses |
Treatment Cmax/MIC ≥ 25 [15] |
41 |
250 mg (150–500) 3.3 mg/kg (1.9–6.5) |
41.7% (10/24) | 58.8% (10/17) |
|
D-Amphotericin B 2 courses |
Treatment Cmax/MIC ≥ 4.5 [16] |
2 |
22.5 mg (10–35) 0.5 mg/kg (0.2–0.7) |
0% (0/1) | 0% (0/1) |
AUC0-24 area under the plasma concentration–time curve from zero to 24 h; fAUC0-24 free AUC0-24; MIC minimum inhibitory concentration; Cmin minimum observed plasma concentration; Cmax maximum observed plasma concentration
aTwice-daily dosing
bNot defined, treatment target was used
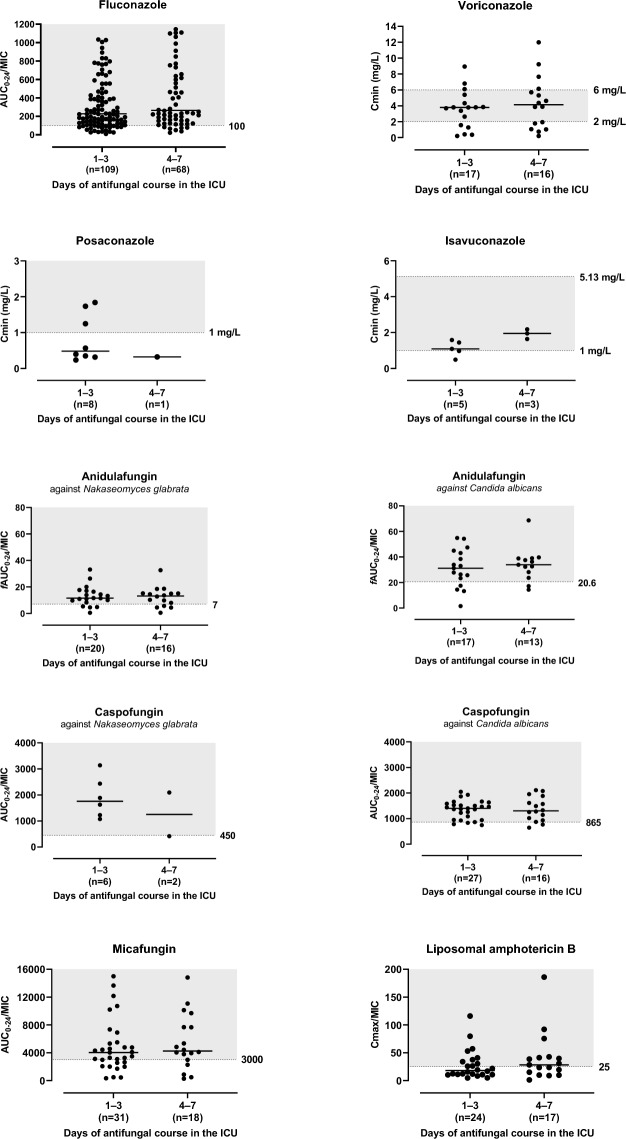
Fig. 1.
PK/PD target-related exposures per occasion of the study antifungals prescribed for treatment. The shaded area represents the PK/PD target used in this study. For fluconazole, 15 data points are outside the y-axis limits (max AUC0-24/MIC value was 3250.3); ICU intensive care unit; AUC0-24 area under the plasma concentration-time curve from zero to 24 h; fAUC0-24 free AUC0-24; MIC minimum inhibitory concentration; Cmin minimum observed plasma concentration; Cmax maximum observed plasma concentration
Attainment of target concentrations and exposures
Target attainment could not be determined for 46 (7.9%) courses (19 on occasion 1/27 on occasion 2) due to insufficient sampling (less than 3 samples) to estimate area under the curve (AUC)0-24. The data describing PK/PD target attainment are described in Table 2. Generally, target attainment was higher for patients receiving prophylaxis with most drugs having > 80% of those patients attaining target drug exposures. For patients receiving antifungal treatment, low target attainment was noted for voriconazole (57.1%), posaconazole (63.2%), micafungin (64.1%), and amphotericin B (41.7%).
Associations between endpoints and patient characteristics
Univariate associations between endpoints and patient characteristics for those prescribed treatment on occasion 1 are presented in Table 3, including overall and individual antifungal analyses. Consistent associations were observed between both clinical failure and 30-day mortality with higher sequential organ failure assessment (SOFA) scores, as well as between 30-day mortality and clinical failure, across both overall and individual antifungal analyses. Univariate associations for patients prescribed treatment on occasion 2 are presented in electronic supplementary table 6.
Table 3.
