Abstract
Nidogen 1 and 2 are basement membrane glycoproteins, and previous biochemical and functional studies indicate that they may play a crucial role in basement membrane assembly. While they show a divergent expression pattern in certain adult tissues, both have a similar distribution during development. Gene knockout studies in mice demonstrated that the loss of either isoform has no effect on basement membrane formation and organ development, suggesting complementary functions. Here, we show that this is indeed the case. Deficiency of both nidogens in mice resulted in perinatal lethality. Nidogen 1 and 2 do not appear to be crucial in establishing tissue architecture during organ development; instead, they are essential for late stages of lung development and for maintenance and/or integrity of cardiac tissue. These organ defects are not compatible with postnatal survival. Ultrastructural analysis suggests that the phenotypes directly result from basement membrane changes. However, despite the ubiquitous presence of nidogens in basement membranes, defects do not occur in all tissues or in all basement membranes, suggesting a varying spectrum of roles for nidogens in the basement membrane.
Basement membranes are specialized extracellular matrices found underlying all epithelia and endothelia and surrounding many mesenchymal cells, in particular, myocytes and Schwann cells. Basement membranes play fundamental roles in differentiation, proliferation, survival, and migration of cells during embryonic development but also serve as selective barriers and structural scaffolds. Further basement membranes act as reservoirs for cytokines and growth factors (38-40). All basement membranes contain at least one representative from each of the laminin, nidogen, collagen type IV, and proteoglycan families. Gene deletion studies in mice have shown that while collagen type IV is needed for the stability of the basement membrane, it is not required for the very early stages of basement membrane formation, an event dependent on the presence of laminin (24, 30, 37).
The nidogen family in mammals consists of two members, nidogen 1 and nidogen 2 (18, 41), also known as entactin 1 and 2 (6, 17), which are expressed by distinct genes located on different chromosomes (9, 35, 47). Both isoforms are ubiquitous basement membrane components with a similar distribution in various organs during embryonic development; however, in the case of nidogen 2, this becomes more restricted in some adult tissues, for instance, in striated muscles where only a faint staining of the muscle basement membranes can be detected (18, 26, 28, 33, 35). Biochemical and structural studies with nidogen 1 have shown binding to a wide spectrum of basement membrane-associated proteins, and it has been proposed that nidogens act as connecting elements between the collagen IV and laminin networks and integrate other basement membrane components, in particular, perlecan, into this specialized extracellular matrix (3, 11, 13, 29, 32). More recently, recombinant mouse nidogen 2 has been shown to possess binding properties comparable to those of nidogen 1 (33).
The high-affinity nidogen-binding site has been localized to a single laminin-type epidermal growth factor-like module, γ1III4, on the laminin γ1 chain (21, 31) and is therefore present in most laminin isoforms. Antibodies against the γ1III3-5 module, which contains the nidogen-binding module γ1III4, perturb early kidney, lung, and salivary gland development in organ culture (10, 14), indicating that binding of nidogens or other proteins to this module could be required for development. Furthermore, it has been shown that the presence of competing recombinant γ1III3-5 disrupts basement membrane development (5, 42). However, mice lacking the γ1III4 module, produced in gene-targeting experiments, while having severe disruption of early kidney development and some abnormalities in late lung morphogenesis, failed to show defects in all basement membranes (12, 44).
While inactivation of the genes for the laminin β1 or γ1 chain in mice showed that laminin per se is a prerequisite for basement membrane formation (24, 37), and although signaling roles are highly significant in the function of certain laminin isoforms, many of the changes induced by the loss of basement membrane molecules appear to be related to a pure loss in mechanical stability (2, 7, 12, 22, 30, 44). Nidogen 1 and 2 knockout mice show surprisingly mild phenotypes. In nidogen 1-deficient animals, most basement membranes appear ultrastructurally unaltered, and there is little change in cellular or tissue morphology. The null animals appear generally healthy, have a normal life span, and are fertile (28). However, they show neurological phenotypes, in particular, a mild hind-limb ataxia and spontaneous seizure activity (8; N. Smyth and R. Nischt, unpublished data). While nidogen 1 is found in all basement membranes, the expression of its homologue, nidogen 2, is more limited, being found only in trace amounts in the basement membranes of skeletal and cardiac muscles. In nidogen 1-deficient animals, nidogen 2 is increased in these tissues by between three- and sevenfold as determined by radioimmunoassays (25) and is redistributed in its staining pattern, suggesting complementary functions of these two isoforms and shared binding partners in vivo (25, 28, 33). There is no alteration in the staining pattern of other basement membrane proteins. The nidogen 2-null animals show no phenotype and no obvious alteration in the expression pattern of other basement membrane molecules, including nidogen 1 (35).
As in the mouse, loss of the only known nidogen gene in Caenorhabditis elegans does not lead to obvious basement membrane defects, but altered axon guidance was noted in some nerves (15, 16). Furthermore, nidogen, together with collagen XVIII, appears to play an important role in synapse organization in the worm (1).
These in vivo results and the finding that nidogens have a broadly complementary binding repertoire suggest redundancy within this protein family. To study this further and to discover the importance of nidogens in development, compound nidogen 1/nidogen 2 mutant mice were generated and analyzed.
MATERIALS AND METHODS
Generation and genotyping of mice lacking both nidogen 1 and 2.
The mutation in the NID1 gene was generated by deleting exon 3 as described previously (28). The mutation in the NID2 gene was introduced by insertion of a gene trap vector in intron 4 (35, 36). Mice lacking both nidogen isoforms were produced by crossing nidogen 1- and nidogen 2-null animals. Wild-type and compound mutant NID1 and NID2 alleles were assessed by Southern blot hybridization and/or PCR from DNA isolated from mouse tail biopsies, from yolk sacs, or from embryonic tissues as described previously (28, 35).
Histological analysis and immunofluorescence staining of tissues.
Embryos and newborn mice were dissected, fixed for 2 to 4 h in 4% (wt/vol) paraformaldehyde-phosphate-buffered saline, and then embedded in paraffin wax, sectioned, and stained using hematoxylin-eosin according to standard protocols. For immunostaining, tissues were snap-frozen in optimal-cutting-temperature compound, and either cryosections (7 to 12 μm) or sections (5 to 10 μm) from paraffin-embedded tissues were used. Immunostaining was performed as described previously (28, 37) using polyclonal rabbit antisera directed against nidogen 1 (11), nidogen 2 (18), laminin 1 (20), collagen type IV (kindly provided by the late R. Timpl, Max Planck Institute for Biochemistry, Martinsried, Germany), and perlecan (34) and against human surfactant protein B (SP-B) (Chemicon International). Immunoreactivity was detected with a Cy3-conjugated goat anti-rabbit immunoglobulin G polyclonal serum (Jackson Immunodiagnostics). For double immunostainings, a rat monoclonal antibody directed against mouse CD31 (PECAM-1) was used (Pharmingen). Immunoreactivity was then detected with Alexa Fluor 488 goat anti-rat (green) and Alexa Fluor 568 goat anti-rabbit (red) immunoglobulin G polyclonal sera (Molecular Probes).
