Abstract
Murine embryo fibroblasts are readily transformed by the introduction of specific combinations of oncogenes; however, the expression of those same oncogenes in human cells fails to convert such cells to tumorigenicity. Using normal human and murine embryonic fibroblasts, we show that the transformation of human cells requires several additional alterations beyond those required to transform comparable murine cells. The introduction of the c-Myc and H-RAS oncogenes in the setting of loss of p53 function efficiently transforms murine embryo fibroblasts but fails to transform human cells constitutively expressing hTERT, the catalytic subunit of telomerase. In contrast, transformation of multiple strains of human fibroblasts requires the constitutive expression of c-Myc, H-RAS, and hTERT, together with loss of function of the p53, RB, and PTEN tumor suppressor genes. These manipulations permit the development of transformed human fibroblasts with genetic alterations similar to those found associated with human cancers and define specific differences in the susceptibility of human and murine fibroblasts to experimental transformation.
The study of specimens derived from human tumors has facilitated the identification of many genetic alterations critical for cancer development. However, the karyotypes of most human epithelial cancers are characterized by widespread alterations involving the amplification, deletion, and rearrangement of multiple chromosomes. As the tools to perform whole genome analysis of human tumors become available, further progress in understanding the mechanisms that program the initiation and maintenance of human cancers will require increasingly sophisticated experimental models.
The use of in vitro experimental models of murine and human cell transformation suggests that a limited set of genetic changes cooperate to program the malignant state. For example, the introduction of cooperating pairs of oncogenes such as the adenoviral E1A protein and an activated allele of H-RAS (RAS) or c-Myc (Myc) and RAS suffices to transform rodent cells (27, 42). Experiments involving transgenic mice engineered to express Myc and RAS revealed that these oncoproteins also cooperate in vivo to drive tumor growth (48, 50). Loss of function of the p53 pathway facilitates murine cell transformation induced by these introduced oncogenes (24, 32), suggesting that a small number of cooperating mutations suffice to transform primary murine cells.
In contrast, the coexpression of Myc and RAS fails to transform human cells (49), suggesting that the experimental transformation of human cells requires additional changes than are necessary to transform murine cells (36). After extended passage in culture (22) or the expression of an activated oncogene such as RAS (45), primary human cells cease dividing and enter a state termed senescence (11). The overexpression of hTERT, the catalytic subunit of human telomerase (7, 51), or the simultaneous inactivation of the p53 and retinoblastoma (RB)/p16INK4A tumor suppressor pathways (45, 47) allows some cells to bypass senescence. Since telomere biology differs between human and murine cells (1, 9, 26, 30, 39), these observations suggest that telomeres and telomerase explain, in part, this species-specific difference in cell transformation.
Recent studies from several laboratories have identified sets of introduced genes that cooperate to transform several types of human cells (14, 19, 29, 40, 44, 52, 56). Such experiments provide experimental models with which to study the contributions of a particular gene of interest or signaling pathway in experimental transformation. However, since the combination of genetic alterations required for transformation is influenced by the specific cell types (40) and experimental systems utilized (14, 29, 44), further studies are necessary to identify and define combinations of genetic changes that suffice to permit transformation in particular types of cells.
Here we compare the transformation of normal diploid murine and human cells and find that whereas Myc and RAS transform murine embryo fibroblasts in the setting of loss of p53 function, these same alterations together with the constitutive expression of hTERT fail to transform human cells. Instead, the transformation of several strains of normal human fibroblasts requires the additional ablation of the RB and PTEN tumor suppressor pathways. These observations identify specific genetic differences in the experimental transformation of human and murine cells.
MATERIALS AND METHODS
Vectors and retroviral infection.
Retroviral vectors (pBabe and pWZL) (33) were used to introduce specific genes into human and murine cells. To ensure that human and murine cells were infected at comparable efficiency, the ecotropic receptor (3) was introduced into human cells by using pBabe-Zeo-EcoR. pWZL-BLAST-Myc was created by introducing the c-Myc cDNA into pWZL-BLAST. We introduced RAS into cells by using pBabe-Puro-RAS (45) (Fig. 1 and 2) or pBabe-HcRed-RAS (Fig. 3 to 5), which was created by replacing the puromycin gene in pBabe-Puro-RAS with HcRed from pcDNA3.1-HcRed1 (a gift from H. Widlund). We introduced ST into cells by using the pBabe-GFP-ST vector. The following vectors have been described previously: pBabe-Hygro-hTERT (13), pBabe-Neo-DD (dominant-negative allele of p53) (20) and pBabe-Puro-DK (56), which encodes the CDK4R24C-cyclin D1 fusion protein (DK) (41).
FIG. 1.
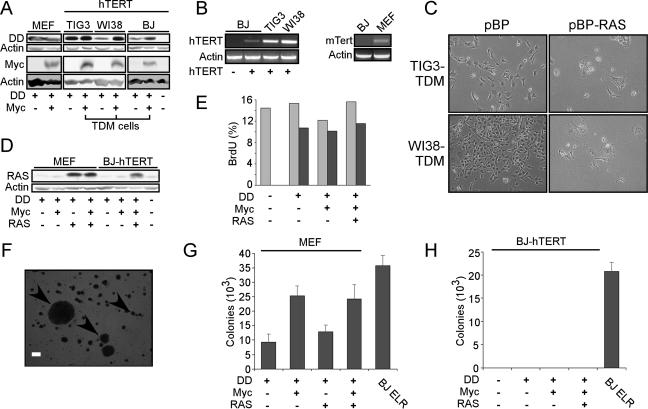
Expression of hTERT fails to cooperate with the expression of DN-p53 (DD), Myc, and RAS to transform human fibroblasts. (A) Immunoblotting to confirm expression of DD and Myc in murine and human cells. Cell lines include MEFs and TIG3, WI38, and BJ strains of human fibroblasts expressing hTERT. Human cells expressing hTERT, DD, and Myc are designated TDM cells. A total of 50 μg of total cell protein (DD) or total cell lysate corresponding to approximately 2 × 105 cells (Myc) was separated on a 7.5 to 15% gradient gel (DD) or a 10% gel (Myc) and immunoblotted for indicated proteins. (B) Expression of hTERT and mTert in asynchronously dividing human and murine cells, respectively. RT-PCR for introduced hTERT or endogenous mTert was performed on total RNA (500 ng). Since asynchronously dividing cells were used, these experimental conditions do not permit the detection of S-phase-specific hTERT expression in human cells. (C) Induction of RAS-induced senescence in TIG3-TDM and WI38-TDM cells. Micrographs depict nonsenescent or senescent morphology of TDM cells infected with pBabe-Puro (pBP) or pBabe-Puro-RAS (pBP-RAS), respectively, shown at ×100 magnification. (D) Immunoblotting to confirm expression of RAS in matched MEF-DM and BJ-TDM cell lines. Immunoblotting was performed as in panel A for DD expression. (E) Assessment of p53 function. BJ-hTERT cells expressing the indicated constructs were subjected to ionizing radiation (5 Gy), labeled with BrdU, and subjected to fluorescence-activated cell sorting. Light gray bars indicate nonirradiated cultures, and dark gray bars indicate irradiated cultures. This experiment was performed in duplicate, and representative results are shown. (F) Micrograph demonstrating the types of colonies scored in these experiments. Bar, 200 μm. (G) Anchorage-independent growth of MEFs expressing the indicated genes. The data are expressed as the mean ± the standard deviation (SD) of triplicate determinations. (H) Anchorage-independent growth of BJ-hTERT cells expressing the indicated genes. No significant colony growth of any BJ-hTERT cell lines was observed after 6 weeks, compared to transformed BJ ELR cells that express hTERT, SV40 LT, ST, and RAS (19).
FIG. 2.