Univariate analysis results for associations between target attainment, clinical outcome, and 30-day mortality with characteristics in patients prescribed treatment on occasion 1, both overall and by antifungal agent
| Characteristic | Overall n = 281 |
Fluconazole n = 117 |
Anidulafungin n = 39 |
Caspofungin n = 36 |
Micafungin n = 32 |
Amphotericin B* n = 24 |
Voriconazole n = 19 |
Posaconazole n = 8 |
Isavuconazole n = 5 |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Target achievers were older [63 (53–71) vs 58 (41–70), p = 0.019] | – | Target achievers were older [66 (59–73) vs 56 (42–58), p = 0.004] | – | – | – | – | – | – |
| Sex | – | – | – | – | – | Clinical cure rates were higher in females [77.8% vs 33.3%, p = 0.035] | – | – | – |
| Height (cm) | – | – | – | – | Clinical failure rates were higher in those with higher height [170 (165–179.4) vs 165 (155–168), p = 0.040] | – | Target achievers had higher height [170 (168–176) vs 160 (159.5–165.2), p = 0.026] | – | – |
| Weight (kg) | – | Weight was lower in those who died [70 (61–85) vs 80 (70–90), p = 0.044] | – | Target achievers had lower weight [80 (70–81) vs 95 (88–147), p = 0.020] | Clinical failure rates were higher in those with higher weight [85.5 (73.8–88.7) vs 70 (60–76), p = 0.025] | – | – | – | – |
| Admission diagnosis | Clinical cure rates were higher in post-operative patients [77% vs 65.6, p = 0.043], and mortality rates were lower [31.9% vs 49.4%, p = 0.004] | – | – | – | – | Clinical cure rates were higher in post-operative patients [77.8% vs 33.3%, p = 0.035] | Mortality was higher in non-operative patients [81.2% vs 0%, p = 0.005] | – | – |
| SOFA | SOFA scores were higher in those with clinical failure [11 (7–13) vs 6 (4–9), p < 0.001] and in those who died [10 (6–13) vs 6 (4–8), p < 0.001] | SOFA scores were higher in those with clinical failure [10 (6–12) vs 6 (4–9), p = 0.001] and in those who died [9 (5–12) vs 6 (4–8), p = 0.001] | – | SOFA scores were higher in those with clinical failure [15 (9–16) vs 6 (4–10), p = 0.001] and in those who died [14 (9–16) vs 6 (4–8), p = 0.001] | SOFA scores were higher in those with clinical failure [12 (10–12) vs 8 (3–11), p = 0.022] | SOFA scores were higher in those with clinical failure [10 (8–14) vs 7 (6–8), p = 0.008] | SOFA scores were higher in those with clinical failure [12 (10–14) vs 4 (3–5), p = 0.005] and in those who died [10 (4–14) vs 4 (2–5), p = 0.048] | Target achievers had lower SOFA scores [1 (1–4) vs 6 (5–13), p = 0.034] | – |
| Albumin (g/dL) | – | Target achievers had lower albumin levels [2 (1.6–2.5) vs 2.3 (2.2–2.8), p = 0.006] | – | – | – | – | – | – | – |
| Renal function | Mortality was higher in those receiving CRRT [58% vs 39.8%, p = 0.019] | Target achievers had lower SCr levels [74 (53–126.4) vs 111.4 (63.9–322.6), p = 0.036] | Mortality was higher in those receiving CRRT [87.5% vs 32.3%, p = 0.005] | Target achievers had lower CrCL [72.6 (40.3–114.4) vs 131.2 (127–267.8), p = 0.045] | – | SCr levels were lower in survivors [53.5 (48.6–75.8] vs 99.9 (64.5–185.6), p = 0.017] | – | Clinical failure and mortality were higher in those receiving CRRT [100% vs 16.7%, p = 0.035, for both outcomes] | – |
| Liver enzymes | – | – | – | – | – | AST/ALT rations were lower in survivors [0.8 (0.8–0.9) vs 1.3 (0.9–1.8), p = 0.020] | AST/ALT rations were lower in survivors [0.6 (0.4–0.6) vs 1.3 (0.8–1.5), p = 0.016] | – | – |
| Vasopressors/inotropes | Target attainment was higher in those receiving vasopressors/inotropes [80.4% vs 63.8%, p = 0.002], but clinical failure [37.8% vs 21.8%, p = 0.003] and mortality [50% vs 35.3%, p = 0.013] were more common | Target attainment was higher in those receiving vasopressors/inotropes [89.3% vs 69.8%, p = 0.011] | – | – | – | – | Clinical failure rates were higher in those receiving vasopressors/inotropes [85.7% vs 25%, p = 0.011] | Clinical failure rates and mortality were higher in those receiving vasopressors/inotropes [100% vs 16.7%, p = 0.0035, for both outcomes] | – |
| Weight-based daily dose (mg/kg) | – | Target achievers received a higher daily dose [5.2 (4.2–7.3) vs 4.3 (2.7–5.3), p = 0.009] | – | – | Clinical cure rates were higher in those with a higher daily dose [1.4 (1.3–1.7) vs 1.2 (1.1–1.4), p = 0.025] | – | – | – | – |
| Initiation of antifungal therapy within 24 h of the first sample collection | Mortality was higher when antifungal therapy was started within 24 h of the first sample collection [52.3% vs 39%, p = 0.037] | – | – | Clinical cure rates were higher when antifungal therapy was not commenced within 24 h of the first sample collection [84.2% vs 52.9%, p = 0.042] | – | – | – | – | – |
| Fungus identification | – | Target attainment was higher when a fungus was identified [88.1% vs 70%, p = 0.019] | – | – | – | – | – | – | Mortality rates were lower when a fungus was identified [0% vs 100%, p = 0.025] |
| Directed prescription | – | Target attainment was higher with directed antifungal therapy prescribed [90.6% vs 69.6%, p = 0.007] | – | – | – | Clinical cure rates were higher in those prescribed directed antifungal therapy [77.8% vs 33.3%, p = 0.035] | – | – | Mortality rates were lower when directed antifungal therapy prescribed [0% vs 100%, p = 0.025] |
| Site of infection | Target attainment was higher in those with intra-abdominal infections (81.7% vs 68.3%, p = 0.024]. Clinical failure was more common in patients with infections outside than blood, intra-abdominal and urinary tract sites [37.6% vs 25.7%, p = 0.040] | – | – | – | – | – | – | – | – |
| Clinical outcome | Mortality was higher in those with clinical failure [91.8% vs 21.9%, p < 0.001] | Mortality was higher in those with clinical failure [82.8% vs 19.3%, p < 0.001] | Mortality was higher in those with clinical failure [100% vs 18.5%, p < 0.001] | Mortality was higher in those with clinical failure [90.9% vs 16%, p < 0.001] | Mortality was higher in those with clinical failure [100% vs 29.2%, p < 0.001] | Mortality was higher in those with clinical failure [91.7% vs 25%, p < 0.001] | Mortality was higher in those with clinical failure [100% vs 40%, p = 0.005] | Mortality was higher in those with clinical failure [100% vs 0%, p = 0.005] | – |
SOFA sequential organ failure assessment; SCr serum creatinine; CRRT continuous renal replacement therapy; CrCL Cockcroft-Gault creatinine clearance; mortality refers to 30-day mortality
*Liposomal amphotericin B (only one patient received deoxycholate amphotericin B)
In patients prescribed prophylaxis on occasion 1, for the overall cohort, associations were found between PK/PD target non-attainment with CRRT (p = 0.019), and between 30-day mortality and clinical failure (p = 0.027). For individual drugs, associations were observed for fluconazole PK/PD target non-attainment with higher weight (p = 0.038) and CRRT (p = 0.030); for caspofungin 30-day mortality with lower weight (p = 0.020) and higher SOFA scores (p = 0.024); for micafungin 30-day mortality with administration of vasopressors/inotropes (p = 0.005); and for posaconazole PK/PD target attainment with non-operative admission diagnosis (p = 0.039).