Ultrastructural analysis.
Kidney, heart, and lung of embryos and newborn mice were dissected. For morphological investigation, 2-mm3 tissue fragments were isolated. Fixation, preparation, and staining for ultrastructural analysis have been described previously (26).
Protein analysis.
Total protein was extracted from kidneys and lungs from three control and nidogen double-null embryos at embryonic day 18.5 (E18.5). For immunoblotting, 20 μg total protein was separated under reducing conditions on 4 to 12% sodium dodecyl sulfate (SDS)-polyacrylamide gels, transferred onto nitrocellulose membranes (Amersham Pharmacia Biotech), and incubated with a polyclonal rabbit antiserum directed against the laminin γ1 chain (1:1,000). Equal protein loadings of lanes was verified by incubating the blots with a mouse monoclonal antibody directed against actin (1:6,000; MP Biomedicals). Detection was done using a horseradish peroxidase-conjugated goat anti-rabbit (1:1,000) or swine anti-mouse (1:2,000) antiserum (DAKO), respectively, followed by chemiluminescence detection (ECL; Amersham Pharmacia Biotech). Urine collected from double-homozygous mutants and control littermates was subjected to microelectrophoresis. Proteins from 10 μl of urine were separated on a 10% SDS-polyacrylamide gel, and the protein bands were then visualized by Coomassie blue staining according to standard protocols and analyzed by densitometry.
RESULTS
Mice lacking both nidogen isoforms develop until birth.
To test whether nidogen 1 and nidogen 2 can compensate for each other during development, double-null mice lacking both isoforms were generated. Nidogen 1-deficient (NID1−/−) animals (28) were bred to nidogen 2-null (NID2−/−) animals (35) to produce double heterozygotes, which were normal and fertile. F1 double heterozygotes were intercrossed to generate mice lacking nidogen 1 and 2. Genotyping of more than 1,000 offspring of heterozygous breeding pairs at weaning stage failed to reveal any surviving compound homozygous mutant (NID1−/− NID2−/−) animals. However, dead pups were observed within 24 h of birth, many of which were nidogen double-null mutants, suggesting that nidogen-deficient animals died after birth. Genotyping of 62 mice from eight litters at birth showed that three nidogen double-null animals were born, corresponding approximately to the expected Mendelian frequency of 1/16. To increase the number of nidogen double-null animals, either NID1−/− NID2+/− or NID1+/− NID2−/− mice were intercrossed. Both mutants have a normal life span and are fertile, showing that a single copy of one of the two NID genes is sufficient for development and adulthood. However, NID1−/− NID2+/− animals show the same neurological phenotypes with hind-limb ataxia and seizure activity as described previously by Dong et al. (8) for NID1−/− animals. These mutants appear to be more severely affected than mice carrying only two mutated NID1 alleles (Smyth and Nischt, unpublished data). Genotype analyses of these intercrosses yielded about 15% nidogen double-null newborn animals (60% of the expected ratio). Although cannibalism of dead or dying double-null pups by their mother certainly reduced the numbers found at birth, there also seems to be a slight reduction of double-null mutants present at different embryonic stages (Table 1).
TABLE 1.
Transmission frequencies of NID1 NID2 genotypes from compound NID1+/− NID2−/− breeding pairs
| Stage | No. (%) of genotypes at embryonic stages and postnatally
|
|||
|---|---|---|---|---|
| NID1+/+ NID2−/− | NID1+/− NID2−/− | NID1−/− NID2−/− | Total | |
| E12.5 | 17 (25.8) | 33 (50.7) | 15 (23.0) | 65 |
| E13.5-17.5 | 45 (28.3) | 86 (52.1) | 34 (20.6) | 165 |
| E18.5-19.5 | 39 (26.4) | 77 (52.0) | 32 (21.6) | 148 |
| P0a-P21b | 3a + 43b = 46 (28.3) | 7a + 82b = 89 (56.0) | 24a (15.1) | 159 |
| >P21 | 185 (34.8) | 347 (65.2) | 0 (0.0) | 532 |
Offspring which are found dead in the cage at P0, phenotypically aberrant, and/or died during day P0.
Their viable siblings at P21.
Phenotypic analysis did not show overt gross anatomical abnormalities in mutant embryos, except for syndactyly of variable severity (data not shown); however, double-null neonates were smaller, varying in size from normal to 70% of the size of control littermates.
Impaired heart morphogenesis and integrity in nidogen double-null mutants.
Nidogen double-null mutants reaching parturition became cyanotic and, despite obvious efforts to breathe, died within 24 h after birth. Therefore, possible causes of mortality in these mice could be cardiovascular and/or respiratory failure. As both proteins are expressed in the heart (18, 35), and as the myocardial basement membrane of nidogen 1-deficient mice showed a marked redistribution and upregulation of nidogen 2 (25, 28), we first analyzed the cardiac structure in the double-null mice. Hearts isolated from nidogen double-null mice surviving birth generally showed no gross anatomical abnormalities, with the heart, the valvular structures, and the great vessels appearing normal. However, the hearts occasionally showed dilatation (data not shown). Mutant hearts isolated from embryos at E17.5 exhibited a diminished overall size, which varied in its severity (Fig. 1a, A and E). The weight of the smallest heart was 0.07 g, as opposed to an average weight of 0.09 g in control littermates.
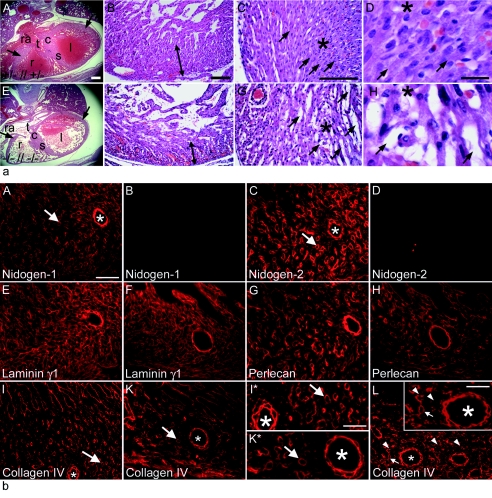
FIG. 1.
