Abstract
Previous studies have indicated that the stem cell leukemia gene (SCL) is essential for both embryonic and adult erythropoiesis. We have examined erythropoiesis in conditional SCL knockout mice for at least 6 months after loss of SCL function and report that SCL was important but not essential for the generation of mature red blood cells. Although SCL-deleted mice were mildly anemic with increased splenic erythropoiesis, they responded appropriately to endogenous erythropoietin and hemolytic stress, a measure of late erythroid progenitors. However, SCL was more important for the proliferation of early erythroid progenitors because the predominant defects in SCL-deleted erythropoiesis were loss of in vitro growth of the burst-forming erythroid unit and an in vivo growth defect revealed by transplant assays. With respect to erythroid maturation, SCL-deleted proerythroblasts could generate more mature erythroblasts and circulating red blood cells. However, SCL was required for normal expression of TER119, one of the few proposed target genes of SCL. The unexpected finding that SCL-independent erythropoiesis can proceed in the adult suggests that alternate factors can replace the essential functions of SCL and raises the possibility that similar mechanisms also explain the relatively minor defects previously observed in SCL-null hematopoietic stem cells.
The production of mature hematopoietic cells involves a step-wise process from the multipotent hematopoietic stem cell, to lineage-committed progenitors, and finally to mature blood cells (18). In the case of erythropoiesis, the erythroid-committed progenitor, the burst-forming erythroid unit (BFU-E) arises from a megakaryocyte-erythroid progenitor (MEP) (22). BFU-Es give rise to the progenitors of more limited proliferative capacity, termed erythroid CFUs (CFU-Es), which in turn, generate proerythroblasts, the earliest postmitotic erythroid cell. Subsequent erythroid maturation from the proerythroblast to late normoblast just prior to enucleation can be monitored by expression of the cell surface markers CD71 and TER119 (32). In response to erythroid stress, erythropoietin (EPO) acts at multiple levels of erythropoiesis to induce red blood cell production (11). The glucocorticoid pathway is also important for normal erythroid response to hemolytic stress (39).
The stem cell leukemia gene (SCL) encodes a basic helix-loop-helix (bHLH) protein first cloned from a leukemic translocation (3). SCL is critical for the formation of primitive erythropoiesis in embryonic development (25, 27, 28, 31). In adult hematopoiesis, SCL is also believed to be essential for erythropoiesis. SCL is expressed in erythroid progenitors and maintained throughout erythroid development, with levels peaking at the CFU-E stage (4). Enforced expression of SCL favored erythroid proliferation and differentiation in cell lines and primary hematopoietic progenitors (7, 33). Continued expression of SCL in erythroid cells appears to be essential for erythropoiesis, as an SCL transgene expressed in hematopoietic progenitors but not erythroid cells failed to rescue erythropoiesis in SCL knockout embryos (30). More recently, analyses of conditional SCL knockout mice have supported the hypothesis that SCL is critical for adult erythropoiesis. Immediately after loss of SCL expression, in vitro and in vivo growth of erythroid progenitors was absent (8, 20). Furthermore, competitive transplant assays suggested that SCL was essential for mature red blood cell formation with a block in maturation at the CD71pos TER119low proerythroblast stage (5, 20). Thus, analyses of SCL-conditional knockout mice suggest that SCL is essential for maturation beyond the proerythroblast stage.
Within erythroid cells, SCL was identified within large protein complexes comprising not only its E-protein partner, but also LMO2, Lbd-1, GATA-1, pRb, and Sp1 (15, 36, 38). This SCL complex regulates transcription at promoters containing E-box GATA motifs and has been reported to positively and negatively modulate expression of target genes. Proposed erythroid target genes of this complex include the gene encoding the receptor for stem cell factor c-kit and the red blood cell membrane proteins glycophorin A and protein 4.2 (12, 14, 15, 41). Other potential erythroid targets of SCL include GATA-1 and EKLF, a transcription factor essential for expression of adult globin (1, 37). Thus, SCL is predicted to regulate erythroid commitment, proliferation, and maturation.
In this study, we have examined the long-term consequences for erythropoiesis of deleting SCL by using conditional SCL knockout mice. In light of the absence of BFU-E and a block in differentiation at the proerythroblast stage in SCL-deleted mice, we predicted that SCL would be essential for the production of mature red blood cells (5, 8, 20). Surprisingly, we found that SCL was important but not essential for continuing adult erythropoiesis, including a response to erythropoietic stress. This unexpected finding suggests that unlike in development, alternate factors or pathways can replace the essential functions of SCL in adult erythropoiesis.
MATERIALS AND METHODS
Generation of SCL-deleted mice.
Mice with a loxP-targeted SCL allele (SCLloxP) were generated as previously described (8). Mice with the lacZ reporter gene knocked in to the SCL locus were used as a source of an SCL null allele (SCL−) (6). The MxCre transgenic mice have been previously reported (13). Deletion of the SCLloxP allele was achieved by injecting 300 μg poly(I:C) (Sigma Chemical Company, St Louis, MO) dissolved in normal saline intraperitoneally (i.p.) on alternate days for three doses. Poly(I:C)-treated MxCre SCL−/loxP and MxCre SCL+/loxP mice generated SCL-deleted (SCL−/Δ) and control (SCL+/Δ)mice, respectively. All analyses were performed at least 4 weeks after administration of poly(I:C). Deletion of the SCLloxP allele was determined by Southern blotting by using an SCL probe that distinguished loxP, Δ, wild-type, and null SCL alleles (8).
Peripheral blood analysis.
For whole-blood counts, 250 μl of blood was collected from the retro-orbital plexus into tubes containing potassium EDTA (Sarstedt, Nümbrecht, Germany), and blood counts were analyzed with an Avidia 120 automated hematological analyzer (Bayer, Tarrytown, NY). Serum bilirubin was measured using the modified Pearlman and Lee method. Serum lactate dehydrogenase was measured by a photometric UV method. Red blood cell survival was measured by using direct in vivo biotinylation of erythrocytes (9). To induce erythropoiesis, mice were either given a single i.p. injection of darbepoetin (30 μg/kg of body weight; Amgen, Inc., Thousand Oaks, CA) or two i.p. injections of phenylhydrazine (60 mg/kg; Sigma) on consecutive days.
Flow cytometry and sorting.