Table 4 highlights the characteristics significantly associated with the endpoints from the multivariate regression analysis for the overall antifungal analyses and for each antifungal individually on occasion 1. Additional details of these analyses are provided in electronic supplementary tables 7-10. The multivariate regression analysis for patients prescribed treatment on occasion 2 can be found in electronic supplementary table 11. No significant factors associated with any of the endpoints were identified in patients prescribed prophylaxis on occasion 1.
Table 4.
Multivariate regression analysis results in patients prescribed treatment on occasion 1, overall and by antifungal agent, focusing on the outcomes of target attainment, clinical failure and 30-day mortality
| Characteristic | Overall n = 281 |
Fluconazole n = 117 |
Anidulafungin n = 39 |
Caspofungin n = 36 |
|---|---|---|---|---|
| Age (years) | – | – | Increasing age ↑ likelihood of target attainment (OR 1.096, 95% CI 1.018–1.181; p = 0.015) | – |
| SOFA | Higher SOFA scores ↑ likelihood of clinical failure (OR 1.434, 95% CI 1.240–1.659; p < 0.001) and mortality (OR 1.136, 95% CI 1.021–1.263; p = 0.019) | Higher SOFA scores ↑ likelihood of clinical failure (OR 1.220, 95% CI 1.067–1.395; p = 0.004) | – | Higher SOFA scores ↑ likelihood of clinical failure (OR 1.355, 95% CI 1.073–1.713; p = 0.011) |
| Renal function | – | – | CRRT ↑ likelihood of mortality (OR 15.858, 95% CI 1.518–165.702; p = 0.021) | – |
| Vasopressors/inotropes | Vasopressors/inotropes ↑ likelihood of target attainment (OR 1.967, 95% CI 1.075–3.600; p = 0.028) | Vasopressors/inotropes ↑ likelihood of target attainment (OR 5.973, 95% CI 1.095–32.571; p = 0.039) | – | – |
| Site of infection | Sites of infection other than blood, intra-abdominal and urinary tract ↓ likelihood target attainment (OR 0.422, 95% CI, 0.231–0.771; p = 0.005) and ↑ likelihood of clinical failure (OR 4.381, 95% CI 1.860–10.318; p < 0.001) | – | – | – |
| Clinical outcome | Clinical failure ↑ likelihood of mortality (OR 34.915, 95% CI 14.331–85.065; p < 0.001) | Clinical failure ↑ likelihood of mortality (OR 17.625, 95% CI 5.402–57.503; p < 0.001) | – | Clinical failure ↑ likelihood of mortality (OR 25.107, 95% CI 2.194–287.340; p = 0.010) |
↑ increased; ↓ decreased; OR odds ratio; CI confidence interval; SOFA sequential organ failure assessment; CRRT continuous renal replacement therapy; mortality refers to 30-day mortality
Discussion
Key findings
This study represents the first large-scale, international prospective investigation into PK/PD target attainment and dosing adequacy of antifungal agents across a large number of ICUs. The findings reveal that early in the treatment course, more than 25% of patients did not meet the predefined PK/PD target antifungal exposures. Voriconazole, posaconazole, micafungin, and amphotericin B were among the antifungal agents with the lowest target attainment (≥ 35%). This suggests that contemporary dosing regimens of these antifungal agents do not effectively achieve optimal therapeutic exposure necessary for treating or preventing fungal infections in adult critically ill patients. These observations underscore the need for better-dosing regimens to achieve optimal exposures in the ICU. It is likely that the current “standard” doses prescribed for treatment do not consider important PK variations in critically ill patients [5, 6], potentially leading to suboptimal outcomes.
Furthermore, sites of infection other than the blood, intra-abdominal, and urinary tract (i.e., vascular access, lung, skin, and central nervous system abscess) were linked to lower target attainment and increased clinical failure across the overall antifungal cohort (Table 4). This may reflect differences in severity, with less aggressive dosing for less critical infections (e.g., skin). The use of vasopressors/inotropes was associated with increased target attainment (Table 4), but further research is needed to understand the nature and implications of this association. Consistent associations were found between both clinical failure and 30-day mortality with higher SOFA scores, as well as between 30-day mortality and clinical failure.
