Abstract
The calcineurin-nuclear factor of activated T cells (NFAT) signaling pathway has been shown to be of critical importance in regulating the growth response of cardiac myocytes. We have previously demonstrated that calcineurin Aβ (CnAβ) mRNA and protein are increased in response to growth stimulation, although the precise regulatory mechanism underlying CnAβ upregulation is not clear. Here, we isolated the mouse CnAβ promoter and characterized its responsiveness to growth stimuli in vitro and in vivo. A 2.3-kb promoter fragment was strongly activated by phenylephrine and endothelin-1 stimulation and by cotransfection with constitutively active CnA, NFATc4, and GATA4. Using chromatin immunoprecipitation, sequence regions were identified within the 2.3-kb promoter that associated with NFAT and GATA4, as well as with acetylated histone H3, following agonist stimulation. Consistent with the chromatin immunoprecipitation experiments, deletion of the distal half of the CnAβ promoter severely reduced NFAT, GATA4, and hypertrophic agonist-mediated activation. To investigate in vivo activity, we generated β-galactosidase (LacZ) containing transgenic mice under the control of the CnAβ 2.3-kb promoter. CnAβ-LacZ mice showed expression in the heart that was cyclosporine sensitive, as well as expression in the central nervous system and skeletal muscle from early embryonic stages through adulthood. CnAβ-LacZ mice were subjected to cardiac pressure overload stimulation and crossbreeding with mice containing cardiac-specific transgenes for activated calcineurin and NFATc4, which revealed inducible expression in the heart. These results indicate that the CnAβ 2.3-kb promoter is specifically activated by hypertrophic stimuli through a positive feedback mechanism involving NFAT and GATA4 transcription factors, suggesting transcriptional induction of CnAβ expression as an additional means of regulating calcineurin activity in the heart.
Calcineurin (PP2B) is a calcium-calmodulin activated serine-threonine protein phosphatase that is conserved from yeast to humans in its overall structure and biochemistry. Calcineurin exists as a heterotrimer between a 57- to 61-kDa catalytic A subunit (CnA) and two smaller, 16- to 19-kDa EF-hand-containing calcium binding proteins referred to as calcineurin B (CnB) and calmodulin (27). In vertebrates, three unlinked loci encode the catalytic subunit (CnAα, CnAβ, and CnAγ), while two loci encode the regulatory subunit (CnB1 and CnB2) (44). The CnAα, CnAβ, and CnB1 genes are each expressed in a ubiquitous pattern throughout the body, while CnAγ and CnB2 expression is more restricted to the brain and testis (5, 26, 39, 47). Once activated, calcineurin directly dephosphorylates a family of transcription factors referred to as nuclear factor of activated T cells (NFAT) within the cytoplasm, exposing a nuclear localization sequence and promoting translocation into the nucleus where they participate in the transcriptional induction of diverse genes in a wide array of cell types (25). There are four calcineurin-regulated NFAT transcription factors, NFATc1 to -c4, each of which is expressed in the heart (52).
Calcineurin-NFAT signaling plays diverse regulatory roles in multiple vertebrate cell types. In the heart, calcineurin has been implicated as a pivotal regulator of the myocyte hypertrophic growth response (38). Calcineurin-NFAT was originally implicated as a cardiac effector through a search for factors that interacted with the cardiac tissue-enriched zinc finger containing transcription factor GATA4, which identified NFATc4 as a critical cofactor (38). NFATc4 and GATA4 synergistically activated the brain natriuretic peptide (BNP) promoter, which is normally upregulated by stimuli that elicit a hypertrophic growth response. Expression of constitutively active forms of CnA or NFATc4 in the mouse heart by transgenesis produced profound cardiac hypertrophy, demonstrating the sufficiency of this pathway in regulating the hypertrophic growth response (38). The necessity of this pathway in mediating cardiac growth responses has been confirmed through the use of the calcineurin inhibitory agents cyclosporine A (CsA) and FK506, as well as by gene targeting of the CnAβ gene and by transgene-mediated expression of calcineurin inhibitory peptides in the heart (4, 36, 37).
While calcineurin-NFAT signaling regulates the cardiac hypertrophic growth response, the mechanism whereby calcium activates this pathway in a cell type experiencing cyclical release of calcium is uncertain. Previous studies have established the requirement for sustained increases in intracellular calcium concentration to mediate calcineurin activation and the nuclear localization of NFATs (13-15, 48, 49). In addition to changes in calcium concentration, calcineurin phosphatase activity can be altered through changes in the activity or concentration of calcineurin modulatory factors or by changes in coregulatory signaling pathways. We have previously observed an increase in CnAβ mRNA and protein expression in hypertrophied neonatal cardiac myocytes and hypertrophied adult hearts, with no coincident change in CnAα levels (20, 46), supporting the existence of yet another way of upregulating calcineurin in the heart. However, the underlying mechanism whereby CnAβ expression is augmented in cardiac myocytes undergoing hypertrophy has not been evaluated.
MATERIALS AND METHODS
Cloning, plasmids, and mutagenesis.
A 2,322-bp DNA fragment from the mouse CnAβ gene was amplified by PCR using the Herculase enhanced polymerase blend (Stratagene) from mouse genomic DNA with primers containing an XhoI site in the forward primer and an HindIII site in the reverse primer (forward, 5′-CTTTTGCTCGAGCTTGCTCTTTTCTTCTGGGG-3′; reverse, 5′-CGATATAAGCTTCTACCAGAGCCAAGCGGCGG-3′). These primers were designed according to mouse chromosome 14 genomic sequences (GenBank accession no. NT 039596). The 2,322-bp fragment was subcloned into XhoI-HindIII-cleaved pGL3-basic (Invitrogen) containing the luciferase cDNA (pGL3-CnAβ2322). A series of CnAβ promoter deletion fragments were generated by PCR as XhoI-HindIII fragments and subcloned into the pGL3 reporter plasmid. The integrity of all deletion constructs was confirmed by sequencing.
Cell culture, DNA transfection, and reporter gene assays.
Preparation of primary cultures of neonatal rat cardiac myocytes was described previously (10). Briefly, 1- to 2-day-old Sprague-Dawley rat neonates were sacrificed by CO2 inhalation, the hearts were collected, atria were removed, and the ventricles were cut into four pieces and subjected to 10 rounds of enzymatic digestion with 0.05% pancreatin and 84 U/ml of collagenase (Worthington). The cells were differentially plated for 1 h in cell culture dishes to remove contaminating nonmyocytes, then plated on gelatinized 12-well cell culture dishes, and cultured overnight in M199 medium supplemented with 15% fetal bovine serum (FBS), penicillin/streptomycin (100 units/ml), and l-glutamine (2 mM). The following day, the cells were washed in phosphate-buffered saline (PBS) and cultured in serum-free M199 medium containing penicillin/streptomycin and l-glutamine. Primary cultured cardiac myocytes were transiently transfected with 0.3 to 1.0 μg/well of pGL3-CnAβ reporter constructs using FuGENE 6 reagent (Roche Molecular Biochemicals) or Lipofectamine 2000 (Invitrogen). pGL3-Basic was used as a promoterless control, while pGL3-Control (Invitrogen) was used as a simian virus 40 (SV40)-driven luciferase fusion control that is highly expressed in most cell types. For cotransfection assays, 0.3 μg/well of pGL3-CnAβ reporter construct was transfected with 0.3 μg/well of pcDL-SRa-ΔCnA, pREP-NFATc4, and pcDNA1-GATA4, either alone or in combination. The total amount of DNA per well was adjusted to 1.2 μg using pcDNA3 empty vector. Stimulation with 50 to 100 μM phenylephrine (PE) or 100 nM endothelin-1 (Endo-1) was performed 24 h after transfection. Adenoviral infections with Adβgal, AdCain, AdVIVIT, Ad-dominant negative mitogen-activated protein kinase (MAPK) kinase 1 (MEK1), and Ad-dominant negative MAPK kinase 3 were performed 24 h before transfection and allowed to incubate for an additional 24 h before being harvested for determination of luciferase activity. BAPTA-AM (Sigma) in dimethyl sulfoxide was used at concentrations of 1 and 10 μM and harvested 4 h afterwards. Cells were harvested with lysis buffer (100 mM KH2PO4 [pH 7.8], 0.5% Igepal [Sigma], 1 mM dithiothreitol) 4 to 48 h after transfection, and luciferase activities were measured in reaction buffer (1 mM luciferin [Promega], 100 mM Tris-HCl [pH 7.8], 10 mM Mg acetate, 1 mM EDTA) using a Berthold MicroLumat LB96P luminometer. C3H 10T1/2 (10T1/2) cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS, and transfections were performed at 80% confluence in 12-well culture dishes. All experiments were performed in triplicate and repeated at least three times, although only a single representative experiment is shown.