For reticulocyte analysis and expression of TER119 on peripheral blood red blood cells, 5-μl aliquots of blood were incubated in 1 ml of thiazole orange (1 μg/ml; Sigma) and a saturating concentration of TER119-PE (BD Pharmingen, San Diego, CA) for 30 min in the dark and then analyzed with a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA). For analyses of red blood cell maturation, single-cell suspensions of bone marrow were stained with saturating concentrations of CD71-fluorescein isothiocyanate (FITC); TER119-PE; biotinylated nonerythroid lineage markers Mac-1α, B220, CD3 and Gr-1; and streptavidin-allophycocyanin conjugate (BD Pharmingen, San Diego, CA). Apoptosis of erythroid subpopulations were analyzed by costaining with annexin V-FITC (BD Pharmingen) and 7-AAD (Sigma). Immature cell populations were identified using CD34-FITC, CD16/CD32-PE, and biotinylated lineage markers including TER119, biotinylated interleukin 7 (IL-7) receptor α-chain, biotinylated Sca-1, and c-kit-allophycocyanin. Biotinylated antibodies were recognized with streptavidin-peridinin chlorophyll protein, and samples were analyzed with an LSR flow cytometer (BD Biosciences). Cells for Southern and gene expression analyses were sorted with a FACSVantage SE system (BD Biosciences); after sorting, cell purity of >90% was confirmed.
Real-time PCR.
Real-time PCR analysis of gene expression was conducted using a Rotorgene 2000 instrument (Corbett Research, Sydney). Amplification of cDNA products was followed using the fluorescent DNA-binding dye SybrGreen (Molecular Probes, Oregon) at a dilution of 1:10,000. Gene expression of erythroid genes was normalized to expression of hypoxanthine phosphoribosyltransferase, and data are expressed as a percentage of the wild type. Gene-specific primer sequences are available on request.
BFU-E and CFU-E assays.
Single-cell suspensions of bone marrow and spleen were plated in 0.9% methylcellulose, 20% fetal calf serum, Iscove's modified Dulbecco medium plus either 10 ng/ml murine interleukin 3 (R&D Systems, Minneapolis, MN), 50 ng/ml rat stem cell factor (Amgen, Inc.), and 4 U/ml human EPO (Amgen, Inc.) (for BFU-Es) or 4 U/ml EPO alone (for CFU-Es). Colonies were scored at day 7 for BFU-Es and day 2 for CFU-Es with an inverted phase-contrast microscope. Plates were stained with a solution of diaminobenzidine and hydrogen peroxide to confirm erythroid lineage.
In vivo assays.
Bone marrow recipients were lethally irradiated with 2 doses of 550 rad administered 3 h apart from a 60Co gamma source at a dose rate of 45 rad/min 2 to 4 h before transplantation. Bone marrow cells for transplant were harvested by flushing femurs and tibias with phosphate-buffered saline-5% fetal bovine serum using a 21-gauge needle. Viability of single-cell suspensions was checked by trypan blue exclusion. The CFU-S assay was performed by injection of 3 × 104 bone marrow cells per recipient, and spleen colonies were analyzed on day 12. For competitive repopulation assays, age- and sex-matched B6-Hbbd (Ptprca [Ly5.1]) congenic mice were used as a source of competitor marrow cells and as recipients. Mice used as a source of competitor cells were not exposed to poly(I:C). Transplanted mice were fed antibiotic-treated water and maintained in hooded cages for the first 4 weeks. A minimum of three recipients were used for each donor cell population. The total donor cell dose was 2 × 106 cells. Hemoglobin electrophoresis was used to determine the donor erythroid contribution (40).
RESULTS
SCL is important but not essential for long-term adult erythropoiesis.
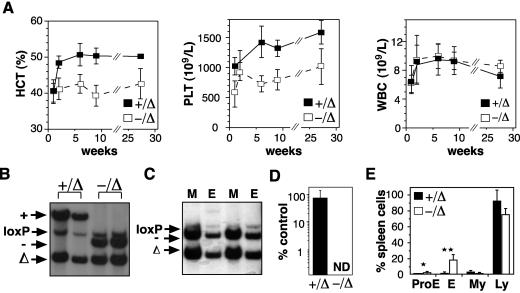
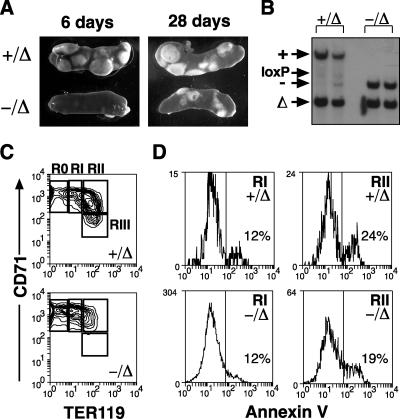
We have used a conditional knockout of SCL to examine its function in adult hematopoiesis (8). Six days after deletion of SCL, when no SCL transcript was detectable, we observed a complete absence of BFU-E and a total lack of erythroid contribution to CFU-S12 (8). These findings, together with previous reports, suggested that SCL was essential for effective erythropoiesis. We predicted that deletion of SCL in the adult mouse would lead to progressive anemia and death of the animal within weeks or months. Remarkably, SCL-deleted mice (SCL−/Δ) remained alive for >6 months after loss of SCL expression, with only mild anemia and thrombocytopenia (Fig. 1A). White blood cell number was not affected by loss of SCL.
FIG. 1.
SCL is not required for adult erythropoiesis. (A) Peripheral blood counts of control (+/Δ) and SCL-deleted (−/Δ) mice following administration of poly(I:C). A minimum of six mice for each time point were used to calculate the means and standard deviations. (B) Spleens from mice 4 weeks after administration of poly(I:C) were analyzed by Southern blotting for the presence of wild-type (+), null (−), loxP-flanked (loxP), and loxP-deleted (Δ) SCL alleles. (C) Southern blot of purified Mac-1+ (M) and TER119+ (E) cells from SCL-deleted mice. (D) Expression of SCL mRNA measured by real-time PCR in bone marrow cells from control (+/Δ) and SCL-deleted (−/Δ) mice harvested 4 weeks after administration of poly(I:C) compared with bone marrow cells from wild-type mice. The means and standard deviations were calculated for three mice of each genotype. ND, not detected. (E) Comparison of cellular content of spleens from control (+/Δ) and SCL-deleted (−/Δ) mice at least 4 weeks after poly(I:C). Cell types were counted for four mice of each genotype. ProE, proerythroblast; E, normoblast; My, granulocyte; Ly, lymphocyte. *, P < 0.05; **, P < 0.01.
