Abstract
Most behavioral experiments within circadian research are based on the analysis of locomotor activity. This paper introduces scientists to chronobiology by explaining the basic terminology used within the field. Furthermore, it aims to assist in designing, carrying out, and evaluating wheel-running experiments with rodents, particularly mice. Since light is an easily applicable stimulus that provokes strong effects on clock phase, the paper focuses on the application of different lighting conditions.
Keywords: Photoperiod, Chronobiology, Circadian Rhythm, Mice
Introduction
Life of almost all organisms is governed by various biological rhythms that are defined as physiological and behavioral oscillations. These rhythms are distinguished by their period length (τ) with circadian (lat: circa diem, around a day) rhythms displaying a τ of approximately 24 hours that evolved in adaptation to the daily rotation of the earth around its axis. They are found in many organisms from unicellular fungi and bacteria to higher organisms such as insects and mammals, including men. The bases of these oscillations are internal molecular clocks that maintain their rhythm even in the absence of external timing signals.
The circadian clockwork has evolved to improve an organism’s adaptation to its environment and to ensure timed coordination of life-sustaining activities such as feeding, sleeping as well as the coordination of physiological and biochemical mechanisms.
With the availability of manipulative in vivo techniques and with the rise of forward and reverse genetic approaches in the field of chronobiology it became increasingly interesting to investigate circadian behavioral phenotypes of wild type and mutant animals under different conditions. Since light is the most potent timing signal of the circadian system – and lighting conditions are relatively easy to control – this paper aims to provide a general guidance for testing and evaluating circadian wheel-running behavior under different lighting conditions.
Definitions
Actogram
Circadian locomotor activity rhythms are frequently represented as a graph called actogram (Fig. 1A), where each horizontal line represents one day. Black vertical bars plotted side-by-side represent the activity, or number of wheel revolutions. The height of each vertical bar indicates the accumulated number of wheel revolutions for a given interval (e.g. 5 min). Aligning the same actogram twice so that two consecutive days are plotted one after the other and the second day being re-plotted in the right half of the successive line results in a so-called double plotted actogram (Fig. 1B). This way of representation often facilitates the identification of existing rhythms. Once the presence of a – not necessarily circadian – rhythm has been established, the chronobiologist divides the cycle into an activity (alpha) and a rest (rho) phase. Sometimes, scattered activity can be observed in the rho-phase because the animal interrupts its sleep for a short time. One advantage of recording wheel-running revolutions as compared to using light beam interruptions is the activity noise during the rest phase. Obviously, under normal light/dark conditions alpha- and rho-phases are at opposite times in regard to the 24 hours solar cycle in diurnal and nocturnal organisms (1).
Fig. 1. Schematic single and double plotted actograms and masking. (A).
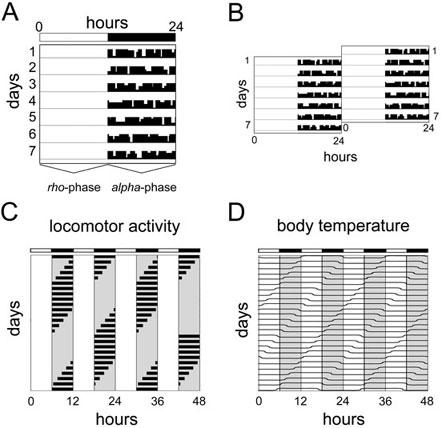
Wheel-running activity is plotted as an actogram with each horizontal line corresponding to one day. Black vertical bars plotted side-by-side represent the activity, or number of wheel revolutions. The height of each vertical bar indicates the accumulated number of wheel revolutions for a given interval (e.g. 5 min). The rho- and alpha-phase marked at the bottom of the actogram refer to rest and activity, respectively. The white and black bar at the top of the scheme depicts light (12 h) and darkness (12 h), respectively. (B) To better visualize behavioral rhythms, actograms are often double plotted by aligning two consecutive days horizontally (e.g. day 1 left and day 2 right). (C) Schematic actogram of a nocturnal animal kept in very short photoperiods (LD 6:6). Since the animal is only showing activity during the dark phases it seems to entrain to the prevailing LD cycle. (D) Parallel monitoring of body temperature reveals that this apparent entrainment is only masking. Although the readout parameter "activity" seemingly adapts to the new schedule, body temperature continues to cycle with its free-running period length implicating that the circadian clock of the animal is not entrained. LD, light-dark cycle.
