Abstract
The most common cause of cystic fibrosis (CF) is deletion of phenylalanine 508 (ΔF508) in the CF transmembrane conductance regulator (CFTR) chloride channel. The ΔF508 mutation produces defects in folding, stability, and channel gating. To identify small-molecule correctors of defective cellular processing, we assayed iodide flux in ΔF508-CFTR–transfected epithelial cells using a fluorescent halide indicator. Screening of 150,000 chemically diverse compounds and more than 1,500 analogs of active compounds yielded several classes of ΔF508-CFTR correctors (aminoarylthiazoles, quinazolinylaminopyrimidinones, and bisaminomethylbithiazoles) with micromolar potency that produced greater apical membrane chloride current than did low-temperature rescue. Correction was seen within 3–6 hours and persisted for more than 12 hours after washout. Functional correction was correlated with plasma membrane expression of complex-glycosylated ΔF508-CFTR protein. Biochemical studies suggested a mechanism of action involving improved ΔF508-CFTR folding at the ER and stability at the cell surface. The bisaminomethylbithiazoles corrected ΔF508-CFTR in ΔF508/ΔF508 human bronchial epithelia but did not correct a different temperature-sensitive CFTR mutant (P574H-CFTR) or a dopamine receptor mutant. Small-molecule correctors may be useful in the treatment of CF caused by the ΔF508 mutation.
Introduction
Cystic fibrosis (CF) is one of the most common inherited diseases, afflicting 1 in approximately 2,500 white individuals (1). The primary cause of morbidity and mortality in CF is chronic lung infection and deterioration of lung function. CF is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, which encodes a cAMP-regulated chloride channel expressed at the apical membrane of epithelial cells in the airways, pancreas, testis, and other tissues (2, 3). The most common CFTR mutation producing CF is deletion of phenylalanine at residue 508 (ΔF508) in its amino acid sequence, which is present in at least 1 allele in approximately 90% of CF subjects (1). The ΔF508-CFTR protein is misfolded and retained at the ER, where it is degraded rapidly (4–7). When allowed to traffic out of the ER by overexpression or by low-temperature incubation, ΔF508-CFTR also has a reduced half-life in the plasma membrane compared with wild-type CFTR (8, 9). The misfolding of ΔF508-CFTR is thought to be mild because it can be “rescued” in cell culture models by incubation for 18 hours or more at reduced (<30°C) temperature (4) or with chemical chaperones such as glycerol (10) or phenylbutyrate (11), which results in partial restoration of ΔF508-CFTR plasma membrane expression. However, channel gating of the plasma membrane–rescued ΔF508-CFTR protein remains defective such that its open probability after cAMP stimulation is reduced by more than 3-fold compared with that of wild-type CFTR (12, 13).
Small-molecule correctors of defective ΔF508-CFTR folding/cellular processing (“correctors”) and channel gating (“potentiators”) may provide a strategy for therapy of CF that corrects the underlying defect. A potential advantage of pharmacotherapy for defective ΔF508- CFTR processing and gating is that it minimizes concerns about treating the wrong cells or losing physiological CFTR regulation, as might occur with gene therapy or activation of alternative chloride channels. Small-molecule ΔF508-CFTR potentiators with relatively low potency (>10–50 μM) have been characterized, including the flavone genistein and the xanthine isobutylmethylxanthine (14–17). Recently, our laboratory identified by high-throughput screening tetrahydrobenzothiophene, phenylglycine, and sulfonamide potentiators that correct defective ΔF508-CFTR gating at concentrations below 100 nM by a mechanism involving increased channel open time (18, 19). These potentiators were effective in human CF airway epithelial cells expressing ΔF508-CFTR and so may be useful in the therapy of CF caused by the ΔF508 mutation when combined with a corrector of defective cellular processing or as monotherapy for a subset of patients having some ΔF508-CFTR plasma membrane expression.
Here, we report the identification and characterization of ΔF508-CFTR correctors by screening of a large collection of diverse small molecules. The discovery of useful correctors was anticipated to present a substantially greater challenge than the discovery of CFTR inhibitors (20, 21) or activators (18, 19, 22) because protein folding and trafficking is a complex, multistep process involving multiple cellular targets, some of which may be cell type specific. As depicted in Figure 1A, our screening assay utilized Fischer rat thyroid (FRT) epithelial cells coexpressing ΔF508-CFTR and the yellow fluorescent protein (YFP) halide indicator YFP-H148Q/I152L at 37°C in a 96-well-plate format. Test compounds at 10 μM concentration (and negative/positive controls) were added to each well for 18–24 hours. ΔF508-CFTR–facilitated iodide influx was then determined from the kinetics of decreasing YFP fluorescence following addition of extracellular iodide in the presence of the potentiators genistein and forskolin. “Hits” from the primary high-throughput screen were verified, optimized by screening of structural analogs, and analyzed for mechanism of action and efficacy in human airway epithelia.
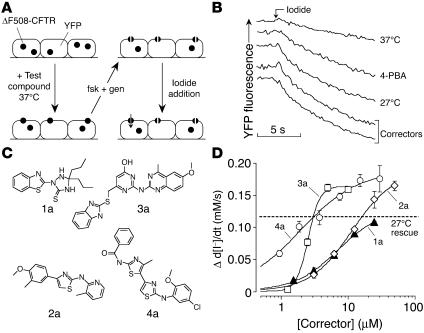
Figure 1.