Relationship to previous studies
Lower antifungal exposures in critically ill patients compared to the general patient population or healthy adults have been documented for fluconazole [9], anidulafungin [9, 17, 18], caspofungin [19, 20], micafungin [21–26], posaconazole [27, 28] and isavuconazole [29, 30], indicating a need for specific dosing regimens for ICU patients. The significant variability (> 30%) in antifungal exposure observed in this study (Supplementary Table 12) aligns with previous reports for fluconazole [9, 31–34], anidulafungin [9, 35, 36], caspofungin [9, 20, 34, 37–41], micafungin [22, 25, 26], liposomal amphotericin B [42–44], voriconazole [45–50], and posaconazole [28, 51, 52]. This variability can impact dosing regimen effectiveness and safety and support the need for antifungal therapeutic drug monitoring (TDM).
Azoles—The DALI study found that 33% of patients receiving fluconazole did not achieve the PK/PD target [9], consistent with other studies showing subtherapeutic exposures [31–33, 53]. While there is room to improve fluconazole dosing by adjusting for weight and renal function [31, 53–56], caution is needed as the absence of a defined toxicity threshold does not eliminate the risk of adverse events at higher doses. Patients on voriconazole for prophylaxis achieved higher target attainment (≥ 75%) compared to those receiving treatment (< 53%) (Table 2) despite the treatment group receiving significantly higher doses reflecting the higher trough concentration target for treatment. This pattern of low voriconazole target attainment has been observed in previous studies [34, 46, 47, 49, 50, 57–60]. Dosing adjustments should be approached with caution as the PK/PD target range accommodates both over- and underexposure. Posaconazole prophylaxis led to > 80% of patients reaching the PK/PD target, compared to 37.5% of those receiving treatment despite similar dosing. Previous research has shown insufficient target attainment in critically ill patients prescribed posaconazole for treatment [51, 52]. Additionally, understanding the PK of available posaconazole formulations is crucial to optimizing dosing regimens [61, 62]. Previous studies on isavuconazole have reported ⁓70% of trough concentrations within 1–5.13 mg/L [63], while others found ⁓20–32% of troughs < 1 mg/L [29, 64]. There are a limited number of studies on isavuconazole in critically ill patients, but dosing adjustments [65, 66] and higher loading doses have been recommended. No associations between patient characteristics and endpoints were found in this study, likely due to the small number of patients administered isavuconazole.
Echinocandins—In this study, CRRT was associated with an increased risk of 30-day mortality. However, this finding likely reflects the well-established association between CRRT and higher mortality in critically ill patients, rather than an effect of antifungal drug concentrations. Previous research has reported minimal impact of CRRT on anidulafungin elimination [67]. Contemporary anidulafungin dosing has been linked with low target attainment [36], leading to recommendations for dose escalations in heavier patients [68]. Simulations of caspofungin contemporary dosing have shown inadequate target attainment in critically ill patients [20, 39, 69, 70]. Despite a fixed 100 mg daily dose of micafungin, target attainment was higher in treatment patients (> 67%) than in those on prophylaxis (> 42%), likely due to significantly higher weight-based doses (p < 0.05). Previous studies have also associated standard micafungin dosing with suboptimal plasma exposure [22, 23, 71].
Lastly, liposomal amphotericin B was exclusively prescribed for treatment and exhibited the largest variability in exposure among the study antifungals. Factors contributing to this variability are not well-defined [42–44, 72, 73], and in the absence of a clear PK/PD target, more data are needed before drawing definitive conclusions about target attainment.
Variations between the findings of this study and previous research may stem from differences in analysis methods, case mix of patients, and sample sizes.
Implications
This study has shown that contemporary antifungal doses are likely to be sufficient for susceptible species with lower MICs. Standard dosing is commonly insufficient for those pathogens with higher MICs. In light of these findings, clinicians are advised to identify the causative pathogen and determine individual MICs in order to inform the magnitude of dosing. However, the availability of MIC data in this study was limited, with only 26% of cases having MICs available. This underscores the need for future studies to prioritize broader MIC data collection in order to better evaluate the relationship between MICs and antifungal target attainment as well as patient outcome. Such data would also strengthen the generalizability of findings in critically ill patients. Furthermore, while most studies indicate the need for higher-than-standard doses in ICU patients, it is uncertain whether this recommendation can be generalized. Higher doses may benefit patients at risk of underexposure but could also lead to overexposure, increasing the risk of toxicity. This is salient for antifungals like voriconazole, which have a defined toxicity threshold. Overexposure can lead to adverse events and further complications. Hence, careful dosage management in clinical practice is essential.
Sufficient loading doses are also important for achieving early adequate exposure. Higher target attainment was often observed during occasion 2. Differences between exposures within occasions could be attributed to concentrations reaching steady state, especially since nearly 30% of the courses sampled on occasion 1 were commenced in the 24 h before the first sample collection. This highlights the need for increased attention to loading doses, which are not widely applied or sufficiently large, particularly for echinocandins [40]. The significant PK variability observed underscores the importance of TDM for antifungal therapy in critically ill patients.
