Abstract
The relative roles of the types 1 and 2 iodothyronine deiodinases (D1 and D2) in extrathyroidal 3,5,3′-triiodothyronine (T3) production in humans are unknown. We calculated the rate of thyroxine (T4) to T3 conversion by intact cells transiently expressing D1 or D2 at low (2 pM), normal (20 pM), and high (200 pM) free T4 concentrations. Deiodinase activities were then assayed in cell sonicates. The ratio of T3 production in cell sonicates (catalytic efficiency) was multiplied by the tissue activities reported in human liver (D1) and skeletal muscle (D2). From these calculations, we predict that in euthyroid humans, D2-generated T3 is 29 nmol/d, while that of D1-generated T3 is 15 nmol/d, from these major deiodinase-expressing tissues. The total estimated extrathyroidal T3 production, 44 nmol/d, is in close agreement with the 40 nmol T3/d based on previous kinetic studies. D2-generated T3 production accounts for approximately 71% of the peripheral T3 production in hypothyroidism, but D1 for approximately 67% in thyrotoxic patients. We also show that the intracellular D2-generated T3 has a greater effect on T3-dependent gene transcription than that from D1, which indicates that generation of nuclear T3 is an intrinsic property of the D2 protein. We suggest that impairment of D2-generated T3 is the major cause of the reduced T3 production in the euthyroid sick syndrome.
Introduction
The monodeiodination of thyroxine (T4) to 3,5,3′-triiodothyronine (T3) activates the major secretory product of the iodine-sufficient human thyroid gland, producing approximately 80% of the circulating T3 in humans. The types 1 and 2 iodothyronine deiodinases (D1 and D2) are members of a family of oxidoreductases that catalyze this reaction (1). These integral membrane proteins contain the rare amino acid selenocysteine (encoded by a UGA) in their active site. Both D1 and D2 require an as-yet-unidentified cofactor for the reaction. Several decades ago, D1 was identified in the liver and the kidney of rats and humans, and it is often assumed that this enzyme is the source of most of the plasma T3 in humans. The more recent discovery of D2 mRNA and activity in human skeletal muscle suggests that D2 could also be a significant source for plasma T3 production in humans, but this has not been quantitatively defined (1).
The biochemical and molecular properties of D2 seem ideal for extrathyroidal T3 production. Its activity is tightly controlled by the concentration of its preferred substrate, T4, since catalysis accelerates the ubiquitination of this enzyme, inactivating it and accelerating its degradation in proteasomes (2). The half-life of D2 in normal cells is 20–30 minutes in the presence of T4. The transcription of the type 2 deiodinase (DIO2) gene is also negatively regulated by T3 (3). In contrast, the D1 protein has a long half-life (>12 hours), and the transcription of the human type 1 deiodinase (DIO1) gene is markedly stimulated by T3, just the opposite of what would be expected in a typical feedback loop (4, 5).
Hepatic T3 production via D1 in the 1.5-kg human liver has been estimated to be only approximately 8 nmol/day, much less than the total daily extrathyroidal T3 production of approximately 40 nmol (6). If accurate, this would predict the likelihood of a significant contribution by D2. It has not been possible to perform similar calculations for T3 production by skeletal muscle D2 for 2 reasons. First, there was a paucity of data, which was only recently remedied with a report indicating that mean D2 activity is approximately 1 fmol T4/mg protein/min in this tissue (7). Second, the catalytic efficiency of D2-catalyzed T3 production is not known at physiological cofactor and free T4 (FT4) concentrations. The Vmax/Km ratio of the ping-pong reaction catalyzed by D1 is independent of the concentration of cofactor, but this is not the case for the sequential kinetics of D2-catalyzed T4 5′-deiodination. In fact, there also is no formal estimate of how efficient D1-catalyzed T3 production is at the euthyroid FT4 concentration of 20 pM.
A second important issue is to determine why in D2-expressing cells a significant portion of the T3 produced enters the cell nucleus, where it binds to specific high-affinity thyroid hormone receptors (TRs), whereas most T3 generated from the D1-catalyzed reaction remains in the cytoplasm or exits the cell (8). The first recognition of D2 activity was due to its catalysis of pituitary T4 to T3 conversion by a 6-n-propylthiouracil–resistant (PTU-resistant) process (9). This agent is a potent inhibitor of D1. The fact that the D2 is located in the ER, while D1 is in the plasma membrane, suggests an explanation, although the catalytic centers of both enzymes are in the cytosol (10).
To accurately define the relative roles of D1 versus D2 in extrathyroidal T3 production in humans, the deiodination process must be analyzed at physiological cofactor and FT4 concentrations. Since we do not know the identity of this cofactor, much less its concentration, we designed an in vitro model in which human D1 or D2 is transiently expressed in the same cell type and compared their catalytic efficiencies at FT4 concentrations spanning the hypothyroid to thyrotoxic range. We also developed a method to quantify the transcriptional potency of the intracellular T3 produced by D1 versus D2. Our results predict that D2-catalyzed T3 production is the major source of plasma T3 in euthyroid humans, while D1 is the major contributor when FT4 is present at thyrotoxic concentrations. Furthermore, at equal rates of production, the T3-generated by D2-catalyzed T4 monodeiodination is 2- to 3-fold more effective in activating T3-dependent gene transcription than that generated by D1, which indicates that this property is intrinsic to the D2 protein.
Results
Quantifying T3 production in cells transiently expressing D1 or D2.
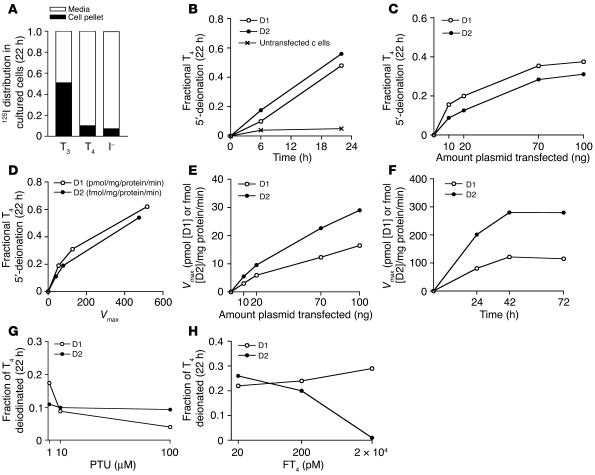
The production of 125I– from outer ring–labeled T4 in intact cells can be analyzed by measuring the level of either [125I]T3 or 125I– in the medium, since equimolar amounts of both products are generated. The D1 or D2 activities were measured in cell sonicates harvested at the completion of the incubation. In human embryonic kidney epithelial (HEK 293) cells incubated in 0.1% BSA medium, approximately 50% of the labeled T3 remains in the medium, and 50% is found in the cell pellet. The T4 distribution differs, with a medium-to–cell pellet ratio of 9:1 (Figure 1A). Therefore, we based the calculation of T4 to T3 conversion on the rate of 125I– release. Cells were transfected with 100 ng of D1- or D2-expressing plasmid and incubated for 22 hours. The rate of T4 deiodination by D1- or D2-catalyzed 5′-deiodination in intact cells was linear over the 22 hours, and less than 5% of the labeled T4 appeared as iodide in untransfected cells (Figure 1B). Enzyme activities increased progressively, though not linearly, with increasing amounts of plasmid transfected in whole cells and cell sonicates (Figure 1, C–E). The rates of fractional T4 deiodination by D1 and D2 in whole cells were quite similar (Figure 1D). The activities of both enzymes increased over the first 24 hours but were stable over the next 24 hours (Figure 1F).