Discovery of ΔF508-CFTR correctors by high-throughput screening. (A) Screening procedure. FRT cells coexpressing ΔF508-CFTR and a halide-sensitive YFP were incubated with test compounds (10 μM) at 37°C. ΔF508-CFTR function was assayed at 18–24 hours in a plate reader according to YFP fluorescence quenching by iodide in the presence of forskolin (20 μM) plus genistein (fsk + gen) (50 μM). (B) Representative traces showing iodide influx under control conditions (37°C) or after 24 hours incubation at 27°C or with 4-PBA (4 mM) or correctors (10 μM; 37°C). (C) Chemical structures of correctors of 4 chemical classes. See Supplemental Table 1 for full listing of correctors and their activities. (D) Dose-response data for indicated correctors (SEM; n = 5). The dashed line indicates the level of activity of the low-temperature rescue used as a positive control.
Results
Discovery and characterization of ΔF508-CFTR correctors.
FRT cells coexpressing ΔF508-CFTR and a fluorescent YFP halide sensor were incubated with test compounds (10 μM) for 18–24 hours at 37°C in a 96-well format (Figure 1A). ΔF508-CFTR–facilitated iodide influx was then assayed after compound washout and addition of forskolin and genistein. Primary screening of 150,000 compounds produced 45 compounds that increased iodide influx (Δ d[I–]/dt) by more than 0.10 mM/s and 15 compounds that increased iodide influx by more than 0.20 mM/s. Examples of original data from individual wells are shown in Figure 1B. The negative control was vehicle (DMSO) alone (labeled “37°C”), and the positive controls were 4-phenylbutyrate (4-PBA) and reduced-temperature (27°C) incubation. Examples of 2 active compounds are shown. Active compounds were retested, and ΔF508-CFTR specificity was verified by inhibition of the increased iodide influx by a CFTR inhibitor (CFTRinh-172) and lack of corrector effect on FRT-null cells.
Correctors were initially optimized by screening of commercially available analogs. Three rounds of optimization with more than 1,500 compounds gave active correctors of the aminobenzothiazole, aminoarylthiazole, quinazolinylaminopyrimidinone, and bisaminomethylbithiazole chemical classes (Figure 1C). Dose-response data are shown in Figure 1D, referenced against 27°C rescue, which is shown as the dashed line. A listing of 37 correctors and their potencies (acid ionization constant [Ka] and Vmax) is provided in Supplemental Table 1 (available online with this article; doi:10.1172/JCI24898DS1), establishing a structure-activity data set. Figure 2A summarizes Vmax data for several correctors, comparing them with positive and negative controls. Several compounds resulted in greater Vmax than did 27°C rescue, and additive effects of compounds and 27°C rescue were found.
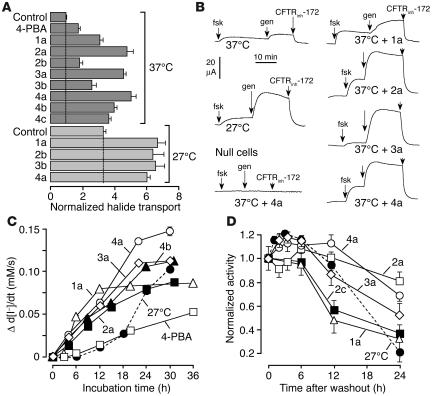
Figure 2.
Properties of ΔF508-CFTR correctors. (A) Maximal iodide influx (normalized to 37°C control) in ΔF508-CFTR–expressing FRT cells incubated at 37°C or 27°C (SEM; n = 5). Iodide influx increased significantly (P < 0.01) for all compounds compared with control. (B) Apical membrane chloride current measured in Ussing chambers after basolateral membrane permeabilization and in the presence of a chloride gradient (see Methods). The concentrations were: forskolin, 20 μM; genistein, 50 μM; CFTRinh-172, 10 μM. Measurements were performed on ΔF508-CFTR–expressing FRT cells, except in the experiment represented in the lower-left panel, which was performed on FRT-null cells. (C) Time course of correction. Cells incubated for different times at 27°C or with indicated correctors or 4-PBA (4 mM) at 37°C. ΔF508-CFTR activity was assayed in the presence of foskolin/genistein. (D) Persistence of correction. Cells were treated for 24 hours with correctors (or at 27°C). ΔF508-CFTR activity was assayed at different times after washout (or return from 27°C to 37°C).
Figure 2B shows results of Ussing chamber experiments in which apical membrane chloride current was measured in FRT cells after basolateral membrane permeabilization and in the presence of a chloride gradient (apical, 65 mM; basolateral, 130 mM). After measurement of apical membrane chloride current at baseline, high concentrations of forskolin (20 μM) and then genistein (50 μM) were added; CFTRinh-172 (10 μM) was added at the end of each experiment. The electrophysiological studies confirmed the data obtained from the fluorescence assay. On the left in Figure 2B is shown the much greater current in ΔF508-CFTR–expressing cells grown at 27°C versus 37°C (top and middle curves) and the lack of corrector effect on FRT-null cells (bottom). Incubation of ΔF508-CFTR–expressing cells with correctors at 37°C for 24 hours produced increased forskolin/genistein-stimulated and CFTRinh-172–inhibited chloride currents (Figure 2B, right), comparable to or greater than those produced by 27°C rescue.
Figure 2C summarizes the time course of correction for 5 correctors (incubated at 37°C), with data for 27°C rescue and 4-PBA shown for comparison. Correction was seen as early as 3 hours after compound addition, with maximal effect after 12–30 hours, whereas correction by 27°C incubation or 4-PBA had a relatively slower onset. Data for the persistence of correction after compound washout (or return of temperature from 27°C to 37°C) are summarized in Figure 2D. Correction persisted beyond 12 hours for most compounds after washout, with substantial activity remaining for 2 of the correctors at 24 hours. In contrast, little correction persisted at 24 hours for cells rescued at 27°C.