Histology (a) and immunofluorescence microscopy (b) of cardiac tissues from nidogen double-null embryos and neonates. (a) Hematoxylin-and-eosin-stained transverse sections of hearts from control littermates (A to D) and double-null mice (E to H) at E17.5 (A and E) and P0 (B to D and F to H) are shown. The nidogen-deficient hearts (E) were slightly smaller in size than the hearts of control embryos (A). (A, B, E, and F) Arrows, compact zones of the ventricular wall; (C, D, G, and H) asterisks, areas shown at different magnifications; arrows, cell-cell contacts. (A and E) c, endocardial cushion; l, left ventricle; r, right ventricle; ra, right atrial chamber; s, interventricular septum; t, tricuspid valve. Bars, 200 μm (A and E), 50 μm (B, C, F, and G), and 10 μm (D and H). (b) Immunofluorescence was performed on embryonic heart sections at E18.5 using rabbit antisera against nidogen 1 (A and B), nidogen 2 (C and D), laminin γ1 (E and F), perlecan (G and H), and collagen type IV (I, I*, K, K*, and L). Deposition of all basement membrane components is detectable in control sections (A, C, E, G, I, and I*), whereas in mutant cardiac tissues, the staining intensities appeared to be reduced (F, H, K, K*, and L). Areas of the cardiac muscle tissue with smaller (arrows) and larger (asterisks) blood vessels in I and K are shown at a higher magnification in I* and K*. In some sections from nidogen-deficient hearts, collagen IV staining of basement membranes of capillaries (L) appears to vary, with some showing a normal (arrow) and others showing an irregular (arrowheads) or sometimes patchy staining pattern. Bars, 50 μm (A to K, K*, I*, and L) and 25 μm (inset in L).
Histologically, the hearts of nidogen double-null embryos and neonates demonstrated signs of trabecular hypoplasia and a thinning of the ventricular wall (Fig. 1a, A, B, E, and F). In the mildest cases, where a reduced compaction of the mutant ventricular myocardium occurred, a loss in the regularity of the muscle sheets was noticed, while in others, a severe separation of the individual muscle fibers was evident (Fig. 1a, C, D, G, and H). Histology showed that hemorrhaging often occurred within the walls of nidogen double-null hearts. This ranged from bleeding into focal areas within the ventricular wall to diffuse bleeding in both ventricles and at the base of the atria (Fig. 1a, B, C, F, and G). Intramyocardial hemorrhage was typically confined to the subepicardial regions and was rarely observed in the interventricular septum. Notably, in double-null mutants, the epicardium and pericardium appeared intact, and no pericardial hemorrhage was observed (Fig. 1a, A and E).
At earlier embryonic stages of heart development, prior to E14, the myocardial and endocardial cell layers were apparent and the myocardial walls of each chamber initially appeared to develop normally. Furthermore, the endocardial cushions, the anlage of the future valves, were developed as in the control hearts. However, at later stages, stretches of the endocardium in double-null embryos frequently separated from the underlying myocardial cells, whereas the endocardium of control hearts remained in tight contact with the myocard, presumably being maintained there in part by the intervening basement membrane (data not shown).
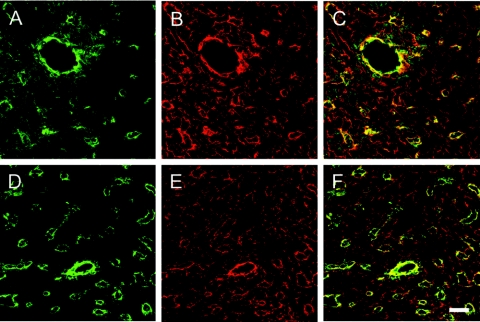
Immunofluorescence microscopy for the major basement membrane proteins laminin, perlecan, and collagen type IV in mutant and control hearts showed the typical localization in the basement membrane zone of the myocardium and cardiac blood vessels in control double-heterozygous embryos at E18.5 (Fig. 1b, A, C, E, G, I, and I*). In contrast, the deposition of these proteins appeared to be reduced to various degrees in nidogen double-null hearts (Fig. 1b, F, H, K, and K*). Immunostaining of larger blood vessels was comparable in nidogen double-null and control hearts (Fig. 1b, E to L). However, while some of the capillaries in nidogen-deficient hearts showed a normal staining pattern (arrows), others had an irregular (arrowheads) or sometimes patchy staining pattern for collagen type IV (Fig. 1b, L). A similar staining pattern for larger and smaller blood vessel in the heart was also observed by confocal microscopy using laminin antibodies (Fig. 2). Double immunofluorescence with antibodies directed against CD31, an endothelial cell marker, revealed that all capillaries show laminin immunoreactivity, although the costaining of some vessels appear significantly weaker in nidogen-deficient hearts (Fig. 2, D to F) than in control hearts (Fig. 2, A to C).
FIG. 2.
Expression and distribution pattern of laminin γ1 (red signal) and PECAM-1 (green signal) in cryosections from hearts of control (A to C) and nidogen double-null (D to F) embryos at E18.5. Cryosections were processed for indirect immunofluorescence and visualized by confocal microscopy. Both color channels were merged to demonstrate codistribution (yellow signal) of both immunofluorescence staining signals in the corresponding sections (C and F). Bar, 20 μm.
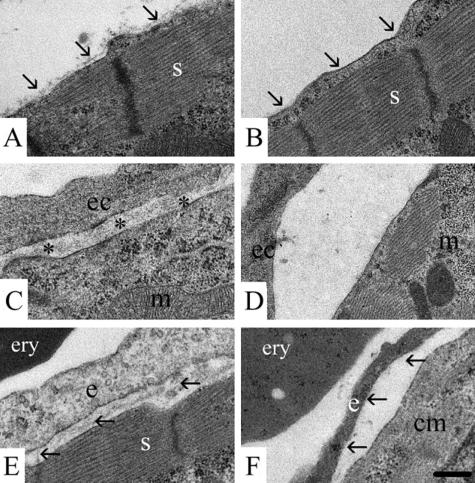
At the ultrastructural level, correct myofilament organization occurred in the double-null animals, suggesting normal differentiation of cardiomyocytes. However, unlike the controls, where a continuous deposition of basement membrane material was seen over the sarcolemma at E18.5, there were only small patches of electron-dense extracellular matrix evident (Fig. 3A and B). Furthermore, at E16.5, there was no distinct basement membrane underlying either the epicardium (data not shown) or the endocardium (Fig. 3C and D) in nidogen double-null animals nor was there evidence of a distinct endothelial basement membrane in mutant cardiac capillaries (Fig. 3E and F).
FIG. 3.
Electron micrographs of ultrathin sections of hearts of control littermates (A, C, E) and of nidogen double-null mutants (B, D, E) at E18.5 (A, B, E, and F) and at E16.5 (C and D). Heart cardiomyocytes with sarcomer (s) of a control embryo with the basement membrane marked by arrows (A) and of a double-null mutant where the basement membrane is lacking (B) are shown. For the endocardium, the endocardial basement membrane (asterisks) is present between the endocardial cell (ec) and the cardiomyocyte with a mitochondrium (m) in control embryos (C). In the double-null embryos, the basement membrane is missing (D). Capillaries in the heart muscle of a control embryo and a double-null mutant with an erythrocyte (ery) are shown. The basement membrane is present (arrows) underneath the endothelial cell (e) adjacent to a cardiomyocyte (cm) with a sarcomer (s) in the littermate control (E), whereas in the nidogen double-null mutant, the basement membrane is lacking (arrows). Bar, 0.25 μm.
Lung development is delayed in nidogen-deficient mice.