To determine whether the ongoing erythropoiesis in SCL-deleted mice was due to expansion of nondeleted, SCL-expressing erythroid progenitors, we analyzed bone marrow and spleen samples from SCL-deleted mice by Southern blotting at least 4 weeks after poly(I:C)-induced deletion. This demonstrated the presence of approximately 10% nondeleted cells (SCL−/loxP) in the spleens of SCL-deleted mice (Fig. 1B). However, a similar proportion of nondeleted cells (SCL+/loxP) were observed in the spleens of control mice, suggesting that the presence of nondeleted cells was not due to selective expansion. To formally exclude the possibility that nondeleted cells in the spleen were contributing significantly to erythropoiesis in SCL-deleted mice, we analyzed fluorescence-activated cell sorter (FACS)-purified erythroblasts by Southern blotting. The majority of TER119pos and Mac-1pos cells from the spleen had an SCL-deleted genotype (SCL−/Δ), indicating that most erythroblasts were truly SCL deleted (Fig. 1C). FACS-purified CD71neg TER119pos normoblasts also had an SCL-deleted genotype, showing that erythroid maturation was not blocked at an early stage (data not shown), as previously suggested (20). Finally, quantitative real-time PCR of whole bone marrow (Fig. 1D) and spleen cells (data not shown) from SCL-deleted mice demonstrated no detectable SCL mRNA. The absence of SCL mRNA despite the presence of nondeleted cells (Fig. 1B) is most likely explained by failure of the MxCre transgene to excise the SCL-targeted locus in cell types, such as stromal cells or lymphocytes, which do not express SCL. Together, these results demonstrated that SCL was not required for ongoing adult erythropoiesis.
Although SCL was not essential for adult erythropoiesis, SCL was required for normal erythropoiesis: SCL-deleted mice were anemic with enlarged spleens (175 ± 60 mg compared with 109 ± 29 mg in controls; 24 mice) containing sixfold more erythroblasts (Fig. 1E). While red blood cell number and hemoglobin were reduced, red blood cell size and hemoglobin content were normal, suggesting that iron metabolism and globin production were not impaired (Table 1). There was also no evidence of a significant hemolytic component to the anemia: the reticulocyte percentage and serum bilirubin and lactate dehydrogenase levels were not significantly elevated in SCL-deleted animals (Table 1). Furthermore, red blood cell survival assayed by in vivo biotinylation was not significantly reduced in SCL-deleted mice (Table 1). From these analyses, defects in iron metabolism, globin production, or the red blood cell membrane are unlikely to be a significant contributors to the anemia observed with SCL-deleted mice.
TABLE 1.
Red blood cell parameters of SCL-deleted mice
| Genotype | Parameter
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| RCC (106/μl) | HGB (g/dl) | HCT (%) | MCV (fl) | MCHC (g/dl) | Reticulocytes (%) | Bilirubin (μM) | LDa | Red blood cell survivalb | |
| Δ/+ | 10.1 ± 0.4c | 15.8 ± 0.5 | 50.0 ± 2.3 | 49.5 ± 1.9 | 31.6 ± 0.8 | 3.0 ± 0.5 | 6.7 ± 4.7 | 708 ± 141 | 20 |
| Δ/− | 8.5 ± 0.6 | 13.1 ± 0.9 | 41.2 ± 2.7 | 48.5 ± 2.0 | 31.9 ± 0.5 | 3.8 ± 0.6 | 9.7 ± 4.0 | 672 ± 184 | 17 |
| P valued | <0.01 | <0.01 | <0.01 | NS | NS | NS | NS | NS | NS |
LD, lactate dehydrogenase.
Number of days for 50% loss of biotinylated red blood cells.
Mean ± standard deviation calculated from at least 15 mice of each genotype between 5 and 8 weeks after administration of poly(I:C).
Statistical comparison by two-way unpaired Student's t test.
SCL is not required for a normal erythroid stress response.
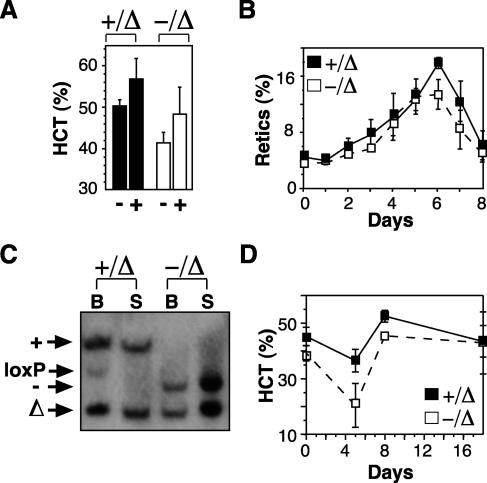
Our analyses of SCL-deleted mice demonstrated that SCL was not essential for steady-state red blood cell or platelet production. However, it was possible that SCL was required for emergency erythropoiesis. Therefore, we tested the response of SCL-deleted mice to two different types of erythropoietic stress: pharmacologic levels of erythropoietin and acute hemolysis. Surprisingly, SCL-deleted mice responded normally to administration of erythropoietin (darbepoetin alfa), with an elevation in hematocrit to the normal range (Fig. 2A). The kinetics of the response, assessed by reticulocyte count, was also similar to that of controls (Fig. 2B). Southern blotting of bone marrow and spleen from SCL-deleted mice demonstrated that the expansion of the erythron was due solely to increased numbers of SCL-deleted erythroid cells (Fig. 2C). Response to phenylhydrazine-induced hemolysis, a stress requiring rapid proliferation of late erythroid progenitors mediated by the glucocorticoid receptor (39), was also preserved (Fig. 2D). These results indicate that SCL was dispensable not only for steady-state erythropoiesis but was not required for a functional erythroid stress response.
FIG. 2.
SCL is not required for an erythroid stress response. (A) Control mice (+/Δ) and SCL-deleted mice (−/Δ) were injected with saline (−) or a single dose of darbepoetin alfa (+), and hematocrit (HCT) was measured on day 8. The means and SD of samples from six mice for each group are shown. (B) Mice injected with darbepoetin alfa were bled each day to assess the reticulocyte response. (C) Southern blot of bone marrow (B) and spleen (S) from darbepoetin alfa-treated mice probed for wild type (+), null (−), loxP-flanked (loxP), and loxP-deleted (Δ) SCL alleles. (D) Hematocrit levels in mice (n = 3) injected with phenylhydrazine on days 0 and 1.
SCL is not essential for maturation of proerythroblasts.