Strengths and limitations
This was a large international study with strong compliance of a robust protocol. However, this study has limitations. First, selecting PK/PD targets for either treatment or prophylaxis can be controversial as some of these targets, such as for amphotericin B and isavuconazole, have not been robustly defined or clinically validated, relying solely on pre-clinical studies. Consequently, results might be biased depending on the chosen PK/PD target. Furthermore, assumptions were necessary in cases where MIC data were unavailable, and the pathogen was unidentified. Additionally, some ECOFFs are not well established as seen with caspofungin. The estimation of the PK parameters was based on limited samples which might affect its accuracy. However, this approach has been implemented in comparable studies [8]. Additionally, free (unbound) plasma drug concentrations were not measured, and the protein binding rate reported for anidulafungin was used. Only calculated eGFRs were available, which may fail to detect augmented renal clearance. The correlation with clinical data should be considered exploratory as in most cases, there was no correlation between target attainment and clinical outcomes. The choice of antifungal agent and dosing regimens was at the discretion of the treating clinicians. While this reflects real-world practice, it introduces variability in local dosing strategies that may have contributed to differences in target attainment. Further studies should focus on collecting data on local dosing practices to better understand their role in target attainment. It is important to note that fungal infections in ICU patients rarely occur in isolation. They may occur with bacterial infections or arise following preceding infections during critical illness. While this study specifically evaluates antifungal dosing, the broader clinical context, including the management of concurrent bacterial infections, is crucial for interpreting overall patient outcomes. Data on actual drug toxicity were not available. Future studies should incorporate toxicity monitoring to comprehensively evaluate antifungal therapy outcomes. Given the study’s international multicenter design, it’s important to note that the analyses did not consider factors, such as ethnicity, patient’s inflammatory status, or burn injuries, all of which could alter PK. Lastly, the number of patients receiving certain drugs was small after stratification based on prescription, leading to a potential for type II error when exploring associations between patient characteristics and endpoints.
Conclusion
This international multicenter PK study highlights the varying degrees of PK/PD target attainment observed across different antifungal agents in critically ill patients. Although a significant proportion of patients achieved the predefined PK/PD targets, considerable variability and subtherapeutic exposures was present. Specifically, agents, such as voriconazole, posaconazole, micafungin, and amphotericin B, showed lower rates of target attainment, supporting the need for optimized dosing regimens.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
JA Roberts would like to acknowledge funding from the Australian National Health and Medical Research Council for a Centre of Research Excellence (APP2007007) and an Investigator Grant (APP2009736) as well as an Advancing Queensland Clinical Fellowship. JJ De Waele is supported by a Sr Clinical Research Grant from the Research Foundation Flanders (FWO, Ref. 1881020N)
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funding for this study was provided by the Royal Brisbane and Women’s Hospital (RBWH) Foundation, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), the ESCMID PK/PD of Anti-Infectives Study Group (EPASG), and the International Society of Antimicrobial Chemotherapy (ISAC) Infections in the ICU and Sepsis Working Group. This project was supported in part by Gilead Australia Fellowship to Fekade Sime (14500). The University of Queensland funded sample transport from the participating sites and assaying of the samples.
Data availability
Requests for data should be made to the corresponding author. Each request requires a research proposal with a clear research question and proposed analysis plan. Requests will be considered on an individual basis and will be reviewed by the SAFE-ICU steering committee, as well as relevant human research ethics committees.
Declarations
Conflicts of interest
JA Roberts has consulted or provided lectures for Qpex, Gilead, Advanz Pharma, Sandoz, Pfizer, MSD, Gilead and Cipla. JJ De Waele has consulted for Biomerieux, Menarini, Monlycke, MSD, Pfizer, Roche Diagnostics, ThermoFisher and Viatris (fees and honoraria paid to institution).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kullberg BJ, Arendrup MC (2015) Invasive candidiasis. Nat Rev Dis Prim 373:1445–1456. 10.1038/nrdp.2018.27 [DOI] [PubMed] [Google Scholar]
- 2.Thompson GR, Young J-AH (2021) Aspergillus infections. N Engl J Med 385:1496–1509. 10.1056/nejmra2027424 [DOI] [PubMed] [Google Scholar]
- 3.Roberts JA, Kumar A, Lipman J (2017) Right dose, right now: customized drug dosing in the critically ill. Crit Care Med 45:331–336. 10.1097/CCM.0000000000002210 [DOI] [PubMed] [Google Scholar]