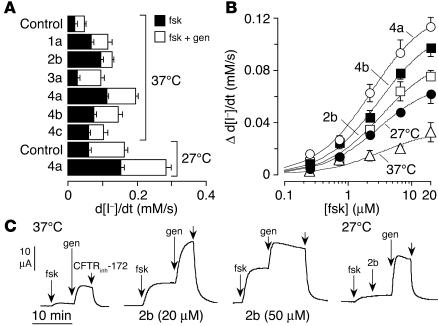
Experiments were performed to investigate whether the correctors might alter the properties of ΔF508-CFTR, such as the sensitivity to cAMP-elevating agents or to potentiators. Figure 3A summarizes Vmax in the presence of forskolin alone (at 20 μM) versus forskolin plus genistein (50 μM). Interestingly, the fractional Vmax produced by forskolin alone versus forskolin plus genistein was greater in cells treated with some correctors than that produced by low-temperature rescue. Thus, several correctors increased ΔF508-CFTR activation by forskolin alone. Corrector 2b (corr-2b) was most effective, with the forskolin response representing approximately 80% of Vmax. Figure 3B shows dose-response data for forskolin (in the absence of genistein). Although Vmax differed, Ka for the forskolin response was approximately 3 μM in each case. As seen in Ussing chamber experiments (Figure 3C), corr-2b at 20 μM (and to an even greater extent at 50 μM) increased the relative amplitude of the forskolin response. This was not due to intrinsic potentiator activity of corr-2b, as it was unable to stimulate CFTR activity in cells rescued at 27°C, with genistein as positive control (Figure 3C, far right).
Figure 3.
Increased ΔF508-CFTR sensitivity to activators after incubation with correctors. (A) Effect of forskolin (20 μM) or forskolin plus genistein (50 μM) in ΔF508-CFTR–expressing FRT cells kept at 37°C or 27°C with or without correctors. (B) Forskolin dose-response relationships. Cells stimulated with forskolin (in the absence of genistein) after incubation with correctors (at 37°C) or at 27°C. (C) Apical membrane chloride current after incubation for 24 hours at 37°C with DMSO vehicle (left curve) or corr-2b (middle curves). The curve at the far right was obtained from an experiment on cells grown at 27°C with compounds added as shown (20 μM forskolin, 20 μM corr-2b, 50 μM genistein).
Mechanism of action of ΔF508-CFTR correctors.
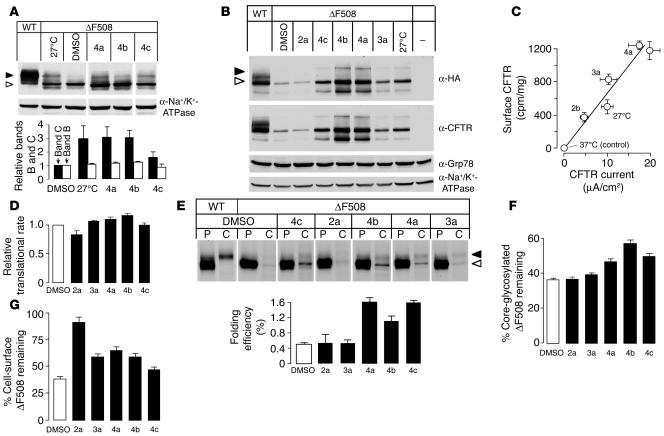
Biosynthetic processing of ΔF508-CFTR by correctors was assayed by immunoblot analysis from the accumulation of mature, complex-glycosylated ΔF508-CFTR with a C-terminal HA tag (ΔF508-CFTR-CtHA) in baby hamster kidney (BHK) cells. Incubation of cells with correctors for 16–24 hours at 37°C resulted in the accumulation of complex-glycosylated ΔF508-CFTR (Figure 4A). This was indicated by the slower electrophoretic mobility (apparent mol wt, approximately 170 kDa) of the complex-glycosylated ΔF508-CFTR–immunoreactive band (C band) compared with its core-glycosylated counterpart (apparent mol wt, approximately 150 kDa), the predominant form in nontreated cells. Similar results were obtained in ΔF508-CFTR–expressing FRT cells (Figure 4B); however, the accumulation of C band was less prominent, which in part reflects the lower CFTR expression capacity of FRT cells (compare with wild-type CFTR in Figure 4, A and B). Interestingly, the accumulation of complex-glycosylated ΔF508-CFTR in corr-4–treated cells was comparable to or greater than that produced by low temperature incubation without correctors (Figure 4, A and B). Correctors did not produce an unfolded protein response (UPR) in BHK or FRT cells, as indicated by the unaltered expression of the UPR markers Grp78/Bip and GRP94 (23) (Figure 4B and data not shown). Plasma membrane ΔF508-CFTR expression was confirmed through use of an anti-HA antibody binding assay based on the recognition of an extracellular HA epitope (24). Incubation with correctors increased the abundance of plasma membrane ΔF508-CFTR significantly, with an approximately proportionate increase in forskolin/genistein-stimulated apical membrane chloride current measured in FRT cells (Figure 4C). Semiquantitative transport measurements in YFP-transfected BHK cells verified functional correction of ΔF508-CFTR processing, with 2.5-, 5.2-, 2.6-, and 5.9-fold increases in I– influx in cells treated with correctors 1a, 2a, 3a, and 4a, respectively, compared with cells treated with vehicle (DMSO) alone (data not shown).
Figure 4.