Entrainment and masking
Many environmental variables including light, temperature, humidity, food availability, and even social cues oscillate with a 24-hour period. Even though the endogenous circadian clock functions in the absence of external time cues, it periodically measures some of these environmental parameters to synchronize internal and external time under natural conditions to so-called diurnal rhythms. This mechanism of synchronization is called entrainment (2-6) and the environmental signal that can phase-set circadian clocks is called Zeitgeber (1, 5). All of the mentioned Zeitgebers may act as entraining agents although the daily 24 hour light-dark (LD) cycle is the most prominent one divided into a dark period or so-called scotophase and a light period or so-called photophase. Furthermore, organisms can only entrain to synchronizers cycling with a period close to 24 hours (7) (see T-cycles below). If the entraining period is too short or too long thereby exceeding the range of entrainment the circadian system cannot follow the Zeitgeber anymore and starts to free-run (see below). Since a strong Zeitgeber defines the rhythm of the clockwork, time is expressed as Zeitgeber time (ZT). Within a lighting schedule of 12 hours of light and 12 hours of darkness (LD 12:12), ZT0 is defined as “lights on,” the beginning of the light phase, and ZT12 corresponds to “lights off,” the end of the light phase.
The time difference [h] between the entraining external and the displayed internal rhythm, e.g. the onset of an animal’s activity or the peak blood concentration of an endocrine factor, is called phase angle difference (Ψ). True entrainment is characterized by a stable phase angle difference between two synchronized rhythms. The value of this phase angle, however, may vary with the strength of the applied Zeitgeber stimulus (e.g. the light intensity or its wave length).
A certain rhythm may often only apparently be entrained to a Zeitgeber while the internal clock at the same time is not affected. This phenomenon is called masking (8). It can be observed when the Zeitgeber exerts - besides its influence on the clock - an additional dominant effect on the chosen readout parameter. One example is the suppressing influence of bright light on the activity of nocturnal rodents. In very short photoperiods (see below) mice seem to entrain to the dark phases, as shown in the schematic drawing (Fig. 1C). The rest time during the light phases, however, is only a masking effect as revealed by parallel monitoring of the same animal’s body temperature (Fig. 1D). Consequently, this masking problem can often be overcome by changing the readout parameter (e.g. here temperature).
Free-run
Organisms kept under constant conditions by shielding them from external time cues display so-called free-running or circadian rhythms that may persist indefinitely. The period length of these free-running rhythms is often no longer equal to 24 and differs from species to species. Therefore, time cannot be expressed in ZT but is expressed in circadian time (CT) units. One circadian cycle is divided into 24 equally sized circadian units (or circadian hours) with one unit being defined as the division of the internal period length (τ) by 24 hours. In nocturnal organisms and constant darkness conditions (DD), one circadian unit usually is less than 1 hour because the internal rhythm of these animals is typically shorter than 24 hours. Wild type mice (C57BL/6Tyrc-Brd × 129S7) for example have an internal period length of 23.7 ± 0.1 h (9) and thus 1 CT equals 59.25 min. CT0 designates the beginning of the subjective day (the rest phase in nocturnal rodents) and CT12 that of the subjective night (their activity phase). The same is true in diurnal animals only that they have their activity phase starting at CT0 and their rest phase at CT12, respectively. Since many experiments are carried out under constant conditions, CT calculations will be described later in the materials and methods section.
To monitor circadian rhythms some researchers use dim red light instead of complete darkness. The advantage of using dim red light is ease of animal handling. However, one should keep in mind that the range of wavelengths, to which the visual receptors of nocturnal animals respond, varies from species to species. Djungarian hamsters, for example, have a very high photosensitivity to red light (10). A recent publication suggests not using dim red light because it can increase the circadian period in mice compared to constant darkness (11). Therefore, it is recommended to use complete darkness protocols and to handle the animals using night vision goggles equipped with an infrared beam. However, in case one wants to use dim red light, it is better to constantly illuminate the chamber rather than switching on a red light while checking the animals.