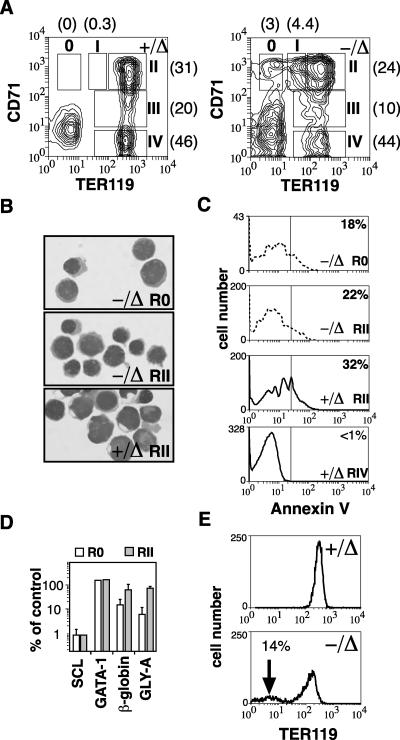
The ability to form erythroid cells in the absence of SCL allowed us to examine its function in erythroid maturation. Erythroid maturation was examined by flow cytometry using the cell surface markers CD71 and TER119, which have been used to characterize four distinct stages of erythroid differentiation (32). Using these markers, proerythroblasts are CD71pos TER119lo (region I), and maturation proceeds through to late erythroblasts that are CD71neg TER119pos (region IV). Loss of SCL consistently resulted in a two- to fourfold reduction in TER119 expression on TER119pos erythroid cells (regions II to IV) (Fig. 3A). In addition, there was the appearance of erythroid cells with the phenotype CD71pos TER119neg (designated region 0). This population was never observed with nondeleted controls. The means and standard deviation (SD) of the proportion of erythroid cells in each region demonstrated a shift toward more immature erythroid cells in SCL-deleted mice (Fig. 3A), which was consistent with the presence of proerythroblasts within the spleen (Fig. 1B). Cytospin preparations of sorted CD71pos TER119neg cells from SCL-deleted mice (region 0) demonstrated a proerythroblast morphology similar to cells located in region II of controls and SCL-deleted mice (Fig. 3B). SCL was not required for survival of erythroblasts because there were comparable numbers of apoptotic cells in control proerythroblasts (region II) and SCL-deleted proerythroblasts (regions 0 and II) (Fig. 3C). To further characterize the CD71pos TER119neg SCL-deleted cells, we examined gene expression by real-time PCR. Region 0 cells were compared with region II cells from SCL-deleted and control mice. GATA-1 levels were similar in all three cell populations; however, expression of two more mature erythroid genes, β major globin and glycophorin A, in purified cells from region 0 were approximately 10% of that observed with CD71pos TER119pos cells from control or SCL-deleted mice, suggesting that these cells were more primitive (Fig. 3D).
FIG. 3.
SCL is not required for maturation of proerythroblasts. (A) Expression of CD71 and TER119 on bone marrow cells from control (+/Δ) and SCL-deleted (−/Δ) mice. The regions corresponding to erythroid maturation (regions I to IV) according to Socolovsky et al. (32) are shown. Note the approximate fourfold decrease in intensity of staining with TER119 compared with control cells and the appearance of a CD71pos TER119neg population (region 0). The proportion of red blood cells in each region is shown in parentheses and was calculated from six mice of each genotype. (B) Cytospins of sorted CD71pos TER119neg cells (region 0) and CD71pos TER119pos (region II) were stained with May-Grunwald-Giemsa. (C) Annexin V staining of control erythroid cells (+/Δ) and SCL-deleted erythroid cells (−/Δ) from region 0 (SCL-deleted only) and region II. The proportion of annexin-positive cells is shown. (D) Gene expression of purified cells from regions 0 and II of SCL-deleted bone marrow compared with control erythroid cells from region II. (E) Peripheral blood stained with TER119 demonstrating the presence of TER119neg erythrocytes in SCL-deleted mice.
However, as demonstrated in Fig. 1C, SCL was not essential for maturation of proerythroblasts into TER119pos erythroblasts. Examination of TER119 expression on mature circulating red blood cells also revealed features consistent with an SCL-deleted phenotype. The majority of circulating red blood cells of SCL-deleted mice had reduced expression of TER119 compared with control red blood cells (Fig. 3E). In addition, there was a minor population (10 to 20%) of red blood cells with undetectable expression of TER119. These results provide an alternate explanation for the presence of CD71pos TER119neg cells found in SCL-deleted mice (Fig. 3A) and mice transplanted with SCL-deletable bone marrow cells (20): SCL may be important for the appropriate expression of TER119 rather than for maturation of proerythroblasts as previously suggested (20).
Abnormal in vitro growth of SCL-deleted erythroid progenitors.
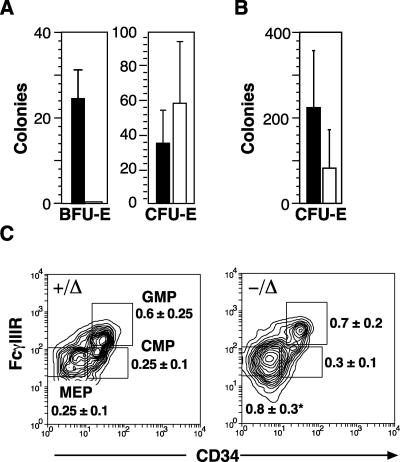
Despite ongoing erythropoiesis in SCL-deleted mice that was sufficient to maintain a near normal hematocrit and respond to erythroid stress, the in vitro progenitor growth from SCL-deleted bone marrow and spleen was markedly abnormal. While the numbers and size of CFU-Es were normal, cultures stimulated with IL-3, SCF, and EPO did not generate detectable BFU-Es (Fig. 4A). Supramaximal concentrations of cytokines, addition of dexamethasone, and culture on primary marrow stroma all failed to support erythroid colony growth from SCL-deleted bone marrow and spleen cells (data not shown). BFU-Es were also absent following erythroid stress. CFU-Es following phenylhydrazine-induced hemolysis increased in number compared with the steady state but were two- to fourfold smaller than CFU-Es from control mice (Fig. 4B). While it is impossible to enumerate BFU-Es other than by in vitro growth assays, the immediate precursor of the BFU-E, the common MEP, can be identified in the bone marrow by its characteristic cell surface phenotype, Lin− Sca-1− IL-7Rα− c-kit+ FcγRlow CD34 (22). Examination of SCL-deleted bone marrow cells by flow cytometry revealed a threefold increase in the number of MEP cells (Fig. 4C). In contrast, the numbers of common myeloid and granulocyte-macrophage progenitors were unaltered. Sorted MEP cells from SCL-deleted mice cultured in IL-3, SCF, and EPO did not generate BFU-Es or CFU-Es (data not shown). Thus, cells with the characteristic MEP phenotype were increased in the SCL-deleted mice but, like BFU-E, did not generate erythroid cells in standard in vitro growth conditions.