Figure 1.
Model for quantification of T3 production in cells transiently expressing D1 or D2. (A) Distribution of [125I]T3, [125I]T4, and I– in HEK 293 cells incubated in 0.1% BSA medium for 22 hours. (B–H) Cells were transfected with 3–100 ng of D1- or D2-expressing plasmids and incubated for 22 hours in 1 ml 0.1% BSA DMEM, to which were added the indicated concentrations of T4, including approximately 100,000 cpm/ml of [125I]T4. Whole-cell T4 to T3 conversion rates were determined from the fraction of [125I]T4 appearing as 125I–. The Vmax for each deiodinase was determined in cell sonicates, harvested after completion of the incubation. (B) Fractional T4 deiodination over time. (C) Correlation between the quantity of plasmid transfected and fractional deiodination. (D) Correlation between Vmax and fractional deiodination. (E) D1 and D2 Vmax versus the quantity of plasmid transfected. (F) Time course of transient deiodinase expression over 72 hours. Effect of PTU (G) or T4 (H) on fractional T4 deiodination in D1- and D2-expressing cells. All data are expressed as the mean of duplicate samples in 3 independent experiments.
The addition of PTU (1–100 μM) caused a dose-dependent decrease in D1 activity but did not affect that of D2 (Figure 1G). On the other hand, an increase in FT4 from 2 to 200 pM reduced the rate of fractional deiodination by D2 but did not affect that by D1 (Figure 1H). These differences are consistent with the PTU sensitivity of D1- but not D2-catalyzed T4 deiodination and the posttranslational substrate-induced inactivation of D2 but not D1. We wished to express an amount of deiodinase that would not consume more than 30% of the T4 over 24 hours but was as close as possible to the level in the most important tissues. This was achieved with 10–20 ng of D1- or D2-expressing plasmids.
Analyses of the deiodination products of cells expressing D1 and D2.
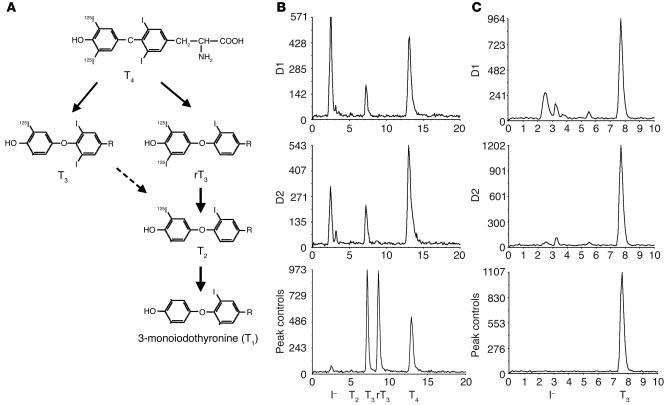
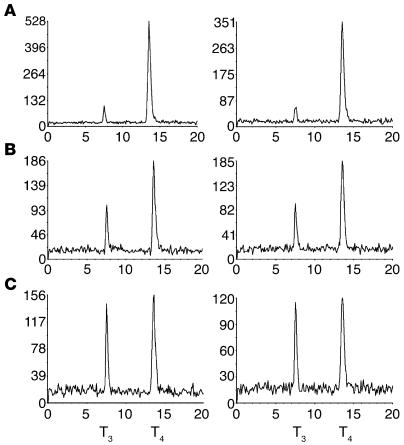
We estimated T4 to T3 conversion from the rate of appearance of enzymatically generated 125I–. Since T4 is labeled only in the outer ring, the labeled T3 and I– produced have one half the specific activity of the parent compound (Figure 2A). Since D2 catalyzes only outer-ring deiodination of T4, the T3 production by D2 is equal to the labeled 125I– production multiplied by 2 to correct for the specific activity reduction. For D1, the calculation is more complex, since the Vmax/Km ratios for 5′- and 5-deiodination of T4 are approximately equal (11). Therefore, 1 mol of T3 and of 3,3′,5′-triiodothyronine (reverse T3 [rT3]) will be generated for every 2 molecules of T4 deiodinated. Because of the much higher Vmax/Km ratios for D1-catalyzed 5′-monodeiodination of rT3 and its products 3,3′-diiodothyronine (T2) and 3′-monoiodothyronine (T1) than for T4, the rT3 is rapidly deiodinated, which leads to the release of all the 125I– (Figure 2A). This being the case, the [125I]T3 production should be equal to one-third of the 125I– generated multiplied by 2. We confirmed this experimentally by exposing D1-expressing cells to approximately 2 × 106 cpm [125I]T4 for 24 hours. The ratio of 125I– (corrected for nonspecific 125I– released) to T3 production was 3.3 ± 0.6 in 3 separate experiments (see D1 in Figure 2B). Note the absence of rT3, T2, or T1 in the chromatogram. A further consideration in D1-expressing cells is the slow rate of inner-ring deiodination of T3, which would generate 125I– from the subsequent 5′-monodeiodination of the T2 product (Figure 2A). Incubation of outer ring–labeled T3 with D1-expressing cells for 24 hours caused the appearance of 15–20% of the T3 label as 125I–, which indicates this is a detectable but inefficient deiodination pathway. This accounts for the slightly higher I–/T3 ratio observed after incubation of [125I]/T4 with D1 than the expected value of 3.0 (Figure 2B). Note that there is no deiodination of T3 by D2.
Figure 2.
Chromatographic analysis of the products of D1- and D2-catalyzed deiodination. (A) Pathway of T4 deiodination by D1. (B and C) HEK 293 cells transfected with 100 ng of D1 or D2 were homogenized in the medium after 24 hours incubation with labeled T4 or T3 and the deiodination products analyzed by HPLC. (B) Chromatographic patterns of the products of D1- or D2-catalyzed [125I]T4 deiodination. (C) Patterns of the products of outer ring–labeled [125I]T3 deiodination by D1- or D2-expressing cells.
Assessing the efficiencies of T3 production by D1 and D2 at varying free T4 concentrations.
