Summary
To investigate the role of hepatocyte genes in fasting metabolism, it is essential to analyze their expression across the entire 24-h circadian cycle. Here, we present a protocol to evaluate fasting metabolism and its relationship to the core circadian clock in mice. We describe steps for time-course fasting/feeding experiment setup and performing staggered fasting and refeeding for 24 h. We then detail procedures for evaluating oxidative substrate selection and rescuing defective oxidative metabolism through FGF21 and CPI-613 injections.
For complete details on the use and execution of this protocol, please refer to Sun et al.1
Subject areas: genetics, metabolism, model organisms
Graphical abstract

Highlights
-
•
Instruction for an in vivo staggered fast/feed time-course experiment
-
•
Steps for designing and performing downstream fasting rescue experiments
-
•
Guidance on applying indirect calorimetry to pharmacological complementation assays
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
To investigate the role of hepatocyte genes in fasting metabolism, it is essential to analyze their expression across the entire 24-h circadian cycle. Here, we present a protocol to evaluate fasting metabolism and its relationship to the core circadian clock in mice. We describe steps for time-course fasting/feeding experiment setup and performing staggered fasting and refeeding for 24 h. We then detail procedures for evaluating oxidative substrate selection and rescuing defective oxidative metabolism through FGF21 and CPI-613 injections.
Before you begin
Organisms adapt to utilize distinct energy sources under highly dynamic dietary, food availability, and temporal conditions to facilitate daily activity and survival.2 In particular, fasting/feeding behavior is to be optimally aligned with the daily rest/active phases in a cyclic pattern3 and, to that end, several genes controlling hepatocyte metabolism exhibit rhythmic expression patterns.4 When investigating rhythmically expressed metabolic genes, it is crucial to evaluate the downstream pathways and corresponding phenotypes throughout the circadian clock to gain a comprehensive understanding of the interaction between time and nutrient input in regulating any metabolic pathway.
Here, we use the fibroblast growth factor 21 to pyruvate dehydrogenase kinase 4 (Fgf21-Pdk4) regulatory axis as an example of how we examine the metabolic role of hepatocyte Period 1 (Per1) in mediating fasting metabolism. The goals achieved in this protocol are to: i) understand circadian gene expression under fasting and ad libitum (ad lib) feeding throughout the 24 h circadian clock and Per1 induction in relationship to fasting; ii) evaluate the oxidative substrate selection during dark-phase fasting; iii) reverse defective fasting oxidative metabolism through in vivo pharmacological complementation. In summary, this protocol provides a step-by-step instruction on how to systematically analyze fasting-mediated gene expression and pathways wherein circadian interactions are postulated.
Institutional permissions
All in vivo experimental procedures were performed in strict accordance with Institutional Animal Care and Use Committee (IACUC) guidelines at Washington University School of Medicine.
Reconstitution of FGF21 and CPI-613
-
1.Prepare stock solution for FGF21 and CPI-613.
-
a.FGF21: for a stock of 100 μg/vial at concentration of 0.706 mg/mL, add 60 μL saline to make a final concentration of 0.5 mg/mL. Store working stocks at −80°C and avoid multiple freeze/thaw cycles.
-
b.CPI-613: for a 25 mg vial, add 1.6 mL of DMSO to make a 15 mg/mL working stock. Store working stocks at −20°C.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| FGF21 recombinant protein | Bio-Techne | Cat#8409-FG |
| 6,8-Bis(benzylthio)-octanoic acid (CPI-613) | Sigma-Aldrich | Cat#SML0404 |
| 0.9% sodium chloride injection, USP | Hospira | Uos NDC#00409-4888 |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | Cat#D8418 |
| Experimental models: Organisms/strains | ||
| Mouse: Per1fl/fl; 8 weeks; male | Sun et al. | Sun et al. |
| Mouse: Bmal1fl/fl; 8 weeks; male | Jackson Laboratory | Strain#007668 RRID:IMSR_JAX:007668 |
| Mouse: C57BL/6J; 8 weeks; male | Jackson Laboratory | Strain#000664 RRID:IMSR_JAX:000664 |
| Mouse: Alb-Cre; 8 weeks; male | Jackson Laboratory | Strain#003574 RRID:IMSR_JAX:003574 |
| Software and algorithms | ||
| GraphPad Prism 9 software | GraphPad | N/A |
| Graphic abstract and schematics in Figure 1 were created using biorender.com | https://www.biorender.com/ | N/A |
| Other | ||
| Insulin syringes with needle 3/10 cc | McKesson | MFR#102-SN310C31516P |
| Indirect calorimetry | TSE Systems | PhenoMaster |
Materials and equipment
To setup PhenoMaster indirect calorimetry, please follow the instruction from the manufacture. Make sure to set the starting light/dark phase correctly. Perform gas calibration before each experiment.