Transients and aftereffects
During entrainment to a new external period or after release into constant conditions, transient cycles (12) can be observed before stable entrainment to the Zeitgeber or free-run occurs (blue bars in Fig. 2 panels A and B). Those transients reflect the disequilibrium or the altered phase angle between the overt rhythm and the Zeitgeber in response to a phase shift (1). A phase shift is a change in the phasing of the rhythm due to a distinct external stimulus (e.g. a light pulse). Transient cycles should be excluded from the determination of the displayed internal period or the phase-angle difference.
Fig. 2. Transients and Aftereffects.
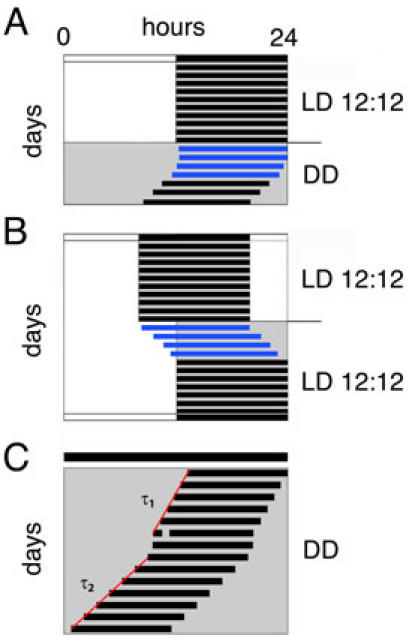
Transients (blue bars) can be caused by various treatments, such as the release of the animal into constant conditions (A) or a shift in the lighting regime (B). These transients usually persist for several days depending on the strength of the provoking signal. The white and black bar at the top of the scheme depicts light (12 h) and darkness (12 h), respectively. (C) An animal kept in constant darkness (DD) displays a stable free-running rhythm with a period length τ1 before it is subjected to a light pulse. This pulse leads to a phase shift and often provokes τ2, which is different from τ1, as an aftereffect. If the animal is left in DD long enough after this treatment, it will again display its old period length τ1. The red regression lines are drawn through the onsets before and after the light pulse to determine τ1 and τ2, respectively. The black bar at the top of the scheme represents constant darkness (24 h) conditions. DD, constant darkness; LD, light-dark cycle.
Even though the rhythm of a free-run can be remarkably precise, a certain plasticity of individual period length is a result of prior entrainment conditions. These so called aftereffects (12) can persist for several weeks and are also observed frequently after phase shifting stimuli (see below). Figure 2C shows the two different period lengths that can be observed before (τ1) and after (τ2) the stimulus.
T-cycles
To determine the stability range of entrainment, light cycles of different periods can be applied (4). Those T-cycles (with L + D = T) have periods deviating from the natural 24-hour period. For example, 22 hour or 26 hour T-cycles (LD 11:11 and LD 13:13, respectively), may be used to define the limits of stable entrainment. This limit differs from species to species and is depending on the nature of the applied Zeitgeber. In order to entrain to a 22 hour T-cycle (Fig. 3A), the organism has to constantly accelerate its internal rhythm whereas it decelerates its internal clock in response to a 26 hour T-cycle (Fig. 3E). This adaptation is only feasible within a close range. The mammalian circadian system has a plasticity of approximately 2 hours around its internal τ. Outside this critical range, entrainment is not possible anymore and the clock will start to free-run as indicated in Fig. 3A and E where the white and gray areas represent light and darkness, respectively. As long as the mice entrain to the T-cycle, they synchronize their activity onsets with lights-off (Fig. 3B and D). The phase-angle difference between lights-off and activity onset may increase with increasing deviation of T from the internal τ but will remain stable under given conditions as long as the animal still entrains.
Fig. 3. T-cycles.