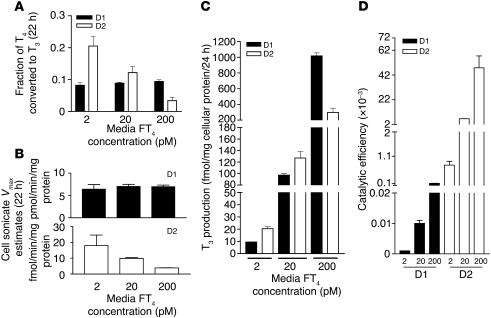
To replicate physiological conditions precisely, it would be ideal to quantitate T3 production when cellular D1 and D2 activities were comparable to those in deiodinase-expressing tissues. For liver, the D1 Vmax(T4) is approximately 8 pmol/mg protein/min, and for skeletal muscle, the D2 Vmax is approximately 1 fmol/mg protein/min (6, 7). While we were able to achieve this level for D1, the T3 production/24 hours in cells expressing D2 at Vmax values of 1–2 fmol/mg protein/min was too low to measure accurately. This is understandable, since the mass of D2-expressing skeletal muscle is in vast excess of that of the D1-expressing liver and kidneys. Accordingly, we expressed D2 at a Vmax of approximately 10 fmol/mg protein/min. To evaluate the effects of thyroid status on the efficiency of D1- and D2-catalyzed T3 production, we incubated cells with FT4 concentrations of 2 pM (typical of severe hypothyroidism), 20 pM (euthyroid state), and 200 pM (severe thyrotoxicosis). The fractional T4 to T3 conversion rates for D1 were unaffected by the FT4 concentration, while those of D2-expressing cells decreased about 4-fold as FT4 increased (Figure 3A). This can be explained by the observed decrease in D2 activity (in cell sonicates) produced by the higher ambient T4 concentration due to catalysis-induced, posttranslational downregulation of D2. In contrast, D1 activity was unchanged during the incubation (Figure 3B). As a consequence, the quantity of T3 produced by D2 decreased from about twice that produced by D1 at 2 pM FT4 to about one-quarter that produced by D1 at 200 pM FT4. It is remarkable that despite the approximately 700-fold higher Vmax estimates for D1 than for D2, the T3 produced via these 2 pathways at 20 pM FT4 was slightly higher with D2 (Figure 3C). The catalytic efficiency, the ratio of T3 production in intact cells to the Vmax for T4 to T3 conversion in sonicates of the same cells, was higher for D2 than D1 at all FT4 concentrations (Figure 3D).
Figure 3.
Human D1- and D2-catalyzed T4 to T3 conversion at different FT4 concentrations. (A) Fractional T4 to T3 conversion in whole HEK 293 cells transiently expressing D1 or D2. (B) Vmax estimates for D1 and D2 in cell sonicates. (C) T3 production in whole cells expressing D1 or D2. (D) Ratios of the whole-cell to cell sonicate T3 production, or catalytic efficiency, of D1 and D2 enzymes.
Deiodination rates in cells expressing endogenous D1 and D2.
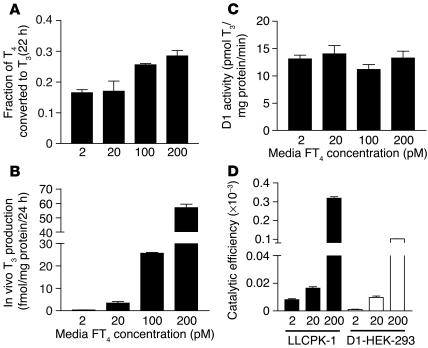
To confirm that endogenous D1 or D2 under control of homologous promoters would show the same characteristics as those found in HEK 293 cells expressing D1 or D2 driven by a heterologous promoter, we evaluated the patterns of deiodination in the porcine kidney tubule LLCPK1 cells for D1 and mesothelioma (MSTO) cells (12) for D2, respectively. Similar to results in HEK 293 cells, the T3 production rate in intact LLCPK1 cells increased proportionately as FT4 concentration increased (Figure 4, A and B), with no changes in D1 Vmax (Figure 4C). In other experiments (data not shown), we found no response to T3 of the T3-sensitive TRE3TKLUC reporter in LLCPK1 cells, which indicates the absence of functional TR in these cells. Therefore we cannot replicate in this system the increase in DIO1 gene transcription anticipated to result from T4 to T3 conversion. The ratio of T3 production in intact cells to cell sonicate activity (catalytic efficiency) was 2- to 3-fold higher for porcine D1 in LLCPK1 cells than for the human D1 transfected in HEK 293 cells (Figure 4D).
Figure 4.
Whole-cell T3 production in LLCPK1 cells expressing endogenous D1 at different FT4 concentrations. (A) Fractional T4 to T3 conversion. (B) Whole-cell T3 production. (C) Vmax estimates for T3 production in cell sonicates. (D) Catalytic efficiency of T4 to T3 conversion by porcine D1 compared with transiently expressed human D1.
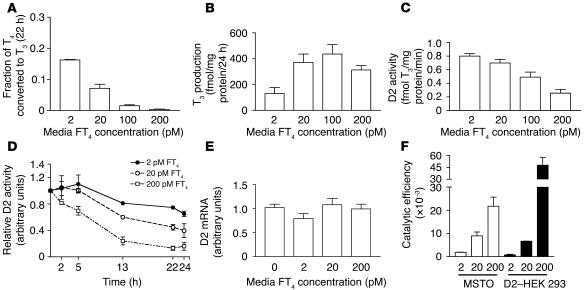
MSTO cells respond to changes in ambient FT4 concentration in a manner similar to D2-expressing HEK 293 cells. The fractional conversion of T4 to T3 decreased progressively with increasing FT4 concentrations, but T3 production in whole cells increased until the FT4 concentration reached 100 pM (Figure 5, A and B). We also examined the time course of T4-induced loss of activity to see how rapidly this occurred. There were decreases in D2 activity over the first 13 hours, after which it reached a plateau (Figure 5D). Finally, since it is not known whether MSTO cells can respond to the T3 produced by D2, we measured D2 mRNA expression after incubation to determine whether it had decreased. There was no effect of increasing FT4 concentrations on the D2 mRNA, which indicated that the loss of D2 activity is completely posttranslational (Figure 5E). The catalytic efficiency of endogenous D2 in MSTO cells was similar to that in D2-expressing HEK 293 cells, except at the highest FT4 concentration (Figure 5F).
Figure 5.
Whole-cell T3 production in MSTO cells expressing endogenous D2 at different FT4 concentrations. (A) The fractional T4 to T3 conversion. (B) Whole-cell T3 production. (C) Vmax estimates for T3 production in cell sonicates. (D) Time course of T4-induced loss of D2 activity. (E) Effects of the FT4 concentrations on D2 mRNA levels. (F) Catalytic efficiency of T4 to T3 conversion in MSTO cells compared with HEK 293 cells transiently expressing human D2.
D2-generated T3 in human skeletal muscle.
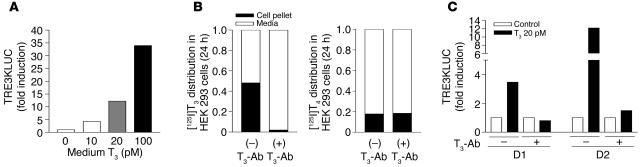
β-Adrenergic–responsive D2 activity is present in human skeletal muscle cells (13). We used primary cultures of human skeletal muscle myoblasts (HSMMs) to examine whether these cells could convert medium [125I]T4 to [125I]T3. HSMMs were incubated for 22 hours with [125I]T4 in medium containing a rabbit anti-T3 Ab (T3-Ab) to block reuptake of [125I]T3 released (see Methods). This results in efficient binding of [125I]T3 plus weak binding of [125I]T4. HPLC was used to determine the T3/T4 ratios of the Ab-bound iodothyronines. In the absence of cells, after correction for recovery, this ratio was 0.016, and the ratio increased to 0.060 in the presence of HSMMs (Figure 6, A and B; see also Methods for calculations). A further increase to 0.090 was observed when cells were exposed to 0.5 mM dibutyryl cAMP (BT2cAMP) to increase the level of D2 (Figure 6C) (13). With the 2-fold correction for loss of specific activity, this represents a 6- and 10-fold increase in the ratio of extracellular T3 to T4 produced by D2 in HSMMs over 22 hours, respectively.