Step-by-step method details
Setup for time-course fasting/feeding experiment
Timing: 1 day
This section shows how we design our experimental information sheet to record important data for the staggered time-course experiment in the following section. This section also includes guidance on preparing mice and cages for the experiment in the following section.
-
1.
Prepare the mouse information sheet before you start the experiment. For the information sheet, prepare columns as follows: Mouse Number, Cage Number, Genotype, Treatment, Treatment Start Time, Treatment End Time, Body Weight @Start, Food Weight @Start, Body Weight @End, Food Weight @End (Table 1).
-
2.
Randomize mice for fasting and feeding groups with similar average body weight. Try to avoid single housing.
CRITICAL: Single housing for mice induces a variable of stress that can affect endpoint measures, so we avoid using singly housed mice wherever possible. We calculate the average food consumption per mouse through dividing the total consumed food weight by the number of mice housed in the cage. For mice used in this experiment, it is important to minimize the age range at enrollment. We recommend minimizing the enrolled age range in the cohort to no more than 1–2 weeks. Setting up more breeding cages at the beginning will help with reducing the age range.
Optional: Data in Figure 1D demonstrate consistent, rhythmic circadian gene expression patterns in wild-type mice in response to ad lib feeding under grouped housing conditions during the staggered feeding/fasting experiment. However, if single housing is preferred and feasible, stress due to single housing can be mitigated prior to the fasting time-course experiment by acclimating mice to single-housing 2–3 weeks prior to the experiment start and throughout the time-course.
-
3.
Put mice in the same treatment group into same cage for the purpose of measuring food consumption.
-
4.
For fasting mice, prepare a corresponding number of cages with Aspen bedding.
Table 1.
Information sheet for time-course 16-h-fasting/feeding experiment
| Mouse | Cage number | Genotype | Treatment | Start time | Body weight | Food weight | SAC time | Body weight | Food weight |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | WT | Feed | ZT8 | ZT0 | ||||
| 2 | WT | Feed | ZT8 | ZT0 | |||||
| 3 | WT | Feed | ZT8 | ZT0 | |||||
| 4 | 2 | WT | Fast | ZT8 |  |
ZT0 |  |
||
| 5 | WT | Fast | ZT8 | ZT0 | |||||
| 6 | WT | Fast | ZT8 | ZT0 | |||||
| 7 | 3 | WT | Feed | ZT12 | ZT4 | ||||
| 8 | WT | Feed | ZT12 | ZT4 | |||||
| 9 | WT | Feed | ZT12 | ZT4 | |||||
| 10 | 4 | WT | Fast | ZT12 |  |
ZT4 |  |
||
| 11 | WT | Fast | ZT12 | ZT4 | |||||
| 12 | WT | Fast | ZT12 | ZT4 | |||||
| 13 | 5 | WT | Feed | ZT16 | ZT8 | ||||
| 14 | WT | Feed | ZT16 | ZT8 | |||||
| 15 | WT | Feed | ZT16 | ZT8 | |||||
| 16 | 6 | WT | Fast | ZT16 |  |
ZT8 |  |
||
| 17 | WT | Fast | ZT16 | ZT8 | |||||
| 18 | WT | Fast | ZT16 | ZT8 | |||||
| 19 | 7 | WT | Feed | ZT20 | ZT12 | ||||
| 20 | WT | Feed | ZT20 | ZT12 | |||||
| 21 | WT | Feed | ZT20 | ZT12 | |||||
| 22 | 8 | WT | Fast | ZT20 |  |
ZT12 |  |
||
| 23 | WT | Fast | ZT20 | ZT12 | |||||
| 24 | WT | Fast | ZT20 | ZT12 | |||||
| 25 | 9 | WT | Feed | ZT0 | ZT16 | ||||
| 26 | WT | Feed | ZT0 | ZT16 | |||||
| 27 | WT | Feed | ZT0 | ZT16 | |||||
| 28 | 10 | WT | Fast | ZT0 |  |
ZT16 |  |
||
| 29 | WT | Fast | ZT0 | ZT16 | |||||
| 30 | WT | Fast | ZT0 | ZT16 | |||||
| 31 | 11 | WT | Feed | ZT4 | ZT20 | ||||
| 32 | WT | Feed | ZT4 | ZT20 | |||||
| 33 | WT | Feed | ZT4 | ZT20 | |||||
| 34 | 12 | WT | Fast | ZT4 |  |
ZT20 |  |
||
| 35 | WT | Fast | ZT4 | ZT20 | |||||
| 36 | WT | Fast | ZT4 | ZT20 |
Figure 1.