- 4.Blot SI, Pea F, Lipman J (2014) The effect of pathophysiology on pharmacokinetics in the critically ill patient - concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 77:3–11. 10.1016/j.addr.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 5.Morales Castro D, Dresser L, Granton J, Fan E (2023) Pharmacokinetic alterations associated with critical illness. Clin Pharmacokinet 62:209–220. 10.1007/s40262-023-01213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdul-Aziz MH, Alffenaar JWC, Bassetti M et al (2020) Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med 46:1127–1153. 10.1007/s00134-020-06050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappas PG, Kauffman CA, Andes DR et al (2015) Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis 62:e1–e50. 10.1093/cid/civ933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts JA, Paul SK, Akova M et al (2014) DALI: Defining antibiotic levels in intensive care unit patients: are current ß-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. 10.1093/cid/ciu027 [DOI] [PubMed] [Google Scholar]
- 9.Sinnollareddy MG, Roberts JA, Lipman J et al (2015) Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: data from multinational defining antibiotic levels in intensive care unit (DALI) patients study. Crit Care 19:1–7. 10.1186/s13054-015-0758-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts JA, Sime F, Lipman J et al (2023) A protocol for an international, multicentre pharmacokinetic study for Screening Antifungal Exposure in Intensive Care Units: the SAFE-ICU study. Crit Care Resusc 25:1–5. 10.1016/j.ccrj.2023.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) (2022) M10 Bioanalytical Method Validation and Study Sample Analysis. Guidance for Industry
- 12.Martín-Cerezuela M, Maya-Gallegos C, Marqués Miñana M et al (2024) Isavuconazole pharmacokinetics in critically ill patients: relationship with clinical effectiveness and patient safety. Res Sq. 10.21203/rs.3.rs-4027011/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furfaro E, Signori A, Di Grazia C et al (2019) Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J Antimicrob Chemother 74:2341–2346. 10.1093/jac/dkz188 [DOI] [PubMed] [Google Scholar]
- 14.Andes D, Diekema DJ, Pfaller MA et al (2010) In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob Agents Chemother 54:2497–2506. 10.1128/AAC.01584-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seibel NL, Shad AT, Bekersky I et al (2017) Safety, tolerability, and pharmacokinetics of liposomal amphotericin B in immunocompromised pediatric patients. Antimicrob Agents Chemother 61:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez-López A (2020) Antifungal therapeutic drug monitoring: focus on drugs without a clear recommendation. Clin Microbiol Infect 26:1481–1487. 10.1016/j.cmi.2020.05.037 [DOI] [PubMed] [Google Scholar]
- 17.van Wanrooy MJP, Rodgers MGG, Uges DRA et al (2014) Low but sufficient anidulafungin exposure in critically ill patients. Antimicrob Agents Chemother 58:304–308. 10.1128/AAC.01607-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brüggemann RJM, Middel-Baars V, De Lange DW et al (2017) Pharmacokinetics of anidulafungin in critically ill intensive care unit patients with suspected or proven invasive fungal infections. Antimicrob Agents Chemother 61:1–8. 10.1128/AAC.01894-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Elst KCM, Veringa A, Zijlstra JG et al (2017) Low caspofungin exposure in patients in intensive care units. Antimicrob Agents Chemother 61:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurland S, Furebring M, Löwdin E et al (2019) Pharmacokinetics of caspofungin in critically ill patients in relation to liver dysfunction: differential impact of plasma albumin and bilirubin levels. Antimicrob Agents Chemother 63:1–11. 10.1128/AAC.02466-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boonstra JM, Van Der Elst KC, Veringa A et al (2017) Pharmacokinetic properties of micafungin in critically ill patients diagnosed with invasive candidiasis. Antimicrob Agents Chemother 61:1–11. 10.1128/AAC.01398-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jullien V, Azoulay E, Schwebel C et al (2017) Population pharmacokinetics of micafungin in ICU patients with sepsis and mechanical ventilation. J Antimicrob Chemother 72:181–189. 10.1093/jac/dkw352 [DOI] [PubMed] [Google Scholar]
- 23.Grau S, Luque S, Campillo N et al (2015) Plasma and peritoneal fluid population pharmacokinetics of micafungin in post-surgical patients with severe peritonitis. J Antimicrob Chemother 70:2854–2861. 10.1093/jac/dkv173 [DOI] [PubMed] [Google Scholar]
- 24.Kapralos I, Mainas E, Neroutsos E et al (2020) Population pharmacokinetics of micafungin over repeated doses in critically ill patients: a need for a loading dose? J Pharm Pharmacol 72:1750–1760. 10.1111/jphp.13353 [DOI] [PubMed] [Google Scholar]
- 25.Garbez N, Mbatchi LC, Wallis SC et al (2021) Prospective cohort study of micafungin population pharmacokinetic analysis in plasma and peritoneal fluid in septic patients with intra-abdominal infections. Clin Pharmacokinet 65:1–13. 10.1007/s40262-021-01062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lempers VJ, Schouten JA, Hunfeld NG et al (2015) Altered micafungin pharmacokinetics in intensive care unit patients. Antimicrob Agents Chemother 59:4403–4409. 10.1128/AAC.00623-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray J, Campbell L, Rudham S et al (2011) Posaconazole plasma concentrations in critically ill patients. Ther Drug Monit 33:387–392. 10.1097/FTD.0b013e31821fb197 [DOI] [PubMed] [Google Scholar]
- 28.Sime FB, Stuart J, Butler J et al (2018) Pharmacokinetics of intravenous posaconazole in critically Ill patients. Antimicrob Agents Chemother 62:1–7. 10.1128/AAC.00242-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikulska M, Melchio M, Signori A et al (2024) Lower blood levels of isavuconazole in critically ill patients compared with other populations: possible need for therapeutic drug monitoring. J Antimicrob Chemother 79:835–845. 10.1093/jac/dkae037 [DOI] [PubMed] [Google Scholar]