FIG. 4.
Absence of BFU-E in SCL-deleted mice. (A) Bone marrow cells from control (+/Δ; solid bars) and SCL-deleted mice (−/Δ; open bars) at least 28 days after poly(I:C) were grown in methylcellulose to determine erythroid progenitor cell numbers (CFU-E and BFU-E). The mean numbers and SD for three independent experiments are shown. (B) CFU-E numbers from control and SCL-deleted mice 5 days after administration of phenylhydrazine. (C) CD34 and FcγR expression on Lin− Sca-1− IL-7Rα− c-kit+ bone marrow cells from control (+/Δ) and SCL-deleted (−/Δ) mice. The regions used to calculate the number of common myeloid progenitors (CMP), granulocyte-macrophage progenitors (GMP), and MEPs relative to the total number of bone marrow mononuclear cells are indicated in the dot plots, and the mean percentage of BMMC and standard deviation were calculated from results from eight mice in each genotype.
Erythropoiesis generated from SCL-deleted CFU-S.
In light of the inability to grow BFU-E, we focused on in vivo assays to more clearly define the role of SCL in erythropoiesis. Previously, we demonstrated that 6 days after poly(I:C)-induced deletion of SCL, CFU-S12 colonies were small, pale and contained no significant erythropoiesis (8). However, when bone marrow cells were assayed 28 days after deletion of SCL, the size of CFU-S12 cells had significantly recovered compared with 6 days after deletion (Fig. 5A). Recovery of erythropoiesis was not complete, with the majority of SCL-deleted colonies 1 to 2 mm in diameter compared with >2 mm for control CFU-S12. Overall, cell numbers within SCL-deleted CFU-S12 cells were approximately fourfold lower than those for control CFU-S12 when assayed at least 28 days after deletion of SCL (data not shown). The number of CFU-S12 was also reduced twofold, consistent with a defect in short-term repopulating cells (5). Southern blotting confirmed that single CFU-S12 colonies were derived from SCL-deleted, rather than nondeleted, progenitors, thus confirming the ability of erythropoiesis to continue in the absence of SCL (Fig. 5B). Flow cytometric analysis of single colonies demonstrated a delay in maturation of SCL-deleted erythroid cells, with the majority (62%) of SCL-deleted erythroid cells in region 0 or region I compared with 19% of control erythroid cells (Fig. 5C). However, the reduced number of cells in region II was not explained by increased cell death because annexin V+ cells in regions I and II were not increased in SCL-deleted CFU-S12 colonies (Fig. 5D). Taken together, the CFU-S12 assay demonstrated significant, although not complete, recovery of erythroid contribution to SCL-deleted CFU-S12 cells during the period between 6 days and 4 weeks after poly(I:C)-induced deletion. Furthermore, contrary to previous suggestions, SCL was not required for survival or maturation of proerythroblasts.
FIG. 5.
Erythropoiesis generated from SCL-deleted CFU-S12. (A) Representative CFU-S12 generated from control (+/Δ) and SCL-deleted (−/Δ) bone marrow cells 6 days or 28 days after poly(I:C) treatment. (B) Individual CFU-S12 from control and SCL-deleted mice were picked and analyzed by Southern blot for the presence of wild-type (+), null (−), loxP-flanked (loxP), and loxP-deleted (Δ) SCL alleles. (C) Individual CFU-S12 colonies from control and SCL-deleted bone marrow cells were analyzed by flow cytometry for the expression of CD71 and TER119. Regions 0 to III are shown. (D) Expression of annexin V on cells in regions I and II. The mean percentage of positive cells is shown (three CFU-S12 colonies for each genotype).
Erythropoiesis generated from SCL-deleted hematopoietic stem cells.
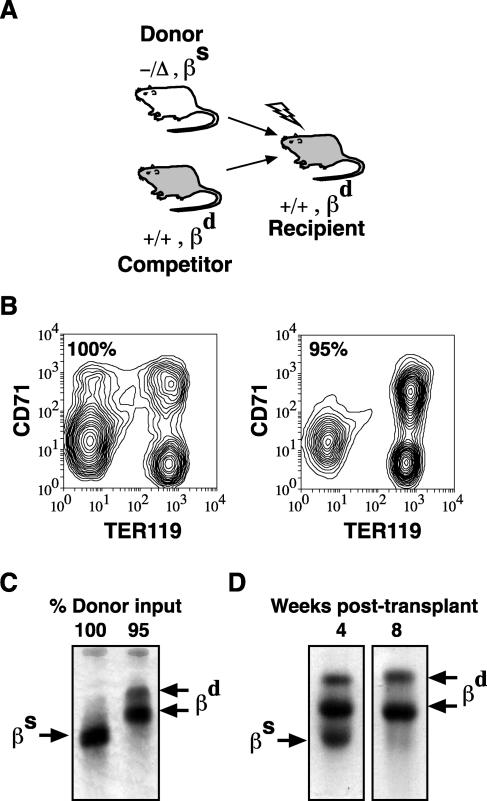
To demonstrate that SCL-independent erythropoiesis was cell intrinsic, we transplanted SCL-deleted bone marrow cells into SCL wild-type recipients (Fig. 6A). Characteristics and origin of erythroid reconstitution were analyzed by flow cytometry, hemoglobin electrophoresis, and Southern blotting. Transplant with SCL-deleted bone marrow cells in the absence of competitor cells yielded erythroid reconstitution similar to that seen with SCL-deleted mice: recipient mice were mildly anemic (data not shown), and expression of CD71 and TER119 was similar to that seen with SCL-deleted mice (Fig. 6B). Hemoglobin electrophoresis (Fig. 6C) and Southern blotting (data not shown) confirmed the SCL-deleted origin of mature red blood cells in mice transplanted in the absence of competitor cells. However, when mice were transplanted with a mixture of 95% SCL-deleted cells and 5% competitor cells, erythroid maturation 16 weeks after transplant resembled wild-type erythropoiesis (Fig. 6B) and hemoglobin (Hb) gel electrophoresis confirmed an SCL-wild type origin of mature red blood cells (Fig. 6C). The absence of SCL-deleted red blood cells in mice transplanted with competitor cells could not be explained by a stem cell defect (5), because SCL-deleted hematopoietic stem cells contributed 50 to 80% of myeloid and lymphoid reconstitution (data not shown). Thus, SCL-independent erythropoiesis was cell intrinsic but was only apparent in the absence of SCL wild-type hematopoiesis.
FIG. 6.