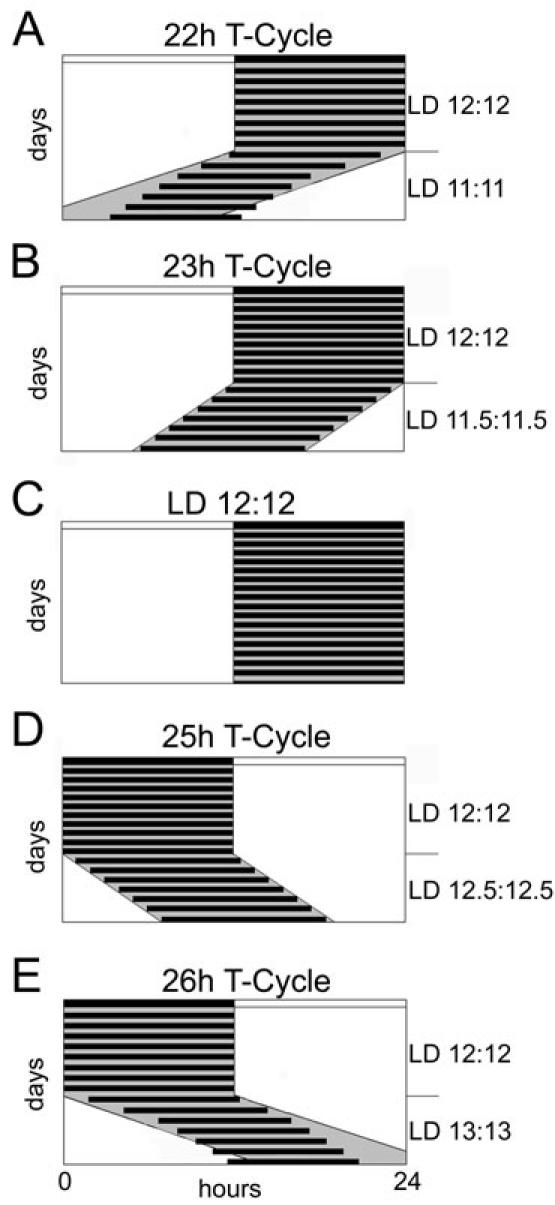
T-cycles are LD cycles with a period length other than 24 hours (T = L + D). Mice are able to entrain to T-cycles of 23 (B) and 25 (D) hours but not to T-cycles of 22 (A) and of 26 (E) hours. Panel C represents a normal 24 hours LD 12:12 cycle. All schemes are plotted on 24 hours scale where the white area represents lights on and the gray area lights off, respectively. The white and black bar at the top of the scheme depicts light and darkness of the LD 12:12 cycle the animals were entrained to in the beginning of each panel. Each species has a distinct range of entrainment; the schemes here represent the range for mice. The black horizontal bars display the active time of the animals. LD, light–dark cycle; T, period or cycle time of a Zeitgeber; h, hours.
Photoperiods and dim light ramps
Seasonal variations influencing circadian behavior can be simulated by applying different experimental photoperiods. A photoperiod is described by the ratio of light to darkness during a 24-hour cycle. Under laboratory conditions, summertime light conditions are typically represented by cycles of 18 hours light and 6 hours dark or of 14 hours light and 10 hours dark (LD 18:6 and LD 14:10, respectively), whereas winter light conditions are mimicked by an LD 6:18 cycle or an LD 10:14 cycle. In photoperiod experiments the external time of the Zeitgeber rhythm is specified as ExT (External Time) with ExT12 corresponding to the middle of the light-phase (Fig. 4) (13, 14).
Fig. 4. Schematic representation of external time (ExT) in photoperiods and T-cycles.
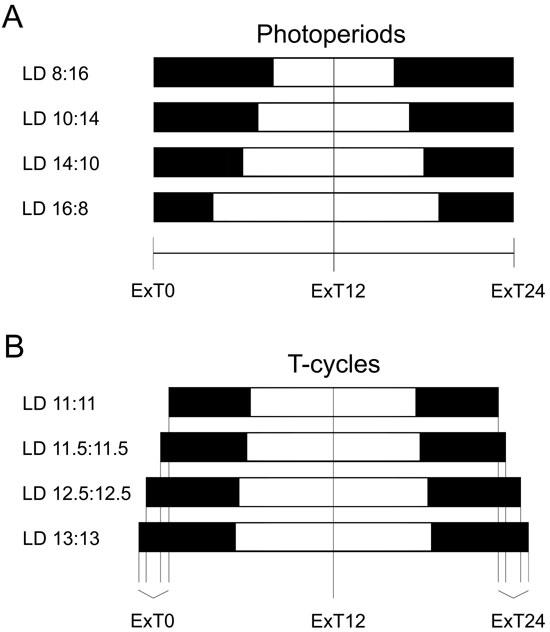
External time (ExT) subdivides any light-dark cycle into 24 units using the following formula: number of hours*24/T elapsed since the middle of the dark period. ExT0 (= ExT24) is determined as the middle of the dark period. ExT12 corresponds to the middle of the light phase. Vertical black lines indicate the corresponding external time. Black bars represent the dark period whereas the white bars correspond to lights on. (A) Schematic representation of ExT for photoperiods. (B) Schematic representation of ExT for T-cycles. ExT, external time; LD, light–dark cycle; T, period or cycle time of a Zeitgeber; h, hours.