Although many nidogen double-null mutants were found dead in the cages, presumably dying shortly after birth, others survived for a few hours. These mice often showed some indications of cyanosis. Given that the lung forms by branching morphogenesis, a process which has been linked to the laminin-nidogen interaction (10, 14), we examined lung development in embryos and neonates surviving birth for some hours.
In respect to the number of lobes and their separation, the lungs had developed normally and were of similar sizes, and the diaphragm had formed (data not shown). Though the major bronchial trees were present in double-null mice, the lungs were more compact than those of the controls (Fig. 4a). Histology of nidogen double-null embryos at E17.5 (Fig. 4a, B) and E18.5 (Fig. 4a, D) showed that lung development was delayed, with extensive mesenchyme remaining. At both developmental stages (Fig. 4a, B and D), the mutant lungs still showed pseudoglandular and canalicular stage-like morphology with terminal lung buds surrounded by extensive mesenchyme, whereas lungs from control littermates were already in the saccular stage at E18.5 (Fig 4a, C). However, early signs of forming saccules can be seen in nidogen-deficient lungs (Fig. 4a, D). Formation of the thin and extensive air-blood barrier is essential for gas exchange and hence crucial for survival postnatally. At birth, lungs from control littermates show typically dilated peripheral airway saccules (Fig. 4a, E and G). Nidogen double-null mice examined at birth had improperly expanded sacculi with very small alveolar spaces and thicker septa (Fig 4a, F and H). At higher magnification, it became apparent that the prealveolar sacculi were immature and poorly inflated, and mesenchymal thickening between the terminal airspaces was observed (Fig. 4a, H). In those mice managing to survive a few hours past birth, the lung showed greater signs of inflation; however, there were some 50% fewer inflated sacculi than in control littermates, and they generally had thicker septae.
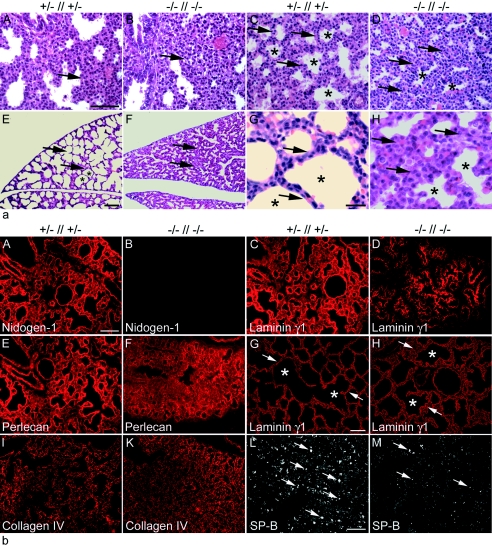
FIG. 4.
Histological analysis of lung tissue from embryos and neonates. (a) Hematoxylin-and-eosin-stained transverse sections of lungs from comparable regions at E17.5 (A and B), E18.5 (C and D), and P0 (E to H) are shown. Development of the lung tissue from the mutant (B and D) appears delayed compared to that of control littermates (A and C). At birth, neonatal lungs from control pups show typically dilated peripheral airway saccules (E and G), whereas nidogen-deficient lungs appear immature (F and H). Arrows, mesenchyme; asterisks, saccules. Bars, 50 μm (A to F) and 10 μm (G and H). (b) Immunofluorescence of lung sections from control (A, C, E, I, and L) and nidogen double-null (B, D, F, K, and M) embryos at E18.5 and (G and H) at P0. Rabbit antisera against the basement membrane components nidogen 1 (A and B), laminin γ1 (C, D, G, H), perlecan (E and F), collagen type IV (I and K), and SP-B (L and M) were used. Prominent deposition of all basement membrane proteins is detectable in control sections (A, C, E, G, I), whereas deposition appears less pronounced at E18.5 in sections from nidogen-deficient mutants (D, F, K). However, sections from lungs of mutant animals after birth showed a laminin staining of lung basement membranes comparable to that seen in control littermates (G and H). Immunostaining for SP-B appears significantly reduced in mutant (M) compared to control (L) lungs. (G and H) Arrows and asterisks mark terminal airways. Bars, 50 μm.
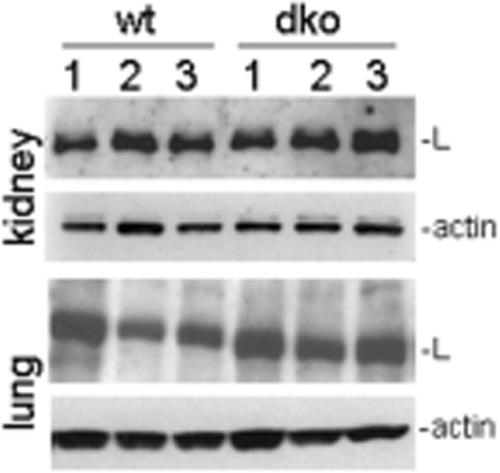
The expression of the basement membrane proteins laminin, collagen IV, and perlecan was studied during lung development by immunofluorescence microscopy (Fig. 4b). Typical deposition of these proteins was observed in the basement membranes of the lung of control littermates, including the airways, visceral pleura, larger blood vessels, and microvessels (Fig. 4b, A, C, E, G, and I, and data not shown). In nidogen double-null lungs at E18.5, the overall distribution and intensity of the immunostaining for laminin, perlecan, and collagen IV were reduced to various degrees, and their deposition appeared to be more concentrated in areas of developing airways (Fig. 4b, D, F, and K). However, immunoblot analysis of protein extracts isolated at E18.5 from lungs showing reduced laminin levels (Fig. 4b, D) and from the kidneys showing no major changes in laminin immunoreactivity (see Fig. 6C and D) revealed no great variations in the total amount of laminin γ1 present in these tissues compared to control embryos (Fig. 5). Lungs isolated from mice at postnatal day 0 (P0) and immunostained for the laminin γ1 chain displayed a continuous staining pattern at this stage (Fig. 4G and H). SP-B is a product of the cuboidal type II pneumocyte. Its expression first occurs early in the embryonic period of lung formation and rapidly increases prior to birth, when the respiratory tubules form alveoli (43). Immunostaining at E18.5 showed a lower level of expression in double-null mutants than in control littermates (Fig. 4b, L and M), suggesting a delay of pulmonary development in the absence of nidogens.
FIG. 6.
Analysis of kidney formation and function from nidogen-deficient embryos and neonates. Hematoxylin-and-eosin-stained sections of kidneys of control (A) and nidogen double-null (B) mice at P0 are shown. Most nidogen-deficient mice formed kidneys that were histologically normal, and immunostaining for laminin γ1 (C, control; D, nidogen double-null mutant) showed a characteristic linear pattern in the glomerulus and under most tubules. Electron microscopy of kidney at E18.5 revealed a normal glomerulus with the typical dual basement membrane being present and in tight contact with the overlying podocytes (e, glomerular epithelial cell; p, podocyte) in control littermates (E) and nidogen double-null embryos (F). However, the tubular basement membrane was often thickened, discontinuous, or even absent (arrows) in double-null kidneys (H), whereas in control kidneys (G), the tubular basement membrane is present (arrows). t, tubular epithelial cell. Bars, 200 μm (A and B), 50 μm (C and D), and 0.25 μm (E to H). Urine removed from the bladders of littermates shortly after birth was analyzed by polyacrylamide gel electrophoresis, and the gels were scanned (I, double-null mutant; K, control). There was a marginal increase in lower-molecular-weight components in the urine of nidogen double-null mice.