Transplantation of SCL-deleted erythropoiesis. (A) Schematic of transplant assay. Donor cells (SCL deleted; −/Δ) were distinguished from competitor and recipient cells (SCL wild type; +/+) by single (βs) and diffuse (βd) hemoglobin alleles. (B) Expression of CD71 and TER119 on bone marrow cells from recipient mice 16 weeks after transplantation with SCL-deleted bone marrow cells alone (100%) or with a mixture of 95% SCL-deleted and 5% competitor bone marrow cells. (C) Hb electrophoresis of recipient mice 16 weeks after transplant with 100% or 95% SCL-deleted bone marrow cells. (D) Hb electrophoresis of recipient mice 4 and 8 weeks after transplant with 95% SCL-deleted bone marrow cells.
We hypothesized that the failure to detect SCL-deleted red blood cells in competitive transplants was due to a competitive disadvantage of SCL-deleted erythroid progenitors. Indeed, when recipient mice were analyzed 4 weeks after transplantation, SCL-deleted red blood cells were readily detected despite the presence of SCL wild-type erythropoiesis (Fig. 6D). However, 8 weeks after transplantation, SCL-deleted red blood cells were at the lower limits of detection by hemoglobin electrophoresis. Thus, mature SCL-deleted erythroid cells can be formed in the presence of SCL wild-type erythropoiesis but were not detected previously because recipient mice were not analyzed sufficiently early after transplant (5, 20).
DISCUSSION
Deletion of SCL in the embryo leads to death of the animal at embryonic day 8.5 (E8.5) due to complete absence of all hematopoietic cells (28, 31). Furthermore SCL-null embryonic stem (ES) cells are unable to form primitive or definitive erythropoiesis in vitro and do not contribute to hematopoiesis in mouse chimeras (25, 27). The inability to form hematopoietic cells is due to a block at a transitional stage of development between mesoderm and hematopoietic cells (29). To circumvent the absence of hematopoietic progenitor formation in the embryo, Sanchez et al. used a selective SCL transgene to rescue early hematopoietic progenitors in SCL−/− ES cells (30). However, these transgenic mice still died with severe anemia, suggesting that continued expression of SCL in erythropoiesis was essential. Another method of circumventing the developmental defect of SCL deletion is conditional deletion of SCL in adult hematopoiesis (8, 20). Surprisingly, SCL was not essential for self-renewal or multipotent activity of hematopoietic stem cells (20). However, SCL-null progenitors did not contribute to the erythroid cell lineage in short- or long-term reconstitution assays, and erythroid progenitors did not grow in vitro (5, 8, 20). Thus, SCL has been considered to be an essential transcription factor for all erythropoiesis. In this study, we demonstrate that SCL was not essential for adult erythropoiesis; surprisingly, the absence of SCL did not significantly impair the ability of mice to respond to darbepoetin alfa or hemolytic stress (Fig. 1 and 2). Despite ongoing erythropoiesis, in vitro growth of the major erythroid progenitor BFU-E was severely limited. We presume that the growth defect reflects inadequate or inappropriate culture conditions for SCL-deleted erythroid progenitors that can be largely overcome in vivo. Definitive evidence that SCL was intrinsically not essential for erythropoiesis came from the clonal CFU-S12 assay where Southern blotting of individual CFU-S demonstrated an SCL-deleted genotype (Fig. 5). Furthermore, transplantation of SCL-deleted bone marrow cells demonstrated that the ability to form red blood cells in the absence of SCL was cell intrinsic and was not previously recognized because SCL-deleted erythropoiesis was out-competed by wild-type erythropoiesis (Fig. 6). Thus, unlike studies of the developing embryo, SCL was not essential for ongoing adult erythropoiesis.
Although the erythroid compartment functioned adequately, SCL is still required for normal erythropoiesis because mice are moderately anemic. Characteristics of the red blood cell parameters, including markers of hemolysis, suggest that increased red blood cell destruction is not a major contributor to the anemia. In contrast, the small size of SCL-deleted CFU-S12 cells in the absence of significant apoptosis and the competitive disadvantage of SCL-deleted erythropoiesis support the conclusion that the anemia is predominantly due to a defect in erythroid proliferation. The absence of BFU-E suggests that SCL acts at the level of immature erythroid progenitors, which can be overcome iby some as-yet-undefined mechanism in vivo. We do not believe that the BFU-Es are bypassed directly to the CFU-Es in vivo because FACS-isolated MEP cells did not form CFU-Es (data not shown). The relative preservation of the CFU-E is consistent with the ability of SCL-deleted mice to respond to erythroid stress, which is mediated predominantly by expansion of CFU-Es (19). Expression of an SCL DNA-binding mutant in CD34+ cells suggested that SCL DNA binding was important for proliferation of early erythroid progenitors (BFU-E) but not the more mature CFU-E (26). Consistent with defects in DNA binding, SCL-deleted red blood cells have reduced expression of TER119. Although the antigen recognized by TER119 antisera has not been cloned, evidence suggests that it is glycophorin A (2, 10), a recently proposed DNA-binding target gene of SCL (14).
Redundancy in mouse knockouts is usually explained by the presence of a functionally related gene. For example, normal platelet production in GATA-1 knockout cells has been attributed to coexpression of GATA-2 in megakaryocytes (23). In the case of SCL, it seems likely that another bHLH factor may be replacing many of the functions of SCL in hematopoiesis. The most likely candidate is Lyl-1 because the bHLH domain of Lyl-1 rescued the hematopoiesis of SCL−/− ES cells (24). Lyl-1 is expressed in cell types similar to SCL and has dimerization properties similar to those of SCL (17, 35) In addition, the predicted DNA-binding preference for Lyl-1-E2a heterodimers was almost identical to that of SCL-E2a heterodimers (21).
It has been proposed that SCL belongs to a class of transcription factors required for the formation of hematopoietic stem cells, but once formed, SCL is no longer required for hematopoietic stem cell activity (20). Similarly, it is possible that SCL is also not required for erythropoiesis once erythroid progenitors are formed. More likely, alternate factors such as Lyl-1 could replace the function of SCL in erythropoiesis. If correct, then expression of Lyl-1 or another bHLH may also explain the lack of a major stem cell defect in the SCL-deleted mice.