Staggered 16-h-fast/ad lib-feed experiment measures the relationship between fasting and circadian clock
(A) Schematic of the time-course experiment.
(B) Correlation test result between normalized Per1 expression and food deficit. Dotted line denotes 95% confidence interval for the simple linear regression calculation.
(C) Liver Per1 expression in ad lib-fed/16-h-fasted mice throughout the time-course experiment. Expression is normalized to the ad lib-fed mice harvested at the same time point, n = 3–4. Data expressed as mean ± SEM; ∗ p<0.05, ∗∗ p<0.01, ∗∗∗ p<0.001 by student’s t-test.
(D) Expression of circadian genes in ad lib-fed/16-h-fasted wild-type livers, n = 3–4. Data expressed as mean ± SEM. Figure reprinted and adapted with permission from Sun et al., 2024.
Perform staggered 16-h fasting and ad lib feeding for 24 h
Timing: 2 days
This staggered time-course experiment will study how target genes behave during the 24 h-circadian clock under fasting/feeding conditions (Figure 1A).
-
5.Record initial values at the starting time point:
-
a.Record body weight.
-
b.Record food weight for ad lib fed group.
-
a.
-
6.
Transfer mice in the fasting group to Aspen cages and withdraw food at the starting time point as listed in the information sheet.
Note: In our experiment we withdraw food every 4 h for a total of 6 time points. This can be adjusted according to different experiments.
CRITICAL: To minimize the disruption of the circadian clock, all experiments should be performed in a 12 h: 12 h light/dark room. For fasting cages, it is important to utilize Aspen bedding (or equivalent bedding like pulp cellulose), since regular corncob bedding are less effective in fasting.5
-
7.Sacrifice mice at the end-point.
-
a.Record body weight.
-
b.Record food weight for ad lib fed group.
-
c.Harvest tissues of interest.
-
a.
Note: In our experiment we perform 16-h fasting. This can be adjusted based on specific experimental protocol. If longer fasting is needed, we recommend preparing 30% glucose solution dissolved in saline prior to fasting. When mice exhibit any signs of hypoglycemia including cold body temperature or seizure, inject 100 μL of 30% glucose to rescue the mice or higher volume until mice restore normal activity.
-
8.
Perform downstream analysis.
Note: To understand the relationship between relative food deficit and gene expression, a correlation plot between the food consumption in the ad lib feeding group at corresponding time and the induction of the target gene in fasting group normalized to the feeding group at the same time point can be evaluated by simple linear regression calculation (Figure 1B).
Optional: Wild-type mice used in this protocol serves as a negative control group. We also demonstrated previously that Bmal1LKO mice, which lack a functional liver clock, respond to fasting by upregulating hepatic Per1.1 As an additional control, the experimentalist should consider assaying Bmal1-deficient mice within their protocol to assess the extent to which fasting regulation in the gene of interest is clock-dependent, fasting-regulated, or both.
Pause point: We recommend evaluating data generated in the time-course experiment first to better understand the expression of the target gene throughout the 24 h clock. We normalized expression of target genes to the ad lib-fed wild-type mice that are sacrificed at the same time point (Figures 1B and 1C) or at ZT0 (Figure 1D). In our experiment, we identify that liver Per1 is significantly upregulated at all fasting time points, but with the greatest amplitude at ZT12-ZT4 fasting (Figure 1C). We therefore perform all subsequent fasting experiments at ZT12-ZT4 to understand the function of Per1.
Evaluate oxidative substrate selection during fasting
Timing: 5–7 days
To understand how mice oxidize different substrates during fasting, and how their activity or thermogenesis change, we utilized indirect calorimetry to record individual physiological data. This section demonstrates how we setup the indirect calorimetry recording and collect the data.
-
9.
Equilibrate mice in indirect calorimetry for at least 48 h before recording the data.
-
10.For ad lib feeding experiment, record data for at least a full 24 h cycle.