Biochemical analysis of corrector mechanism of action. (A) Effect of the indicated correctors (10 μM) on the expression pattern of ΔF508-CFTR-CtHA in BHK cells. Cells were cultured for 24 hours at 37°C with or without correctors or at 27°C. Top: CFTR was visualized using anti-HA primary and HRP-conjugated secondary antibodies. Filled arrowhead, complex-glycosylated form (band C); open arrowhead, core-glycosylated form (band B). Bottom: Quantification of bands B and C (SEM; n = 4–5). (B) Effect of the indicated correctors (10 μM) on the expression pattern of ΔF508-CFTR in FRT epithelia. Confluent monolayers were treated as described in A, and immunoblot analysis was performed with the indicated primary antibodies. (C) Correlation between cell-surface density and PKA-activated chloride current. Cell-surface density of ΔF508-CFTR was determined using the radioactive anti-HA antibody binding assay and plotted against ΔF508-CFTR apical membrane currents in parallel experiments done on FRT cells. (D) Translational rate of ΔF508-CFTR was measured in the presence of correctors (10 μM) by monitoring [35S]methionine/cysteine incorporation into CFTR during a 15-minute pulse labeling (SEM; n = 3). (E) Folding efficiency measured by pulse-chase assay. Top: The amount of newly synthesized ΔF508-CFTR was computed from radioactive incorporation during a 15-minute pulse (P). For measurement of folding efficiency, cells were pulsed for 150 minutes and than chased for 150 minutes (C). The amounts of core- (open arrowhead) and complex-glycosylated (filled arrowhead) forms were determined by phosphorimage analysis. Bottom: Maturation efficiency expressed as the percent of mature, complex-glycosylated ΔF508-CFTR relative to the newly synthesized pool, as shown in the top panel. (F) Stability of the core-glycosylated ΔF508-CFTR was measured following a 15-minute pulse labeling in the presence of the indicated correctors. Remaining radioactivity associated with CFTR was measured after 1 hour chase (SEM; n = 3). (G) Cell-surface stability of the rescued ΔF508-CFTR was measured by the anti-HA antibody binding assay before and after 2 hours chase in the presence of the indicated correctors. Data are expressed as the mean ± SEM of 3 independent experiments.
The ΔF508 mutation impairs ΔF508-CFTR conformational maturation and export competence at the ER and destabilizes complex-glycosylated ΔF508-CFTR in post-Golgi compartments (6, 8, 24). Correctors may facilitate translation or posttranslational folding of newly synthesized ΔF508-CFTR and/or enhance the stability of mature, complex-glycosylated ΔF508-CFTR. We examined these possibilities to establish the cellular basis of corrector action.
First, we determined whether the correctors augmented ΔF508-CFTR transcription and/or translation. Figure 4D shows that the correctors did not alter the amount of newly synthesized ΔF508-CFTR as measured by short metabolic pulse labeling, which provides evidence against altered ΔF508-CFTR transcription/translation. Next, corrector effect on ΔF508-CFTR posttranslational folding efficiency was assessed by measurement of the fractional conversion of newly synthesized, core-glycosylated ΔF508-CFTR into the complex-glycosylated form (Figure 4E, top). When the radioactive pulse labeling was extended from 15 to 150 minutes, the detection sensitivity of the assay was enhanced enough to allow folding efficiency to be estimated. Phosphorimage analysis showed that ΔF508-CFTR had low but measurable maturation efficiency (0.5% ± 0.15%) as compared with wild-type CFTR (31% ± 5%) in BHK cells at 37°C (Figure 4E, bottom). Similar results have been obtained for maturation efficiency of wild-type CFTR in other heterologous expression systems (5, 25). ΔF508-CFTR folding efficiency was increased by 2- to 3-fold by class 4 correctors (Figure 4E). Class 4 correctors increased the folding efficiency of wild-type CFTR by 5–70% (data not shown).
Efficient correctors may function as pharmacological chaperones by improving ΔF508-CFTR posttranslational folding and stabilizing its ER core-glycosylated form. We found that class 4 correctors delayed by 30–50% ER-associated degradation of metabolically labeled core-glycosylated ΔF508-CFTR (Figure 4F).
Slowing of the normally rapid elimination of ΔF508-CFTR from the cell surface may also contribute to its functional rescue. For assessment of the plasma membrane stability of rescued ΔF508-CFTR, ΔF508-CFTR with a 3-HA tag (ΔF508-CFTR-3HA) was accumulated at the cell surface at reduced temperature. ΔF508-CFTR surface density was then monitored by antibody binding after the temperature was returned to 37°C in the presence of correctors. Whereas the cell-surface density of rescued ΔF508-CFTR was reduced to approximately 35% during a 3-hour chase, 50–60% of the ΔF508-CFTR remained at the cell surface in the presence of correctors 3a, 4a, and 4b (Figure 4G). Intriguingly, corr-2a was the most potent stabilizer, though it had no effect on ΔF508-CFTR folding in the ER, which suggests that the primary target of corr-2a is the peripheral quality control machinery that is responsible for targeting misfolded ΔF508-CFTR for lysosomal degradation (24). These results also indicate that class 4 correctors not only improve ΔF508-CFTR folding, but also enhance the residence time of rescued ΔF508-CFTR at the cell surface.
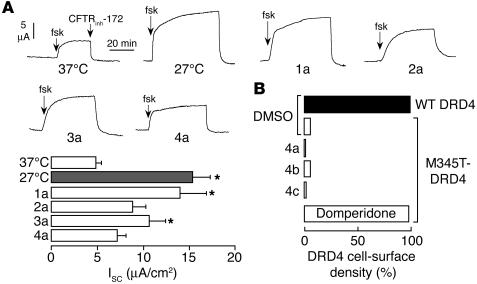
As an initial test of compound specificity for correction of defective ΔF508-CFTR processing, compounds were tested on P574H-CFTR, a mutant that like ΔF508-CFTR is retained at the ER but can be rescued by incubation for 24 hours at reduced temperature (26). Cells were incubated with correctors or at reduced temperature for 24 hours. Apical membrane chloride current was measured in response to forskolin (which fully activates this CFTR mutant; ref. 27) and then CFTRinh-172. Figure 5A shows that corr-4a and -2a, which produced robust chloride currents in ΔF508-CFTR–expressing cells, did not produce significant correction in P574H-CFTR cells, despite a positive low-temperature rescue control. Corr-1a and -3a caused a small but significant increase in CFTR-dependent current (Figure 5A). We further substantiated corrector specificity using a mutant G protein–coupled receptor that is misfolded and retained in the ER (28). While the processing defect of a mutant dopamine receptor 4 (DRD4) was efficiently rescued by the pharmacological chaperone domperidone as assayed by cell surface antibody binding measurements, class 4 compounds did not correct defective DRD4 processing (Figure 5B).