There are several possible explanations for the critical role of SCL in the developing embryo yet the relative redundancy in the adult. First, it is likely that SCL has unique target genes critical for hematopoietic specification. Second, the factors involved in the establishment of the SCL-independent erythroid program in the adult may not be expressed in the required spatiotemporal fashion in the developing embryo. The expression pattern of Lyl-1 in development has not been examined in detail but may differ significantly from SCL. Third, SCL-independent erythropoiesis can occur in the embryo but is insufficient to allow survival of the embryo. The concept that adult hematopoiesis is more resilient than embryonic development to loss of gene function is supported by the phenotype of GATA-1 knockdown mutations (16). The majority of GATA-1lo mice die during embryogenesis, but in the small proportion of mice that survive to adulthood, their hematocrit levels and ability to response to erythroid stress return to normal in the first few weeks of life (34). The recovery of erythropoiesis in adult GATA-1lo mice has been attributed to selection of clones the expressing GATA-1 at the highest levels; however, this cannot explain the recovery of erythropoiesis in SCL-conditional knockout mice. Our demonstration that SCL was not essential for erythropoiesis provides a rationale for generating conditional knockouts of other transcription factors, such as GATA-1, also believed to be essential for adult erythropoiesis.
In summary, contrary to previous claims, our analyses of SCL conditional knockout mice demonstrate that erythropoiesis can occur in the absence of SCL. This unexpected finding suggests that alternate factors or pathways can replace the essential erythropoietic functions of SCL in the adult but not the embryo. Identification of the mechanism of SCL-independent erythropoiesis in the adult will provide valuable insights into the transcriptional regulation of erythropoiesis.
Acknowledgments
The technical assistance and animal husbandry of Sumi Vasudevan, Louise Inglis, Meagan James, Alice Holloway, Thomas Nikolaou, and Jason Corbin is gratefully acknowledged. Thanks also to John Cunningham for helpful advice during preparation of the manuscript.
This work was supported in part by the NHMRC, Australia, and NIH grants PO1 HL53749-03 and RO1 HL69232-01.
REFERENCES
- 1.Anderson, K. P., S. C. Crable, and J. B. Lingrel. 2000. The GATA-E box-GATA motif in the EKLF promoter is required for in vivo expression. Blood 95:1652-1655. [PubMed] [Google Scholar]
- 2.Auffray, I., S. Marfatia, K. de Jong, G. Lee, C. H. Huang, C. Paszty, M. J. Tanner, N. Mohandas, and J. A. Chasis. 2001. Glycophorin A dimerization and band 3 interaction during erythroid membrane biogenesis: in vivo studies in human glycophorin A transgenic mice. Blood 97:2872-2878. [DOI] [PubMed] [Google Scholar]
- 3.Begley, C. G., P. D. Aplan, M. P. Davey, K. Nakahara, K. Tchorz, J. Kurtzberg, M. S. Hershfield, B. F. Haynes, D. I. Cohen, T. A. Waldmann, and I. R. Kirsch. 1989. Chromosomal translocation in a human leukemic stem-cell line disrupts the T-cell antigen receptor delta-chain diversity region and results in a previously unreported fusion transcript. Proc. Natl. Acad. Sci. USA 86:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condorelli, G., L. Vitelli, M. Valtieri, I. Marta, E. Montesoro, V. Lulli, R. Baer, and C. Peschle. 1995. Coordinate expression and developmental role of Id2 protein and TAL1/E2A heterodimer in erythroid progenitor differentiation. Blood 86:164-175. [PubMed] [Google Scholar]
- 5.Curtis, D. J., M. A. Hall, L. J. Van Stekelenburg, L. Robb, S. M. Jane, and C. G. Begley. 2004. SCL is required for normal function of short-term repopulating hematopoietic stem cells. Blood 103:3342-3348. [DOI] [PubMed] [Google Scholar]
- 6.Elefanty, A. G., C. G. Begley, D. Metcalf, L. Barnett, F. Kontgen, and L. Robb. 1998. Characterization of hematopoietic progenitor cells that express the transcription factor SCL, using a lacZ “knock-in” strategy Proc. Natl. Acad. Sci. USA 95:11897-11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elwood, N. J., H. Zogos, D. S. Pereira, J. E. Dick, and C. G. Begley. 1998. Enhanced megakaryocyte and erythroid development from normal human CD34(+) cells: consequence of enforced expression of SCL. Blood 91:3756-3765. [PubMed] [Google Scholar]
- 8.Hall, M. A., D. J. Curtis, D. Metcalf, A. G. Elefanty, K. Sourris, L. Robb, J. R. Gothert, S. M. Jane, and C. G. Begley. 2003. The critical regulator of embryonic hematopoiesis, SCL, is vital in the adult for megakaryopoiesis, erythropoiesis, and lineage choice in CFU-S12. Proc. Natl. Acad. Sci. USA 100:992-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann-Fezer, G., H. Maschke, H. J. Zeitler, P. Gais, W. Heger, J. Ellwart, and S. Thierfelder. 1991. Direct in vivo biotinylation of erythrocytes as an assay for red cell survival studies. Ann. Hematol. 63:214-217. [DOI] [PubMed] [Google Scholar]
- 10.Kina, T., K. Ikuta, E. Takayama, K. Wada, A. S. Majumdar, I. L. Weissman, and Y. Katsura. 2000. The monoclonal antibody TER119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Haematol. 109:280-287. [DOI] [PubMed] [Google Scholar]
- 11.Krantz, S. B. 1991. Erythropoietin. Blood 77:419-434. [PubMed] [Google Scholar]
- 12.Krosl, G., G. He, M. Lefrancois, F. Charron, P. H. Romeo, P. Jolicoeur, I. R. Kirsch, M. Nemer, and T. Hoang. 1998. Transcription factor SCL is required for c-kit expression and c-Kit function in hemopoietic cells. J. Exp. Med. 188:439-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn, R., F. Schwenk, M. Aguet, and K. Rajewsky. 1995. Inducible gene targeting in mice. Science 269:1427-1429. [DOI] [PubMed] [Google Scholar]
- 14.Lahlil, R., E. Lecuyer, S. Herblot, and T. Hoang. 2004. SCL assembles a multifactorial complex that determines glycophorin A expression. Mol. Cell. Biol. 24:1439-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecuyer, E., S. Herblot, M. Saint-Denis, R. Martin, C. G. Begley, C. Porcher, S. H. Orkin, and T. Hoang. 2002. The SCL complex regulates c-kit expression in hematopoietic cells through functional interaction with Sp1. Blood 100:2430-2440. [DOI] [PubMed] [Google Scholar]
- 16.McDevitt, M. A., R. A. Shivdasani, Y. Fujiwara, H. Yang, and S. H. Orkin. 1997. A “knockdown” mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc. Natl. Acad. Sci. USA 94:6781-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellentin, J. D., S. D. Smith, and M. L. Cleary. 1989. lyl-1, a novel gene altered by chromosomal translocation in T cell leukemia, codes for a protein with a helix-loop-helix DNA binding motif. Cell 58:77-83. [DOI] [PubMed] [Google Scholar]
- 18.Metcalf, D. 1984. Haemopoietic colony-stimulating factors. Elsevier, Amsterdam, The Netherlands.