Most laboratories use a rapid transition from light to dark and from dark to light and apply the same wavelength range and light intensity during the whole light period. However, this does not truly reflect natural lighting conditions. To more faithfully mimic the different sunlight conditions in a circadian context, one can use dim light ramps simulating dusk and dawn for light-dark transitions. Dim light conditions may allow a better understanding of daily events taking place in an organism in response to light. This procedure is still not commonly used – mainly due to technical and historical reasons. Moreover, the question of how much influence the dim light has on the establishment of circadian rhythmicity still remains to be answered.
Phase shift
A phase shift (Φ) is defined as the resetting of the organism’s internal rhythm in response to an external stimulus such as nocturnal light exposure (3). Such a phase shift can either result in a phase advance or a phase delay, where the former is the exact opposite of the latter. To specify, this means that a phase advance shifts the activity onset to an earlier position in the circadian cycle, whereas a phase delay shifts it to a later time. As an example, a shift of the activity phase by 2 circadian hours from CT12 to CT10 represents a 2 circadian hour phase advance. To analyze this resetting ability, animals displaying a stable free-running rhythm are kept in constant darkness and a light pulse is administered at a specific CT (Fig. 5A and B). Animals having an unstable free-running rhythm, receive the light at a certain ZT before they are released into DD (Fig. 5C). An overview of the daily variations in an animal’s ability to shift its rhythm in response to a certain stimulus is given by the phase response curve (PRC) whose shape varies depending on species and stimulus (15). Figure 5D shows a typical light PRC of a nocturnal rodent. It can be divided into three parts, a phase delaying zone (CT12 to CT18 in Fig. 5D), a phase advancing zone (CT18 to CT2), and a dead zone (CT2 to CT12), where the stimulus has no or little effect on the activity phase. In a light PRC, this dead zone normally corresponds to the subjective day.
Fig. 5. Real actograms and phase response curve (PRC). (A).
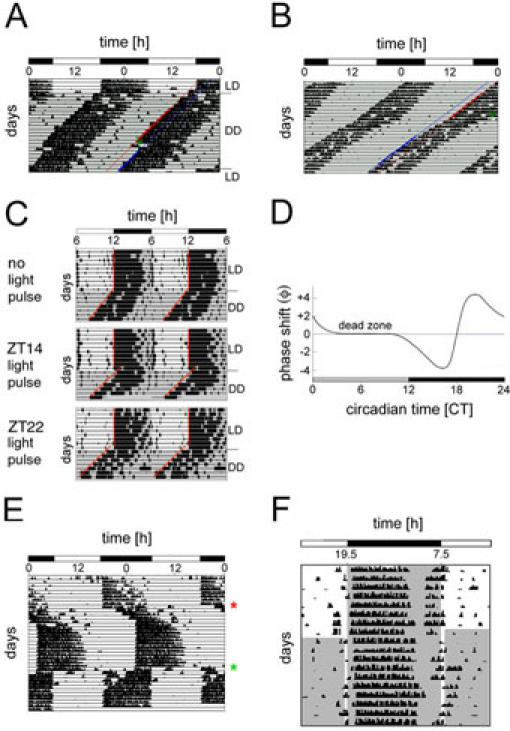
Plot of mouse locomotor activity before and after administering a light pulse (LP) at CT14 provoking a phase delay. The mouse was entrained to an LD 12:12 cycle before it was released into constant darkness (DD). The line above “DD” indicates the transition from LD to DD. When a stable free-running rhythm was established, a LP (green arrow) was administered at CT14 for 15 min. In order to quantify the phase shift, regression lines are drawn through 10 activity onsets before (red) and through 6 onsets after (blue) the LP. The horizontal distance between them corresponds to the phase shift triggered by the LP. (B) Plot of mouse locomotor activity before and after administering a LP at CT22 provoking a phase advance. The mouse was entrained to LD 12:12 (not shown) and then released into DD. As soon as a stable free-running rhythm was established, a LP (green arrow) was administered at CT22 for 15 min. In order to quantify the phase shift, regression lines are drawn through 6 activity onsets before (red) and through 7 onsets after (blue) the LP. The horizontal distance between them corresponds to the phase shift triggered by the LP. (C) Typical actograms of wild-type mice subjected to an Aschoff type II protocol. Mice were entrained to an LD 12:12 cycle (10 days) before releasing them into DD. The line above “DD” indicates the transition from LD to DD. The grey background represents darkness. Upon release into DD, no light pulse was administered to the mouse in the upper panel, whereas light pulses of 15 min were applied to the mouse at ZT14 (middle panel) and ZT 22 (lower panel). Regression lines (red) are drawn through the onsets of