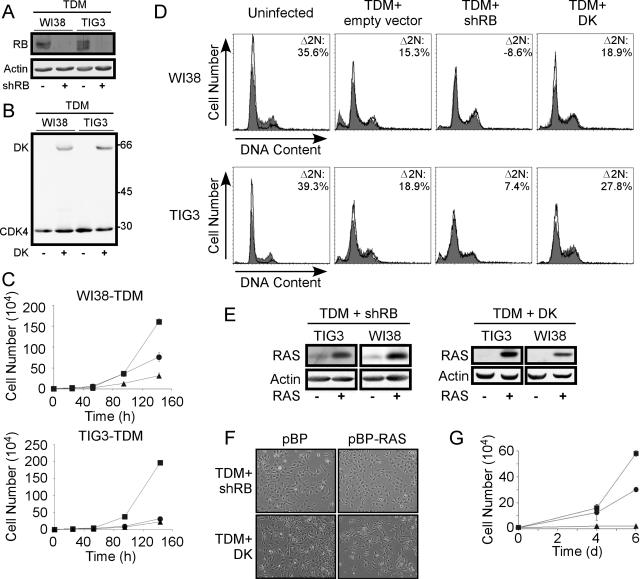
Perturbation of the RB pathway. (A) shRNA-mediated suppression of RB in WI38-TDM and TIG3-TDM cells, as determined by immunoblotting. (B) Expression of the cyclin D1-CDK4R24C fusion protein (41) (DK) in WI38-TDM and TIG3-TDM cells. The CDK4-specific antibody detects both endogenous CDK4 and exogenous DK in immunoblotting. Molecular masses in kilodaltons are shown on the right. (C) Proliferation of WI38-TDM and TIG3-TDM cells expressing a control vector (▴), shRB (•), or DK (▪). For each cell line, 104 cells were plated and counted at the indicated time points. Each point is represented as the mean ± the SD of triplicate determinations. Error bars are shown for each point; however, in some cases the symbol covers the error bars. (D) Disruption of the G1/S checkpoint by shRB. Cells expressing the indicated genes were serum starved (1% serum) for 48 h (WI38 cells) or 24 h (TIG3 cells). Cells were stained with PI and subjected to flow cytometric analysis. Shaded profiles indicate parallel cultures maintained in 10% serum; open profiles represent tracings derived from starved cells. We examined the percentage of cells with 2 N DNA content under each condition to assess the capacity of the each cell population to arrest in G0/G1 in response to starvation. The percentage of cells with 2 N DNA content (%2N) was determined by using BD CellQuest Pro software and Δ2N represents the percentage change between starved and 10% serum conditions, i.e., (%2N starved − %2N serum)/%2N serum × 100. Larger Δ2N values reflect cultures retaining the capacity to arrest in response to serum, while smaller Δ2N values reflect cell populations that have lost the capacity to respond appropriately to starvation. (E) shRB and DK permit cells to express RAS. RAS was introduced into TIG3-TDM and WI38-TDM cells expressing shRB or DK. Cells that expressed shRB or DK did not undergo RAS-induced senescence as in Fig. 1C but instead proliferated with robust levels of RAS expression. (F) Suppression of RAS-induced senescence morphology in WI38-TDM cells expressing shRB or DK. Micrographs depict cells infected with pBabe-Puro (pBP) or pBabe-Puro-RAS (pBP-RAS), respectively, shown at ×100 magnification. Similar results were observed with TIG3-TDM cells (not shown). (G) Suppression of RAS-induced proliferative arrest by shRB or DK. A total of 104 cells were plated in triplicate at day 0 and infected with RAS-expressing virus at both day 1 and day 2. Cells were counted as in panel C at the indicated time points. Symbols are as defined in panel C. Proliferation of WI38 cells is shown; similar results were observed with TIG3 cells (not shown).
FIG. 3.
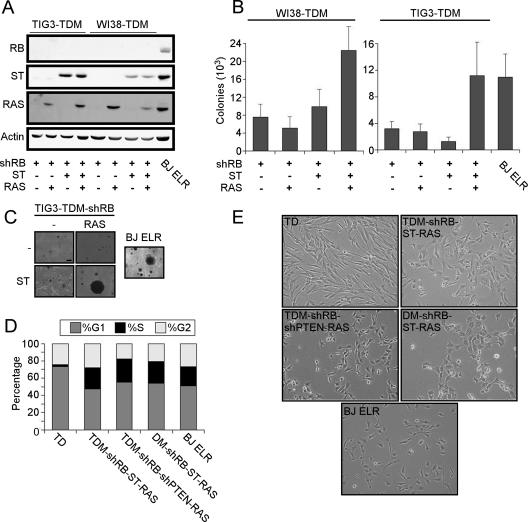
ST cooperates with RAS to transform cells expressing TDM and shRB. (A) Immunoblotting for introduced genes. Suppression of RB and expression of ST and RAS was confirmed in TIG3-TDM and WI38-TDM cell lines engineered to express the indicated proteins by immunoblotting for indicated proteins (50 μg). BJ ELR cells were used as a positive control. (B) Anchorage-independent growth of cell lines expressing the indicated genes, compared to BJ ELR cells. (C) Representative micrographs are shown to demonstrate colony sizes of TIG3-TDM or BJ ELR fibroblasts expressing the indicated constructs. (D) Contact inhibition at confluence. The indicated cells were grown to confluence and maintained for 48 h before labeling with PI. Cell cycle profiles were analyzed by using ModFit Software, and the fraction of cells in each phase is shown as a shaded region of each bar as indicated. (E) Cell morphology. Transformed cells display an altered morphology characterized by irregular-sized cells with a vacuolated appearance, similar to that observed with BJ ELR cells. Representative micrographs are shown depicting TIG3 cells expressing the indicated constructs, shown at ×100 magnification. Similar results were obtained with WI38 cells.
FIG. 5.
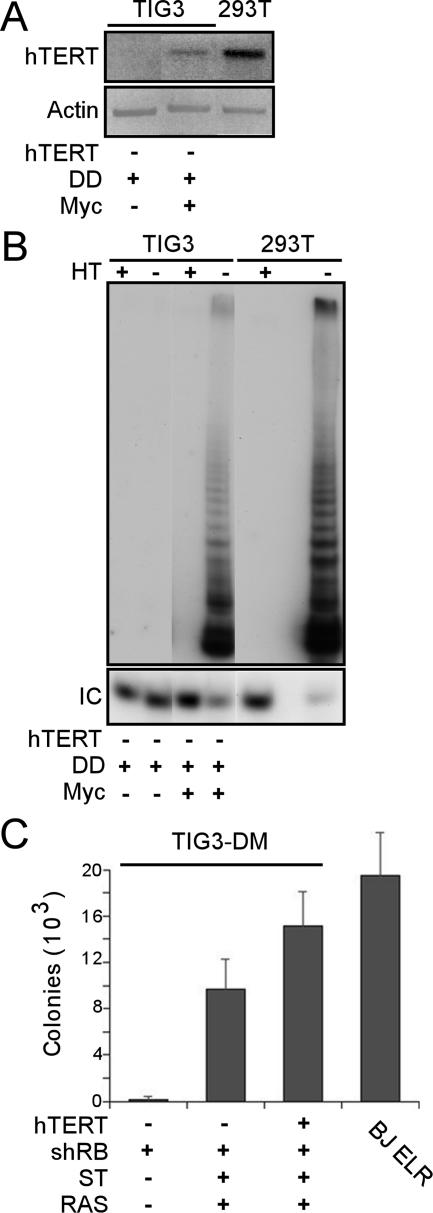
Myc activates hTERT in human cell transformation. (A) Detection of hTERT in Myc-expressing cells. TIG3 cells were infected serially with DD, Myc, shRB, ST, and RAS viruses. RT-PCR was performed on 500 ng of RNA harvested from TIG3-DM-shRB-ST-RAS cells (Myc +) or TIG3-D cells (Myc −) by using primers specific for endogenous hTERT. Cells did not undergo replicative senescence. (B) Detection of telomerase activity in TIG3-DM-shRB-ST-RAS (Myc +) cells. The PCR-based TRAP assay was performed as previously described (25, 30). HT, heat-treated samples; IC, TRAP internal control. Telomerase activity was detectable in all cell lines after the introduction of Myc (data not shown) but not in TIG3-D cells (Myc −). (C) Anchorage-independent growth of DM-shRB-ST-RAS cells compared to TDM-shRB-ST-RAS cells. The results are shown as the mean ± the SD for triplicate determinations.