- 19.Mide, S. M., P. Huygens, C. E. Bozzini, and J. A. Fernandez Pol. 2001. Effects of human recombinant erythropoietin on differentiation and distribution of erythroid progenitor cells on murine medullary and splenic erythropoiesis during hypoxia and post-hypoxia. In Vivo 15:125-132. [PubMed] [Google Scholar]
- 20.Mikkola, H. K., J. Klintman, H. Yang, H. Hock, T. M. Schlaeger, Y. Fujiwara, and S. H. Orkin. 2003. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature 421:547-551. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto, A., X. Cui, L. Naumovski, and M. L. Cleary. 1996. Helix-loop-helix proteins LYL1 and E2a form heterodimeric complexes with distinctive DNA-binding properties in hematolymphoid cells. Mol. Cell. Biol. 16:2394-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Na Nakorn, T., D. Traver, I. L. Weissman, and K. Akashi. 2002. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J. Clin. Investig. 109:1579-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pevny, L., C. S. Lin, V. D'Agati, M. C. Simon, S. H. Orkin, and F. Costantini. 1995. Development of hematopoietic cells lacking transcription factor GATA-1. Development 121:163-172. [DOI] [PubMed] [Google Scholar]
- 24.Porcher, C., E. C. Liao, Y. Fujiwara, L. I. Zon, and S. H. Orkin. 1999. Specification of hematopoietic and vascular development by the bHLH transcription factor SCL without direct DNA binding. Development 126:4603-4615. [DOI] [PubMed] [Google Scholar]
- 25.Porcher, C., W. Swat, K. Rockwell, Y. Fujiwara, F. W. Alt, and S. H. Orkin. 1996. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages Cell 86:47-57. [DOI] [PubMed] [Google Scholar]
- 26.Ravet, E., D. Reynaud, M. Titeux, B. Izac, S. Fichelson, P. H. Romeo, A. Dubart-Kupperschmitt, and F. Pflumio. 2004. Characterization of DNA-binding-dependent and -independent functions of SCL/TAL1 during human erythropoiesis. Blood 103:3326-3335. [DOI] [PubMed] [Google Scholar]
- 27.Robb, L., N. J. Elwood, A. G. Elefanty, F. Kontgen, R. Li, L. D. Barnett, and C. G. Begley. 1996. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 15:4123-4129. [PMC free article] [PubMed] [Google Scholar]
- 28.Robb, L., I. Lyons, R. Li, L. Hartley, F. Kontgen, R. P. Harvey, D. Metcalf, and C. G. Begley. 1995. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc. Natl. Acad. Sci. USA 92:7075-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson, S. M., M. Kennedy, J. M. Shannon, and G. Keller. 2000. A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Development 127:2447-2459. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez, M. J., E. O. Bockamp, J. Miller, L. Gambardella, and A. R. Green. 2001. Selective rescue of early haematopoietic progenitors in Scl(-/-) mice by expressing Scl under the control of a stem cell enhancer. Development 128:4815-4827. [DOI] [PubMed] [Google Scholar]
- 31.Shivdasani, R. A., E. L. Mayer, and S. H. Orkin. 1995. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature 373:432-434. [DOI] [PubMed] [Google Scholar]
- 32.Socolovsky, M., H. Nam, M. D. Fleming, V. H. Haase, C. Brugnara, and H. F. Lodish. 2001. Ineffective erythropoiesis in Stat5a(-/-)5b(-/-) mice due to decreased survival of early erythroblasts. Blood 98:3261-3273. [DOI] [PubMed] [Google Scholar]
- 33.Valtieri, M., A. Tocci, M. Gabbianelli, L. Luchetti, B. Masella, L. Vitelli, R. Botta, U. Testa, G. L. Condorelli, and C. Peschle. 1998. Enforced TAL-1 expression stimulates primitive, erythroid and megakaryocytic progenitors but blocks the granulopoietic differentiation program. Cancer Res. 58:562-569. [PubMed] [Google Scholar]
- 34.Vannucchi, A. M., L. Bianchi, C. Cellai, F. Paoletti, V. Carrai, A. Calzolari, L. Centurione, R. Lorenzini, C. Carta, E. Alfani, M. Sanchez, G. Migliaccio, and A. R. Migliaccio. 2001. Accentuated response to phenylhydrazine and erythropoietin in mice genetically impaired for their GATA-1 expression. Blood 97:3040-3050. [DOI] [PubMed] [Google Scholar]
- 35.Visvader, J., C. G. Begley, and J. M. Adams. 1991. Differential expression of the LYL, SCL and E2A helix-loop-helix genes within the hemopoietic system. Oncogene 6:187-194. [PubMed] [Google Scholar]
- 36.Vitelli, L., G. Condorelli, V. Lulli, T. Hoang, L. Luchetti, C. M. Croce, and C. Peschle. 2000. A pentamer transcriptional complex including tal-1 and retinoblastoma protein downmodulates c-kit expression in normal erythroblasts. Mol. Cell. Biol. 20:5330-5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vyas, P., M. A. McDevitt, A. B. Cantor, S. G. Katz, Y. Fujiwara, and S. H. Orkin. 1999. Different sequence requirements for expression in erythroid and megakaryocytic cells within a regulatory element upstream of the GATA-1 gene. Development 126:2799-2811. [DOI] [PubMed] [Google Scholar]
- 38.Wadman, I. A., H. Osada, G. G. Grutz, A. D. Agulnick, H. Westphal, A. Forster, and T. H. Rabbitts. 1997. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J. 16:3145-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wessely, O., E. M. Deiner, H. Beug, and M. von Lindern. 1997. The glucocorticoid receptor is a key regulator of the decision between self-renewal and differentiation in erythroid progenitors. EMBO J. 16:267-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitney, J. B., III. 1978. Simplified typing of mouse hemoglobin (Hbb) phenotypes using cystamine. Biochem. Genet. 16:667-672. [DOI] [PubMed] [Google Scholar]
- 41.Xu, Z., S. Huang, L. S. Chang, A. D. Agulnick, and S. J. Brandt. 2003. Identification of a TAL1 target gene reveals a positive role for the LIM domain-binding protein Ldb1 in erythroid gene expression and differentiation. Mol. Cell. Biol. 23:7585-7599. [DOI] [PMC free article] [PubMed] [Google Scholar]