- 30.Mertens B, Elkayal O, Dreesen E et al (2023) Isavuconazole exposure in critically ill patients treated with extracorporeal membrane oxygenation: two case reports and a narrative literature review. Antibiotics 12:1–19. 10.3390/antibiotics12071085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boonstra JM, Märtson AG, Sandaradura I et al (2021) Optimization of fluconazole dosing for the prevention and treatment of invasive candidiasis based on the pharmacokinetics of fluconazole in critically ill patients. Antimicrob Agents Chemother 65:1–11. 10.1128/AAC.01554-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Daele R, Wauters J, Lagrou K et al (2021) Pharmacokinetic variability and target attainment of fluconazole in critically ill patients. Microorganisms 9:1–12. 10.3390/microorganisms9102068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bienvenu AL, Pradat P, Matusik E et al (2023) Suboptimal exposure to fluconazole in critically ill patients: pharmacokinetic analysis and determinants. Infect Dis Now 53:2–4. 10.1016/j.idnow.2022.10.002 [DOI] [PubMed] [Google Scholar]
- 34.Shekar K, Abdul-Aziz MH, Cheng V et al (2023) Antimicrobial exposures in critically ill patients receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med 207:704–720. 10.1164/rccm.202207-1393OC [DOI] [PubMed] [Google Scholar]
- 35.Liu P, Ruhnke M, Meersseman W et al (2013) Pharmacokinetics of anidulafungin in critically ill patients with candidemia/invasive candidiasis. Antimicrob Agents Chemother 57:1672–1676. 10.1128/AAC.02139-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapralos I, Mainas E, Apostolopoulou O et al (2021) Population pharmacokinetics of anidulafungin in ICU patients assessing inter- and intrasubject variability. Br J Clin Pharmacol 87:1024–1032. 10.1111/bcp.14457 [DOI] [PubMed] [Google Scholar]
- 37.Nguyen TH, Hoppe-Tichy T, Geiss HK et al (2007) Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J Antimicrob Chemother 60:100–106. 10.1093/jac/dkm125 [DOI] [PubMed] [Google Scholar]
- 38.Muilwijk EW, Schouten JA, van Leeuwen HJ et al (2014) Pharmacokinetics of caspofungin in ICU patients. J Antimicrob Chemother 69:3294–3299. 10.1093/jac/dku313 [DOI] [PubMed] [Google Scholar]
- 39.Martial LC, Brüggemann RJM, Schouten JA et al (2016) Dose reduction of caspofungin in intensive care unit patients with Child Pugh B will result in suboptimal exposure. Clin Pharmacokinet 55:723–733. 10.1007/s40262-015-0347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailly S, Gautier-Veyret E, Lê MP et al (2020) Impact of loading dose of caspofungin in pharmacokinetic-pharmacodynamic target attainment for severe candidiasis infections in patients in intensive care units: the CASPOLOAD study. Antimicrob Agents Chemother. 10.1128/AAC.01545-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adembri C, Villa G, Rosi E et al (2020) Caspofungin PK in critically ill patients after the first and fourth doses: suggestions for therapeutic drug monitoring? J Chemother 32:124–131. 10.1080/1120009X.2020.1737783 [DOI] [PubMed] [Google Scholar]
- 42.Heinemann V, Bosse D, Jehn U et al (1997) Pharmacokinetics of liposomal amphotericin B (AmBisome) in critically ill patients. Antimicrob Agents Chemother 41:1275–1280. 10.1128/aac.41.6.1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Daele R, Wauters J, Elkayal O et al (2022) Liposomal amphotericin B exposure in critically ill patients: a prospective pharmacokinetic study. Med Mycol 60:1–8. 10.1093/mmy/myac074 [DOI] [PubMed] [Google Scholar]
- 44.Stott KE, Moyo M, Ahmadu A et al (2023) Population pharmacokinetics of liposomal amphotericin B in adults with HIV-associated cryptococcal meningoencephalitis. J Antimicrob Chemother 78:276–283. 10.1093/jac/dkac389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grensemann J, Pfaffendorf C, Wicha SG et al (2021) Voriconazole pharmacokinetics are not altered in critically ill patients with acute-on-chronic liver failure and continuous renal replacement therapy: an observational study. Microorganisms 9:1–13. 10.3390/microorganisms9102087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Wanrooy MJP, Rodgers MGG, Span LFR et al (2016) Voriconazole therapeutic drug monitoring practices in intensive care. Ther Drug Monit 38:313–318. 10.1097/FTD.0000000000000284 [DOI] [PubMed] [Google Scholar]
- 47.Ruiz J, Gordon M, Villarreal E et al (2019) Impact of voriconazole plasma concentrations on treatment response in critically ill patients. J Clin Pharm Ther 44:572–578. 10.1111/jcpt.12817 [DOI] [PubMed] [Google Scholar]
- 48.Khan-Asa B, Punyawudho B, Singkham N et al (2020) Impact of albumin and omeprazole on steady-state population pharmacokinetics of voriconazole and development of a voriconazole dosing optimization model in thai patients with hematologic diseases. Antibiotics 9:1–14. 10.3390/antibiotics9090574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myrianthefs P, Markantonis SL, Evaggelopoulou P et al (2010) Monitoring plasma voriconazole levels following intravenous administration in critically ill patients: an observational study. Int J Antimicrob Agents 35:468–472. 10.1016/j.ijantimicag.2009.12.021 [DOI] [PubMed] [Google Scholar]
- 50.Wang T, Miao L, Shao H et al (2022) Voriconazole therapeutic drug monitoring and hepatotoxicity in critically ill patients: a nationwide multi-centre retrospective study. Int J Antimicrob Agents 60:1–9. 10.1016/j.ijantimicag.2022.106692 [DOI] [PubMed] [Google Scholar]
- 51.König C, Göpfert M, Kluge S, Wichmann D (2023) Posaconazole exposure in critically ill ICU patients: a need for action. Infection 51:1767–1772. 10.1007/s15010-023-02078-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Daele R, Wauters J, Dreesen E et al (2022) Exposure to intravenous posaconazole in critically ill patients with influenza: a pharmacokinetic analysis of the POSA-FLU study. Mycoses 65:656–660. 10.1111/myc.13446 [DOI] [PubMed] [Google Scholar]