Figure 6.
Chromatographic analysis of T4 to T3 conversion in HSMMs. Control medium incubated with no cells (A) or HSMMs (B and C) incubated in duplicate 60-mm dishes for 22 hours in 2 ml 0.1% BSA DMEM with approximately 1 μCi/ml [125I]T4 plus specific T3-Ab (1:1,000) in the absence (B) or presence (C) of dibutyryl cAMP (0.5 mM).
To determine whether D2 is expressed at equal levels in different skeletal muscles, we obtained fresh samples of human sternocleidomastoid (n = 7), rectus abdominis (n = 20), and vastus lateralis (n = 3) muscle during surgery and assayed them for D2 activity at 0.15 nM T4. No differences in D2 activity were found among these (0.021 ± 0.001 vs. 0.024 ± 0.001 vs. 0.023 ± 0.001 fmol/mg protein/min, respectively; P = 0.79).
The T3 derived from D2-catalyzed T4 to T3 conversion is more potent than that from D1 in stimulating T3-dependent gene expression.
D1 is expressed in plasma membrane and D2 in the ER. If this is the explanation for the greater contribution of D2- than D1-generated T3 to TR-bound T3, it should be possible to demonstrate a greater effect of the intracellular T3 generated by D2 than by D1 on T3-dependent transcription. To evaluate this, we transfected HEK 293 cells with D1- or D2-expressing plasmids, a TRα-expressing plasmid, and the T3-responsive TRE3TKLUC construct. This was a highly T3-responsive system (Figure 7A). Since we wished to monitor the effects of intracellular T3 production, we prevented cellular reentry of the T3 produced from T4 without interfering with T4 uptake by addition of T3-Ab to the medium. In the presence of a 1;1,000 dilution of rabbit T3-Ab, more then 98% of the labeled T3 remained in the medium, whereas there was no effect on T4 uptake (Figure 7B). The effectiveness of the T3-Ab in preventing cellular T3 uptake was confirmed by its blockade of the transcriptional response of TRE3TKLUC to medium T3 in D1- or D2-expressing cells (Figure 7C).
Figure 7.
A T3-responsive system for evaluating the contribution of D1- and D2-generated T3 to nuclear T3. (A–C) HEK 293 cells were transfected with D1- or D2-expressing plasmids, a TRα-expressing plasmid, and the T3-responsive TRE3TKLUC construct (see Methods). (A) The TRE3TKLUC reporter responded to T3 in a dose-dependent manner. (B) The addition of specific T3-Ab prevents cellular uptake of T3 but not T4. (C) T3-Ab blocks the transcriptional response to 20 pM T3.
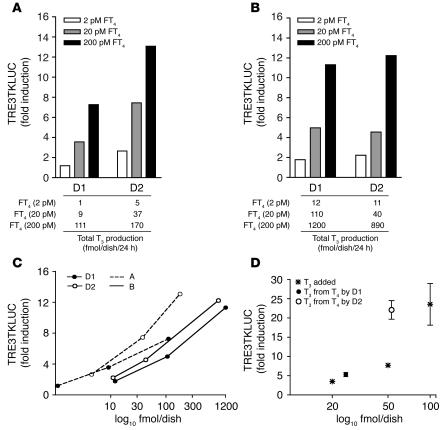
As a control for the potential T4 induction of TRE3TKLUC, which could occur at high FT4 concentrations, the same concentrations of FT4 were added to cells transfected with a plasmid expressing an inactive D2 construct (see Methods). In the first experiment, performed after transfection of 100 ng D1 or D2 plasmids, luciferase (LUC) induction in cells expressing D1 was negligible at 2 pM, whereas a 3.6- and 7.3-fold increase occurred at 20 and 200 pM FT4, respectively (Figure 8A). The mean LUC induction in D2-expressing cells was higher at all FT4 concentrations — 2.7-, 7.5-, and 13.1-fold, respectively — but so was the T3 production at comparable FT4 concentrations (Figure 8, A and B). Nonetheless, note that 111 fmol of T3 produced by D1 triggered a LUC induction similar to that observed with 37 fmol of T3 generated by D2 (Figure 8A), which suggests that the D2-catalyzed T3 production had an approximately 3-fold greater effect on gene transcription than that catalyzed by D1 (Figure 8C). We confirmed that this was indeed the case by increasing the level of transfected D1 4-fold, thereby increasing T3 production by D1 to amounts comparable to those produced by D2 at the same FT4 concentrations. Despite the higher T3 production by D1, the TRE3TKLUC induction was the same with D2 (Figure 8B). The ratio of fold induction of TRE3TKLUC to T3 production by D2 was again shifted to the left of that for D1 (Figure 8C). In a third experiment, we compared the effects of T3 generated from 20 pM FT4 by D1 or D2 with those of T3 added directly to the medium, the latter in the absence of T3-Ab (Figure 8D). While T3 production by D2 was only twice that by D1, the induction of TRE3TKLUC was equivalent to that with a 5-fold higher quantity of T3. Thus the transcriptional stimulation by D2-generated T3 is consistently 2- to 3-fold greater than that from D1-generated T3.
Figure 8.
The T3 derived from D2-catalyzed T4 to T3 conversion is more potent than that from D1 in stimulating T3-dependent gene expression. (A and B) TRE3TKLUC induction in D1- and D2-expressing HEK 293 cells. At any rate of T3 production (see tables below graphs), D2-catalyzed T3 production causes greater increases in T3-dependent luciferase (LUC) expression than T3 derived from the D1-catalyzed T4 to T3 reaction. (C) TRE3TKLUC induction versus T3 production (log10). D2-derived T3 caused greater LUC induction than did D1-derived T3. LUC expression was normalized to growth hormone as transfection efficiency control. (D) Effects of added T3 and D1- or D2-derived T3 on TRE3TKLUC induction. Cells transfected with inactive D2 were incubated with 1 ml 0.1% BSA DMEM, and the indicated amounts of T3 were added. D1- and D2-expressing cells were incubated in the same medium containing T3-Ab (1:1,000) and 20 pM FT4.
Discussion
There are no prior studies addressing the relative roles of the 2 outer-ring human iodothyronine deiodinases in the peripheral conversion of T4 to T3. There are several reasons for this, including the fact that the putative thiol cofactor necessary for both D1- and D2-catalyzed reactions has not yet been identified. In addition, the assay conditions require the use of in vivo FT4 of 20 pM, and the rate would be so low as to be difficult to quantitate. To address this issue, we modified a system used by us and others in which D1 or D2 are transiently expressed in HEK 293 cells at near physiological levels and exposed to a range of FT4 concentrations that occur clinically (14, 15). This allows stable reactions over 18–24 hours at physiological substrate and cofactor concentrations (Figure 1).