Figure 5.
Specificity for corrector action on ΔF508-CFTR cellular misprocessing. (A) Apical membrane chloride current in FRT cells expressing the temperature-sensitive mutant P574H-CFTR. Experiments were carried out as described in Figure 2B. ISC, short-circuit current. (B) CHO cells expressing Flag-tagged wild-type or mutant (M345T) dopamine receptor (DRD4) were treated for 16 hours with the indicated correctors (5 μM corr-4c and -4b; 10 μM corr-4a) or 10 μM domperidone, a dopamine antagonist. The cell-surface density of DRD4 was measured using anti-Flag primary and HRP-conjugated goat anti-mouse antibodies. Data represent the mean of 2 independent experiments, each performed in triplicate.
Correction of defective ΔF508-CFTR processing in human airway epithelium.
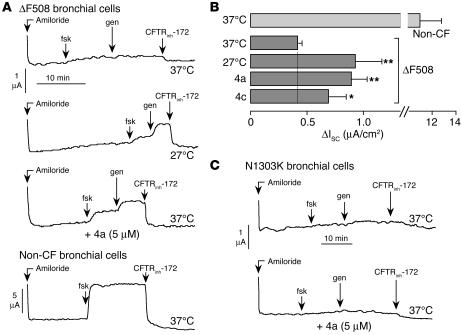
Last, functional measurements of corrector action were performed on well-differentiated primary cultures of human bronchial epithelial cells from ΔF508-CFTR–homozygous subjects. Cells polarized on permeable supports were mounted in Ussing chambers for measurement of chloride secretion by short-circuit current analysis. After Na+ current was blocked with amiloride, cells treated with DMSO vehicle alone showed little response to forskolin, genistein, or CFTRinh-172 (Figure 6A, top panel). Incubation at 27°C for 24 hours resulted in the appearance of significant chloride current as indicated by the increased current after addition of forskolin and genistein and inhibition by CFTRinh-172 (Figure 6A, middle panels). Incubation with corr-4a at 37°C for 24 hours increased chloride current comparably. For comparison, data for non-CF bronchial epithelial cells are shown (Figure 6A, bottom panel). Figure 6B summarizes the CFTRinh-172–sensitive short-circuit for the measurements used in Figure 6A, including data for a second bisaminomethylbithiazole. Corr-4a produced a significant increase in the CFTR-dependent Cl– secretion, which was equivalent to 8% of that measured in non-CF epithelia (Figure 6B). The other compounds tested (corr-1a, -2a, and -3a) did not significantly increase CFTR-dependent Cl– secretion (data not shown). As a negative control for these studies, the active correctors were tested on human bronchial epithelial cells derived from a CF subject homozygous for the N1303K-CFTR mutation, which also manifests defective CFTR processing (29). Figure 6C shows that there was no significant correction of short-circuit current for the N1303K-CFTR bronchial cells when measured with the same compounds and conditions used for the ΔF508-CFTR cells measured in Figure 6A.
Figure 6.
Correction of ΔF508-CFTR misprocessing in human bronchial epithelial cells. (A) Representative short-circuit current recordings on primary cultures of human bronchial epithelial cells from a ΔF508/ΔF508 subject (top 3 curves) and a non-CF subject (bottom curve). ΔF508 cells maintained at 37°C for 24 hours in the presence of DMSO vehicle (top curve) or corr-4a (third curve) or incubated at 27°C (second curve). Concentrations were: amiloride, 10 μM; forskolin, 20 μM; genistein, 50 μM; CFTRinh-172, 10 μM. (B) Summary of CFTRinh-172–inhibited short-circuit current (ΔIsc) for a series of experiments as described in A (SEM; n = 12–14). *P < 0.05; **P < 0.01. (C) Short-circuit current recordings of primary cultures of human bronchial epithelial cells from a homozygous N1303K-CFTR subject taken under conditions as described in A.
Discussion
High-throughput screening yielded several classes of small molecules that corrected ΔF508-CFTR cellular misprocessing and restored plasma membrane expression and halide permeability to levels greater than those achieved by low-temperature rescue. We verified correction by electrophysiological and biochemical measurements, as well as using control (non–ΔF508-CFTR–expressing) cells and a CFTR-selective inhibitor. The micromolar potency of the correctors is orders of magnitude better than the millimolar potency reported for correction of ΔF508-CFTR misprocessing by 4-PBA (11). The identification of small-molecule ΔF508-CFTR correctors presented a greater conceptual difficulty than that of ΔF508-CFTR potentiators or CFTR activators/inhibitors because correction of cellular misprocessing could involve multiple targets, whereas the primary target for potentiators, activators, and inhibitors is CFTR itself.
The cell line used for primary screening was chosen to be of epithelial origin (to resemble native CFTR-expressing cells), to permit rapid assessment of chloride currents in cell monolayers, and to give a robust low-temperature rescue response. Additional requirements for the cell line for high-throughput screening included rapid growth in multiwell plates, stable and bright YFP-H148Q/I152L expression, and low basal halide permeability. The YFP-H148Q/I152L fluorescent halide indicator was developed by our lab (30) to have bright cellular expression and ultra-high iodide sensitivity. The transfected FRT cell line used here was selected after many transfected and natively expressed epithelial cell lines were screened, and well as more than 100 ΔF508-CFTR/YFP-H148Q/I152L–transfected FRT cell clones (18). Although primary screening using natively expressed human airway cells may be preferable on theoretical grounds, practical considerations of stable cell phenotype, relatively high ΔF508-CFTR expression, and robust signal (Z′ factor > 0.5) precluded the use of available human ΔF508-CFTR cell lines.