-
a.VCO2 and VO2.
 CRITICAL: VCO2 and VO2 are the most important data to record. Respiratory exchange ratio can be calculated based on these two values: RER = VCO2/VO2. RER value is an indicator of glucose and fatty oxidation. For RER close to 1.0, it suggests the organism utilize glucose as the major energy source. For RER close to 0.7, it suggests the organism utilize fatty acid as the major energy source. For intact circadian clock, you should see the RER is high during dark phase and low during light phase (Figures 2A and 2B).
CRITICAL: VCO2 and VO2 are the most important data to record. Respiratory exchange ratio can be calculated based on these two values: RER = VCO2/VO2. RER value is an indicator of glucose and fatty oxidation. For RER close to 1.0, it suggests the organism utilize glucose as the major energy source. For RER close to 0.7, it suggests the organism utilize fatty acid as the major energy source. For intact circadian clock, you should see the RER is high during dark phase and low during light phase (Figures 2A and 2B). -
b.Food consumption.
-
c.Activity.
-
d.Heat.
-
a.
-
11.
For fasting experiments, switch the bedding to Aspen and withdraw food from the chamber at ZT12. Record the data as in ad lib feeding experiment.
CRITICAL: To perform most effective fasting, changing bedding before fasting is necessary, as occult, pulverized chow could be mixed with the old bedding.
Optional: Glucose and fatty acid oxidation rate can be calculated by VCO2 and VO2 (Figures 2C and 2D).6
Note: If more data are needed to record after the fasting experiment, all the mice should recover (ad lib fed) for at least one week.
Pause point: After collecting the RER data, plot the data in relationship to time and record the time point where the RER diverges between the control mice and the mice lacking a gene of interest. This time point or time window will be helpful in performing downstream rescue experiments. For example, we noticed that in our mice lacking hepatocyte Per1 (Per1LKO), RER started diverging from the control mice between ZT18-ZT20, therefore we chose to inject FGF21 and CPI-613 within this window to rescue the defective oxidative metabolism (Figures 2A and 2B).
Figure 2.
Respiratory exchange ratio, fat and glucose oxidation rate measurement during ad lib feeding and fasting
(A) Respiratory exchange ratio in ad lib-fed (left) and 16-h-fasted (right) mice with or without hepatocyte Per1, n = 6.
(B) Quantification of ad lib fed RER (left) and fasting RER (right) from A. Error bar = 10-90 percentile; ∗∗∗ p<0.001, ∗∗∗∗ p<0.0001 by 2-way ANOVA.
(C) Fat (left) and glucose (right) oxidation rate during ad lib feeding calculated from A.
(D) Fat (left) and glucose (right) oxidation rate during 16-h fasting calculated from A. Data expressed as mean ± SEM. Figure reprinted and adapted with permission from Sun et al., 2024.
Rescue the defective oxidative metabolism through FGF21 and CPI-613 injections
Timing: 10–14 days
We observed defective oxidative metabolism and impaired Fgf21-Pdk4 expression in mice lacking hepatocyte Per1.1 To further validate if this axis is accountable for the phenotype we observed, we performed in vivo rescue experiments using FGF21 supplementation and CPI-613 to inhibit pyruvate dehydrogenase – a direct inhibitory target of Pdk4. This section serves as an example of how to perform pharmacologic complementation experiments in indirect calorimetry. Please adjust accordingly for different studies.
-
12.
Measure body weight of mice and equilibrate mice in indirect calorimetry (2 Days). Start recording the data.
-
13.Prepare solutions for injection:
-
a.CPI-613: 15 mg/mL in DMSO.
-
b.FGF21: 0.5 mg/mL in saline.
-
a.
-
14.Determine the injection volume for each mouse:
-
a.CPI-613: 25 mg/kg body weight.
-
b.FGF21: 1 mg/kg body weight.
-
c.For vehicle controls, use DMSO for CPI-613 and saline for FGF21.
-
a.
-
15.
Start fasting at ZT12. Preload the solution for injection and keep FGF21 solution on ice.
-
16.
Inject mice intraperitoneally (IP) at desired time point and record the data (Figure 3A).