The system developed in these experiments allowed us to compare the catalytic efficiencies of these enzymes under conditions that obtain in vivo. The characteristic properties of the 2 deiodinases, PTU sensitivity (D1) or resistance (D2) and posttranslational downregulation by T4 (D2), were preserved (Figure 1). Another advantage to this system is that after the incubations, deiodinase activities can be quantitated in the same cells under conditions used to assay deiodinase activity in human tissues (Figure 3). This facilitates extrapolation of the results to the in vivo state. The system was further validated by the fact that it yielded results similar to those for cells expressing endogenous D1 or D2 (Figures 4 and 5).
The D1 activity in the cell sonicates in these transient expression studies was approximately 7 pmol/mg protein/min, virtually identical to that in human liver (6). To obtain reliable reaction rates, we studied D2-mediated T4 to T3 conversion at D2 activities approximately 10 fmol/mg protein/min (Figure 3B), about 10-fold that in human skeletal muscle (7). The striking results were that at a euthyroid FT4 concentration of 20 pM, the D2-catalyzed T3 production rate was slightly greater than that catalyzed by D1 despite the fact that the Vmax in cell sonicates was estimated to be 700-fold higher for D1 than for D2 (Figure 3, B and C). This is explained by the roughly 700-fold higher catalytic efficiency of D2 (Figure 3D).
What factors contribute to the remarkable difference in the catalytic efficiency of these 2 enzymes? First, each molecule of T4 deiodinated via D2 produces 1 of T3. Because D1 catalyzes the inner- and outer-ring deiodination of T4 equally well, as predicted from Vmax/Km estimates, for each 2 moles of T4 deiodinated by D1, only 1 mole of T3 and a second of rT3 is produced (Figure 2A) (6). However, this can only explain a catalytic efficiency difference of 2. The rest of the difference is primarily due to the much slower rate of D1-catalyzed T4 to T3 conversion under physiological conditions than under typical assay conditions. The assay conditions for D1 amplify the apparent Vmax to a much greater extent than those used for D2. This can be explained in part by differences in the kinetics of the enzyme: D1-catalyzed deiodination is a ping-pong reaction with the thiol cofactor acting as the second substrate, while the kinetics of D2 are sequential, with the cofactor thought to bind at the same time as the substrate. Relatively massive concentrations of the DTT cofactor, in our case 20 mM, are used for D1 and D2 assays. The catalytic activity of D1 decreases approximately 1,000-fold when the cofactor is reduced from 20 mM DTT to the more “physiological” 5 mM reduced glutathione (16). The Vmax is also reduced for D2 at lower cofactor concentrations, but by a much lower fraction (data not shown). Nonetheless, even considering these differences, D2 appears to be a more efficient enzyme than D1. In fact, D2 is so efficient that it is nearly impossible to quantitate the D2 protein by Western blotting, and only prolonged exposure of cell sonicates from 75Se-labeled cells allows recognition of its presence (12). On the other hand, Western blotting or bromoacetyl [125I]iodothyronine labeling allows ready quantitation of D1 (17, 18). Thus, we cannot establish as yet how much higher the turnover rate is for D2 than that reported for D1. The estimated Km(T4) for D2, which is approximately 1,000-fold lower than that for D1, could also contribute to the higher catalytic efficiency of T4 to T3 conversion by D2 versus D1 at normal FT4 concentrations.
These arguments pertain at euthyroid or hypothyroid FT4 concentrations. As mentioned, D2 activity, but not that of D1, falls as FT4 concentration increases due to substrate-induced ubiquitination (2). This is the explanation for the fact that at 200 pM FT4, the D2 activity at the end of the incubation was about 10% of that found at 2 pM FT4 (Figure 3B). For this reason, D1 would be predicted to be more important in T4 to T3 conversion in the thyrotoxic patient. This concept is supported by the comparisons of the effect of thyroid status on the sensitivity of T4 to T3 conversion in humans to inhibition by the D1-specific inhibitor PTU. We showed several decades ago that in hyperthyroid patients with Graves disease, there was a dose-dependent decrease of up to 50% in T4 to T3 conversion by administering up to 1,200 mg/d of PTU (19). On the other hand, 2 other studies showed that when even higher doses of PTU were used, a maximum decrease of 25% in serum T3 occurred in athyreotic T4-replaced, euthyroid individuals (20, 21). This is in excellent agreement with the predictions based on the current studies (Figure 3D). In addition to the posttranslational reduction of D2 by high FT4 concentrations shown in this study, it has also been shown that transcription of the DIO2 gene is reduced by about 50% by a high level of T3, while the human DIO1 gene is transcriptionally induced by T3 (3, 5). In the present studies, the transcriptional effects of thyroid hormone on D1 and D2 expression were not seen either in the transiently expressing cells or in cells expressing endogenous deiodinase, since these do not express functional TR (Figures 4C and 5E.).
Another correlation between the predictions of these experiments and in vivo data with respect to the effective thyroid status comes from early work by Inada et al. (22). These studies showed that in hypothyroid patients, the fractional whole body conversion rate of T4 to T3 was 42%, while in the same patients made euthyroid with levothyroxine, this rate fell to 21%. We saw the same decrease in fractional T4 to T3 conversion by D2 as the medium FT4 concentration was increased from 2 to 20 pM (Figure 3A). Lum et al. (23) also observed sustained elevations in serum T3/T4 ratios in athyreotic subjects partially withdrawn from T4 therapy. Since T4 concentrations are so low, this does not apply to total T3 production, which was markedly reduced at 2 pM FT4 (Figure 3C). The increased efficiency of T4 to T3 conversion in the hypothyroid state is expected from an increase in D2 activity due to the longer half-life of the D2 protein, increased DIO2 gene transcription, and reduced DIO1 gene transcription (2, 3, 5).
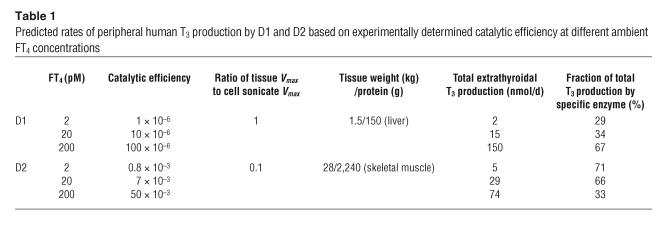
To test the applicability of these catalytic efficiency estimates to the in vivo state, we extrapolated these data to predict the quantities of T3 from T4 produced by D1 and D2 in humans in various thyroid states. This can be done if the ratios of intact cell to cell sonicate T3 production are applied to deiodinase estimates in human tissues. D1 activity in human liver is approximately 8 pmol/mg protein/min, similar to that achieved in this study after correction for differences in assay conditions (5 vs. 20 mM DTT and microsomal vs. total cellular protein) (6, 24). The D2 activity estimate of approximately 1 fmol/mg protein/min is from a recent study in which D2 activity was measured in the sternocleidomastoid or the rectus abdominis muscle in euthyroid subjects (7). The present studies are the first to our knowledge to demonstrate that human skeletal muscle cells produce extracellular T3 from T4 (Figure 6). Other assumptions regarding the mass of human liver and skeletal muscle and the protein content/wet weight of these tissues are shown in Table 1. The conversion efficiencies for D1 and D2 are as reported in Figure 3.