CFTR cellular processing involves translation, folding at the ER, Golgi transport, posttranslational glycosylation, and apical plasma membrane targeting (6). Plasma membrane CFTR is internalized by endocytosis and then recycled to the plasma membrane or targeted for lysosomal degradation (9, 24). ΔF508-CFTR folding is inefficient, with 99.5% of newly synthesized ΔF508-CFTR in BHK cells targeted for degradation without reaching the Golgi apparatus. Near-complete ER retention of ΔF508-CFTR was reported in other model systems as well (2, 6).
Screening of 150,000 chemically diverse compounds produced several classes of candidate correctors of defective ΔF508-CFTR cellular processing. Functional and biochemical analysis identified the class 4 bisaminomethylbithiazoles as the most promising class of compounds for further development. Corr-4a and -4c increased ΔF508-CFTR folding efficiency nearly 3-fold without affecting translational rate, which suggests that the correctors could partially overcome the posttranslational folding barrier. Based on recent structural studies, small molecules might facilitate the folding of the nucleotide binding domain 2 (NBD2) and/or transmembrane domains (7, 31). The simplest interpretation of the peripheral stabilizing effect is that the conformationally stabilized mutant is less susceptible to the ubiquitin-dependent peripheral quality control mechanism and lysosomal degradation than the low temperature–rescued ΔF508-CFTR (24). The lack of effect of some ΔF508-CFTR correctors on the temperature-sensitive mutant P574H-CFTR and the mutant DRD4-M345T suggests their specificity for the ΔF508 mutation, though further studies are needed to evaluate the numerous potential off-target specificities.
Our results thus provide proof-of-principle for discovery of small-molecule correctors of ΔF508-CFTR cellular misprocessing. While some correctors, such as those of class 4, have favorable properties, including efficacy in human airway epithelia, partial correction of the gating defect, apparent ΔF508-CFTR specificity, and a mechanism involving improved ΔF508-CFTR protein folding, additional analysis and compound optimization are needed prior to the initiation of clinical studies, to include in vivo testing of compound pharmacology, toxicity, and efficacy. The effect of class 4 correctors on Cl– secretion in human airway CF epithelial cells was small (approximately 8%) compared with that in non-CF epithelia. However, the correction conferred by class 4 compounds is comparable to that obtained by low-temperature rescue and within the range considered to be therapeutically beneficial. It has been estimated that 6–10% of normal CFTR activity might prevent or significantly reduce lung pathology in CF (32), although this remains the subject of debate. Finally, the general paradigm of small molecule discovery of correctors of mutant protein misfolding may be applicable to a wide range of protein folding diseases such as Alzheimer disease, Parkinson disease, nephrogenic diabetes insipidus, and α1-antitrypsin deficiency (33, 34).
Methods
Cell lines.
FRT epithelial cells stably coexpressing human ΔF508-CFTR and the high-sensitivity halide-sensing green fluorescent protein YFP-H148Q/I152L (30) were generated as described previously (22, 35). A similar approach was used to generate BHK cells coexpressing ΔF508-CFTR and YFP-H148Q/I152L and FRT cells stably expressing P574H-CFTR. FRT cells were cultured on plastic in Coon’s modified Ham’s F12 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Generation of BHK cells expressing wild-type CFTR or ΔF508-CFTR-CtHA (8). The extracellular triple HA (3HA) tag, consisting of SLEYPYDVPDYASYPYDVPDYAYPYDVPDYAA residues, was inserted after amino acid residue 897 in wild-type CFTR and ΔF508-CFTR using engineered XhoI/NheI sites. For primary screening, FRT cells were plated using a LabSystems multidrop dispenser into black, 96-well microplates (Costar; Corning Inc.) at 50,000 cells/well. Screening was done at 18–24 hours after plating. For short-circuit current measurements, cells were cultured on Snapwell permeable supports (Costar; Corning Inc.) at 500,000 cells/insert. Human nasal epithelial cells, which were obtained from ΔF508/ΔF508 CF subjects (or an N1303K/N1303K subject) with informed consent, were cultured on Snapwell inserts and allowed to differentiate in a hormone-supplemented medium as described previously (36). CHO cells expressing the Flag-tagged wild-type and mutant DRD4 (28) were kindly provided by H.H.M. Van Tol (University of Toronto).
Compounds.
A total of 150,000 diverse drug-like compounds (>90% with a molecular size of 250–500 Da; ChemDiv Inc. and ChemBridge Corp.) was used for initial screening. For optimization, more than 1,500 commercially available analogs of active compounds identified in the primary screen were tested. Plates containing 1 or 4 compounds per well were prepared for screening (1 mM in DMSO). Compounds for secondary analysis were confirmed by NMR and liquid chromatography/mass spectrometry.
Screening procedures.