To introduce short hairpin RNAs (shRNAs), we used the pMKO.1P retroviral vector (30) and the following oligonucleotides for RB: forward, 5′-CCGGCAGAGATCGTGTATTGAGATTCTCGAGAATCTCAATACACGATCTCTGTTTTTG-3′; and reverse, 5′-AATTCAAAAACAGAGATCGTGTATTGAGATTCTCGAGAATCTCAATACACGATCTCTG-3′. Similarly, we introduced the following oligonucleotides specific for PTEN: forward, 5′-CCGGCGTATACAGGAACAATATTGCTCGAGCAATATTGTTCCTGTATACGC-3′; and reverse, 5′-AATTGCGTATAC AGGAACAATATTGCTCGAGCAATATTGTTCCTGTATACG-3′. The sequences specific for the 3′ untranslated region (UTR) of RB or the coding sequence of PTEN are indicated in boldface. The puromycin resistance gene was replaced with a simian virus 40 (SV40) promoter-green fluorescent protein (GFP) cassette from pBabe-GFP to create pMKO-GFP-PTEN. Retroviruses were generated as previously described (20). Infections were performed serially by using drug selection or fluorescence-activated cell sorting to purify cell populations 48 h after infection. The drug concentrations used were as follows: zeocin, 200 μg/ml; hygromycin, 100 μg/ml; neomycin (G418), 400 μg/ml; puromycin, 1 μg/ml; and blasticidin, 2.5 μg/ml.
Cell culture.
BJ cells are human diploid foreskin fibroblasts (7), WI38 and TIG3 cells are human diploid embryonic lung fibroblasts. BJ and WI38 cells were obtained from the American Type Culture Collection (Manassas, VA), and TIG3 cells were obtained from the Health Science Research Resource Bank (Osaka, Japan) (31). Transformed BJ cells expressing hTERT, SV40 early region (ER) and RAS (19) are termed BJ ELR cells. BJ fibroblasts were maintained in a 4:1 mixture of Dulbecco modified Eagle medium to M199 medium supplemented with 15% heat-inactivated fetal calf serum. Murine embryo fibroblasts (MEFs) and WI38 and TIG3 cells were maintained in Dulbecco modified Eagle medium supplemented with glutamine and 10% fetal calf serum. Proliferation curves were generated by plating cells in triplicate in six-well dishes and counting cells at the indicated time points using a Z1 Coulter particle counter (Beckman-Coulter, Miami, FL). The analysis of proliferation after the introduction of RAS-induced senescence was performed by infecting cells in six-well dishes in triplicate and counting at the indicated time points. For irradiation experiments, cells were treated with trypsin, exposed to ionizing radiation (5 Gy), and replated for 48 h. To assess the cell cycle distribution of cell populations, bromodeoxyuridine (BrdU; 100 μM) was added to culture media for 6 h, and cells were costained with propidium iodide (PI; 15 nM). For cell cycle analysis, WI38 and TIG3 cells were maintained in media containing 1% serum for 48 h (WI38) or 24 h (TIG3). To analyze AKT phosphorylation (P-AKT) status, cells were starved in 0.1% serum overnight, followed by stimulation with 10% serum for 1 h before lysis and immunoblotting. To determine contact inhibition, cells were allowed to grow for 48 h after reaching confluence, followed by BrdU incorporation and PI staining as indicated above.
Immunoblotting, telomere repeat amplification protocol (TRAP), and reverse transcription-PCR (RT-PCR).
Cells were lysed in a lysis buffer containing 1.25% NP-40, 1.25% sodium dodecyl sulfate [SDS], 12.5 mM Na2PO4 [pH 7.2], 2 mM EDTA, 50 mM NaF, 0.5 mM sodium vanadate, and 1 pellet of Complete protease inhibitor/10 ml (Roche, Indianapolis, IN). After sonication, lysates were cleared of insoluble material by centrifugation at 16,000 × g. Proteins (50 μg) were subjected to 7.5 to 15% gradient SDS-PAGE, followed by immunoblotting. For the analysis of Myc protein levels, 2.5 × 105 cells were plated, incubated overnight, washed with phosphate-buffered saline, and lysed directly on the plate using 2× SDS sample buffer (125 mM Tris-base, 138 mM SDS, 10% β-mercaptoethanol, 20% glycerol, bromophenol blue [pH 6.8]). Lysates were boiled for 10 min, cleared of insoluble material by centrifugation at 16,000 × g, and subjected to SDS-10% polyacrylamide gel electrophoresis (PAGE).
We used the following antibodies: anti-p53, monoclonal antibody 421 (a gift from M. Ewen); anti-Myc, 9E10 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-pRB, G3-245 (BD Biosciences Pharmingen, San Diego, CA); anti-RAS, c-20 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-P-AKT (Ser473), 9271 (Cell Signaling, Beverly, MA); anti-AKT, 9272 (Cell Signaling); anti-CDK4, H-22 (Santa Cruz Biotechnology); and anti-ST (12).
To detect telomerase activity, we performed a PCR-based TRAP as previously described (25, 30). RT-PCR was performed on total RNA (500 ng) isolated from the indicated cell lines by using TRIzol (Invitrogen, Carlsbad, CA). hTERT was detected by using WH33 and AS1310 primers (Fig. 1B) (30), which amplify retrovirally introduced hTERT, or LT5 and LT6 primers (Fig. 5A), which amplify endogenous hTERT (35). mTert was detected by using the following primers: 5′-ACTCAGCAACCTCCAGCCTA-3′ and 5′-CAGACACCACCTTGCAGAGA-3′.
Anchorage-independent growth and tumorigenicity assays.
Growth of cells in soft agar was determined by plating 5 × 104 cells in triplicate in 0.4% Noble agar. Colonies greater than 100 μm in diameter were counted microscopically 6 to 8 weeks after plating. For tumor xenograft experiments, immunodeficient mice (C.Cg/AnNTac-Foxn1nunu; Taconic, Germantown, NY) were used. A total of 2 × 106 cells were injected subcutaneously into mice anesthetized with isofluorane. Tumors were measured biweekly.
RESULTS
Transformation of MEFs.
Although the introduction of Myc and RAS transforms murine cells, several lines of evidence indicate that the expression of these oncogenes coselects for loss of function of the p53/ARF tumor suppressor pathway (16, 24). To achieve murine cell transformation without selection for spontaneous inactivation of the p53 pathway, we expressed a dominantly interfering allele of p53 (DD) in MEFs (Fig. 1A). This p53 mutant stabilizes and inactivates endogenous p53 (46). We then infected DD-expressing cells serially with retroviruses encoding Myc or RAS, followed by drug selection to eliminate uninfected cells. These manipulations required approximately 50 population doublings (PDs) and generated polyclonal populations of cells expressing these genes singly or in combination (Fig. 1A and D).
In parallel, we also introduced DD and Myc into human diploid fibroblasts rendered immortal by the prior introduction of the catalytic subunit of telomerase, hTERT. To ensure that our results were relevant beyond a single fibroblast strain, we used three different types of human fibroblasts: TIG3 and WI38 embryonic lung fibroblasts and BJ foreskin fibroblasts. We used human cells constitutively expressing hTERT for these experiments since prior work indicated that murine cells constitutively express mTert (39) and harbor long telomeres (26). As shown in Fig. 1B, these human and murine cells expressed hTERT and mTert transcripts. In these experiments, we used human cells that stably expressed the ecotropic receptor to allow us to infect murine and human cells with the same viral particles in parallel.
We infected TIG3-hTERT, WI38-hTERT, and BJ-hTERT cells serially with control retroviruses or retroviruses encoding DD or Myc (cells expressing hTERT, DD, and Myc are hereafter referred to as TDM) and confirmed the expression of each of these introduced genes by immunoblotting (Fig. 1A). We then characterized these human diploid fibroblasts for their capacity to tolerate RAS overexpression. As expected (45, 54), the expression of RAS in human cells expressing either hTERT alone or hTERT and DD yielded growth-arrested cells that displayed morphological similarity to those undergoing RAS-induced senescence (data not shown). Expression of Myc in BJ cells expressing hTERT and DD permitted these cells to avoid the growth arrest induced by the subsequent expression of RAS and enabled us to generate a population of human cells expressing hTERT, DD, Myc, and RAS (Fig. 1A and D).