Table 1.
Predicted rates of peripheral human T3 production by D1 and D2 based on experimentally determined catalytic efficiency at different ambient FT4 concentrations
In a 70-kg euthyroid human, the hepatic D1-catalyzed T4 to T3 conversion is predicted to produce approximately 15 nmol of T3/d and that from skeletal muscle D2 approximately 29 nmol/d (Table 1). The total, 44 nmol/d, is quite close to the predicted extrathyroidal T3 production rate of 40 nmol/d according to many in vivo kinetic studies (25). In the euthyroid state, two-thirds of this is predicted to be derived from D2-catalyzed T4 monodeiodination. In the hypothyroid state, D2 is predicted to account for at least 70% of the extrathyroidal T4 to T3 conversion, but it would be the source of a much lower fraction, approximately 30%, in the thyrotoxic state (Table 1). It should be noted that the contribution of D1 is overestimated in the hypothyroid state and underestimated in the hyperthyroid state since the catalytic efficiency figures do not take into account the transcriptional effects of thyroid status, namely, reduction and enhancement of DIO2 and DIO1 gene expression, respectively.
The following assumptions were made. We ignored D1 in kidney and the D2 in brain and skin and assumed that cellular T4 uptake in all tissues is the same as it is in HEK 293 cells. We also assumed that the catalytic efficiencies of D1 and D2 in vivo are the same as those in tissue culture and that the effect of the cofactor present in HEK 293 cells is not significantly different from that supporting D1- and D2-mediated catalysis in liver and skeletal muscle. This assumption is supported by the comparisons of the results in transfected cells with those in cells expressing endogenous D1 and D2 (Figures 4 and 5). The D2 activity at the start of the incubation was higher than that at the end of the experiment due to posttranslational downregulation (Figure 3B). It appears that this reaches equilibrium value by about 12 hours of incubation (Figures 3C and 4D). Thus, T3 production would be somewhat higher in the first than in the second half of the incubation. Our model indicates that the estimated production by D2 was only about 10–15% higher than it would have been if the terminal levels of D2 had been present throughout the incubation period. Last, the in vivo concentration of FT4 is maintained at a constant level by the hypothalamic pituitary thyroid axis. In our experiments, the fraction of total T4 converted to T3 in the system was less than 30% (except in the LLCPK1 experiments). Thus, depletion of substrate does not significantly reduce the T3 production in this system (Figures 3A, 4A, and 5A).
The other major difference between D1- and D2-catalyzed T3 production that we addressed in these studies is the explanation for the unique characteristic of D2-expressing tissues, namely, that D2-catalyzed T4 to T3 contributes a substantial fraction to the TR-bound T3 (8). We have postulated that the location of D2 in the ER, as opposed to that of D1 in the inner surface of the plasma membrane, could improve the access of T3 produced from T4 to the nucleus (10). However, other explanations are also possible; for example, the rate of T4 to T3 conversion by D2 may be more rapid than that by D1. Yet, another possibility would be that in D2-expressing cells, there is a specific mechanism translocating D2-generated T3 from the perinuclear ER into the nucleus. Specific cytosol-to-nuclear plasma T3 transport mechanisms have been demonstrated (26).
We were able to modify the system to study transcriptional effects of the T3 produced by the D1 or D2 pathway and at the same time estimate the rate of total T3 production so that this could be taken into account (Figure 8). This required trapping the T3 produced during the overnight incubation in the medium so that it could not induce transcription. These studies showed that when the induction of T3-dependent gene expression is plotted as a function of T3 production rates, D2-generated T3 produces a 2- to 3-fold greater effect on the transcription of a T3-dependent gene than does D1-generated T3 (Figure 8C). Since these experiments are performed in the same cells, this difference cannot be explained by any cell type–specific property or by a faster rate of T3 production by D2. They indicate that the effect of D2 in providing more nuclear T3 than D1 very likely reflects its subcellular location in the ER as opposed to the plasma membrane.
In conclusion, our results argue that D2 is the major source of human extrathyroid-produced T3 in the euthyroid state. It contributes a much lower fraction of the T3 in the thyrotoxic patient, accounting for the fact that PTU inhibits T4 to T3 conversion to a greater extent in thyrotoxic than in euthyroid individuals. A major source of T3 in the hyperthyroid patient, of course, is direct secretion by the thyroid gland, which may account for as much of the T3 production as does D1-catalyzed deiodination (19). The important role of D2 in the euthyroid state and its half-life of less than 45 minutes would argue that the rapid decrease in T3 in the sick patient is more likely to result from a decrease in D2-catalyzed T4 to T3 conversion than from the modest decrease in hepatic D1 activity. This is supported by the complete absence of D2 activity in skeletal muscle in patients dying in an intensive care unit (27). However, in chronic severe illness at least, this decrease is also supplemented by an increase in the inner-ring deiodination of T4 and T3 by type 3 iodothyronine deiodinase in the liver and skeletal muscle (27).
Methods
Reagents.
Reagents were from Calbiochem-Novabiochem or Sigma-Aldrich. Outer ring–labeled [125I]T3 or T4 (specific activity, 4,400 Ci/mmol) was from PerkinElmer. Purification of [125I]T3 and [125I]T4 was performed on LH-20 columns just before use to reduce 125I– to less than 1%.
Transfection and expression studies.
MSTO, LLCPK1, and HEK 293 lines were obtained from ATCC. Cells were grown and maintained in DMEM supplemented with 10% FBS. HSMMs were obtained from Cambrex Corp. and cultured according to the manufacturer’s instructions using Cambrex-supplied SkGM2 medium. NH2-terminal FLAG-tagged wild-type human D1 and D2 plasmids were used (17). The Flag tag does not change kinetic properties.
For studies of catalytic efficiency, D1 or D2 expressing plasmids (3–100 ng) were transiently expressed in HEK 293 cells using CaPO4 precipitation (28). A TK-hGH plasmid was used to control for transfection efficiency (28). At 48 hours after transfection, cells in 6-well plates were washed twice with sterile PBS and then cultured for 18–24 hours in 1 ml serum-free 0.1% BSA (resulting in an FT4 concentration of 2.7%; ref. 29) in DMEM plus variable T4 concentrations ([total T4], 74–7,400 nM; [FT4], 2–200 pM) including approximately 100,000 cpm/ml [125I]T4. Experiments were performed in duplicate or triplicate for each condition.
Assay of 5 ′-deiodinase activity in intact transfected cells and in cell sonicates.
Deiodinase activity in intact cells was assayed as described previously with the following modifications (14, 15). At completion of the experiment, 300 μl of medium was removed and added to 200 μl horse serum and protein precipitated by the addition of 100 μl 50% trichloroacetic acid (TCA) followed by centrifugation at 12,000 g for 2 minutes. The 125I– generated was expressed as the fraction of the total T4 counts minus the nonspecific deiodination in untransfected control cells (<5% of the total [125I]T4 counts) and corrected for the 50% reduction in the specific activity relative to T4.