Electrophoretic mobility shift assay (EMSA).
The NFATc4-rel homology domain (RHD) and full-length GATA4 protein were each generated using a coupled transcription-translation system (Promega) using the plasmids pT7-NFATc4-RHD and pcDNA1-GATA4 as templates. Double-stranded oligonucleotides corresponding to putative NFAT and GATA binding sequences were generated so that the core consensus was surrounded by seven additional base pairs on both the 5′ and 3′ ends, one strand of which was labeled with [γ-32P]ATP with T4 polynucleotide kinase (New England Biolabs) before being annealed to make the double-stranded site (Table 1). Labeled oligonucleotides were incubated with 3 μl of programmed reticulocyte lysate and 1 μg of poly(dI-dC)-(dI-dC) with 4 μl of 5× binding buffer (10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 10% glycerol, 0.5% dithiothreitol) in a volume of 20 μl for 30 min at room temperature. The protein-DNA complexes were separated by electrophoresis on a 5% polyacrylamide gel in 0.5× Tris borate/EDTA buffer at 25 mA for approximately 2 h. A consensus NFAT binding motif from the interleukin-4 (IL-4) promoter was used as a positive control (38). Unprogrammed reticulocyte lysate without plasmid was used as a negative control for each of the binding sites analyzed.
TABLE 1.
Sense sequence of the oligonucleotides used for EMSAa
| Name | Location | Sequence or probe for EMSA |
|---|---|---|
| N1* | −96 | 5′-TGGACTGTTTCCGTTTCTG-3′ |
| N2* | −199 | 5′-GTCCTTTTTTCCCCTTGCA-3′ |
| N3* | −236 | 5′-TCTTCATTTTCCTGGGCTC-3′ |
| N4* | −268 | 5′-AGGCCTGGGAAATTCTGAG-3′ |
| N5* | −394 | 5′-ATTAAAGGGAAAATTTCAT-3′ |
| N6* | −554 | 5′-TTGGACTTTTCCAAACTCT-3′ |
| N7* | −584 | 5′-TTAGACTTTTCCAGCTGAA-3′ |
| N8* | −680 | 5′-CAATGGCTTTCCAGGGGAA-3′ |
| N9 | −820 | 5′-TTCCTTCTTTCCATTGTCT-3′ |
| N10 | −896 | 5′-TCTCTGCTTTCCATTTTTT-3′ |
| N11 | −926 | 5′-GAACTAATTTCCTATTTTC-3′ |
| N12* | −1449 | 5′-ATTGGGTTTTCCCTCCTTA-3′ |
| N13* | −1720 | 5′-CAGTCTCTTTCCTTTGAAA-3′ |
| N14* | −1794 | 5′-CCTTTCCTTTCCCCTTAAG-3′ |
| N15* | −1799 | 5′-TGCTCCCTTTCCTTTCCCC-3′ |
| N16* | −1866 | 5′-TGGTGCCTTTCCTCTCTGC-3′ |
| N17* | −2076 | 5′-AAGAGTCTTTCCCTCTTGT-3′ |
| G1* | −185 | 5′-TTGCATTTTATCAACAGCCC-3′ |
| G2* | −299 | 5′-ATTTTGTAGATAAATTGTCA-3′ |
| G3* | −317 | 5′-CATAGTATTATCACTTAGAT-3′ |
| G4* | −377 | 5′-TTTTAAATGATAATGCCACT-3′ |
| G5* | −747 | 5′-AGCACCTTTATCCATTGAAC-3′ |
| G6* | −940 | 5′-TGTACAAAGATAAGGAACTA-3′ |
| G7 | −1208 | 5′-CACAATTCTATCTTTTTGGA-3′ |
| G8* | −1260 | 5′-CCTTAGTAGATATGTTGGCC-3′ |
| G9* | −1325 | 5′-AGTAATAAGATACAGAGGGC-3′ |
| G10 | −1652 | 5′-TGTTCATGTATCGGACCCAG-3′ |
| G11 | −1754 | 5′-ATGAGAGCTATCTTCACCCA-3′ |
| G12 | −1766 | 5′-ATCTCTGTGATAATGAGAGC-3′ |
| G13 | −1775 | 5′-AGTCAGAATATCTCTGTGAT-3′ |
| G14 | −2044 | 5′-GTGTTAACTATCCCTCCCCT-3′ |
| G15 | −2091 | 5′-AGTCAACATATCTTTAAGAG-3′ |
| IL4* | 5′-TACATTGGAAAATTTTATTAC-3′ |
*, used in EMSA. The core consensus DNA binding element is highlighted in boldface text. Oligonucleotides with names beginning with N represent putative NFAT sites, while oligonucleotides beginning with G represent putative GATA binding sites.
Chromatin immunoprecipitation assay.
Cardiomyocytes (4 × 106) on 10-cm plates were stimulated with 50 μM PE or infected with AdGFP-NFATc3, AdΔCnA, and AdGATA4 for 48 h. Cells were then treated with 1% formaldehyde for 10 min at 37°C. Chromatin immunoprecipitation was performed with anti-acetylated histone H3, anti-NFATc3 (p-20), or anti-GATA4 (c-20) antibody (Santa Cruz Biotechnology) using an acetyl-histone H3 immunoprecipitation assay kit (Upstate Biotechnology). After immunoprecipitation, the eluted protein-DNA cross-links were reversed by being heated at 65°C for 4 h, after which the DNA was purified with a QIAGEN PCR Quick spin column. Rat calcineurin Aβ promoter fragments (#1 to #4) were PCR amplified from immunoprecipitated and nonimmunoprecipitated chromatin using the following primers: #1 (−2134, 5′-ATGGAGTCCACATGTCCTTATG-3′; and −1578, 5′-TCAGTGAGACCAAGATCTGGAG-3′), #2 (−1601, 5′-ACTCCAGATCTTGGTCTCAC-3′; and −1226, 5′-AGATGGCTCAGGCTGTAAAG-3′), #3 (−1242, 5′-TACAGCCTGAGCCATCTTAG-3′; and −640, 5′-GAGACTGTCTCAACAAACGG-3′), #4 (−659, 5′-CCGTTTGTTGAGACAGTCTC-3′; and −162, 5′-AGGGTGCAGACTAGACTTAG-3′). Atrial natriuretic factor (ANF) was amplified with 5′-AAGGAATCCTGAGGCGAGCGC-3′ and 5′-GCGGCGGCCAGGAGAAGATGC-3′, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified with 5′-TCACCTTTGGTCAATCCCTGG-3′ and 5′-CTCATCGCGATATTCAATTGG-3′. PCR consisted of 1 cycle at 100°C for 2.5 min, and 30 or 35 cycles, each at 96°C for 30 s, 55°C for 30 s, and 72°C for 50 s. PCR products were resolved by 2% agarose gel electrophoresis and imaged using a Typhoon 9400 variable mode imager (Amersham Biosciences).
Generation of CnAβ promoter-lacZ transgenic mice.
The CnAβ 2,322-bp promoter was PCR amplified with primers containing NotI-BamHI linkers and subcloned into NotI and BamHI sites of the promoterless β-galactosidase reporter plasmid AUG-β-gal (35) to create the plasmid CnAβ2322-lacZ for generation of transgenic mice. The Bluescript II (Stratagene) plasmid backbone was removed by NotI-SalI digestion, and the isolated CnAβ2322-LacZ fragment was gel purified and injected into newly fertilized FVBN oocytes, which were transferred to the oviducts of pseudopregnant FVBN recipients. Transgenic founders were identified by PCR using LacZ cDNA-specific primers 5′-GTCACACTACGTCTGAACGT-3′ and 5′-CTGCACCATTCGCGTTACG-3′.
Staining and measurement of LacZ activity.
LacZ expression in embryos or adult mice was detected by 5-bromo-4-chloro-3-indolyl-β-galactopyranoside (X-Gal) staining as described previously (12). Briefly, embryos, adult mice, and isolated hearts were incubated in X-Gal solution (1 mM potassium ferricyanide, 1 mM potassium ferrocyanide, 2 mM MgCl2, and 1 mg/ml X-Gal in 1× PBS) overnight and then fixed in 4% paraformaldehyde in PBS. For longitudinal sections, embryos were collected at embryonic day 9.5 (E9.5) postcoitum and stained with X-Gal, fixed, mounted in paraffin and sectioned along the longitudinal axis at a thickness of 7 μm by using a microtome. Sections were counterstained with eosin to visualize embryonic structures. To quantify LacZ activity in the heart, protein homogenates in lysis buffer were assayed using the Galacto-Star chemiluminescent reporter system (Tropix). Light units were normalized to protein concentration. Four-month-old female mice with the CnAβ2322-LacZ transgene (line 5.2) were subjected to CsA injection (20 mg/kg of body weight/day; three mice) or vehicle (three mice) for 5 days, and then LacZ activities in the heart were measured.