- 53.Sandaradura I, Marriott DJE, Day RO et al (2021) Current fluconazole treatment regimens result in under-dosing of critically ill adults during early therapy. Eur J Clin Microbiol Infect Dis 40:1521–1528. 10.1007/s10096-021-04201-w [DOI] [PubMed] [Google Scholar]
- 54.Muilwijk EW, de Lange DW, Schouten JA et al (2020) Suboptimal dosing of fluconazole in critically ill patients: time to rethink dosing. Antimicrob Agents Chemother 64:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel K, Roberts JA, Lipman J et al (2011) Population pharmacokinetics of fluconazole in critically ill patients receiving continuous venovenous hemodiafiltration: using Monte Carlo simulations to predict doses for specified pharmacodynamic targets. Antimicrob Agents Chemother 55:5868–5873. 10.1128/AAC.00424-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novy E, Abdul-Aziz MH, Cheng V et al (2024) Population pharmacokinetics of fluconazolein critically ill patients receiving extracorporeal membrane oxygenation and continuous renal replacement therapy: an ASAP ECMO study. Antimicrob Agents Chemother 68:1–13. 10.1128/aac.01201-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi WM, Schoeppler KE, Jaeger J et al (2017) Voriconazole and posaconazole therapeutic drug monitoring: a retrospective study. Ann Clin Microbiol Antimicrob 16:1–14. 10.1186/s12941-017-0235-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H, Li M, Yan J et al (2020) Voriconazole therapeutic drug monitoring in critically ill patients improves efficacy and safety of antifungal therapy. Basic Clin Pharmacol Toxicol 127:495–504. 10.1111/bcpt.13465 [DOI] [PubMed] [Google Scholar]
- 59.Bienvenu AL, Pradat P, Plesa A et al (2021) Association between voriconazole exposure and Sequential Organ Failure Assessment (SOFA) score in critically ill patients. PLoS ONE 16:1–11. 10.1371/journal.pone.0260656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bakir Ekinci P, Kara E, Er AG et al (2022) Challenge in treating COVID-19 associate pulmonary aspergillosis: supratherapeutic voriconazole levels. Br J Clin Pharmacol 88:1387–1391. 10.1111/bcp.14953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mian P, Trof RJ, Beishuizen A et al (2022) Suboptimal plasma concentrations with posaconazole suspension as prophylaxis in critically ill COVID-19 patients at risk of Covid-associated pulmonary aspergillosis. J Clin Pharm Ther 47:383–385. 10.1111/jcpt.13518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen L, Krekels EHJ, Verweij PE et al (2020) Pharmacokinetics and pharmacodynamics of posaconazole. Drugs 80:671–695. 10.1007/s40265-020-01306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cojutti PG, Carnelutti A, Lazzarotto D et al (2021) Population pharmacokinetics and pharmacodynamic target attainment of isavuconazole against Aspergillus fumigatus and Aspergillus flavus in adult patients with invasive fungal diseases: should therapeutic drug monitoring for isavuconazole be considered as. Pharmaceutics 13:1–13. 10.3390/pharmaceutics13122099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Höhl R, Bertram R, Kinzig M et al (2022) Isavuconazole therapeutic drug monitoring in critically ill ICU patients: a monocentric retrospective analysis. Mycoses 65:747–752. 10.1111/myc.13469 [DOI] [PubMed] [Google Scholar]
- 65.Perez L, Corne P, Pasquier G et al (2023) Population pharmacokinetics of isavuconazole in critical care patients with COVID-19-associated pulmonary aspergillosis and Monte Carlo simulations of high off-label doses. J Fungi 9:2–17. 10.3390/jof9020211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jansen AME, Mertens B, Spriet I et al (2023) Population pharmacokinetics of total and unbound isavuconazole in critically ill patients: implications for adaptive dosing strategies. Clin Pharmacokinet 62:1701–1711. 10.1007/s40262-023-01305-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aguilar G, Azanza JR, Carbonell JA et al (2014) Anidulafungin dosing in critically ill patients with continuous venovenous haemodiafiltration. J Antimicrob Chemother 69:1620–1623. 10.1093/jac/dkt542 [DOI] [PubMed] [Google Scholar]
- 68.Luque S, Hope W, Campillo N et al (2019) Population pharmacokinetics of anidulafungin in critically ill patients. Antimicrob Agents Chemother 63:1–5. 10.1128/AAC.00378-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Märtson AG, van der Elst KCM, Veringa A et al (2020) Caspofungin weight-based dosing supported by a population pharmacokinetic model in critically ill patients. Antimicrob Agents Chemother 64:1–10. 10.1128/AAC.00905-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li F, Zhou M, Jiao Z et al (2021) Caspofungin pharmacokinetics and probability of target attainment in ICU patients in China. J Glob Antimicrob Resist 25:238–263. 10.1016/j.jgar.2021.03.011 [DOI] [PubMed] [Google Scholar]
- 71.Maseda E, Grau S, Luque S et al (2018) Population pharmacokinetics/pharmacodynamics of micafungin against Candida species in obese, critically ill, and morbidly obese critically ill patients. Crit Care 22:1–9. 10.1186/s13054-018-2019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foulquier JB, Berneau P, Frérou A et al (2019) Liposomal amphotericin B pharmacokinetics in a patient treated with extracorporeal membrane oxygenation. Med Mal Infect 49:69–71. 10.1016/j.medmal.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 73.Álvarez-Lerma F, Rodriguez M, Soriano MC et al (2013) Effectiveness of liposomal amphotericin B in patients admitted to the ICU on renal replacement therapy. Rev Esp Quimioter 26:360–368 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for data should be made to the corresponding author. Each request requires a research proposal with a clear research question and proposed analysis plan. Requests will be considered on an individual basis and will be reviewed by the SAFE-ICU steering committee, as well as relevant human research ethics committees.