FIG. 5.
Immunoblot analysis of protein extracts from kidneys and lungs isolated from three control (wild type [wt] [lanes 1 to 3]) and three nidogen double-null (dko [lanes 1 to 3]) embryos at E18.5. Twenty micrograms of total protein was separated under reducing conditions on 4 to 12% SDS-polyacrylamide gels and, after transfer, incubated with primary antibodies directed either against laminin γ1 (L) or actin, which was used as a loading control.
Renal differentiation in the absence of nidogens.
Renal formation requires the outgrowth of the ureteric bud into the metanephric mesenchyme in order to induce formation of the metanephric kidney. Where nidogen-laminin binding is genetically abrogated, renal aplasia very commonly occurs due to a defect in the Wolffian duct (44). Therefore, it was surprising to find that grossly normal kidneys did develop in most mice lacking both nidogen isoforms (see Fig. 7A). However, a small percentage of these nidogen-deficient mutants did show either unilateral (6%) (see Fig. 7B) or bilateral (8%) renal aplasia (5 and 7 out of 85 double-null mice, respectively).
FIG. 7.
Most nidogen double-null animals showed little or no gross renal phenotype (A); however, a minority of nidogen-null mice had either renal dysgenesis or agenesis. Agenesis could be unilateral or bilateral (B). While the range of changes occurring in the developed kidneys included hydronephrosis, the remaining kidney in B shows an obvious dilated and tortous ureter (arrows), which could be gross (C), while other kidneys displayed polycystic defects marked by arrows (D). ad, adrenal gland; b, bladder; ep, epididymus; k, kidney; t, testis.
Histologically, most of the nidogen double-null mice had developed functioning kidneys, with all parts of the nephron and collecting system having formed (Fig. 6A and B. The numbers of glomeruli where similar in both control and mutant littermates. However, in some cases, there appeared to be greater amounts of intertubular connective tissue within the renal medulla, and occasionally, the cortex was somewhat thinner than that in controls (data not shown). Kidney function was shown by the presence of urine in the bladders of many embryos obtained either perinatally or at E18.5. Analysis of the urine by gel electrophoresis revealed only a slight increase in total protein levels (Fig. 6I and K). Immunostaining for the laminin γ1 chain (Fig. 6C and D) or perlecan (data not shown) showed a well-defined linear staining in the glomerular basement membrane and Bowman's capsule. While laminin immunoreactivity was detectable under all tubules, some displayed a more diffuse staining. Electron microscopy of the E18.5 kidney in nidogen double-null embryos revealed the fully formed dual basement membrane characteristic of the glomerulus with well-formed podocytes and slit junctions (Fig. 6E and F). However, the basement membrane underlying the tubular epithelium, while in many regions appearing normal, was also sometimes altered, giving a diffuse and thickened pattern (data not shown) or being totally absent (Fig. 6G and H).
As stated above, a small minority of nidogen double-null mice failed to develop one or both kidneys (Fig. 7B). Furthermore, some mice also showed renal dysgenesis which was inconsistent in its severity. In a number of double-null mutant kidneys examined histologically (7/28), evident formation of cysts with variable sizes through the renal medulla and cortex was seen (Fig. 7D). Obvious hydronephrosis occurred in two further kidneys (Fig. 7C), resulting in the thinning and fibrosis of the renal cortex and a dilated and tortuous ureter. In one of these cases, the ureter could be followed back to a cystic structure continuous with the vas deferens. Similar defects were not encountered in any of the control littermates examined.
DISCUSSION
We have generated nidogen-deficient mice, and we demonstrate that the lack of both nidogen isoforms does not prevent embryonic development and is compatible with survival to birth. However, that nidogen-deficient mice have basement membrane defects and developmental abnormalities in certain organs strongly argues for tissue-specific roles of nidogens in basement membrane assembly and function. Here, we focused on the most striking defects: those observed in heart, lung, and kidney. Analysis of the pial basement membrane (12) and of tissues with morphologically more rudimentary basement membranes or no basement membrane, such as thymus, spleen, and cartilage (33), were not included in this study.
Embryonic development is not affected by the loss of nidogens.
Basement membranes are believed to be crucial during embryogenesis for both tissue strengthening and inductive events. The potential of nidogen 1 to bridge the laminin and collagen IV networks and its high-affinity interaction with the perlecan core protein led to the proposal that nidogens play a central role for basement membrane assembly and integrity (3, 11, 13, 40). The importance of the laminin-nidogen interaction for organogenesis was demonstrated in antibody perturbation studies showing impaired branching morphogenesis of kidney and lung organ cultures (10, 14). Therefore, it was unexpected that mice lacking nidogen 1 did not show developmental defects, their basement membranes appearing normal (28). However, in these mice, redistribution and/or upregulation of nidogen 2 was observed (25, 28), suggesting redundancy within the nidogen family. Indeed, nidogen 2 has a highly homologous domain structure and binding repertoire and colocalizes with nidogen 1 in almost all basement membranes during embryonic development (18, 25, 33). Mice lacking nidogen 2 showed no overt abnormalities, and basement membrane defects strengthened this compensation hypothesis (35) and prompted us to generate mice lacking both nidogen isoforms.
These mice reveal that the deposition of basement membranes is not critically dependent on the presence of nidogens. Embryos reach term and are born, indicating that these proteins are dispensable for basement membrane function and for development in most tissues and that the secretion and deposition of other basement membrane components are sufficient for the essential functions of basement membranes during embryogenesis. Of the other ubiquitous basement membrane proteins, i.e., laminin, collagen type IV, and perlecan, only genetic deletion of the laminin 1 subchains in mice resulted in early embryonic lethality at the peri-implantation stage (24, 37), whereas ablation of any other basement membrane component resulted in later defects (7, 22, 28, 30, 35, 44). In vitro laminins are known to self-interact to form a polymer (46), and the in vivo results show that these aggregates formed by laminins are sufficient to provide minimal matrices which enable migration, differentiation, proliferation, and cell survival in the early embryo (24, 37). This suggests that either laminins alone are sufficient for the formation of basement membrane-like structures or the other components can compensate for one another to varying degrees. The lack of one basement membrane component like perlecan, collagen IV, or, as described here, nidogen may still allow formation of an imperfect basement membrane-like matrix which provides the basic functions essential for early embryogenesis. In the nidogen double-null embryos, the major basement membrane components are produced, suggesting that the absence of nidogens does not impair secretion of laminins, perlecan, or collagen type IV. While the variable weaker immunostaining intensities for basement membrane components in the mutants, for instance, in the heart, may be due to decreased synthesis, it is more likely caused by reduced retention of these basement membrane components as indicated by immunoblot analysis. This effect was obvious only in organs where basement membranes appear ultrastructurally altered or absent and suggests that in specific tissues, nidogens have a role in the recruitment to or maintenance of other proteins at the basement membrane zone. Similar findings have been previously described in mice lacking the nidogen-binding module of the laminin γ1 chain (44) or collagen IV (30).