wheel-running activity in order to calculate the phase shift. Adapted from (26). (D) Typical light phase response curve (PRC) for nocturnal rodents. The grey and black bars below the PRC indicate subjective day and night, respectively. The X-axis shows the circadian time (CT) at which the light pulse was applied whereas the Y-axis displays the observed phase shift (f) [h]. Light pulses administered between CT11 and CT18 provoke a phase delay (negative values). Light pulses between CT19 and CT3, on the other hand, generate phase advances (positive values). Between CT4 and CT10, no phase shift can be observed (dead zone). (E) Jet lag can be mimicked in the lab by subjecting entrained animals to a rapid shift in the lighting schedule. This actogram shows the locomotor behavior of a mouse that was first entrained to LD 12:12 with light from 6 am to 6 pm. After 10 days, the lighting schedule was still LD 12:12 but shifted to "lights on" at 2 pm and "lights off" at 2 am (red star). The mouse only entrains after around 7 days of transition to the new LD cycle. 17 days after the first shift, the lighting schedule was again shifted to the original schedule (LD 12:12 from 6 am to 6 pm; green star). (F) Panel F shows an actogram of a mouse subjected to a skeleton photoperiod. The mouse was first entrained to LD 12:12 with lights on from 7:30 am to 7:30 pm for 8 days. The grey background represents lights off, whereas the white area stands for lights on. After day 8, an asymmetrical skeleton photoperiod was applied with a shorter pulse in the evening (dusk) and a longer one in the morning (dawn). Due to the two light pulses, the mouse remains entrained and wheel-running activity does not differ tremendously compared to LD 12:12. Adapted from ref. 31. LD, light-dark cycle; h, hours; DD, constant darkness.
Jet lag
Traveling across several time zones and shift work schedules disturb the circadian clock and lead to fatigue, insomnia, irritability, etc. All these symptoms taken together are referred to as jet lag and are provoked by the transients generated during the resetting of the internal pacemaker to re-synchronize with external time. Since jet lag is generated artificially by our fast-living society, this problem is predominantly observed in humans. However, it can be mimicked in rodents by an abrupt shift in the lighting schedule (Fig. 5E). Depending on the amplitude of the shift, the animal needs several days to re-adjust to the new light regimen. In mice (and in human) back-shifting normally is accomplished considerably faster than forward-shifting of a similar time span, but activity read-outs are often overlaid by the masking influence of the new light phase (Fig. 5E, lower part of the actogram). For example, wild type mice need around 4 days to adapt to a 4 hours phase delay and around 6 days to adapt to a 4 hours phase advance.
Skeleton photoperiods
In nature, nocturnal animals are only foraging during the night and hiding in their burrows during the day where they normally do not perceive any light. The standard LD cycles of the laboratory therefore do only weakly reflect natural conditions. To overcome this and mimic the periodic crepuscular light exposure skeleton photoperiods may be used (4).
Animals subjected to skeleton photoperiods are kept in constant darkness with two short light pulses per circadian cycle. In skeleton photoperiods, one distinguishes three parts: dawn (light), daytime (dark) and dusk (light). One pulse is applied at the beginning and one at the end of an otherwise complete photoperiod (e.g. one 15 min light pulse every 12 hours). The duration of the pulses is arbitrary (16). If the dawn- and the dusk-pulse have the same duration, the skeleton photoperiod is called symmetrical. In contrast, asymmetrical skeleton photoperiods are determined by pulses, where one is longer than the other. In general, the dawn pulse is longer than the dusk pulse (e.g. 3 hour dawn pulse and 30 min dusk pulse).