In contrast to what we observed with BJ fibroblasts, the introduction of RAS into TIG3-TDM and WI38-TDM fibroblasts produced cells that displayed a senescent morphology (Fig. 1C). We attempted to stain these cells for senescence-associated β-galactosidase (β-Gal) activity but observed significant background staining in BJ, WI38, and TIG3 cells expressing Myc, suggesting that Myc expression alone induces senescence-associated β-Gal activity (data not shown). These observations confirm recent work suggesting that BJ human fibroblasts exhibit different responses to senescence-inducing stimuli than other strains of human fibroblasts (4). Since abundant evidence indicates that expression of RAS at high levels induces a growth arrest unless both the p53 and RB pathways are inactivated (38, 45), these results suggest that the expression of Myc in WI38 and TIG3 cells failed to inactivate the RB pathway sufficiently to allow high-level RAS expression. Alternatively, Myc expression itself or a downstream target of Myc may have rendered the cells unable to tolerate high levels of RAS expression.
However, using BJ-TDM-RAS fibroblasts, we investigated the contribution of hTERT to transformation by comparing these cells to MEF-DM-RAS cells. We first confirmed that expression of the DD mutant inactivated the p53 pathway, by exposing these murine and human cells to ionizing radiation (5 Gy) and determining the subsequent proliferation of these cells by measuring the incorporation of BrdU into DNA (Fig. 1E and data not shown). The majority of cells lacking DD entered a proliferative arrest after exposure to ionizing radiation, whereas BJ-hTERT cells expressing DD (TD cells), TDM cells, or TDM-RAS cells continued to proliferate at similar rates to control, nonirradiated cells. These findings provided evidence that the expression of DD sufficed to disrupt p53 function.
We then tested these murine and human cells for their ability to grow in an anchorage-independent manner. After 6 weeks, we counted colonies, scoring colonies larger than 100 μm (arrowheads, Fig. 1F). Although DM-RAS MEFs showed robust colony growth in soft agar (Fig. 1G), BJ TDM-RAS cells failed to form colonies (Fig. 1H). These results confirm that although constitutive expression of telomerase contributes to the immortalization of human cells, further alterations are necessary to achieve the transformation of human fibroblasts.
Contributions of RB pathway inactivation.
Since WI38 and TIG3 human fibroblasts expressing hTERT, DD, and Myc responded differently than BJ fibroblasts to the introduction of RAS, we chose to study WI38-TDM and TIG3-TDM fibroblasts further in order to identify cooperating lesions in human cell transformation that were not specific to a single strain of fibroblasts. Since prior work identified the importance of RB and p53 pathway inactivation in permitting cells to bypass RAS-induced premature senescence, we hypothesized that inactivation of the RB pathway in WI38-TDM and TIG3-TDM cells should allow such cells to tolerate RAS overexpression. To inactivate the RB pathway in human TDM-expressing cells, we used two complementary experimental approaches. First, we suppressed the expression of RB by generating an RB-specific short hairpin RNA (shRB) by targeting the 3′UTR of RB. Introduction of a retroviral vector encoding shRB into TIG3-TDM and WI38-TDM cells stably suppressed the expression of RB (Fig. 2A). Alternatively, we introduced a CDK4R24C-cyclin D1 fusion protein (DK) that is insensitive to inhibition by p16INK4A and constitutively phosphorylates RB (41, 56) into TIG3-TDM and WI38-TDM cells (Fig. 2B).
To determine whether expression of shRB or DK led to functional inactivation of the RB pathway, we first determined the effects of shRB or DK expression on the proliferation of TDM-expressing cells (Fig. 2C). In both cell lines, cells that expressed shRB showed little or no proliferative advantage over cells expressing a control vector, despite lacking detectable RB protein. In contrast, cells expressing the DK fusion protein proliferated more rapidly than cells expressing a control vector or shRB (Fig. 2C). These results are consistent with recent observations that shRNA-mediated suppression of RB fails to affect the proliferation of human fibroblasts (52).
We then determined whether expression of shRB or DK altered the activity of the G0/G1 restriction point in response to serum starvation. We exposed WI38-TDM and TIG3-TDM cells to normal (10%) and starvation (1%) serum conditions for 48 h (WI38) or 24 h (TIG3) and determined the cell cycle distribution of such cells (Fig. 2D). Since cells with an intact G0/G1 restriction point arrest in response to serum starvation, we measured the percentage increase in cells with 2 N DNA content (Δ2N) under starvation conditions compared to parallel cultures maintained in normal conditions. We found that unmanipulated WI38 and TIG3 fibroblasts arrest in G0/G1 in response to starvation and calculated Δ2N values of 35.6 and 39.3%, respectively. Similarly, we found that a high percentage of serum-starved WI38-TDM and TIG3-TDM cells expressing either the control vector or DK arrested in G0/G1 (Δ2N > 15%) (Fig. 2D). This result suggests that the G0/G1 restriction point remains intact in cells expressing DK. In contrast, WI38-TDM and TIG3-TDM cells expressing shRB exhibited little or no change in the percentage of cells with 2 N DNA content (Δ2N < 7.5%). Taken together, these results suggest that suppression of RB expression perturbs the G1/S-phase checkpoint and renders TIG3-TDM and WI38-TDM cells insensitive to serum withdrawal. In contrast, cells expressing DK retain the capacity to respond to serum starvation.
As described previously, the introduction of RAS into TIG3-TDM and WI38-TDM cells resulted in senescent cultures (Fig. 1C). We therefore examined whether expression of shRB or DK permitted high levels of RAS expression. Indeed, the introduction of RAS into TIG3-TDM and WI38-TDM cell lines expressing shRB or DK resulted in a population of cells expressing high levels of RAS (Fig. 2E) without morphological evidence of premature senescence (Fig. 2F). Although the expression of RAS in WI38-TDM cells prevented such cells from continuing to proliferate, cells expressing either shRB or DK continued to proliferate after the introduction of RAS (Fig. 2G). Similar results were observed with TIG3-TDM cells (data not shown). These observations suggest that both shRB and DK disabled the RB pathway sufficiently to allow such cells to bypass RAS-induced senescence. In addition, since DK-expressing cells retained the capacity to respond to serum starvation, our findings suggest that ablation of RB-mediated regulation of the response to serum starvation is not required to bypass RAS-induced senescence.
Since suppression of RB expression more closely recapitulates mutations found associated with some human cancers, we focused on examining cell lines in which we ablated RB functionality by suppressing RB expression. To determine whether TIG3-TDM-shRB-RAS or WI38-TDM-shRB-RAS cells were transformed, we tested whether these cells were able to form colonies in soft agar. Despite high levels of RAS expression, neither TIG3-TDM nor WI38-TDM cells expressing shRB and RAS showed significant anchorage-independent growth (Fig. 3B), suggesting that further alterations were necessary to transform these cells.
Transformation of human embryonic fibroblasts.
We and others have shown that both the SV40 large T (LT) and small t (ST) oncoproteins contribute to human cell transformation (20, 59). Since the expression of shRB and RAS in TIG3-TDM or WI38-TDM human fibroblasts failed to allow these cells to form a significant number of colonies in soft agar, we hypothesized that the expression of ST might render such cells capable of anchorage-independent growth.
To test this hypothesis, we created TIG3-TDM and WI38-TDM cell lines that expressed shRB, RAS and ST (Fig. 3A) and determined whether any of these cell lines were able to form colonies in soft agar. As described above, TIG3-TDM and WI38-TDM cells expressing shRB and RAS formed few colonies in soft agar (Fig. 3B). In contrast, TIG3-TDM and WI38-TDM cells expressing shRB, RAS, and ST grew in an anchorage-independent manner (Fig. 3B) at a frequency comparable to transformed BJ fibroblasts expressing hTERT, LT, ST, and RAS (BJ ELR) (19). WI38-TDM cells expressing shRB, RAS, and ST formed quantitatively more colonies than corresponding control cells (Fig. 3B), whereas TIG3-TDM cells expressing shRB, RAS, and ST formed both quantitatively and qualitatively more colonies than control cells (Fig. 3B and C). We note that many colonies formed by BJ ELR cells were larger than those formed by transformed TIG3 or WI38 cells.