For measurements of activity in cell sonicates, the remaining medium was removed and the cells washed twice with PBS, harvested, and sonicated in 0.25 M sucrose in PE buffer (0.1 M potassium phosphate and 1 mM EDTA) with 10 mM DTT. For deiodinase assays, we used 100–150 μg cell sonicate; 10 μM [125I]T4 (for D1) or 10 nM T4 (for D2); and 20 mM DTT in a final volume of 300 μl PE. These concentrations are approximately 5 times the estimated Km(T4) for each enzyme (2 μM for D1 and 2 nM for D2). Incubation was for 60–120 minutes at 37°C, and 125I– was separated from labeled T4 by TCA precipitation. Results are the mean of values derived from at least 2 separate experiments.
To assess whether T4 to T3 conversion in human skeletal muscle could increase the ratio of extracellular T3 to T4, we incubated HSMMs in 60-mm dishes for 22 hours in 2 ml 0.1% BSA DMEM with approximately 1 μCi/ml [125I]T4 without or with 0.5 mM BT2cAMP. Rabbit polyclonal T3-Ab (1:1,000) was added to the medium to trap released [125I]T3. A sample of the same medium was incubated with no cells as a control. At the end of the incubation, the 2-ml samples of medium were incubated with 50 μl of protein A/G agarose suspension at 4°C overnight and centrifuged at 15,000 g for 5 minutes and the pellet rinsed twice with cold PBS. Ice-cold methanol (150 μl) was added and the tubes vortexed vigorously for 2 minutes and allowed to stand for 2 hours at room temperature. The T3 recovery with this procedure was 40%. T4 displayed a weak cross-reactivity with T3-Ab, resulting in the binding of 6% of the [125I]T4. The methanol extract containing more than 90% of the labeled iodothyronines was subjected to HPLC as described below so that the ratio of [125I]T3 to [125I]T4 in the medium could be determined.
To analyze the effect of D1- versus D2-catalyzed T3 production on T3-dependent gene transcription, HEK 293 cells in 6-well plates were transfected by CaPO4 precipitation with D1- or D2-expressing plasmid, 3 μg pTK-LUC containing 3 copies of the DR-4 5′ TRE from the human DIO1 gene (TRE3TKLUC) (5), 0.2 μg of a plasmid expressing the mouse thyroid hormone receptor α (T3Rα) (30), and 1 μg of TK-hGH plasmid for correction of transfection efficiencies (28). Results are expressed as the mean induction of T4-treated D1- or D2-transfected wells paired with cells transfected with an inactive D2, a COOH-terminal FLAG-tagged human D2 in which Ala replaced Glu163 (31). To prevent reentry of the T3 produced from T4 deiodination after it exited the cell, we added T3-Ab to the medium (1:1,000) (32). Luciferase activity was measured by a commercial kit (Luciferase Assay System; Promega). Results are expressed as the mean of 3 experiments.
Reverse-phase HPLC.
Cells were homogenized in 1 ml medium after 24 hours incubation with appropriate amounts of [125I]T4 or T3 and vortexed vigorously. Aliquots of 150 μl were mixed with ice-cold methanol and centrifuged. The resultant supernatant was mixed with an equal volume of 0.02 M ammonium acetate (pH 4) and the distribution of 125I-labeled products determined by HPLC as described previously (33). The radioactivity of each iodothyronine peak was measured using a Radiomatic 500TR Flow Scintillation Analyzer (PerkinElmer).
Real-time PCR.
Total RNA was extracted using TRIzol reagent (Invitrogen Corp.) and used to synthesize cDNA using SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen Corp.). The generated cDNAs were used in a real-time PCR using the QuantiTect SYBR Green PCR Kit (Bio-Rad Laboratories) in iCycler (Bio-Rad Laboratories). Standard curves representing 5-point serial dilution of mixed cDNA of the experimental and control groups were analyzed and used as calibrators of the relative quantification of product generated in the exponential phase of the amplification curve. The r2 was greater than 0.99, and the amplification efficiency varied between 80% and 100%. Quantification was normalized to cyclophilin A mRNA expression. Oligonucleotides for human D2 (5′-ACTTCCTGCTGGTCTACATTGATG-3′ and 5′-CTTCCTGGTTCTGGTGCTTCTTC-3′) and cyclophilin A (5′-GTCAACCCCACCGTGTTCTTC-3′ and 5′-ACTTGCCACCAGTGCCATTATG-3) were designed using Beacon Designer 2.06 (PREMIER Biosoft International).
Skeletal muscle biopsies.
Biopsies of sternocleidomastoid, rectus abdominis, and vastus lateralis muscles were obtained during routine surgical procedures at Hospital de Clínicas de Porto Alegre. Exclusion criteria included a diagnosis of diabetes mellitus or impaired glucose intolerance, metastatic cancer, and hyper- or hypothyroidism. Samples were immediately snap-frozen in liquid nitrogen and stored at –70°C. The Ethics Committee of the Hospital de Clínicas de Porto Alegre approved the protocol, and patients provided informed consent.
Statistical analysis.
Data are mean ± SEM unless otherwise indicated. Statistical analysis was performed using 2-tailed Student’s t or ANOVA tests, and P < 0.05 was considered significant.
Acknowledgments
We thank Michelle A. Mulcahey for the assistance with the HPLC analysis and J. Miguel Dora for D2 assays in human muscles. We also thank Antonio C. Bianco for valuable discussion and thoughtful review of this manuscript. Grant support was provided by Fundação Coordenação de Aperfeicoamento de Pessoal de Nivel Superior and Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil and NIH grants DK36256, DK064643, DK060494, and DK07529.