Pressure overload and crossbreeding.
Eight-week-old male mice with the CnAβ2322-LacZ transgene were subjected to pressure overload by transverse aortic constriction (TAC). TAC was performed using a 7-0 silk suture that was tied around a 27-gauge constriction at the aortic arch as described previously (51). After 1 week of TAC, heart weight-to-body weight (HW/BW) ratios were measured, followed by measurement of LacZ activity. CnAβ2322-LacZ transgenic mice were also crossbred with cardiac tissue-specific transgenic mice expressing activated CnA or activated NFATc4 (38). Hearts from 4-week-old nontransgenic (NTG), CnAβ2322-LacZ transgenic, and double-transgenic mice were assayed for LacZ activity.
Statistical analysis.
Data were expressed as means ± standard deviation (luciferase assay) and means ± standard error (LacZ assay). Differences between experimental groups were evaluated for statistical significance by using Student's t test for unpaired data. P values of <0.05 were considered to be significant.
RESULTS
Isolation and molecular characterization of the mouse CnAβ promoter.
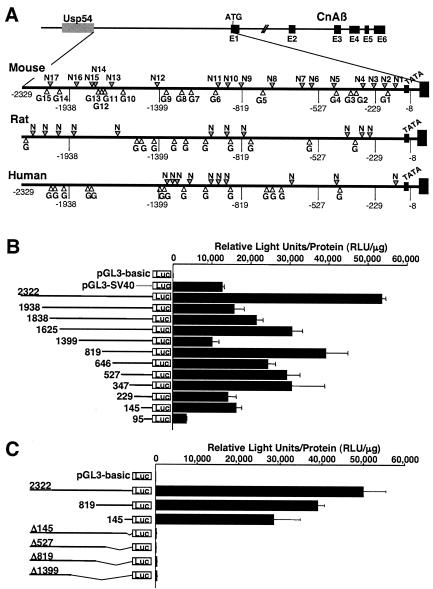
We previously observed that CnAβ mRNA and protein levels are increased in cultured cardiac myocytes subjected to growth stimulation, suggesting the possibility that this gene was subject to inducible transcriptional regulation in the heart (46). To investigate this potential mechanism, we isolated the 5′ upstream regulatory region from the mouse CnAβ gene within chromosome 14 between the first exon of CnAβ and the 3′-most exon from the ubiquitin specific protease 54 (Usp54) gene (Fig. 1A). The region of sequence encoding the 5′ untranslated region and the first 12 amino acids of CnAβ is almost entirely guanines and cytosines, thus hindering our efforts to definitively map the putative transcription start site by primer extension or RNase protection assay. However, we estimated the putative transcription start site to be localized approximately 115 bp upstream from the translation initiation codon in the CnAβ first exon, based on cDNA sequence comparisons between multiple mouse and human expressed sequence tags and weak primer extension assay signals (data not shown). Based on this approximation, a putative TATA box is situated at position −72 (TAATTTT) in the mouse CnAβ promoter, which is also conserved within the rat and human CnAβ proximal promoter sequence (Fig. 1A). The most interesting characteristic of the entire 2,329-bp promoter region is the large number of consensus binding sequences for NFAT and GATA transcription factors that are largely conserved between mouse, rat, and humans (Fig. 1A). Within the mouse promoter, there are 17 putative binding sites for NFAT factors (GGAAA or TTTCC) and 15 putative sites for GATA factors (GATA or TATC) (Table 1), but none for myocyte enhancer factor-2, serum response factor, or Nkx2.5.
FIG. 1.
Characterization of the mouse CnAβ gene 5′ upstream region. (A) The mouse CnAβ gene upstream region contains 17 and 15 consensus sequences for NFAT (gray arrowheads) and GATA (white arrowheads) factors, respectively, which are largely conserved in the homologous regions from the rat and human CnAβ promoter. The putative transcription start site is situated 115 bp upstream of the translation start site and is defined as +1, and the putative TATA box is situated at −72. (B) Transient transfection analysis in neonatal rat cardiac myocytes with luciferase fusion constructs containing the full-length CnAβ2322 promoter or the indicated deletion fragments. The promoterless pGL3-basic and the SV40 promoter-containing pGL3-SV40 were used as controls. All activities were assayed under serum-free conditions. The data are represented as relative light units (RLU) per microgram of protein. (C) Transient transfection analysis in neonatal cardiac myocytes with constructs encoding internal deletions from the 3′ end of the CnAβ promoter, each of which essentially inactivates the promoter. All data are represented as mean values obtained from a representative experiment performed in triplicate, although similar results were obtained in two additional independent experiments.
To characterize the potential promoter activity of the 5′-flanking region from the mouse CnAβ gene, we generated a series of promoter deletions fused to a luciferase reporter for transfection analysis in cultured neonatal rat cardiomyocytes. Results were compared to a construct (pGL3-SV40) that contained the relatively potent SV40 promoter or a construct lacking a promoter (pGL3-basic). The nearly full-length CnAβ2322 promoter construct showed the highest level of luciferase activity in cardiac myocytes, which was considerably stronger than the pGL3-SV40 control construct (Fig. 1B). Each of the subsequent deletions constructs produced less activity than the full-length construct, although each deletion still retained activity comparable to or slightly greater than the SV40 promoter, with the exception of the CnAβ95 construct, whose activity was significantly attenuated (Fig. 1B). CnAβ1399 also showed relatively lower expression, yet CnAβ819 showed relatively robust expression, suggesting the presence of a negative regulatory element between −1399 and −819. However, deletion of only the first 145 bp within the context of the full-length sequence completely inactivated expression, suggesting the importance of the minimal promoter and definitively identifying the TATA box-containing area (Fig. 1C).
The CnAβ promoter is activated by hypertrophic stimulation.
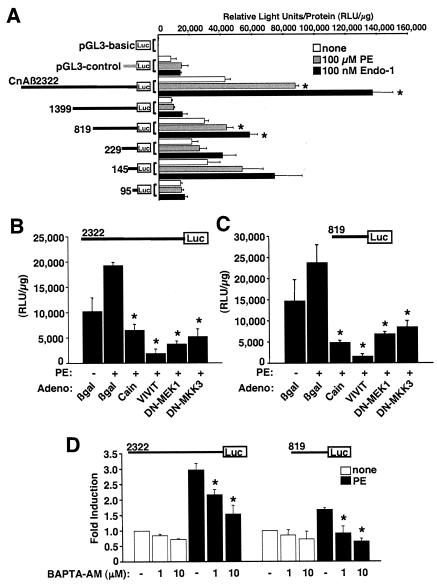
To investigate the ability of the CnAβ promoter to respond to hypertrophic stimulation, PE or Endo-1 was added to cardiac myocytes cultured in serum-free medium 24 h after transient transfection with CnAβ2322, CnAβ1399, CnAβ819, CnAβ229, CnAβ145, or CnAβ95. Twenty-four hours afterwards, cardiac myocytes were harvested for luciferase activity assessment. The construct encoding the full-length promoter showed the most prominent induction by PE and Endo-1 (2- to 3.5-fold), while the remaining constructs showed either no induction or a weaker 1.5- to 2.0-fold induction (Fig. 2A). These results suggest that the CnAβ promoter is responsive to hypertrophic stimulation in cultured cardiac myocytes and that the more distal region of the promoter mediates much of the responsiveness.
FIG. 2.
The CnAβ promoter is induced by agonist stimulation. (A) Transient transfection assays of neonatal cardiomyocyte cultures with the indicated CnAβ promoter-luciferase fusion construct, the control construct pGL3-basic (promoterless), or pGL3-control (SV40 promoter). Twenty-four hours after transfection, myocytes were stimulated in serum-free medium and with 100 μM PE or 100 nM Endo-1 for an additional 24 h (*, P < 0.05 versus unstimulated). (B and C) Transient transfection assays in cultured neonatal cardiac myocytes with the pGL3-CnAβ2322 or pGL3-CnAβ819 promoter-luciferase fusion constructs. Cells were stimulated with 100 μM PE in the presence of the indicated adenovirus (which was infected 24 h previously). Cells were harvested 24 h later, and luciferase activities were measured as relative light units per microgram of protein (*, P < 0.05 versus Adβgal PE stimulated). DN, dominant negative. (D) Transient transfection assays in cultured neonatal cardiac myocytes with the pGL3-CnAβ2322 or pGL3-CnAβ819 promoter-luciferase fusion constructs in the absence or presence of PE and the indicated concentration of BAPTA-AM (*, P < 0.05 versus PE stimulated without BAPTA-AM). All data are represented as mean values obtained from a representative experiment performed in triplicate, although similar results were obtained in two additional independent experiments.