To assay additional phenotypes associated with the transformed state, we examined whether the cells that had obtained the capacity to grow in an anchorage-independent manner displayed contact inhibition. TIG3 cells were grown to confluence and maintained for 48 h. Cells were costained with BrdU and PI to assess both proliferation and the distribution of cells in the cell cycle. In cultures expressing hTERT and DD (TD), we found a high percentage of cells in G1, few cells in S phase, and low levels of BrdU incorporation (Fig. 3D and data not shown), suggesting that these cells exhibited contact inhibition. In contrast, the WI38- and TIG3-TDM-shRB-ST-RAS cells incorporated BrdU at confluence and failed to arrest in G1, indicating that such cells were not subject to contact inhibition (Fig. 3D and data not shown). We note that WI38 and TIG3 cells expressing TDM-shRB-ST-RAS exhibited a sub-G1 (<2N DNA) peak of DNA content at confluence, suggesting that these cells initiated apoptosis at confluence (data not shown).
Furthermore, TIG3-TDM-shRB-ST-RAS cells displayed an altered morphology similar to BJ ELR cells, compared to TIG3-TD cells (Fig. 3E). Specifically, these transformed cells were irregularly shaped and appeared vacuolated. Similar results were obtained with WI38 cells (data not shown). We finally assessed the tumorigenic potential of the transformed TIG3 cells by performing subcutaneous injections in immunocompromised mice. TIG3-TDM-shRB cells, TIG3-TDM-shRB-RAS cells and TIG3-TDM-shRB-ST cells failed to form tumors (zero tumors/six injections for each cell line). In contrast, TIG3-TDM-shRB-ST-RAS cells formed tumors (three tumors/six injections), albeit at a slightly reduced frequency compared to the control BJ ELR cells (five tumors/six injections). Importantly, the short latency of tumor growth of the TIG3-TDM-shRB-ST-RAS cells (mean, 23 days) makes it unlikely that the observed tumor formation was due to selection for additional lesions in vivo. In summary, these experiments suggest that the additional expression of hTERT, shRB, and ST are together sufficient to render human cells expressing DD, Myc, and RAS transformed by several criteria.
Contribution of the PI3K signaling pathway.
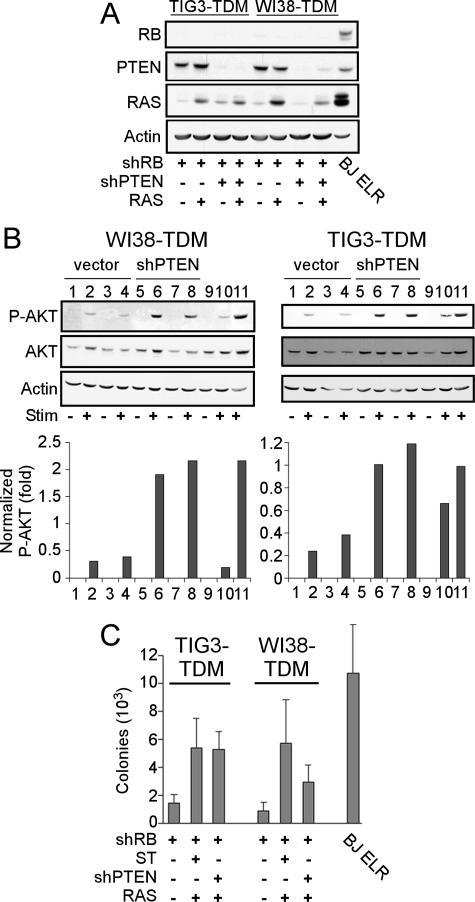
In late-passage human mammary epithelial cells (HMECs), constitutive activation of the phosphatidylinositol 3-kinase (PI3K) pathway functionally replaces ST in transformation (60). This finding suggests that ST promotes transformation of human cells by activating the PI3K signaling pathway. To determine whether activation of the PI3K signaling pathway also replaces ST in the cells described herein, we expressed a short hairpin RNA specific for the PTEN tumor suppressor gene (shPTEN) in TIG3-TDM and WI38-TDM cells expressing shRB. We then introduced RAS and confirmed the expression of each of the introduced constructs by immunoblotting (Fig. 4A). Expression of shPTEN stably suppressed the expression of PTEN in all cell lines tested (Fig. 4A).
FIG. 4.
shPTEN replaces ST in transformation of cells expressing TDM, shRB, and RAS. (A) Suppression of RB and PTEN and expression of RAS were confirmed by immunoblotting. (B) Effects of shPTEN on AKT phosphorylation. WI38-TDM or TIG3-TDM cell lines expressing shRB (lanes 1 and 2), shRB and RAS (lanes 3 and 4); shRB and shPTEN (lanes 5 and 6); shRB, shPTEN, and RAS (lanes 7 and 8); or shRB, ST, and RAS (lanes 9 and 10) were starved overnight in 0.1% serum (Stim −) and then stimulated with 10% serum for 1 h (Stim +). Immunoblotting for P-AKT and AKT was performed on total cell lysates (50 μg). P-AKT levels were normalized to total AKT levels by densitometry. Lane 11 represents a control cell line expressing high levels of activated PI3K under stimulated conditions. (C) Anchorage-independent growth. The results are shown as the mean ± the SD for triplicate determinations.
To determine whether expression of shPTEN also resulted in activation of the PI3K signaling pathway, we starved TIG3-TDM and WI38-TDM cells expressing shRB and either shPTEN or an empty vector control overnight in 0.1% serum and then stimulated these cells with 10% serum for 1 h. We observed that cells expressing a control vector showed only a low level of AKT phosphorylation (P-AKT, S473) after serum stimulation, whereas cells expressing shPTEN showed a more robust level of P-AKT after stimulation (Fig. 4B). These results are reminiscent of our prior work in human mammary and prostate epithelial cells expressing either ST or an activated allele of PI3K (6, 60).
We then tested the shPTEN-expressing cell lines for their ability to grow in an anchorage-independent manner. WI38-TDM cells expressing shRB, shPTEN, and RAS formed more colonies than WI38-TDM-shRB cells but formed fewer colonies than WI38-TDM cells expressing shRB, ST, and RAS (Fig. 4C). However, TIG3-TDM cells expressing shRB, shPTEN, and RAS formed colonies at a similar rate as TIG3-TDM cells expressing shRB, ST, and RAS (Fig. 4C). These cells also failed to display contact inhibition at confluence (Fig. 3D and data not shown) and displayed an altered morphology similar to that previously described for TIG3 TDM-shRB-ST-RAS cells (Fig. 3E). Surprisingly, we failed to observe tumor formation by the TIG3-TDM-shRB-shPTEN-RAS cells (zero of six). However, since the levels of RAS overexpression correlate with the ability of cells to form tumors as xenografts (15), we note that we were unable to generate cells that expressed RAS at levels comparable to TIG3-TDM-shRB-ST-RAS cells (Fig. 4C). Nevertheless, these observations corroborate previous results (60) suggesting that activation of the PI3K pathway functionally replaces ST in human cell transformation. Furthermore, these manipulations generated transformed human cells solely through the use of genetic alterations associated with human tumors.
Myc activates hTERT to transform human cells.
The observations described above suggest that the additional expression of shRB, ST, and RAS are sufficient to transform human cells expressing hTERT, DD, and Myc (TDM). Since previous reports indicated that Myc activates hTERT (17, 18, 53, 58), we ascertained whether the expression of Myc obviated the requirement for hTERT expression in human cell transformation.
To address this question, we generated TIG3 cell lines expressing DD, Myc, shRB, ST, and RAS (TIG3-DM-shRB-ST-RAS cells). Introduction of Myc induced constitutive expression of hTERT (Fig. 5A), confirming that Myc induces hTERT expression in human fibroblasts (53). We next determined whether this level of endogenous hTERT induced by Myc sufficed to reconstitute telomerase activity, as measured by the PCR-based TRAP assay (25). Although we failed to detect telomerase activity in asynchronously dividing TIG3 cells expressing DD, we found that TIG3 cells expressing Myc exhibited readily detectable telomerase activity (Fig. 5B and data not shown). Moreover, although TIG3-DM-shRB cells failed to grow in an anchorage-independent manner, TIG3-DM-shRB-ST-RAS cells formed colonies in soft agar at levels comparable to TIG3-TDM-shRB-ST-RAS cells, in which we had introduced hTERT (Fig. 5C). In addition, such cells failed to exhibit contact inhibition (Fig. 3D) and displayed an altered morphology (Fig. 3E). These results confirm that Myc activates hTERT in TIG3 cells to levels sufficient to contribute to human cell transformation.