Nidogens are important for myocardial basement membrane formation and heart function.
Nidogen double-null mice surviving to birth become cyanotic and die within 24 h, possibly due to either pulmonary or cardiovascular failure. Superficially, hearts isolated from these mice showed little changes, and the valvular structures and great vessels appeared normal, indicating that nidogens are not essential for heart development. However, the myocardium was thinner and less organized, with severe separation of the muscle fibers in some cases. Ultrastructural analysis revealed striking abnormalities in the cardiac basement membranes. There were no distinct basement membranes detectable surrounding cardiomyocytes and cardiac capillaries as well as underlying either the epicardium or endocardium, clearly demonstrating that nidogens are a prerequisite for the formation of ultrastructurally normal basement membranes in the heart. Interestingly, although nidogen is essential for myocardial basement membrane formation, this basement membrane does not appear to be required to maintain the overall integrity of the muscle, as these mice do not develop complete myocardial breakdown leading to pericardial hemorrhage. This indicates that either the residual disorganized basement membrane, other extracellular matrices, and/or cell-cell contacts such as at the intercalated discs, which are normal in nidogen double-null embryos, are sufficient to maintain the gross integrity of the myocardium and to compensate for the basement membrane defects. This is in contrast to findings in mice lacking the other major basement membrane components, perlecan and collagen type IV, which generally die at E10.5 due to a rupture of the myocardium resulting in intrapericardial hemorrhage. While these proteins are also not essential for initiating basement membrane assembly in other organs, they are crucial for the structural and functional integrity of the working heart (7, 30).
Our findings suggest that nidogens have a less significant role in heart development and stabilization of tissue architecture but are required for the formation of ultrastructurally normal basement membranes in this high-stress environment. It may be that other extracellular matrices which are adversely affected by the absence, for instance, of perlecan may be more important for early cardiac development. In later embryonic development, and at birth, heart function is associated with increased contraction and capacity demands. Presumably, the finding of a thinner, irregular muscle wall and postnatal cardiac dilation is evidence for a failure of the mutant heart to withstand these increasing stresses despite other mechanisms maintaining its overall integrity. A similar cardiac phenotype has not been described in mice lacking the nidogen-binding site on the laminin γ1 chain (44), indicating that other nidogen functions rather than laminin binding may be important in the heart.
While intrapericardial hemorrhage due to myocardial rupture was not a feature of nidogen double-null mice, locally restricted bleeding within the heart wall did occur in many mutant embryos. Since distinct cardiac capillary basement membranes were not detected, these hemorrhages are likely to result from blood leakage out of the microvasculature.
Delayed lung development in nidogen-deficient mice.
Nidogens and in particular their interaction with laminin have been suggested to be crucial for branching morphogenesis (10, 14). Our results indicate that early branching morphogenesis of the lung is unaffected by the lack of both nidogen isoforms. The size and lobation of the lungs in nidogen double-null mice were not obviously abnormal, indicating that branching morphogenesis in the pseudoglandular and canalicular stages is also not dramatically disturbed in the absence of nidogens. However, morphological abnormalities are obvious in the later maturation stages. At E17.5 and E18.5, the lungs in nidogen-deficient mice have an appearance comparable to those seen in wild-type mice in the late pseudoglandular and canalicular stages, with extensive mesenchyme remaining. At birth, the prealveolar sacculi were immature, with thick mesenchymal septae, which have normally thinned in preparation for birth. Differentiation of epithelial cells is characteristic of fetal lungs at the saccular stage; ultrastructurally, only very few alveoli were found in the double-knockout mice at E18.5, and most of the smaller bronchioli, while present, lacked a well-defined basement membrane (data not shown). SP-B produced by type II alveolar pneumocytes could be detected by immunostaining in the lungs of mutant mice, indicating that epithelial differentiation of airway cells had proceeded in the absence of nidogens. However, the reduced expression of surfactant protein in the mutants suggests a delay or/and an impairment of the epithelial differentiation process.
Taken together, all these findings are reminiscent of the immature lungs which result in respiratory distress syndrome of premature human infants, suggesting that respiratory failure might have caused the death of most nidogen-deficient pups. Furthermore, these data demonstrate that while nidogens are not essential for embryonic lung development and lung branching morphogenesis, they are required for the morphological and functional maturation of the lung needed at birth. A similar lung phenotype has been described in mice lacking the nidogen-binding site on the laminin γ1 chain (44), indicating that the high-affinity binding of nidogen to laminin might be the critical interaction for normal lung maturation. The importance of basement membrane proteins for lung development has been described in other gene knockout mice lacking the laminin α5 chain (22). Interestingly, ablation of fibulin 1, a minor basement membrane component interacting with nidogens, causes delayed embryonic lung development, resembling nidogen-deficient lungs, with thickened parenchymal septa and improperly expanded saculi at E18.5 (19).
Kidney formation can proceed in the absence of nidogens.
Development of the kidneys also involves branching morphogenesis, and in organ culture, this process can be impaired by blocking the nidogen-binding site on the laminin γ1 chain by antibodies directed against the nidogen-binding module (10, 21, 31). While mice harboring a genetic deletion of the nidogen-binding site on the laminin γ1 chain generally showed a renal aplasia, where kidneys did form, they had a reduced number of glomeruli which were often distorted (44). However, our data show that branching morphogenesis during kidney development occurs in the absence of both nidogen isoforms. In most nidogen double-null mice, the urogenital tract appears normal. The kidneys in most of these mice functioned normally around birth as shown by the analysis of the urine. As glomerular filtration requires an intact basement membrane, this result suggests that filtration is not significantly disturbed by the loss of nidogens. Indeed, ultrastructural analysis revealed a glomerular basement membrane in the mutant kidneys indistinguishable from that of wild-type kidneys with well-formed podocytes and slit junctions. Furthermore, the changes in the glomerular blood vessels seen in mice lacking the nidogen-binding site upon laminin, resulting in varicosities and bleeding into the glomerulus (44), were not obvious in mice lacking both nidogen isoforms.
In contrast to the normal glomerular basement membrane, the tubular basement membrane in nidogen double-null kidneys was sometimes absent or thickened and diffuse, indicating a different structural role for nidogens within the glomerular and tubular basement membranes and possibly the presence of different binding partners within these structures.