To analyze the relationship between CnAβ promoter induction and select intracellular signaling pathways that are known to alter the cardiac hypertrophic response, recombinant adenoviruses that inhibit calcineurin, NFAT, MEK1-extracellular signal-regulated kinases 1 and 2 (ERK1/2) and MAPK kinase 3/6-p38 MAPK signaling were employed. Both the CnAβ2322 and CnAβ819 constructs showed specific inhibition of promoter activity in response to blockade of calcineurin/NFAT, ERK1/2, and p38 signaling following PE stimulation (Fig. 2B and C). Consistent with these results, FBS-stimulated expression of the CnAβ2322 promoter construct was blocked with the calcineurin inhibitory agent CsA and the MEK1-ERK1/2 pathway inhibitor U0126 and partially blocked with the p38 inhibitor SB203580 but not with the c-Jun N-terminal kinase (JNK) inhibitor SP600125 (data not shown). Consistent with AdCain-, AdVIVIT-, and CsA-mediated inhibition of the CnAβ promoter, chelation of intracellular calcium with BAPTA-AM also inhibited expression of the CnAβ2322 and CnAβ819 constructs following PE stimulation, demonstrating the necessity for increased intracellular calcium in augmenting promoter expression, possibly through an autoregulatory feedback mechanism involving calcineurin-NFAT signaling (Fig. 2D) (see Discussion). Collectively, these results suggest that induction of the CnAβ promoter is downstream of the calcium-calcineurin-NFAT, MEK1-ERK1/2, and p38 signaling pathways in the heart.
Identification of NFAT and GATA4 binding sites within the CnAβ promoter.
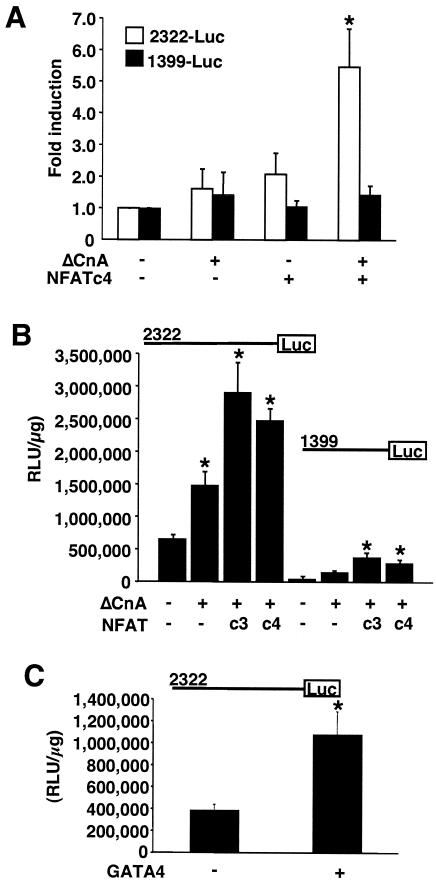
Given the relatively large number of NFAT and GATA DNA binding sites contained within the CnAβ promoter, it was of interest to determine the general region that might respond to these factors. Hence, the CnAβ2322 and CnAβ1399 constructs were cotransfected into neonatal rat cardiomyocytes, along with expression vectors encoding activated calcineurin (ΔCnA) and NFATc4. The data demonstrate that CnAβ2322 but not CnAβ1399 responded to NFATc4 and ΔCnA cotransfection (Fig. 3A). A similar overall paradigm was observed in 10T1/2 cells cotransfected with NFATc4 and ΔCnA (Fig. 3B). Moreover, the CnAβ2322 promoter region also responded to GATA4 cotransfection in cardiomyocytes (data not shown) and 10T1/2 cells (Fig. 3C). Thus, NFATc4 and GATA4 are capable of augmenting expression of the full-length CnAβ2322 promoter, with most of the induction mapping to the more distal region of the promoter.
FIG. 3.
The CnAβ promoter responds to NFATc4 and GATA4. (A) Transient transfection assays in cultured neonatal cardiac myocytes with the pGL3-CnAβ2322 or pGL3-CnAβ1399 promoter-luciferase fusion constructs and constructs encoding NFATc4 or ΔCnA. Cells were harvested 24 h later, and luciferase activities were measured as relative light units per microgram of protein (*, P < 0.05 versus no cotransfection). (B) Essentially the same experiment shown in panel A, except that 10T1/2 fibroblasts were used. (C) Transient transfection assay in cultured 10T1/2 cells with the pGL3-CnAβ2322 promoter-luciferase fusion construct and a construct encoding GATA4 (*, P < 0.05 versus no cotransfection). Similar results were observed in a total of three independent experiments, each performed in duplicate.
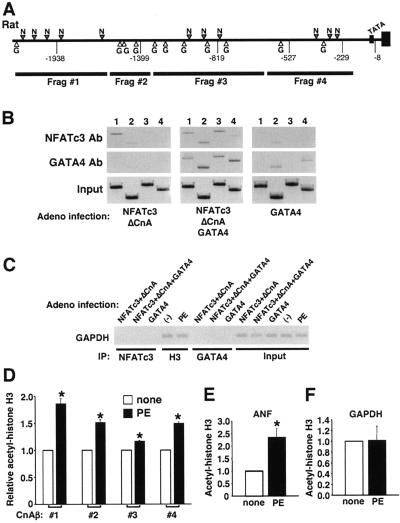
To more definitively implicate NFAT and GATA binding sites in the regulation of the CnAβ promoter in vivo, a series of chromatin immunoprecipitation (ChIP) assays were performed using PCR primers that generated consecutive DNA fragments spanning the rat CnAβ promoter for analysis of rat neonatal cardiomyocyte cultures (Fig. 4A). Cardiomyocytes were infected with three different groups of adenovirus: (i) AdNFATc3 plus AdΔCnA, (ii) AdNFATc3 plus AdΔCnA plus AdGATA4, or (iii) AdGATA4 alone. The DNA and protein was cross-linked, and extracts were immunoprecipitated for NFATc3 or GATA4, followed by PCR for each of the four CnAβ promoter fragments (Fig. 4B). The most distal fragment (#1) readily interacted with NFATc3 when NFATc3 was overexpressed but not when GATA4 alone was overexpressed (Fig. 4B, lane 1) in each grouping in response to NFATc3 immunoprecipitation. In contrast, fragments 2 and 4 associated with GATA4 by immunoprecipitation when GATA4 was overexpressed but not when NFATc3 alone was overexpressed (Fig. 4B, lanes 2 and 4) in each grouping following GATA4 immunoprecipitation. Also of interest, when GATA4 and NFATc3 were overexpressed together, all four fragments from the CnAβ promoter showed specific immunoprecipitation with either GATA4 or NFATc3 antibody (Fig. 4B). As a control, the GAPDH minimal promoter was immunoprecipitated with NFATc3 or GATA4 antibodies from each of the infection groups assayed in Fig. 4B (Fig. 4C). Neither GATA4 nor NFATc3 showed a specific association with the GAPDH promoter, although it was associated with acetylated histone H3 (Fig. 4C).
FIG. 4.
ChIP of CnAβ. (A) Schematic of the rat CnAβ promoter and the four different PCR fragments that span the promoter, each of which was tested by ChIP assay. (B) Neonatal rat cardiomyocytes were infected with the indicated adenoviruses and subjected to ChIP assay for NFATc3 or GATA4, in conjunction with the four fragments. Input DNA is also shown as a control. (C) Control ChIP experiment in which the GAPDH minimal promoter region was precipitated with NFATc3, GATA4, or acetylated histone H3 from cardiomyocytes infected with the indicated adenovirus. Some cultures were also stimulated with PE as a control for the next panel. (D) Neonatal rat cardiomyocytes left unstimulated or stimulated with PE (50 μM) were subjected to ChIP with acetylated histone H3 antibody against each of the four fragments spanning the rat CnAβ promoter (*, P < 0.05 versus unstimulated; three independent experiments). (E) Control ChIP against the known hypertrophic responsive ANF promoter using acetylated histone H3 antibody or (F) the same antibody in conjunction with the relatively unresponsive GAPDH minimal promoter (*, P < 0.05 versus unstimulated; three experiments).
The inducibility of the CnAβ promoter was also characterized by ChIP using acetylated histone H3 antibody. Extracts were generated from cardiomyocyte left untreated or treated with PE (50 μM) for 24 h. All four CnAβ promoter regions showed a significant increase in acetylated histone H3 content, although fragments 1, 2, and 4 were the most robust, corresponding to the ability of these same fragments to interact with either GATA4 or NFATc4 when each factor was overexpressed by itself (Fig. 4D shows the results of three independent experiments). As a control, the hypertrophic responsive ANF promoter also showed a specific increase in acetylated histone H3 content in cardiomyocytes following PE stimulation (Fig. 4E), while GAPDH did not (Fig. 4C and F). These results support the hypothesis that the CnAβ promoter is responsive to hypertrophic agonist stimulation, GATA4, and NFATc3 in vivo.