Screening was carried out using a Beckman Coulter integrated system containing a 3-m robotic arm, CO2 incubator containing microplate carousel, plate-washer, liquid handling work station, bar code reader, delidding station, plate sealer, and 2 FLUOstar fluorescence plate readers (Optima; BMG LABTECH Gmbh), each equipped with dual syringe pumps and 500 ± 10 nm excitation and 535 ± 15 nm emission filters (Chroma Technology Corp.). FRT cells were grown at 37°C (90% humidity; 5% CO2) for 18–24 hours and then incubated for 18–24 hours with 50 μl of medium containing test compounds (10-μM final concentrations). At the time of the assay, cells were washed with PBS and then incubated with PBS containing forskolin (20 μM) and genistein (50 μM). Each well was assayed individually for I– influx in a plate reader by recording fluorescence continuously (200 ms per point) for 2 seconds (baseline) and then for 12 seconds after rapid (<1 second) addition of 165 μl PBS in which 137 mM Cl– was replaced by I–. The I– influx rate was computed by fitting the final 11.5 seconds of the data to an exponential for extrapolation of initial slope and normalizing for background-subtracted initial fluorescence. All compound plates contained negative controls (DMSO vehicle) and positive controls (4-PBA, 4 mM), and separate positive control plates were used for low-temperature rescue (27°C incubation for 18–24 hours). Assay analysis indicated a Z′ factor (37) of more than 0.6.
Short-circuit current measurements.
ΔF508-CFTR–expressing FRT cells were cultured on Snapwell inserts for 7–9 days. Test compounds were added 18–24 hours prior to measurements. The basolateral solution contained 130 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgCl2, 10 mM glucose, and 10 mM Na-HEPES (pH 7.3). In the apical bathing solution, 65 mM NaCl was replaced by Na gluconate, and CaCl2 was increased to 2 mM. Solutions were bubbled with air and maintained at 37°C. The basolateral membrane was permeabilized with 250 μg/ml amphotericin B. For studies of human bronchial epithelial cells, apical and basolateral chambers contained identical solutions: 126 mM NaCl, 0.38 mM KH2PO4, 2.1 mM K2HPO4, 1 mM MgSO4, 1 mM CaCl2, 24 mM NaHCO3 and 10 mM glucose (the basolateral membrane was not permeabilized). Hemichambers were connected to a DVC-1000 voltage clamp (World Precision Instruments Inc.) via Ag/AgCl electrodes and 1 M KCl agar bridges for recording of apical membrane or short-circuit current.
Analysis of corrector mechanisms.
Some biochemical studies were done on BHK cells stably transfected with HA-tagged wild-type CFTR or ΔF508-CFTR. CFTR was tagged at the C-terminal tail (CFTR-CtHA) or in its fourth extracellular loop with 3 HA epitopes (CFTR-3HA) (8, 24). Accumulation of complex-glycosylated CFTR was assayed by immunoblot analysis as described previously (8). Plasma membrane expression was assayed by HA antibody binding (in nonpermeabilized cells) using an iodinated secondary anti-mouse antibody as described previously (24) or an HRP-coupled secondary antibody with Amplex Red (Invitrogen Corp.) as substrate. Nonspecific antibody binding was measured in nontransfected cells (using the same assay conditions) and in transfected cells with primary antibody omitted. The translational rate of ΔF508-CFTR was measured as described previously (7). Folding efficiency was assayed as described previously (7) with modifications. ΔF508-CFTR-CtHA–expressing cells were depleted of endogenous methionine and cysteine in the presence of correctors and then incubated with 0.2 mCi/ml [35S]methionine and [35S]cysteine for 150 minutes and chased in culture medium for an additional 150 minutes. The amount of newly synthesized ΔF508-CFTR during 150 minutes of labeling was computed from radioactive incorporation during a 15-minute pulse, performed in parallel measurement. Radioactively labeled CFTR was isolated by immunoprecipitation, visualized by fluorography, and quantified by phosphorimage analysis as described previously (5). Maturation efficiency was expressed as the percentage of radioactivity associated with the complex-glycosylated form relative to the total amount of newly synthesized ΔF508-CFTR. Considering the metabolic stability of the complex-glycosylated ΔF508-CFTR and its kinetics of biogenesis, we estimated that approximately 35% of the complex-glycosylated ΔF508-CFTR is degraded during the chase. Thus, the calculated maturation efficiencies in the absence of peripheral stabilization are slight underestimates.
Supplementary Material
Acknowledgments
We thank R. Kip Guy and A. Shelat for computational analysis in selecting compounds for primary screening and structure-activity analysis and Hubert H.M. Van Tol for providing the DRD4 expressing cell lines. This work was supported by the Cystic Fibrosis Foundation and by grants DK72517, HL73856, HL59198, EB00415, EY13574, and DK35124 from the NIH. L. Galietta acknowledges grant GP0296Y01 from Telethon-Italy. G. Lukacs was supported in part by the Canadian Institutes of Health Research.