Footnotes
Nonstandard abbreviations used: D1, type 1 iodothyronine deiodinase; DIO2, type 2 deiodinase; FT4, free T4; HSMM, human skeletal muscle myoblast; LUC, luciferase; MSTO, mesothelioma; PTU, 6-n-propylthiouracil; rT3, reverse T3; T2, 3,3′diiodothyronine; T3, 3,5,3′-triiodothyronine; T3-Ab, anti-T3 Ab; T4, thyroxine; TR, thyroid hormone receptor.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology and physiological roles of the iodothyronine selenodeiodinases [review] Endocr. Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 2.Gereben B, Goncalves C, Harney JW, Larsen PR, Bianco AC. Selective proteolysis of human type 2 deiodinase: a novel ubiquitin-proteasomal mediated mechanism for regulation of hormone activation. Mol. Endocrinol. 2000;14:1697–1708. doi: 10.1210/mend.14.11.0558. [DOI] [PubMed] [Google Scholar]
- 3.Kim SW, Harney JW, Larsen PR. Studies of the hormonal regulation of type 2 5′-iodothyronine deiodinase messenger ribonucleic acid in pituitary tumor cells using semiquantitative reverse transcription-polymerase chain reaction. Endocrinology. 1998;139:4895–4905. doi: 10.1210/endo.139.12.6334. [DOI] [PubMed] [Google Scholar]
- 4.Baqui MM, et al. Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J. Biol. Chem. 2003;278:1206–1211. doi: 10.1074/jbc.M210266200. [DOI] [PubMed] [Google Scholar]
- 5.Toyoda N, Zavacki AM, Maia AL, Harney JW, Larsen PR. A novel retinoid X receptor-independent thyroid hormone response element is present in the human type 1 deiodinase gene. Mol. Cell. Biol. 1995;15:5100–5112. doi: 10.1128/mcb.15.9.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visser TJ, Kaptein E, Terpstra OT, Krenning EP. Deiodination of thyroid hormone by human liver. J. Clin. Endocrinol. Metab. 1988;67:17–24. doi: 10.1210/jcem-67-1-17. [DOI] [PubMed] [Google Scholar]
- 7.Canani LH, et al. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2005;90:3472–3478. doi: 10.1210/jc.2004-1977. [DOI] [PubMed] [Google Scholar]
- 8.Silva JE, Larsen PR. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J. Clin. Invest. 1978;61:1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva JE, Larsen PR. Pituitary nuclear 3,5,3′-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science. 1977;198:617–620. doi: 10.1126/science.199941. [DOI] [PubMed] [Google Scholar]
- 10.Baqui MM, Gereben B, Harney JW, Larsen PR, Bianco AC. Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology. 2000;141:4309–4312. doi: 10.1210/endo.141.11.7872. [DOI] [PubMed] [Google Scholar]
- 11.Moreno M, et al. Activation and inactivation of thyroid hormone by type I iodothyronine deiodinase. FEBS Lett. 1994;344:143–146. doi: 10.1016/0014-5793(94)00365-3. [DOI] [PubMed] [Google Scholar]
- 12.Curcio C, et al. The human type 2 iodothyronine deiodinase is a selenoprotein highly expressed in a mesothelioma cell line. J. Biol. Chem. 2001;276:30183–30187. doi: 10.1074/jbc.C100325200. [DOI] [PubMed] [Google Scholar]
- 13.Hosoi Y, et al. Expression and regulation of type II iodothyronine deiodinase in cultured human skeletal muscle cells. J. Clin. Endocrinol. Metab. 1999;84:3293–3300. doi: 10.1210/jcem.84.9.5969. [DOI] [PubMed] [Google Scholar]
- 14.Buettner C, Harney JW, Larsen PR. The role of selenocysteine 133 in catalysis by the human type 2 iodothyronine deiodinase. Endocrinology. 2000;141:4606–4612. doi: 10.1210/endo.141.12.7831. [DOI] [PubMed] [Google Scholar]
- 15.Croteau W, Bodwell JE, Richardson JM, St. Germain DL. Conserved cysteines in the type 1 deiodinase selenoprotein are not essential for catalytic activity. J. Biol. Chem. 1998;273:25230–25236. doi: 10.1074/jbc.273.39.25230. [DOI] [PubMed] [Google Scholar]
- 16.Sharifi J, St. Germain DL. The cDNA for the type I iodothyronine 5′-deiodinase encodes an enzyme manifesting both high Km and low Km activity. Evidence that rat liver and kidney contain a single enzyme which converts thyroxine to 3,5,3′-triiodothyronine. J. Biol. Chem. 1992;267:12539–12544. [PubMed] [Google Scholar]
- 17.Curcio-Morelli C, et al. In vivo dimerization of types 1, 2, and 3 iodothyronine selenodeiodinases. Endocrinology. 2003;144:3438–3443. doi: 10.1210/en.2002-220960. [DOI] [PubMed] [Google Scholar]
- 18.Berry MJ, Kieffer JD, Larsen PR. Evidence that cysteine, not selenocysteine, is in the catalytic site of type II iodothyronine deiodinase. Endocrinology. 1991;129:550–552. doi: 10.1210/endo-129-1-550. [DOI] [PubMed] [Google Scholar]
- 19.Abuid J, Larsen PR. Triiodothyronine and thyroxine in hyperthyroidism. Comparison of the acute changes during therapy with antithyroid agents. J. Clin. Invest. 1974;54:201–208. doi: 10.1172/JCI107744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geffner DL, Azukizawa M, Hershman JM. Propylthiouracil blocks extrathyroidal conversion of thyroxine to triiodothyronine and augments thyrotropin secretion in man. J. Clin. Invest. 1975;55:224–229. doi: 10.1172/JCI107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saberi M, Sterling FH, Utiger RD. Reduction in extrathyroidal triiodothyronine production by propylthiouracil in man. J. Clin. Invest. 1975;55:218–223. doi: 10.1172/JCI107924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inada M, et al. Estimation of thyroxine and triiodothyronine distribution and of the conversion rate of thyroxine to triiodothyronine in man. J. Clin. Invest. 1975;55:1337–1348. doi: 10.1172/JCI108053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lum SM, Nicoloff JT, Spencer CA, Kaptein EM. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J. Clin. Invest. 1984;73:570–575. doi: 10.1172/JCI111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visser TJ, van der Does-Tobe I, Docter R, Hennemann G. Subcellular localization of a rat liver enzyme converting thyroxine into triiodothyronine and possible involvement of essential thiol groups. Biochem. J. 1976;157:479–482. doi: 10.1042/bj1570479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen, P.R., Davies, T.F., Schlumberger, M.-J., and Hay, I.D. 2003. Thyroid physiology and diagnostic evaluation of patients with thyroid disorders. In Williams textbook of endocrinology. P.R. Larsen, H.M. Kronenberg, S. Melmed, and K.S. Polonsky, editors. W.B. Saunders. Philadelphia, Pennsylvania, USA. 331–373.
- 26.Oppenheimer JH, Schwartz HL. Stereospecific transport to triiodothyronine from plasma to cytosol and from cytosol to nucleus in rat liver, kidney, brain and heart. J. Clin. Invest. 1985;75:147–154. doi: 10.1172/JCI111667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peeters RP, et al. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J. Clin. Endocrinol. Metab. 2003;88:3202–3211. doi: 10.1210/jc.2002-022013. [DOI] [PubMed] [Google Scholar]
- 28.Brent GA, Larsen PR, Harney JW, Koenig RJ, Moore DD. Functional characterization of the rat growth hormone promoter elements required for induction by thyroid hormone with and without a co-transfected beta type thyroid hormone receptor. J. Biol. Chem. 1989;264:178–182. [PubMed] [Google Scholar]
- 29.Everts ME, et al. Uptake of triiodothyroacetic acid and its effect on thyrotropin secretion in cultured anterior pituitary cells. Endocrinology. 1994;135:2700–2707. doi: 10.1210/endo.135.6.7988460. [DOI] [PubMed] [Google Scholar]
- 30.Prost E, Koenig RJ, Moore DD, Larsen PR, Whalen RG. Multiple sequences encoding potential thyroid hormone receptors isolated from mouse skeletal muscle cDNA libraries. Nucleic Acids Res. 1988;16:6248. doi: 10.1093/nar/16.13.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callebaut I, et al. The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase-clan GH-A-like structure. J. Biol. Chem. 2003;278:36887–36896. doi: 10.1074/jbc.M305725200. [DOI] [PubMed] [Google Scholar]
- 32.Larsen PR. Direct immunoassay of triiodothyronine in human serum. J. Clin. Invest. 1972;51:1939–1949. doi: 10.1172/JCI107000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richard K, et al. Ontogeny of iodothyronine deiodinases in human liver. J. Clin. Endocrinol. Metab. 1998;83:2868–2874. doi: 10.1210/jcem.83.8.5032. [DOI] [PubMed] [Google Scholar]