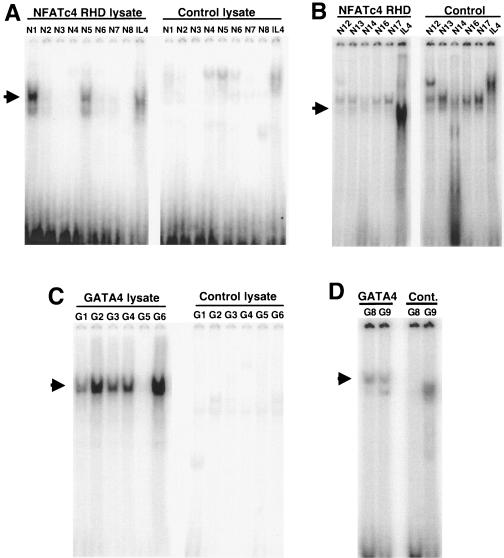
To again implicate NFAT and GATA binding factors as potential regulators of the CnAβ promoter, a series of EMSAs were performed with 13 putative NFAT sites and 8 putative GATA sites taken from the proximal and distal promoter regions that corresponded with the areas of ChIP reactivity (Fig. 4B). The DNA binding RHD from NFATc4 or full-length GATA4 was generated by coupled in vitro transcription-translation reactions in reticulocyte lysates and incubated with double-stranded oligonucleotides corresponding to each of the putative sites (Table 1). Unprogrammed reticulocyte lysate was used as a control, while a consensus NFAT binding site from the IL-4 promoter was used as a control for NFAT DNA binding activity (38). The data demonstrate that the N1 and N5 sites are relatively high-affinity NFAT binding sites compared with the IL-4 site, while N2, N6, N7, N12, N13, N14, and N16 are much weaker binding sites (Fig. 5A and B). With respect to GATA4 binding sites, the G1 to G4, G6, G8, and G9 sites all showed significant binding, while the G5 site was ineffective (Fig. 5C and D). These results indicate that both NFAT and GATA factors can interact with the CnAβ promoter at multiple locations, as shown by an in vitro assay, although some sites are relatively weak.
FIG. 5.
Characterization of NFAT and GATA binding elements in the CnAβ proximal promoter. (A and B) The left-hand panels show EMSA reactions between in vitro-translated NFATc4-RHD and the indicated NFAT sites from the CnAβ proximal promoter. The right-hand panels show the same putative NFAT sites reacted with unprogrammed lysates as a control. The arrows show the position of the NFAT-specific mobility shift. The well-characterized NFAT site from the IL-4 promoter was used as a migration control. (C and D) The left-hand panels show EMSA reactions between in vitro-translated GATA4 and the indicated GATA sites from the CnAβ proximal promoter. The right-hand panels show the same putative GATA sites reacted with unprogrammed lysates as a control. The arrows show the position of the GATA4-specific mobility shift. Similar results were obtained with three additional independent experiments.
Generation and characterization of CnAβ2322-LacZ reporter transgenic mice.
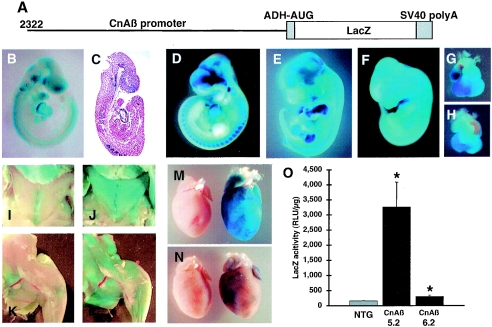
The data collected in cultured cardiac myocytes suggested that the CnAβ promoter was responsive to hypertrophic agonist stimulation and was activated by CnA, NFATc4, and GATA4. However, it was uncertain if such data accurately reflected the regulation that might occur in vivo. To this end, we generated transgenic mice with a construct containing the full-length (2,322-bp) CnAβ promoter fused to a LacZ reporter cDNA (Fig. 6A). Three stable transgenic lines were initially obtained and characterized by X-Gal staining. While one line failed to show significant expression, lines 5.2 and 6.2 each showed expression throughout embryonic development and in the adult mouse. Specifically, line 6.2 showed expression in the heart, brain, neural tube, and somites at E9.5 (Fig. 6B and C). At E11.5 and E13.5 of development, line 6.2 showed more defined expression in the myotome of the somites, the developing musculature in the limbs, forebrain, and the heart (Fig. 6D, E, and G). This overall pattern of developmental expression was remarkably similar to that described for CnAβ in the chicken embryo as assessed by in situ hybridization (30). With respect to line 5.2, LacZ expression was also relatively enriched in the developing heart at E12.5, similar to line 6.2, although the remaining pattern of embryonic expression in line 5.2 was substantially more widespread, consistent with a somewhat more ubiquitous expression pattern (Fig. 6F and H) (see Discussion). At 2 weeks of age, line 5.2 transgenic mice showed expression specifically in skeletal muscle throughout the body (Fig. 6J and L), which was not seen in nontransgenic controls (Fig. 6I and K). More careful analysis of heart expression showed robust LacZ activity in adults from line 5.2 but weaker and patchy LacZ expression in line 6.2 mice (Fig. 6 M and N; the transgenic hearts are on the right side in each panel). Subsequent quantitative analysis of LacZ expression in 2-week-old hearts from each line demonstrated significant LacZ activity, although line 5.2 was much more robust (P < 0.05) (Fig. 6O). These results indicate that the CnAβ promoter is capable of driving expression within the heart of transgenic mice and that these mice could be useful reagents for examining inducible expression in vivo.
FIG. 6.
Generation and characterization of CnAβ-LacZ mice. (A) Scheme of the transgene used to generate transgenic mice, which contains the 2,322-bp upstream regulatory region from the CnAβ gene fused to LacZ. (B) X-Gal staining of a line 6.2 transgenic embryo collected at E9.5. (C) Eosin-strained histological cross-section of the embryo shown in panel B. E11.5 (D) and E13.5 (E) X-Gal-stained embryos from line 6.2. (F) An E12.5 embryo from line 5.2 shows more robust and ubiquitous LacZ activity throughout the embryo, although a significant enrichment is seen within the hearts of line 6.2 (G) and line 5.2 (H). X-Gal staining of the chest and leg musculature in 2-week-old nontransgenic (I and K) and line 5.2 transgenic (J and L) mice. (M and N) X-Gal staining of isolated hearts from line 5.2 and 6.2 taken at 2 weeks of age and compared to NTG hearts. (O) Quantitative comparison of LacZ activity in the hearts of line 5.2, line 6.2, and NTG (six samples per group) taken from mice at 2 weeks of age and normalized to total protein (*, P < 0.05 versus NTG).
Pressure overload activates the CnAβ promoter in the mouse heart.
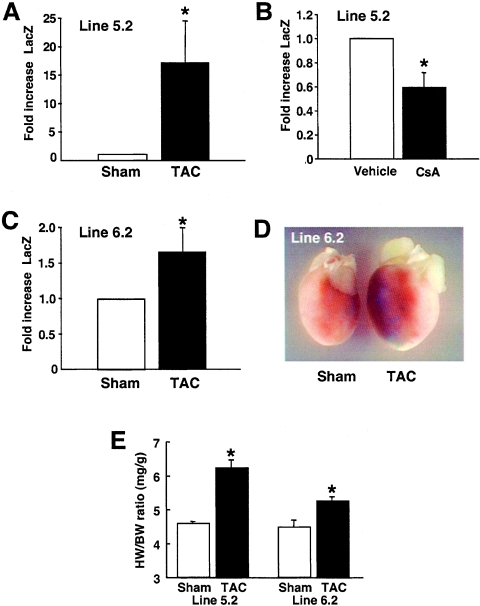
We previously observed an increase in CnAβ protein levels in explanted human hearts that were hypertrophic or in failure, suggesting that CnAβ expression might be transcriptionally induced in vivo (20). To further examine this potential mechanism, both CnAβ2322-LacZ lines 5.2 and 6.2 were subjected to pressure overload stimulation in mice at 8 weeks of age, as induced by TAC (line 5.2, six mice; line 6.2; six mice), or subject to sham operation (line 5.2, three mice; line 6.2, three mice). The data demonstrate that CnAβ promoter activity is significantly increased in each line following pressure overload (P < 0.05) (Fig. 7A and C). Also of interest, CsA treatment of mice reduced baseline cardiac LacZ activity in line 5.2, further implicating a calcineurin-NFAT feedback regulatory circuit in controlling CnAβ promoter activity (Fig. 7B). While TAC stimulation increased the relative amount of LacZ activity in line 6.2, whole-mount histological analysis revealed that the increase in activity was still patchy throughout the heart, although more total cells were positive (Fig. 7D). As a final control, HW/BW ratios were significantly elevated in both lines following 7 days of TAC, demonstrating the effectiveness of the procedure (Fig. 7E). These results suggest that pressure overload stimulation, which is associated with profound cardiac hypertrophy, can activate the CnAβ promoter in vivo.