Renal agenesis did occur in a small percentage of nidogen double-null mutants. Variable penetrance of impaired kidney development has been reported upon deletion of the laminin α5 chain (23), fibulin 1 (19), or the integrin α8 subunit (27). This is most likely due to stochastic events which allow a proportion of mice to overcome the absence of these proteins. These as-yet-unknown variations may also be the reason for the loss of embryos at various stages of development observed in many mouse strains with deficiencies of extracellular matrix proteins or integrin receptors (4, 7, 19, 45).
What is most striking is the very high penetrance of renal aplasia (∼90%) in mice lacking the nidogen-binding site in the laminin γ1 chain (44). This is believed to occur due to a failure in the outgrowth of the Wolffian duct, and presumably, this is the underlying change producing renal aplasia in the small number of nidogen-deficient mice. Indeed, no ureter was present in these animals. The vast difference in the penetrance between the nidogen double-null and the nidogen-binding site-lacking mouse lines could be caused by free nidogen in the latter, sequestering other matrix and matrix-associated molecules away from the basement membrane region and therefore having a more severe effect on Wolffian duct outgrowth. Alternatively, although nidogen binding is the only known activity of this region of the laminin γ1 chain, it is possible that the observed kidney defects in mice lacking the nidogen-binding site are the result of altering other, unidentified activities of the laminin γ1 subunit.
In summary, this work shows that the previously held theory that nidogens are the major molecular link between the laminin and collagen IV networks and are therefore crucial for basement membrane formation is false. Nidogens are not needed for early embryogenesis nor are they required for most events in organogenesis; rather, they have specific roles in the formation and maintenance of certain basement membranes.
Acknowledgments
This work was supported by the grant Ba 1327/3-2 from the Deutsche Forschungsgemeinschaft through the priority program SPP 1086 (B.L.B.), by the Center of Molecular Medicine, University of Cologne (N.S.), and by the Deutsche Forschungsgemeinschaft through the SFB 589 at the University of Cologne (R.N.). N.M. is funded by the Deutsche Forschungsgemeinschaft (Mi 573/3-1).
We thank Jay W. Fox for the generous gifts of antibodies. We gratefully acknowledge the excellent technical assistance by Marion Reibetanz (Department of Dermatology) and Christian Frie (Institute for Biochemistry II). Finally, we gratefully acknowledge the contribution of the late Rupert Timpl to this work.
REFERENCES
- 1.Ackley, B. D., S. H. Kang, J. R. Crew, C. Suh, Y. Jin, and J. M. Kramer. 2003. The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in C. elegans. J. Neurosci. 23:3577-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arikawa-Hirasawa, E., H. Watanabe, H. Takami, J. R. Hassell, and Y. Yamada. 1999. Perlecan is essential for cartilage and cephalic development. Nat. Genet. 23:354-358. [DOI] [PubMed] [Google Scholar]
- 3.Aumailley, M., C. Battaglia, U. Mayer, R. Nischt, R. Timpl, and J. W. Fox. 1993. Nidogen mediates the formation of ternary complexes of basement membrane components. Kidney Int. 43:7-12. [DOI] [PubMed] [Google Scholar]
- 4.Bader, B. L., H. Rayburn, D. Crowley, and R. Hynes. 1998. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking αv integrins. Cell 95:507-519. [DOI] [PubMed] [Google Scholar]
- 5.Breitkreutz, D., N. Mirancea, C. Schmidt, R. Beck, U. Werner, H.-J. Stark, M. Gerl, and N. Fusenig. 2004. Inhibition of basement membrane formation by a nidogen-binding laminin γ1-chain fragment in human skin-organotypic cocultures. J. Cell Sci. 117:2611-2622. [DOI] [PubMed] [Google Scholar]
- 6.Carlin, B., R. Jaffe, B. Bender, and A. E. Chung. 1981. Entactin, a novel basal lamina-associated glycoprotein. J. Biol. Chem. 256:5209-5214. [PubMed] [Google Scholar]
- 7.Costell, M., E. Gustafsson, A. Aszodi, M. Morgelin, W. Bloch, E. Hunziker, K. Addicks, R. Timpl, and R. Fässler. 1999. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 147:1109-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, L., Y. Chen, M. Lewis, J.-C. Hsieh, J. Reing, J. R. Chaillet, C. Y. Howell, M. Melhem, S. Inoue, J. R. Kuszak, K. DeGeest, and A. E. Chung. 2002. Neurological defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab. Investig. 82:1617-1630. [DOI] [PubMed] [Google Scholar]
- 9.Durkin, M. E., U. M. Wever, and A. E. Chung. 1995. Exon organization of the mouse entactin gene corresponds to the structural domains of the polypeptide and has regional homology to the low-density lipoprotein receptor gene. Genomics 26:219-228. [DOI] [PubMed] [Google Scholar]
- 10.Ekblom, P., M. Ekblom, L. Fecker, G. Klein, H. Y. Zhang, Y. Kadoya, M. L. Chu, U. Mayer, and R. Timpl. 1994. Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development 120:2003-2014. [DOI] [PubMed] [Google Scholar]
- 11.Fox, J. W., U. Mayer, R. Nischt, M. Aumailley, D. Reinhardt, H. Wiedemann, K. Mann, R. Timpl, T. Krieg, J. Engel, and M. L. Chu. 1991. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 10:3137-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halfter, W., S. Dong, Y.-P. Yip, M. Willem, and U. Mayer. 2002. A critical function of the pial basement membrane in cortical histogenesis. J. Neurosci. 22:6029-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopf, M., W. Gohring, E. Kohfeldt, Y. Yamada, and R. Timpl. 1999. Recombinant domain IV of perlecan binds to nidogens, laminin-nidogen complex, fibronectin, fibulin-2 and heparin. Eur. J. Biochem. 259:917-925. [DOI] [PubMed] [Google Scholar]
- 14.Kadoya, Y., K. Salmivirta, J. F. Talts, K. Kadoya, U. Mayer, R. Timpl, and P. Ekblom. 1997. Importance of nidogen binding to laminin γ1 for branching epithelial morphogenesis of the submandibular gland. Development 124:683-691. [DOI] [PubMed] [Google Scholar]
- 15.Kang, S. H., and J. M. Kramer. 2000. Nidogen is nonessential and not required for normal type IV collagen localization in Caenorhabditis elegans. Mol. Biol. Cell 11:3911-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, S., and W. G. Wadsworth. 2000. Positioning of longitudinal nerves in C. elegans by nidogen. Science 288:150-154. [DOI] [PubMed] [Google Scholar]
- 17.Kimura, N., T. Toyoshima, T. Kojima, and M. Shimane. 1998. Entactin-2: a new member of basement membrane proteins with high homology to entactin/nidogen. Exp. Cell Res. 241:36-45. [DOI] [PubMed] [Google Scholar]