DISCUSSION
Although cooperating oncogenes such as Myc and RAS efficiently transform primary murine cells (27), similar attempts to transform human cells have repeatedly failed (36, 49). We previously reported that human cells are converted to tumorigenicity through the introduction of the SV40 early region, hTERT, and RAS (19). However, since such experiments utilized the LT and ST viral oncoproteins, the specific pathways whose disruption was necessary to achieve cell transformation remained undefined. We show here that normal human fibroblasts are rendered transformed by the coexpression of Myc, RAS, and hTERT, together with the loss of function of the RB, PTEN, and p53 tumor suppressor pathways (Fig. 6).
FIG. 6.
Summary of findings. DN-p53, Myc, and RAS suffice to transform MEFs. In contrast, the additional expression of hTERT, RB-shRNA, and either ST or PTEN-shRNA are sufficient to transform human embryonic lung fibroblasts. Alternatively, Myc activates hTERT to levels sufficient to permit transformation.
Previous observations indicate that several aspects of telomere maintenance differ in human and murine cells. Normal human cells only transiently express hTERT at levels that are insufficient to maintain telomere length (1, 9, 30). In contrast, most murine cells constitutively express telomerase (39) and maintain telomere lengths that are 3 to 10 times longer than those in comparable human cells (26). Thus, we hypothesized that the capacity of Myc and RAS to transform murine cells but not human cells might be explained by species-specific differences in telomere maintenance. However, the observations presented here indicate that even constitutive expression of telomerase fails to cooperate with the expression of Myc and RAS in a setting of loss of p53 function to achieve human cell transformation. Thus, differences in telomere biology alone do not fully explain the differential susceptibility of human and murine cells to transformation.
Others have reported that overexpression of Myc activates hTERT expression in some human cells (17, 18, 53, 58). In TIG3 cells, we confirmed that Myc expression constitutively activates telomerase to levels that cooperate with other genetic alterations to transform human cells. These findings corroborate a recent report (17). Thus, although constitutive telomere maintenance alone does not explain the difference in transformation between human and murine cells, these observations are consistent with the notion that expression of hTERT contributes to cell transformation.
High-level expression of RAS induces senescence in both human and murine cells (45). However, inhibition of p53 function permits murine cells to bypass RAS-induced senescence, whereas human cells appear to require loss of both p53 and RB function (45). Interestingly, we found that BJ human foreskin fibroblasts expressing hTERT, DD, and Myc tolerated high levels of RAS expression (Fig. 1D), whereas RAS expression in TIG3, WI38, or MRC5 human fibroblasts expressing hTERT, DD, and Myc elicited a senescent-like state (Fig. 1C and 2G and data not shown). Since Myc has been reported to activate CDK4 (23) and cyclin D2 (8), the expression of Myc may partially disable the RB pathway. Indeed, expression of Myc in human prostate epithelial cells induces hTERT expression and disables the RB pathway, permitting cell immortalization (17). Thus, it remains possible that the levels of Myc expression in BJ and these prostate epithelial cells sufficed to disable RB function and permitted cells to bypass RAS-induced senescence, whereas the levels of Myc expression attained in other cell types failed to reach a threshold necessary to inactivate RB. Alternatively, since TIG3 and WI38 fibroblasts and BJ fibroblasts are derived from different tissues (lung versus foreskin) and different developmental states (embryonic versus newborn), intrinsic differences in fibroblasts derived from these distinct tissues may explain this difference in susceptibility to RAS-induced senescence. Consistent with this notion, it has recently been reported that the expression level of the cyclin-dependent kinase inhibitor p16INK4A correlates with the differential response of human fibroblasts to stimuli that induce senescence (4, 5).
DK expression significantly increased the proliferation of both TIG3-TDM and WI38-TDM cells and yet failed to significantly change the capacity of these cells to respond to serum starvation, whereas the expression of shRB failed to increase cell proliferation but dramatically prevented such cells from responding to serum starvation (Fig. 2C and D). TDM cells expressing a short hairpin RNA specific for p16INK4A behaved identically to DK-expressing cells in regard to proliferation and the response to serum starvation (data not shown). Since the DK fusion protein primarily perturbs the RB pathway at the level of p16INK4A and the shRB construct directly suppresses RB, these observations corroborate recent reports that suggest that loss of function of p16INK4A and loss of RB function are not equivalent (55).
The finding that an shRNA specific for the PTEN tumor suppressor gene was capable of replacing ST in transformation corroborates our recent study that showed that in late-passage HMECs constitutive activation of the PI3K pathway replaced the capacity of ST to induce transformation (60). Since the interaction of ST with the protein phosphatase 2A (PP2A) family of serine-threonine phosphatases is required for human cell transformation (20, 34), these observations suggest that ST expression modulates the activity of PI3K through its effects on PP2A. Accumulating biochemical evidence suggests that PP2A signaling regulates the activity of the PI3K pathway (2, 57, 60). Short-hairpin RNA-mediated suppression of PP2A regulatory subunits or suppression of PP2A activity by treating cells with okadaic acid induces phosphorylation of AKT (2; W. Chen, J. Arroyo, and W. C. Hahn, submitted for publication). The relevant PP2A substrate(s) that lead to activation of the PI3K pathway are unclear but may include AKT itself or downstream effector molecule(s) such as p70S6K (57).
Although AKT activation may be one consequence of perturbing PP2A function, we did not observe a strict correlation between the absolute levels of P-AKT and the number of colonies formed in soft agar (Fig. 4B and C). There are several possible explanations for this observation. One possibility is that there exists a threshold level of AKT activation sufficient to transform human cells and that exceeding this level of AKT phosphorylation does not further increase the capacity for anchorage-independent growth. Alternatively, although PI3K pathway activation is one consequence of expressing ST in human cells, ST perturbs other pathways which may independently contribute to its ability to transform human cells (43).
TIG3-TDM-shRB-ST-RAS cells formed colonies in soft agar (Fig. 3B), failed to exhibit contact inhibition (Fig. 3D), displayed an altered morphology (Fig. 3E), and formed tumors in immunocompromised hosts, collectively indicating that these cells were both transformed and tumorigenic. TIG3-TDM-shRB-shPTEN-RAS cells formed colonies in soft agar (Fig. 4C), failed to exhibit contact inhibition (Fig. 3D), and displayed an altered morphology (Fig. 3E) but failed to form tumors in immunocompromised hosts. The reasons for this observation are unclear, but previous reports suggest that tumor formation depends critically on the level of RAS overexpression (15). Indeed, the level of RAS overexpression we were able to achieve in TIG3-TDM-shRB-shPTEN-RAS compared to control cells was significantly less than in TIG3-TDM-shRB-ST-RAS cells compared to control cells (Fig. 3A and 4A, compare lanes 3 and 4 in each panel). Thus, these observations suggest that the suppression of PTEN rendered cells unable to tolerate sufficiently high levels of RAS to allow such cells to form tumors in immunocompromised animals.
To determine whether other alterations in the PI3K pathway could similarly replace ST in the transformation of human cells, we also tested the capacity of a constitutively active PI3K allele (myr-p110α) to transform TIG3- and WI38-TDM-shRB-RAS cells (data not shown). Although we found constitutive phosphorylation of AKT under both starved and stimulated conditions, these cells were unable to grow in an anchorage-independent manner (data not shown). In summary, these observations suggest that PTEN loss and PI3K activation by this mutant allele do not act equivalently in this system of human cell transformation.