FIG. 7.
The CnAβ promoter transgene is activated by pressure overload in the heart. (A) Cardiac LacZ activity from CnAβ2322-LacZ transgenic line 5.2 mice after 1 week of pressure overload stimulation induced by TAC (line 5.2; six mice) or following sham operation (three mice) (*, P < 0.05 versus sham). (B) LacZ activity from the hearts of mice treated with vehicle or CsA at 20 mg/kg/day for 5 days (three mice each) (*, P < 0.05 versus vehicle). (C) Cardiac LacZ activity from CnAβ2322-LacZ transgenic line 6.2 after 1 week of pressure overload stimulation induced by TAC (line 6.2; six mice) or following sham operation (three mice) (*, P < 0.05 versus sham). (D) Whole-mount histological stain for LacZ expression in the heart from line 6.2 mice subjected to sham or TAC operation. (E) After 1 week of TAC stimulation, the hearts were removed and cardiac hypertrophy was verified by measurement of HW/BW ratios (*, P < 0.05 versus sham).
Activated CnA and NFATc4 transgenes enhance cardiac CnAβ promoter activity in vivo.
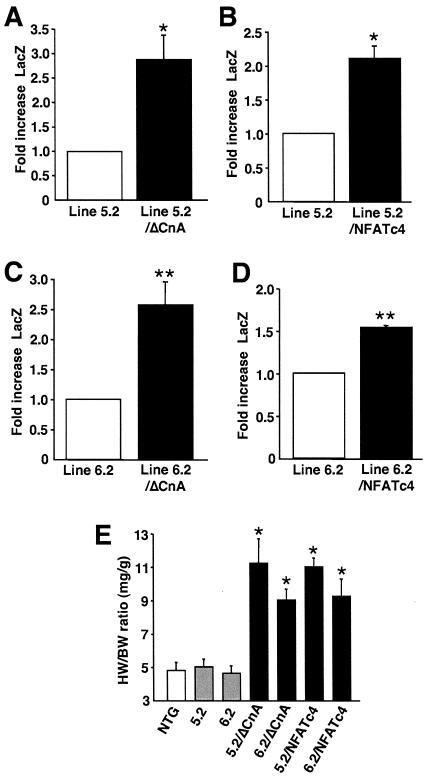
To further examine the underlying mechanism associated with CnAβ promoter induction, CnAβ2322-LacZ transgenic lines 5.2 and 6.2 were each crossed with mice containing a cardiac-specific transgene encoding activated CnA or NFATc4 (38). At 4 weeks of age, mice from lines 5.2 and 6.2 showed significant induction of expression in response to the activated CnA and NFATc4 transgenes, compared with single 5.2 or 6.2 transgene mice alone (Fig. 8A to D). HW/BW ratios in the double-transgenic mice were significantly increased, demonstrating the effectiveness of the activated CnA and NFATc4 transgenes (Fig. 8E). Collectively, these data suggest that calcineurin and NFAT signaling can induce CnAβ promoter expression in vivo, further suggesting a mechanism involving positive transcriptional feedback in the heart.
FIG. 8.
Crossing CnAβ2322-LacZ transgenic mice with ΔCnA and NFATc4 transgenic mice. CnAβ2322-LacZ line 5.2 transgenic mice (A and B) or line 6.2 transgenic mice (C and D) were crossed with cardiac tissue-specific transgenic mice expressing either activated CnA or activated NFATc4. Hearts were removed at 4 weeks of age, and LacZ activity was measured and normalized to total protein concentrations (*, P < 0.05 versus CnAβ2322-LacZ line 5.2; **, P < 0.05 versus CnAβ2322-LacZ line 6.2) (line 5.2, three mice; line 5.2/ΔCnA, three mice; line 6.2, three mice; line 6.2/ΔCnA, three mice; line 5.2/NFATc4, four mice; line 6.2/NFATc4, three mice). (E) HW/BW ratios were significantly increased in double-transgenic mice compared to NTG or CnAβ2322-LacZ transgenic mice (*, P < 0.05 versus NTG or CnAβ2322 single transgenic mice).
DISCUSSION
The CnAβ promoter is regulated by NFAT and GATA factors.
Cardiac myocytes directly respond to a wide array of stress and neuroendocrine stimuli by undergoing hypertrophic enlargement without cell division. This hypertrophic program is characterized by the reexpression of a number of fetus-encoded genes including skeletal α-actin, β-myosin heavy chain, ANF, and BNP. The mechanisms underlying the hypertrophic transcriptional upregulation of each of these genes has been previously characterized through extensive promoter dissection analyses. Such studies have implicated a few transcriptional effectors, such as GATA4 and NFAT, which program, in part, hypertrophic inducibility of gene expression. For example, in the promoter region of the β-myosin heavy chain gene or angiotensin II type Ia receptor gene, cis-acting elements specific for GATA transcription factors have been shown to mediate pressure overload-inducible expression (21, 23). More germane to the present study, three separate reports have previously shown NFAT- and GATA-dependent synergistic or cooperative gene activation in cardiac myocytes on specific promoters. We were the first to demonstrate such a mechanism in the regulation of the BNP promoter. Specifically, GATA4 and NFATc4 were shown to directly interact with one another in cardiac myocytes, resulting in synergistic activation of the BNP promoter through an NFAT site located at −927 (38). More recently, the adenylsuccinate synthase-1 gene was shown to be a target for NFAT transcription factors in the heart (50, 53), which may cooperate with a GATA4 binding site in the same promoter (53). Lastly, the proximal endothelin-1 promoter was also shown to contain an NFAT and GATA binding element that synergistically responded to NFAT and GATA factors in cultured cells (40). These results are consistent with the known induction in both NFAT and GATA DNA binding activities within the hypertrophic heart itself (6, 29, 51).
Here, we identified a number of putative NFAT and GATA binding sites within the CnAβ promoter. While many of these sites likely play important roles in mediating CnAβ inducible expression in vivo, transient transfection experiments and ChIP analysis in cultured neonatal cardiac myocytes revealed that the distal promoter region appears to confer most of the inducible activity in response to hypertrophic stimulation and NFATc3 and GATA4 overexpression. While the most proximal portion of the CnAβ promoter (229 bp) contained a high-affinity NFAT site (N1) and two good GATA sites, mutagenesis of these three sites alone or in combination did not diminish hypertrophic inducibility or NFAT and GATA4 inducibility within the context of the full-length promoter (data not shown). Thus, we favor the interpretation that the more distal CnAβ regulatory sequences are important for inducibility (−2322 to −1399). Of interest, the role that NFAT and GATA sites play in potentially regulating CnAβ expression outside the heart is unknown, although there is precedent for upregulation of CnAβ expression in other tissues following stress or disease stimulation (see below).
In vivo, both lines of CnAβ2322-LacZ transgenic mice showed increased expression in the heart in response to activated CnA and activated NFATc4 transgenes. Moreover, both lines of transgenic mice showed a significant increase in promoter activation within the adult heart following pressure overload stimulation. These results are consistent with the in vitro transfection experiments that together suggest an additional mechanism for controlling calcineurin activity in the heart through transcriptional induction of the CnAβ gene. A minor concern with the CnAβ2322-LacZ transgenic mice is that one or both of the lines may be partially influenced by the site of transgene genomic integration. However, the fact that both lines showed inducible expression in response to multiple hypertrophic stimuli, although at relatively different overall levels, suggests some degree of fidelity. Indeed, line 6.2 shows a pattern of embryonic expression that is nearly identical to in situ hybridization experiments performed with chicken embryos (30). While line 5.2 shows enriched expression in the heart, it also has substantially higher levels of overall expression, so that embryonic staining appears much more ubiquitous compared with line 6.2, not unlike in situ hybridization experiments performed with CnAβ in mouse embryos (K. E. Yutzey, Cincinnati Children's Hospital Medical Center, personal communication).
Regulation of calcineurin signaling by calcium.
Calcineurin activity is strictly dependent on calcium levels within a cell. As calcium levels progressively rise, calmodulin and CnB become fully saturated, thus altering the conformation of CnA, displacing the autoinhibitory domain, and permitting access of the active site to substrates such as NFAT. Using NFAT translocation as a surrogate for calcineurin activation, sustained increases in calcium, but not transient oscillatory calcium, were shown to be necessary for calcineurin activation (13-15, 48, 49). For example, calcineurin activity in skeletal muscle is likely regulated by the tonic rate of motor nerve activity, so that predominantly slow fiber-containing muscles with high rates of tonic nerve firing have more NFAT activity and expression of slow fiber-specific genes than fast fibers that have more transient rates of motor nerve activity (7, 34, 41). Indeed, increased firing rates in cultured adult skeletal muscle cells directly correlated with the degree of NFAT nuclear translocation (34). In T lymphocytes, engagement of the T-cell receptor leads to sustained calcium entry in the form of capacitative or store-operated currents (ICRAC), which is necessary for NFAT nuclear localization and proper cytokine expression (13, 15, 48).