CRITICAL: When injecting protein or drug, make sure to perform the injection before the cage air is being sampled. For the PhenoMaster protocol used for indirect calorimetry, data from each cage are recorded sequentially and cyclically. The sensor records one cage at any given time. Typical sampling period lasts ∼3 min (set by the operator). Recording for all 16 cages can take ∼30 min (2 sensors, depending on how many cages are recorded). Therefore, to obtain accurate readings from all cages, inject mice consistently in sequence, and within the same time duration as the recording time for each cage. For example, inject the mouse in cage 2 while the program is sampling and recording gas content for cage 1. Do not open the cage and inject the mouse in cage 3 until the program cycles to record cage 2, etc.
CRITICAL: Injection should be performed only under red light to avoid perturbing the dark phase. Make sure to seal the cage well after injection. Gas extravasation from the semi-closed circuit can lead to inaccurate readings.
Optional: For protein/drugs with short half-life, multiple injections will help extend the function of the protein/drug. If longer assessment of drug injections is needed (e.g. to assess potential longer-term physiologic and behavioral effects of drug injection), mice can be maintained in indirect calorimetry for several days following injection prior to sacrifice.
-
17.
Perform downstream analysis.
Note: The goal for analyzing the RER values during fasting with or without drug injections is to evaluate if supplementing FGF21 or inhibiting pyruvate dehydrogenase activity through CPI-613 are sufficient to terminate the high glucose oxidation rate in Per1LKO mice. For different pathways or targets, FGF21 and CPI-613 can be replaced by other drugs. In our rescue experiment, we showed that FGF21 supplementation or pharmacological glucose oxidation inhibition by CPI-613 are both sufficient to suppress the hyperactive glucose oxidation in Per1LKO mice during fasting (Figures 3B and 3C). We used Prism software to graph the glucose oxidation and RER graphs. We used 2-way ANOVA with Sidak’s post hoc test to delineate differences in RER, and to understand the statistical interaction between light/dark phase and genotype (Figure 2B), or the differences among different drug treatments during fasting (Figures 3B and 3C).
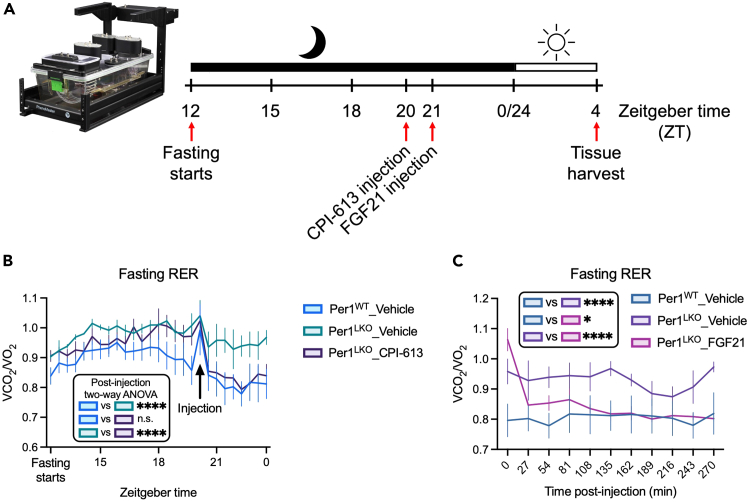
Figure 3.
In vivo CPI-613 and FGF21 treatments rescue defective fasting oxidative metabolism
(A) Schematic of in vivo FGF21 and CPI-613 injection in indirect calorimetry.
(B) RER measured during 16-h fasting in Per1fl/fl or Per1LKO mice injected with either vehicle or CPI-613 at ZT20, n = 3. Data expressed as mean ± SEM; ∗∗∗∗ p<0.0001 by 2-way ANOVA.
(C) RER measured in fasted Per1WT and Per1LKO mice injected with either vehicle or FGF21 at ZT21, n = 3. Data expressed as mean ± SEM; ∗ p<0.05, ∗∗∗∗ p<0.0001 by 2-way ANOVA. Figure reprinted and adapted with permission from Sun et al., 2024.
Expected outcomes
For ad lib fed control mice in the time-course experiment, core circadian genes should show rhythmic expression (Figure 1D). RER level in ad lib fed mice throughout the light/dark phases should show a rhythmic change (Figure 2A). In addition, during fasting, blood glucose level drops due to the lack of food intake, which leads to the transition from utilizing glucose to fatty acid as the primary energy source. This can be reflected by a decrease in RER during fasting (Figure 2A). Given Fgf21 and Pdk4 are both established as fasting-induced genes that facilitate fatty acid oxidation,7,8 treatment of FGF21 or pyruvate dehydrogenase inhibitor CPI-613 should inhibit glucose oxidation and reduce the RER during fasting (Figures 3B and 3C).