Although the coexpression of DD, Myc, and RAS transforms primary murine cells, the transformation of comparable human fibroblasts requires the additional expression of hTERT, shRB, and shPTEN or ST (Fig. 6). These results corroborate and extend a recent report in which mutant LT and RAS alleles were used to study human and murine transformation requirements (40). Whereas mutant LT proteins that disable only the p53 or RB pathways cooperated with RAS to transform murine cells, coexpression of a wild-type LT with the expression of hTERT, ST, and RAS was necessary to transform human cells (20, 40). Similarly, shRNAs specific for p53 and RB cooperate with hTERT, ST, and RAS to transform BJ fibroblasts (52). These findings suggest that LT contributes to human cell transformation solely by inactivating the p53 and RB tumor suppressor pathways. However, Wei et al. (56) found that deletion of p53 by homologous recombination failed to cooperate with DK, hTERT, RAS, and ST to transform LF1 cells, suggesting that the manner in which p53 inactivation is accomplished also contributes to transformation. In the studies presented here, we used the p53 DD mutant (46), which acts in a dominant manner to functionally inactivate the p53 pathway. Since recent work indicates that specific p53 mutations found in tumors derived from patients with Li-Fraumeni syndrome exhibit gain-of-function phenotypes in addition to inactivating p53 (28, 37), it remains possible that such mutants contribute to transformation differently than DD. Nevertheless, these observations highlight the importance of inactivation of both the p53 and RB pathways in human cell transformation.
Murine and human cells also show divergent requirements with respect to the downstream RAS effector pathways that participate in transformation. Although the activation of the Ral-GEF signaling pathway participates in the transformation of multiple types of human cells, activation of the Raf pathway plays a predominant role in the transformation of MEFs (21, 40). Furthermore, although transformation of human fibroblasts requires the activation of the Raf and Ral-GEF RAS effector pathways, the transformation of human embryonic kidney cells requires the perturbation of the PI3K and Ral-GEF pathways, whereas the transformation of HMECs requires the combined activation of the Raf, Ral-GEF, and PI3K pathways (40). These observations demonstrate that different human cell types show disparate requirements for activation of the pathways downstream of RAS. Taken together, these studies highlight pathways that differentially govern the transformation of human and murine cells.
The studies presented here provide a strategy for the construction of additional human cell lines transformed with mutations found associated with human tumors. Drayton et al. have used a complementary approach to dissect the additional alterations that cooperate with Myc and RAS in the transformation of human cells (14). These authors used p16INK4A-deficient cells derived from a patient harboring a homozygous deletion in the INK4A/ARF locus (Leiden cells). Although these cells fail to express p16INK4A, they produce a truncated protein that retains many functions associated with p14ARF (10). Coexpression of hTERT, Myc, and RAS in Leiden cells permitted such cells to form tumors at long latency and low frequency (14), suggesting that an additional unknown lesion(s) participates in tumor formation in these cells. Both studies, however, reinforce the notion that inactivation of the RB pathway is necessary to achieve human cell transformation. Taken together, these studies provide a framework for studying the pathways involved in malignant transformation in cancer-susceptible patients.
The experimental models described here suggest that similar manipulations in specific types of human cells will permit the development of experimental cancer cell models that recapitulate particular stages of human tumors. Because cell culture models permit the rapid generation of closely matched cell lines that differ by the expression of single alleles, such experimental models complement efforts to develop human cancer models in mice. Moreover, these models will facilitate both forward and reverse genetic approaches to understanding the combinatorial associations that lead to cancer.
Acknowledgments
We thank Jason Arroyo, Kenkichi Masutomi, and Richard Possemato for helpful comments on the manuscript and all of the members of the Hahn and Cichowski laboratories for advice and encouragement.
This study was supported in part by grants from the U.S. National Cancer Institute (K01 CA94223 [W.C.H.] and PO1 CA50661 [W.C.H.])and the Doris Duke Charitable Foundation (W.C.H.).
REFERENCES
- 1.Allsopp, R. C., H. Vaziri, C. Patterson, S. Goldstein, E. V. Younglai, A. B. Futcher, C. W. Greider, and C. B. Harley. 1992. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 89:10114-10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andjelkovic, M., T. Jakubowicz, P. Cron, X. F. Ming, J. W. Han, and B. A. Hemmings. 1996. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc. Natl. Acad. Sci. USA 93:5699-5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, B. W., D. Boettiger, E. Spooncer, and J. D. Norton. 1992. Efficient retroviral-mediated gene transfer into human B lymphoblastoid cells expressing mouse ecotropic viral receptor. Nucleic Acids Res. 20:5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beausejour, C. M., A. Krtolica, F. Galimi, M. Narita, S. W. Lowe, P. Yaswen, and J. Campisi. 2003. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 22:4212-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benanti, J. A., and D. A. Galloway. 2004. Normal human fibroblasts are resistant to RAS-induced senescence. Mol. Cell. Biol. 24:2842-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger, R., P. G. Febbo, P. K. Majumder, J. J. Zhao, S. Mukherjee, S. Signoretti, K. T. Campbell, W. R. Sellers, T. M. Roberts, M. Loda, T. R. Golub, and W. C. Hahn. 2004. Androgen-induced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 64:8867-8875. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 8.Bouchard, C., K. Thieke, A. Maier, R. Saffrich, J. Hanley-Hyde, W. Ansorge, S. Reed, P. Sicinski, J. Bartek, and M. Eilers. 1999. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 18:5321-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broccoli, D., J. W. Young, and T. de Lange. 1995. Telomerase activity in normal and malignant hematopoietic cells. Proc. Natl. Acad. Sci. USA 92:9082-9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brookes, S., J. Rowe, M. Ruas, S. Llanos, P. A. Clark, M. Lomax, M. C. James, R. Vatcheva, S. Bates, K. H. Vousden, D. Parry, N. Gruis, N. Smit, W. Bergman, and G. Peters. 2002. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. EMBO J. 21:2936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campisi, J. 2001. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 11:S27-31. [DOI] [PubMed] [Google Scholar]
- 12.Chen, W., R. Possemato, K. T. Campbell, C. A. Plattner, D. C. Pallas, and W. C. Hahn. 2004. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5:127-136. [DOI] [PubMed] [Google Scholar]
- 13.Counter, C. M., W. C. Hahn, W. Wei, S. D. Caddle, R. L. Beijersbergen, P. M. Lansdorp, J. M. Sedivy, and R. A. Weinberg. 1998. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl. Acad. Sci. USA 95:14723-14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drayton, S., J. Rowe, R. Jones, R. Vatcheva, D. Cuthbert-Heavens, J. Marshall, M. Fried, and G. Peters. 2003. Tumor suppressor p16INK4a determines sensitivity of human cells to transformation by cooperating cellular oncogenes. Cancer Cell 4:301-310. [DOI] [PubMed] [Google Scholar]
- 15.Elenbaas, B., L. Spirio, F. Koerner, M. D. Fleming, D. B. Zimonjic, J. L. Donaher, N. C. Popescu, W. C. Hahn, and R. A. Weinberg. 2001. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 15:50-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finlay, C. A., P. W. Hinds, and A. J. Levine. 1989. The p53 proto-oncogene can act as a suppressor of transformation. Cell 57:1083-1093. [DOI] [PubMed] [Google Scholar]
- 17.Gil, J., P. Kerai, M. Lleonart, D. Bernard, J. C. Cigudosa, G. Peters, A. Carnero, and D. Beach. 2005. Immortalization of primary human prostate epithelial cells by c-Myc. Cancer Res. 65:2179-2185. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg, R. A., R. C. O'Hagan, H. Deng, Q. Xiao, S. R. Hann, R. R. Adams, S. Lichtsteiner, L. Chin, G. B. Morin, and R. A. DePinho. 1999. Telomerase reverse transcriptase gene is a direct target of c-Myc but is not functionally equivalent in cellular transformation. Oncogene 18:1219-1226. [DOI] [PubMed] [Google Scholar]