While calcineurin is exquisitely regulated by calcium, the calcineurin-NFAT signaling module is subject to a number of additional levels or regulation. For example, the overall effectiveness of translocation is diminished by direct phosphorylation within the N-terminal regulatory domain of NFAT proteins through the action of a diverse array of calcium-independent kinases. Glycogen synthase kinase 3β, JNK1/2, p38 MAPK, protein kinase A, and casein kinase 1 each phosphorylate NFAT factors, thus antagonizing calcineurin-mediated dephosphorylation and nuclear translocation (2, 8, 16, 42, 45, 54, 55). In cardiac myocytes, glycogen synthase kinase 3β, JNK1/2, and p38 have all been implicated as critical negative regulators of NFAT translocation, thus antagonizing the cardiac hypertrophic response (1, 3, 19, 28).
Calcineurin activity is also regulated by the modulatory calcineurin-interacting proteins (MCIP, also known as calcipressin/DSCR1/ZAKI-4), A kinase anchoring protein 79, and Cain/Cabin-1 (11, 24, 33). Transgenic mice expressing the calcineurin inhibitory domain from modulatory calcineurin-interacting protein 1, A kinase anchoring protein 79, and Cain/Cabin-1 have each been shown to have reduced cardiac hypertrophy in response to stress stimulation (9, 43). Thus, alterations in the activity or functional interactions with one or more calcineurin-interacting protein could influence signaling of this pathway partially independent of calcium.
Finally, calcineurin-NFAT signaling might also be influenced by alterations in expression levels or stability of calcineurin isoforms. Indeed, we have previously demonstrated that total CnA protein levels are elevated in hypertrophic adult hearts (31, 32). More careful examination of this effect revealed that the CnAβ isoform, but not the CnAα isoform, is specifically upregulated in response to hypertrophic stimulation in either cultured cardiac myocytes or the adult heart (20, 46). These results suggested a mechanism whereby hypertrophic stimulation produced a positive feedback loop that secondarily increased CnA protein levels within the heart, thus leading to greater NFAT activation and a propagation of the hypertrophic response. Indeed, CnAβ-deficient mice showed a 60 to 80% decrease in enzymatic activity in the heart that was associated with a significant impairment in cardiac hypertrophy following pressure overload or neuroendocrine stimulation (4).
While CnAβ levels are increased in hypertrophic cardiac myocytes, the mechanism underlying this increase in expression was not known. Here we demonstrated that the CnAβ promoter is directly responsive to hypertrophic stimulation in vitro and in vivo, which suggests that CnAβ levels are also regulated, in part, at the level of transcription. However, the initiating stimulus that leads to CnAβ transcriptional augmentation is likely complex, given the observation that both NFAT and GATA factors can regulate expression and that calcium chelation reduces expression. NFAT induction would require prior activation of calcineurin, suggesting a more proximal requirement for alterations in intracellular calcium concentration in initiating the positive feedback loop. Alternatively, GATA4 transcriptional activity is directly and acutely regulated by ERK1/2- and p38 MAPK-mediated phosphorylation (6, 29), suggesting a mechanism whereby MAPK signaling pathways can contribute to induction and propagation of the hypertrophic response through the CnAβ promoter. Indeed, we observed that inhibition of MEK1-ERK1/2 and p38 signaling subsequently blocked agonist-induced CnAβ promoter activity in cardiac myocytes.
That the CnAβ promoter shows abundant NFAT and GATA sites, binding factor families that are fairly ubiquitously expressed, suggests that CnAβ expression might be inducible in other tissues. Indeed, total CnA expression was previously shown to increase over time in the kidneys of diabetic rats undergoing progressive degeneration (17), which was later shown to include both CnAα and CnAβ isoforms (18). More provocatively, CnAβ mRNA was shown to be specifically upregulated in the brains of Alzheimer's patients (22). Taken together with our observations, these results suggest that CnA expression, especially that of CnAβ, is subject to transcriptional modulation as an additional means of altering total calcineurin activity in select tissues. However, even if transcriptional regulation of CnA subunit genes serves as a mechanism for controlling total calcineurin activity in cardiac cells, it is not mutually exclusive with the well-established calcium control paradigm known to be required for calcineurin activation.
Acknowledgments
This work was supported by grants from the National Institutes of Health, by an American Heart Association Established Investigator Grant to J.D.M., and a Postdoctoral Fellowship grant (0425393B) to T.O.
REFERENCES
- 1.Antos, C. L., T. A. McKinsey, N. Frey, W. Kutschke, J. McAnally, J. M. Shelton, J. A. Richardson, J. A. Hill, and E. N. Olson. 2002. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 99:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1934. [DOI] [PubMed] [Google Scholar]
- 3.Braz, J. C., O. F. Bueno, Q. Liang, B. J. Wilkins, Y. S. Dai, S. Parsons, J. Braunwart, B. J. Glascock, R. Klevitsky, T. F. Kimball, T. E. Hewett, and J. D. Molkentin. 2003. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Investig. 111:1475-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bueno, O. F., B. J. Wilkins, K. M. Tymitz, B. J. Glascock, T. F. Kimball, J. N. Lorenz, and J. D. Molkentin. 2002. Impaired cardiac hypertrophic response in calcineurin Aβ-deficient mice. Proc. Natl. Acad. Sci. USA 99:4586-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buttini, M., S. Limonta, M. Luyten, and H. Boddeke. 1995. Distribution of calcineurin A isoenzyme mRNAs in rat thymus and kidney. Histochem. J. 27:291-299. [DOI] [PubMed] [Google Scholar]
- 6.Charron, F., G. Tsimiklis, M. Arcand, L. Robitaille, Q. Liang, J. D. Molkentin, S. Meloche, and M. Nemer. 2001. Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev. 15:2702-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin, E. R., E. N. Olson, J. A. Richardson, Q. Yang, C. Humphries, J. M. Shelton, H. Wu, W. Zhu, R. Bassel-Duby, and R. S. Williams. 1998. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 12:2499-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow, C. W., M. Rincon, J. Cavanagh, M. Dickens, and R. J. Davis. 1997. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science 278:1638-1641. [DOI] [PubMed] [Google Scholar]
- 9.De Windt, L. J., H. W. Lim, O. F. Bueno, Q. Liang, U. Delling, J. C. Braz, B. J. Glascock, T. F. Kimball, F. del Monte, R. J. Hajjar, and J. D. Molkentin. 2001. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 98:3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Windt, L. J., H. W. Lim, T. Taigen, D. Wencker, G. Condorelli, G. W. Dorn II, R. N. Kitsis, and J. D. Molkentin. 2000. Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: An apoptosis-independent model of dilated heart failure. Circ. Res. 86:255-263. [DOI] [PubMed] [Google Scholar]
- 11.Dodge, K. L., and J. D. Scott. 2003. Calcineurin anchoring and cell signaling. Biochem. Biophys. Res. Commun. 311:1111-1115. [DOI] [PubMed] [Google Scholar]
- 12.Dodou, E., S. M. Xu, and B. L. Black. 2003. mef2c is activated directly by myogenic basic helix-loop-helix proteins during skeletal muscle development in vivo. Mech. Dev. 120:1021-1032. [DOI] [PubMed] [Google Scholar]
- 13.Dolmetsch, R. E., R. S. Lewis, C. C. Goodnow, and J. I. Healy. 1997. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386:855-858. [DOI] [PubMed] [Google Scholar]
- 14.Dolmetsch, R. E., K. Xu, and R. S. Lewis. 1998. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392:933-936. [DOI] [PubMed] [Google Scholar]
- 15.Feske, S., J. Giltnane, R. Dolmetsch, L. M. Staudt, and A. Rao. 2001. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2:316-324. [DOI] [PubMed] [Google Scholar]
- 16.Gomez del Arco, P., S. Martinez-Martinez, J. L. Maldonado, I. Ortega-Perez, and J. M. Redondo. 2000. A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J. Biol. Chem. 275:13872-13878. [DOI] [PubMed] [Google Scholar]