- 18.Kohfeldt, E., T. Sasaki, W. Göhring, and R. Timpl. 1998. Nidogen-2: a new basement membrane protein with diverse binding properties. J. Mol. Biol. 282:99-109. [DOI] [PubMed] [Google Scholar]
- 19.Kostka, G., R. Giltay, W. Bloch, K. Addicks, R. Timpl, R. Fässler, and M.-L. Chu. 2001. Perinatal lethality and endothelial cell abnormalities in several vessel compartments of fibulin-1-deficient mice. Mol. Cell. Biol. 21:7025-7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kücherer-Ehret, A., J. Pottgießer, G. W. Kreutzberg, H. Thoenen, and D. Edgar. 1990. Developmental loss of laminin from the interstitial extracellular matrix correlates with decreased laminin gene expression. Development 110:1285-1293. [DOI] [PubMed] [Google Scholar]
- 21.Mayer, U., R. Nischt, E. Pöschl, K. Mann, K. Fukuda, M. Gerl, Y. Yamada, and R. Timpl. 1993. A single EGF-like motif of laminin is responsible for high affinity nidogen binding. EMBO J. 12:1879-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miner, J. H., J. Cunnigham, and J. R. Sanes. 1998. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin α5 chain. J. Cell Biol. 143:1713-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miner, J. H., and C. Li. 2000. Defective glomerulogenesis in the absence of laminin α5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev. Biol. 217:278-289. [DOI] [PubMed] [Google Scholar]
- 24.Miner, J. H., J. L. Mudd, G. Go, and A. E. Sutherland. 2004. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development 131:2247-2256. [DOI] [PubMed] [Google Scholar]
- 25.Miosge, N., T. Sasaki, and R. Timpl. 2002. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol. 21:611-621. [DOI] [PubMed] [Google Scholar]
- 26.Miosge, N., C. Klenczar, R. Herken, M. Willem, and U. Mayer. 1999. The organization of the myotendenious junction is dependent on the presence of α7β1 integrin. Lab. Investig. 79:1591-1599. [PubMed] [Google Scholar]
- 27.Müller, U., D. Wang, S. Denda, J. J. Meneses, R. A. Pedersen, and L. F. Reichardt. 1997. Integrin α8β1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell 88:603-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murshed, M., N. Smyth, N. Miosge, J. Karolat, T. Krieg, M. Paulsson, and R. Nischt. 2000. The absence of nidogen 1 does not affect murine basement membrane formation. Mol. Cell. Biol. 20:7007-7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulsson, M., M. Aumailley, R. Deutzmann, R. Timpl, K. Beck, and J. Engel. 1987. Laminin-nidogen complex. Extraction with chelating agents and structural characterization. Eur. J. Biochem. 166:11-19. [DOI] [PubMed] [Google Scholar]
- 30.Pöschl, E., U. Schlotzer-Schrehardt, B. Brachvogel, K. Saito, Y. Ninomiya, and U. Mayer. 2004. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131:1619-1628. [DOI] [PubMed] [Google Scholar]
- 31.Pöschl, E., J. W. Fox, D. Block, U. Mayer, and R. Timpl. 1994. Two non-contiguous regions contribute to nidogen binding to a single EGF-like motif of the laminin γ1 chain. EMBO J. 13:3741-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinhardt, D., K. Mann, R. Nischt, J. W. Fox, M.-L. Chu, T. Krieg, and R. Timpl. 1993. Mapping of nidogen binding sites for collagen type IV, heparan sulfate proteoglycan and zinc. J. Biol. Chem. 268:10881-10887. [PubMed] [Google Scholar]
- 33.Salmivirta, K., J. F. Talts, M. Olsson, T. Sasaki, R. Timpl, and P. Ekblom. 2002. Binding of mouse nidogen-2 to basement membrane components and cells and its expression in embryonic and adult tissues suggest complementary functions of the two nidogens. Exp. Cell Res. 279:188-201. [DOI] [PubMed] [Google Scholar]
- 34.Schulze, B., K. Mann, R. Battistutta, H. Wiedemann, and R. Timpl. 1995. Structural properties of recombinant domain III-3 of perlecan containing a globular domain inserted into an epidermal-growth-factor-like motif. Eur. J. Biochem. 231:551-556. [DOI] [PubMed] [Google Scholar]
- 35.Schymeinsky, J., S. Nedbal, N. Miosge, E. Pöschl, C. Rao, D. R. Beier, W. C. Skarnes, R. Timpl, and B. Bader. 2002. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol. Cell. Biol. 22:6820-6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skarnes, W. C., J. E. Moss, S. M. Hurtley, and R. S. Beddington. 1995. Capturing genes encoding membrane and secretory proteins important for mouse development. Proc. Natl. Acad. Sci. USA 92:6592-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smyth, N., H. S. Vatansever, P. Murray, M. Meyer, C. Frie, M. Paulsson, and D. Edgar. 1999. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J. Cell Biol. 144:151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smyth, N. R., and M. Paulsson. 1998. Basement membranes, p. 117-141. In W. Birchmeier, and C. Birchmeier (ed.), Epithelial morphogenesis in development and disease. Harwood Reading, Academic Publishers, United Kingdom.
- 39.Timpl R. 1996. Macromolecular organization of basement membranes. Curr. Opin. Cell Biol. 8:618-624. [DOI] [PubMed] [Google Scholar]
- 40.Timpl, R., and J. C. Brown. 1996. Supramolecular assembly of basement membranes. BioEssays 18:123-132. [DOI] [PubMed] [Google Scholar]
- 41.Timpl, R., M. Dziadek, S. Fujiwara, H. Nowack, and G. Wick. 1983. Nidogen: a new, self-aggregating basement membrane protein. Eur. J. Biochem. 15:455-465. [DOI] [PubMed] [Google Scholar]
- 42.Tunggal, J., M. Wartenberg, M. Paulsson, and N. Smyth. 2003. Expression of the nidogen-binding site of the laminin γ1 chain disturbs basement membrane formation and maintenance in F9 embryoid bodies. J. Cell Sci. 116:803-812. [DOI] [PubMed] [Google Scholar]
- 43.Whitsett, J. A., and T. E. Weaver. 2002. Hydrophobic surfactant proteins in lung function and disease. N. Engl. J. Med. 374:2141-2148. [DOI] [PubMed] [Google Scholar]
- 44.Willem, M., N. Miosge, W. Halfter, N. Smyth, I. Jannetti, E. Burghart, R. Timpl, and U. Mayer. 2002. Specific ablation of the nidogen binding site in the laminin γ1 chain interferes with kidney and lung development. Development 129:2711-2722. [DOI] [PubMed] [Google Scholar]
- 45.Yang, J. T., H. Rayburn, and R. O. Hynes. 1995. Cell adhesion events mediated by the alpha 4 integrins are essential in placental and cardiac development. Development 121:549-560. [DOI] [PubMed] [Google Scholar]
- 46.Yurchenco, P., and Y. S. Cheng. 1993. Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J. Biol. Chem. 268:17286-17299. [PubMed] [Google Scholar]
- 47.Zimmermann, K., S. Hoischen, M. Hafner, and R. Nischt. 1995. Genomic sequences and structural organization of the human nidogen gene. Genomics 27:245-250. [DOI] [PubMed] [Google Scholar]