Footnotes
Nonstandard abbreviations used: BHK, baby hamster kidney; CF, cystic fibrosis; CFTR, CF transmembrane conductance regulator; DRD4, dopamine receptor 4; FRT, Fischer rat thyroid; ΔF508, deletion of phenylalanine at residue 508; ΔF508-CFTR-CtHA, ΔF508-CFTR with a C-terminal HA tag; 4-PBA, 4-phenylbutyrate; YFP, yellow fluorescent protein.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations — correlation with incidence data and application to screening [review] Hum. Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 2.Pilewski JM, Frizzell RA Role of CFTR in airway disease. Physiol. Rev. 1999;79:S215–S255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- 3.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol. Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 4.Denning GM, et al. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 5.Lukacs GL, et al. Conformational maturation of CFTR but not its mutant counterpart (ΔF508) occurs in the endoplasmic reticulum and requires ATP. EMBO J. 1994;13:6076–6086. doi: 10.1002/j.1460-2075.1994.tb06954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopito RR. Biosynthesis and degradation of CFTR. Physiol. Rev. 1999;79:S167–S173. doi: 10.1152/physrev.1999.79.1.S167. [DOI] [PubMed] [Google Scholar]
- 7.Du K, Sharma M, Lukacs GL. The ΔF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat. Struct. Mol. Biol. 2005;12:17–25. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- 8.Sharma M, Benharouga M, Hu W, Lukacs GL. Conformational and temperature-sensitive stability defects of the ΔF508 cystic fibrosis transmembrane conductance regulator in post-endoplasmic reticulum compartments. J. Biol. Chem. 2001;276:8942–8950. doi: 10.1074/jbc.M009172200. [DOI] [PubMed] [Google Scholar]
- 9.Gentzsch M, et al. Endocytic trafficking routes of wild type and ΔF508 cystic fibrosis transmembrane conductance regulator. Mol. Biol. Cell. 2004;5:2684–2696. doi: 10.1091/mbc.E04-03-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato S, Ward CL, Krouse ME, Wine JJ, Kopito RR. Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J. Biol. Chem. 1996;271:635–658. doi: 10.1074/jbc.271.2.635. [DOI] [PubMed] [Google Scholar]
- 11.Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing ΔF508-CFTR. J. Clin. Invest. 1997;100:2457–2465. doi: 10.1172/JCI119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalemans W, et al. Altered chloride ion channel kinetics associated with the ΔF508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 13.Haws CM, et al. ΔF508-CFTR channels: kinetics, activation by forskolin, and potentiation by xanthines. Am. J. Physiol. 1996;270:C1544–C1555. doi: 10.1152/ajpcell.1996.270.5.C1544. [DOI] [PubMed] [Google Scholar]
- 14.Drumm ML, et al. Chloride conductance expressed by ΔF508 and other mutant CFTRs in Xenopus oocytes. Science. 1991;254:1797–1799. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- 15.Hwang TC, Wang F, Yang IC, Reenstra WW. Genistein potentiates wild-type and ΔF508-CFTR channel activity. Am. J. Physiol. 1997;273:C988–C998. doi: 10.1152/ajpcell.1997.273.3.C988. [DOI] [PubMed] [Google Scholar]
- 16.Al-Nakkash L, Hwang TC. Activation of wild-type and ΔF508-CFTR by phosphodiesterase inhibitors through cAMP-dependent and -independent mechanisms. Pflügers Arch. 1999;437:553–561. doi: 10.1007/s004240050817. [DOI] [PubMed] [Google Scholar]
- 17.Hwang TC, Sheppard DN. Molecular pharmacology of the CFTR Cl- channel. Trends Pharmacol. Sci. 1999;20:448–453. doi: 10.1016/s0165-6147(99)01386-3. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, et al. Nanomolar affinity small molecule correctors of defective ΔF508-CFTR chloride channel gating. J. Biol. Chem. 2003;278:35079–35085. doi: 10.1074/jbc.M303098200. [DOI] [PubMed] [Google Scholar]
- 19.Pedemonte N, et al. Phenylglycine and sulfonamide correctors of defective ΔF508- and G551D-CFTR chloride channel gating. Mol. Pharmacol. 2005;67:1797–1807. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- 20.Ma T, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Invest. 2002;110:1651–1658. doi:10.1172/JCI200216112. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muanprasat C, et al. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J. Gen. Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma T, et al. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J. Biol. Chem. 2002;277:37235–37241. doi: 10.1074/jbc.M205932200. [DOI] [PubMed] [Google Scholar]
- 23.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 24.Sharma M, et al. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J. Cell Biol. 2004;164:923–933. doi: 10.1083/jcb.200312018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward CL, Kopito RR. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J. Biol. Chem. 1994;269:25710–25718. [PubMed] [Google Scholar]
- 26.Ostedgaard LS, Zeiher B, Welsh MJ. Processing of CFTR bearing the P574H mutation differs from wild-type and ΔF508-CFTR. J. Cell Sci. 1999;112:2091–2098. doi: 10.1242/jcs.112.13.2091. [DOI] [PubMed] [Google Scholar]
- 27.Sheppard DN, Ostedgaard LS, Winter MC, Welsh MJ. Mechanism of dysfunction of two nucleotide binding domain mutations in cystic fibrosis transmembrane conductance regulator that are associated with pancreatic sufficiency. EMBO J. 1995;14:876–883. doi: 10.1002/j.1460-2075.1995.tb07069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Craenenbroeck K, et al. Folding efficiency is rate-limiting in dopamine D4 receptor biogenesis. J. Biol. Chem. 2005;280:19350–19357. doi: 10.1074/jbc.M414043200. [DOI] [PubMed] [Google Scholar]
- 29.Gregory RJ, et al. Maturation and function of cystic fibrosis transmembrane conductance regulator variants bearing mutations in putative nucleotide-binding domains 1 and 2. Mol. Cell. Biol. 1991;11:3886–3893. doi: 10.1128/mcb.11.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galietta LJ, Haggie PM, Verkman AS. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001;499:220–224. doi: 10.1016/s0014-5793(01)02561-3. [DOI] [PubMed] [Google Scholar]
- 31.Chen EY, Bartlett MC, Loo TW, Clarke DM. The ΔF508 mutation disrupts packing of the transmembrane segments of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2004;279:39620–39627. doi: 10.1074/jbc.M407887200. [DOI] [PubMed] [Google Scholar]
- 32.Johnson LG, et al. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 33.Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 34.Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases [review] Nat. Cell Biol. 2004;3:1054–1056. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 35.Galietta LJ, et al. Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J. Biol. Chem. 2001;276:19723–19728. doi: 10.1074/jbc.M101892200. [DOI] [PubMed] [Google Scholar]
- 36.Galietta LJ, et al. An electrogenic amino acid transporter in the apical membrane of cultured human bronchial epithelial cells. Am. J. Physiol. 1998;275:L917–L923. doi: 10.1152/ajplung.1998.275.5.L917. [DOI] [PubMed] [Google Scholar]
- 37.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in valuation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.