- 17.Gooch, J. L., J. L. Barnes, S. Garcia, and H. E. Abboud. 2003. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am. J. Physiol. Renal Physiol. 284:F144-F154. [DOI] [PubMed] [Google Scholar]
- 18.Gooch, J. L., P. E. Pergola, R. L. Guler, H. E. Abboud, and J. L. Barnes. 2004. Differential expression of calcineurin A isoforms in the diabetic kidney. J. Am. Soc. Nephrol. 15:1421-1429. [DOI] [PubMed] [Google Scholar]
- 19.Haq, S., G. Choukroun, Z. B. Kang, H. Ranu, T. Matsui, A. Rosenzweig, J. D. Molkentin, A. Alessandrini, J. Woodgett, R. Hajjar, A. Michael, and T. Force. 2000. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J. Cell Biol. 151:117-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haq, S., G. Choukroun, H. Lim, K. M. Tymitz, F. del Monte, J. Gwathmey, L. Grazette, A. Michael, R. Hajjar, T. Force, and J. D. Molkentin. 2001. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation 103:670-677. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa, K., S. J. Lee, S. M. Jobe, B. E. Markham, and R. N. Kitsis. 1997. cis-Acting sequences that mediate induction of beta-myosin heavy chain gene expression during left ventricular hypertrophy due to aortic constriction. Circulation 96:3943-3953. [DOI] [PubMed] [Google Scholar]
- 22.Hata, R., M. Masumura, H. Akatsu, F. Li, H. Fujita, Y. Nagai, T. Yamamoto, H. Okada, K. Kosaka, M. Sakanaka, and T. Sawada. 2001. Up-regulation of calcineurin Aβ mRNA in the Alzheimer's disease brain: assessment by cDNA microarray. Biochem. Biophys. Res. Commun. 284:310-316. [DOI] [PubMed] [Google Scholar]
- 23.Herzig, T. C., S. M. Jobe, H. Aoki, J. D. Molkentin, A. W. Cowley, Jr., S. Izumo, and B. E. Markham. 1997. Angiotensin II type 1a receptor gene expression in the heart: AP-1 and GATA-4 participate in the response to pressure overload. Proc. Natl. Acad. Sci. USA 94:7543-7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilioti, Z., and K. W. Cunningham. 2003. The RCN family of calcineurin regulators. Biochem. Biophys. Res. Commun. 311:1089-1093. [DOI] [PubMed] [Google Scholar]
- 25.Hogan, P. G., L. Chen, J. Nardone, and A. Rao. 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17:2205-2232. [DOI] [PubMed] [Google Scholar]
- 26.Jiang, H., F. Xiong, S. Kong, T. Ogawa, M. Kobayashi, and J. O. Liu. 1997. Distinct tissue and cellular distribution of two major isoforms of calcineurin. Mol. Immunol. 34:663-669. [DOI] [PubMed] [Google Scholar]
- 27.Klee, C. B., H. Ren, and X. Wang. 1998. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 273:13367-13370. [DOI] [PubMed] [Google Scholar]
- 28.Liang, Q., O. F. Bueno, B. J. Wilkins, C. Y. Kuan, Y. Xia, and J. D. Molkentin. 2003. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 22:5079-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang, Q., R. J. Wiese, O. F. Bueno, Y. S. Dai, B. E. Markham, and J. D. Molkentin. 2001. The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol. Cell. Biol. 21:7460-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberatore, C. M., and K. E. Yutzey. 2004. Calcineurin signaling in avian cardiovascular development. Dev. Dyn. 229:300-311. [DOI] [PubMed] [Google Scholar]
- 31.Lim, H. W., and J. D. Molkentin. 1999. Calcineurin and human heart failure. Nat. Med. 5:246-247. [DOI] [PubMed] [Google Scholar]
- 32.Lim, H. W., and J. D. Molkentin. 2000. Reply to revisiting calcineurin and human heart failure. Nat. Med. 6:3. [DOI] [PubMed] [Google Scholar]
- 33.Liu, J. O. 2003. Endogenous protein inhibitors of calcineurin. Biochem. Biophys. Res. Commun. 311:1103-1109. [DOI] [PubMed] [Google Scholar]
- 34.Liu, Y., Z. Cseresnyes, W. R. Randall, and M. F. Schneider. 2001. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J. Cell Biol. 155:27-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McFadden, D. G., J. Charite, J. A. Richardson, D. Srivastava, A. B. Firulli, and E. N. Olson. 2000. A GATA-dependent right ventricular enhancer controls dHAND transcription in the developing heart. Development 127:5331-5341. [DOI] [PubMed] [Google Scholar]
- 36.Molkentin, J. D. 2000. Calcineurin and beyond: cardiac hypertrophic signaling. Circ. Res. 87:731-738. [DOI] [PubMed] [Google Scholar]
- 37.Molkentin, J. D. 2004. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc. Res. 63:467-475. [DOI] [PubMed] [Google Scholar]
- 38.Molkentin, J. D., J. R. Lu, C. L. Antos, B. Markham, J. Richardson, J. Robbins, S. R. Grant, and E. N. Olson. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muramatsu, T., P. R. Giri, S. Higuchi, and R. L. Kincaid. 1992. Molecular cloning of a calmodulin-dependent phosphatase from murine testis: identification of a developmentally expressed nonneural isoenzyme. Proc. Natl. Acad. Sci. USA 89:529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nemer, G., and M. Nemer. 2002. Cooperative interaction between GATA5 and NF-ATc regulates endothelial-endocardial differentiation of cardiogenic cells. Development 129:4045-4055. [DOI] [PubMed] [Google Scholar]
- 41.Parsons, S. A., B. J. Wilkins, O. F. Bueno, and J. D. Molkentin. 2003. Altered skeletal muscle phenotypes in calcineurin Aα and Aβ gene-targeted mice. Mol. Cell. Biol. 23:4331-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porter, C. M., M. A. Havens, and N. A. Clipstone. 2000. Identification of amino acid residues and protein kinases involved in the regulation of NFATc subcellular localization. J. Biol. Chem. 275:3543-3551. [DOI] [PubMed] [Google Scholar]
- 43.Rothermel, B. A., T. A. McKinsey, R. B. Vega, R. L. Nicol, P. Mammen, J. Yang, C. L. Antos, J. M. Shelton, R. Bassel-Duby, E. N. Olson, and R. S. Williams. 2001. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 98:3328-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rusnak, F., and P. Mertz. 2000. Calcineurin: form and function. Physiol. Rev. 80:1483-1521. [DOI] [PubMed] [Google Scholar]
- 45.Sheridan, C. M., E. K. Heist, C. R. Beals, G. R. Crabtree, and P. Gardner. 2002. Protein kinase A negatively modulates the nuclear accumulation of NF-ATc1 by priming for subsequent phosphorylation by glycogen synthase kinase-3. J. Biol. Chem. 277:48664-48676. [DOI] [PubMed] [Google Scholar]
- 46.Taigen, T., L. J. De Windt, H. W. Lim, and J. D. Molkentin. 2000. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA 97:1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takaishi, T., N. Saito, T. Kuno, and C. Tanaka. 1991. Differential distribution of the mRNA encoding two isoforms of the catalytic subunit of calcineurin in the rat brain. Biochem. Biophys. Res. Commun. 174:393-398. [DOI] [PubMed] [Google Scholar]
- 48.Timmerman, L. A., N. A. Clipstone, S. N. Ho, J. P. Northrop, and G. R. Crabtree. 1996. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature 383:837-840. [DOI] [PubMed] [Google Scholar]
- 49.Tomida, T., K. Hirose, A. Takizawa, F. Shibasaki, and M. Iino. 2003. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J. 22:3825-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wen, H. Y., Y. Xia, M. E. Young, H. Taegtmeyer, and R. E. Kellems. 2002. The adenylosuccinate synthetase-1 gene is activated in the hypertrophied heart. J. Cell. Mol. Med. 6:235-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkins, B. J., Y. S. Dai, O. F. Bueno, S. A. Parsons, J. Xu, D. M. Plank, F. Jones, T. R. Kimball, and J. D. Molkentin. 2004. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 94:110-118. [DOI] [PubMed] [Google Scholar]
- 52.Wilkins, B. J., L. J. De Windt, O. F. Bueno, J. C. Braz, B. J. Glascock, T. F. Kimball, and J. D. Molkentin. 2002. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol. Cell. Biol. 22:7603-7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia, Y., J. B. McMillin, A. Lewis, M. Moore, W. G. Zhu, R. S. Williams, and R. E. Kellems. 2000. Electrical stimulation of neonatal cardiac myocytes activates the NFAT3 and GATA4 pathways and up-regulates the adenylosuccinate synthetase 1 gene. J. Biol. Chem. 275:1855-1863. [DOI] [PubMed] [Google Scholar]
- 54.Yang, T. T., Q. Xiong, H. Enslen, R. J. Davis, and C. W. Chow. 2002. Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol. Cell. Biol. 22:3892-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu, J., F. Shibasaki, R. Price, J. C. Guillemot, T. Yano, V. Dotsch, G. Wagner, P. Ferrara, and F. McKeon. 1998. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell 93:851-861. [DOI] [PubMed] [Google Scholar]