- 19.Hahn, W. C., C. M. Counter, A. S. Lundberg, R. L. Beijersbergen, M. W. Brooks, and R. A. Weinberg. 1999. Creation of human tumour cells with defined genetic elements. Nature 400:464-468. [DOI] [PubMed] [Google Scholar]
- 20.Hahn, W. C., S. K. Dessain, M. W. Brooks, J. E. King, B. Elenbaas, D. M. Sabatini, J. A. DeCaprio, and R. A. Weinberg. 2002. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol. 22:2111-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamad, N. M., J. H. Elconin, A. E. Karnoub, W. Bai, J. N. Rich, R. T. Abraham, C. J. Der, and C. M. Counter. 2002. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 16:2045-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayflick, L., and P. S. Moorhead. 1961. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25:585-621. [DOI] [PubMed] [Google Scholar]
- 23.Hermeking, H., C. Rago, M. Schuhmacher, Q. Li, J. F. Barrett, A. J. Obaya, B. C. O'Connell, M. K. Mateyak, W. Tam, F. Kohlhuber, C. V. Dang, J. M. Sedivy, D. Eick, B. Vogelstein, and K. W. Kinzler. 2000. Identification of CDK4 as a target of c-MYC. Proc. Natl. Acad. Sci. USA 97:2229-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91:649-659. [DOI] [PubMed] [Google Scholar]
- 25.Kim, N. W., and F. Wu. 1997. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res. 25:2595-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kipling, D., and H. J. Cooke. 1990. Hypervariable ultra-long telomeres in mice. Nature 347:400-402. [DOI] [PubMed] [Google Scholar]
- 27.Land, H., L. F. Parada, and R. A. Weinberg. 1983. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 304:596-602. [DOI] [PubMed] [Google Scholar]
- 28.Lang, G. A., T. Iwakuma, Y. Suh, G. Liu, A. Rao, J. M. Parant, Y. A. Valentin-Vega, T. Terzian, L. C. Caldwell, L. C. Strong, A. K. El-Naggar, and G. Lozano. 2004. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119:861-872. [DOI] [PubMed] [Google Scholar]
- 29.Lazarov, M., Y. Kubo, T. Cai, M. Dajee, M. Tarutani, Q. Lin, M. Fang, S. Tao, C. L. Green, and P. A. Khavari. 2002. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nat. Med. 8:1105-1114. [DOI] [PubMed] [Google Scholar]
- 30.Masutomi, K., E. Y. Yu, S. Khurts, I. Ben-Porath, J. L. Currier, G. B. Metz, M. W. Brooks, S. Kaneko, S. Murakami, J. A. DeCaprio, R. A. Weinberg, S. A. Stewart, and W. C. Hahn. 2003. Telomerase maintains telomere structure in normal human cells. Cell 114:241-253. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo, M., K. Kaji, T. Utakoji, and K. Hosoda. 1982. Ploidy of human embryonic fibroblasts during in vitro aging. J. Gerontol. 37:33-37. [DOI] [PubMed] [Google Scholar]
- 32.Metz, T., A. W. Harris, and J. M. Adams. 1995. Absence of p53 allows direct immortalization of hematopoietic cells by the myc and raf oncogenes. Cell 82:29-36. [DOI] [PubMed] [Google Scholar]
- 33.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mungre, S., K. Enderle, B. Turk, A. Porras, Y. Q. Wu, M. C. Mumby, and K. Rundell. 1994. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small-t antigen in vitro decrease viral transformation. J. Virol. 68:1675-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura, T. M., G. B. Morin, K. B. Chapman, S. L. Weinrich, W. H. Andrews, J. Lingner, C. B. Harley, and T. R. Cech. 1997. Telomerase catalytic subunit homologs from fission yeast and human. Science 277:955-959. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien, W., G. Stenman, and R. Sager. 1986. Suppression of tumor growth by senescence in virally transformed human fibroblasts. Proc. Natl. Acad. Sci. USA 83:8659-8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olive, K. P., D. A. Tuveson, Z. C. Ruhe, B. Yin, N. A. Willis, R. T. Bronson, D. Crowley, and T. Jacks. 2004. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119:847-860. [DOI] [PubMed] [Google Scholar]
- 38.Peeper, D. S., J. H. Dannenberg, S. Douma, H. te Riele, and R. Bernards. 2001. Escape from premature senescence is not sufficient for oncogenic transformation by Ras. Nat. Cell Biol. 3:198-203. [DOI] [PubMed] [Google Scholar]
- 39.Prowse, K. R., and C. W. Greider. 1995. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc. Natl. Acad. Sci. USA 92:4818-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rangarajan, A., S. J. Hong, A. Gifford, and R. A. Weinberg. 2004. Species- and cell type-specific requirements for cellular transformation. Cancer Cell 6:171-183. [DOI] [PubMed] [Google Scholar]
- 41.Rao, R. N., N. B. Stamm, K. Otto, S. Kovacevic, S. A. Watkins, P. Rutherford, S. Lemke, K. Cocke, R. P. Beckmann, K. Houck, D. Johnson, and B. J. Skidmore. 1999. Conditional transformation of rat embryo fibroblast cells by a cyclin D1-cdk4 fusion gene. Oncogene 18:6343-6356. [DOI] [PubMed] [Google Scholar]
- 42.Ruley, H. E. 1983. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature 304:602-606. [DOI] [PubMed] [Google Scholar]
- 43.Rundell, K., and R. Parakati. 2001. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin. Cancer Biol. 11:5-13. [DOI] [PubMed] [Google Scholar]
- 44.Seger, Y. R., M. Garcia-Cao, S. Piccinin, C. L. Cunsolo, C. Doglioni, M. A. Blasco, G. J. Hannon, and R. Maestro. 2002. Transformation of normal human cells in the absence of telomerase activation. Cancer Cell 2:401-413. [DOI] [PubMed] [Google Scholar]
- 45.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 46.Shaulian, E., A. Zauberman, D. Ginsberg, and M. Oren. 1992. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol. Cell. Biol. 12:5581-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shay, J. W., and W. E. Wright. 1989. Quantitation of the frequency of immortalization of normal human diploid fibroblasts by SV40 large T-antigen. Exp. Cell Res. 184:109-118. [DOI] [PubMed] [Google Scholar]
- 48.Sinn, E., W. Muller, P. Pattengale, I. Tepler, R. Wallace, and P. Leder. 1987. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell 49:465-475. [DOI] [PubMed] [Google Scholar]
- 49.Stevenson, M., and D. J. Volsky. 1986. Activated v-myc and v-ras oncogenes do not transform normal human lymphocytes. Mol. Cell. Biol. 6:3410-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson, T. C., J. Southgate, G. Kitchener, and H. Land. 1989. Multistage carcinogenesis induced by ras and myc oncogenes in a reconstituted organ. Cell 56:917-930. [DOI] [PubMed] [Google Scholar]
- 51.Vaziri, H., and S. Benchimol. 1998. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8:279-282. [DOI] [PubMed] [Google Scholar]
- 52.Voorhoeve, P. M., and R. Agami. 2003. The tumor-suppressive functions of the human INK4A locus. Cancer Cell 4:311-319. [DOI] [PubMed] [Google Scholar]
- 53.Wang, J., L. Y. Xie, S. Allan, D. Beach, and G. J. Hannon. 1998. Myc activates telomerase. Genes Dev. 12:1769-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei, S., and J. M. Sedivy. 1999. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 59:1539-1543. [PubMed] [Google Scholar]
- 55.Wei, W., U. Herbig, S. Wei, A. Dutriaux, and J. M. Sedivy. 2003. Loss of retinoblastoma but not p16 function allows bypass of replicative senescence in human fibroblasts. EMBO Rep. 4:1061-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei, W., W. A. Jobling, W. Chen, W. C. Hahn, and J. M. Sedivy. 2003. Abolition of cyclin-dependent kinase inhibitor p16Ink4a and p21Cip1/Waf1 functions permits Ras-induced anchorage-independent growth in telomerase-immortalized human fibroblasts. Mol. Cell. Biol. 23:2859-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Westphal, R. S., R. L. Coffee, Jr., A. Marotta, S. L. Pelech, and B. E. Wadzinski. 1999. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J. Biol. Chem. 274:687-692. [DOI] [PubMed] [Google Scholar]
- 58.Wu, K. J., C. Grandori, M. Amacker, N. Simon-Vermot, A. Polack, J. Lingner, and R. Dalla-Favera. 1999. Direct activation of TERT transcription by c-MYC. Nat. Genet. 21:220-224. [DOI] [PubMed] [Google Scholar]
- 59.Yu, J., A. Boyapati, and K. Rundell. 2001. Critical role for SV40 small-t antigen in human cell transformation. Virology 290:192-198. [DOI] [PubMed] [Google Scholar]
- 60.Zhao, J. J., O. V. Gjoerup, R. R. Subramanian, Y. Cheng, W. Chen, T. M. Roberts, and W. C. Hahn. 2003. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell 3:483-495. [DOI] [PubMed] [Google Scholar]