Limitations
This protocol introduces a systematic study of metabolism in relation to the core circadian clock. It is worth noting that in our staggered fasting/feeding time-course experiment, it is possible that the most proximal food intake prior to fasting could vary, which would then contribute to some variability in fasting duration. To mitigate this potential source of variability, the experimentalist can consider introducing a controlled-content oral gavage of all mice immediately before fasting. In addition, all experiments are performed under 12:12 light-dark conditions, rather than constant light or dark exposure. Whereas a 12:12 light-dark pattern mimics the native light/dark exposure of a colony-housed animal, one should consider that light exposure can mask or interact with circadian rhythm-mediated metabolic effects. In the downstream rescue experiments, the indirect calorimetry measurements are limited by the number of chambers in the machine. If the experiment has multiple treatment groups, distinct cohorts are required.
Troubleshooting
Problem 1
At step 8, a dampened or disrupted expression of circadian genes is found in target tissue.
Potential solution
Equilibrate mice in the procedure room before sacrificing to reduce the pressure from transporting mice. We recommend one-day equilibration prior to performing the experiment.
Problem 2
At step 10, RER is lower than 0.7 or greater than 1.0.
Potential solution
Repeat the gas calibration. If this does not resolve the problem, perform manual calibration under the guidance of the TSE technician (or call for site-based service).
Problem 3
At step 17, high variation exists within same treatment group.
Potential solution
Reduce the measurement time for each cage to minimize the delay in data recording among different cages. Practice intraperitoneal injection to optimize injection timing, injection site, and minimizing stress due to handling in each mouse.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Brian J. DeBosch (bdebosch@iu.edu).
Technical contact
Further information and requests for technical will be fulfilled by technical contact, Jiameng Sun (jiameng@wustl.edu).
Materials availability
Request for unique mouse line in this protocol should be directed to the lead contact.
Data and code availability
This protocol does not include datasets.
Acknowledgments
We thank the Genome Engineering and iPSC Center at Washington University in St. Louis for gRNA validation services. This work was supported by grants from the NIDDK (1R01DK126622 and 1R01DK131009, B.J.D.), NHLBI (1R01HL147968-01A1, B.J.D.), the Washington University Center for Autophagy Therapeutics Research (B.J.D.), and the Longer Life Foundation (B.J.D.).
Author contributions
J.S. performed experiments, interpreted data, and wrote the manuscript. B.J.D. conceptualized the study, acquired funding, directed the research, and finalized the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- 1.Sun J., Zhang Y., Adams J.A., Higgins C.B., Kelly S.C., Zhang H., Cho K.Y., Johnson U.G., Swarts B.M., Wada S.I., et al. Hepatocyte Period 1 dictates oxidative substrate selection independent of the core circadian clock. Cell Rep. 2024;43 doi: 10.1016/j.celrep.2024.114865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rui L. Energy metabolism in the liver. Compr. Physiol. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinke H., Asher G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019;20:227–241. doi: 10.1038/s41580-018-0096-9. [DOI] [PubMed] [Google Scholar]
- 4.Reinke H., Asher G. Circadian Clock Control of Liver Metabolic Functions. Gastroenterology. 2016;150:574–580. doi: 10.1053/j.gastro.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 5.Sveeggen T.M., Isakson B.E., Straub A.C., Bagher P. Bedding as a variable affecting fasting blood glucose and vascular physiology in mice. Am. J. Physiol. Heart Circ. Physiol. 2023;325:H338–H345. doi: 10.1152/ajpheart.00168.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berbee J.F., Boon M.R., Khedoe P.P., Bartelt A., Schlein C., Worthmann A., Kooijman S., Hoeke G., Mol I.M., John C., et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 2015;6:6356. doi: 10.1038/ncomms7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potthoff M.J., Inagaki T., Satapati S., Ding X., He T., Goetz R., Mohammadi M., Finck B.N., Mangelsdorf D.J., Kliewer S.A., Burgess S.C. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pettersen I.K.N., Tusubira D., Ashrafi H., Dyrstad S.E., Hansen L., Liu X.Z., Nilsson L.I.H., Løvsletten N.G., Berge K., Wergedahl H., et al. Upregulated PDK4 expression is a sensitive marker of increased fatty acid oxidation. Mitochondrion. 2019;49:97–110. doi: 10.1016/j.mito.2019.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This protocol does not include datasets.