Skeleton photoperiods – like dim light – can also be used to overcome the masking effects of the LD cycle on the activity onset phase-angle and the alpha phase (Fig. 5F).
Materials and Methods
Why wheel-running?
Monitoring wheel-running activity is only one of several possible ways to track circadian locomotor activity. Compared to infrared beam or emitter-based measurements, wheel-running only registers voluntary movements. In contrast, the two other methods record all the movements including eating, drinking and grooming which increases noise levels. A mentionable advantage of the emitter-based measurement is that many modern systems allow you to easily record locomotor activity and certain physiological parameters such as body temperature or heart beat simultaneously.
Wheel-running facility
Circadian rhythms can be influenced by several environmental cues such as light, noise, vibrations, temperature, humidity, or pheromones. Due to this, it is important to perform any wheel-running experiment under defined environmental conditions. To achieve this, wheel-running experiments in our lab are carried out in an isolated, soundproof and air-conditioned room (17).
Isolation cabinets and cages
Animals are housed individually in plastic cages (280 mm long x 105 mm wide x 125 mm high, Tecniplast 1155M) equipped with a steel running wheel (115 mm in diameter, Trixie GmbH, Article No. 6083) (Fig. 6A). Diameter and type of the running wheel may influence the amount of wheel-running activity of mice (18, 19). The cages are provided with little bedding and one nestlet (5 x 5 cm; EBECO). One should take care not to use excessive amounts of bedding to avoid wheel blockage. Food and water are accessible ad libitum. Wheel revolutions are measured by a small magnet (Fehrenkemper Magnetsysteme, Article No. 34.601300702) embedded in a plastic disk that is fitted to the axis of the wheel (Fig. 6B). Upon rotation of the wheel, the magnet opens and closes a magnetic switch (Reed-Relais 60; Conrad Electronic AG, No. 503835-22), which is fixed outside the cage. Signals are registered on a computer using the ClockLab data aquisitition system (Actimetrics).
Fig. 6. Wheel-running cages and isolation cabinets. (A).
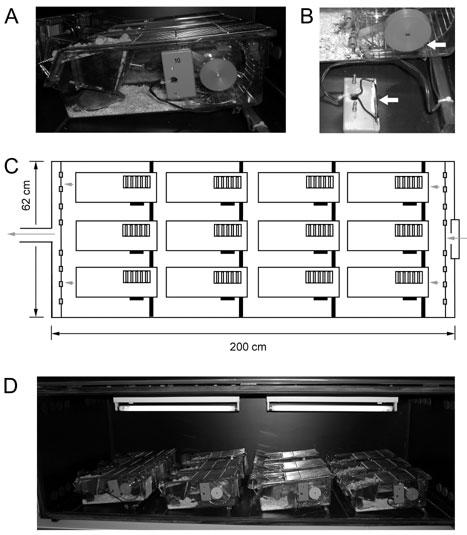
Individually housed mouse in a wheel-running cage connected via a magnetic switch to the system recording wheel revolutions. On each rotation of the running wheel, the magnetic switch is once opened and closed. (B) Detailed view of the magnet (upper arrow) and the magnetic switch (lower arrow). (C) Schematic representation of a ventilated isolation cabinet (200 x 62 cm) offering space for 12 wheel-running cages. The arrows represent the airflow through the cabinet. (D) Picture of a fully occupied isolation cabinet with two light bulbs at the ceiling.
Maximally twelve of these wheel-running cages can be placed in a light-tight box. These isolation cabinets (Fig. 6 panels C and D) are ventilated and contain two fluorescent light bulbs (Mazdafluor Symphony AZURA 965, 18 W) mounted on the ceiling of the cabinet.
The lighting conditions can be adjusted via a timer without opening the box. In order to minimize reflection of light and guarantee comparable lighting conditions for each cage, the interior of the isolation chamber should be black and non-reflective. Although the isolation cabinets are well ventilated, the heat produced by the two light bulbs can’t be eliminated completely by ventilation. Therefore there are diurnal temperature variations of 2-3.5°C (min. 23.5-24°C; max. 26-27°C) within the cabinets. However, light is a stronger and more immediate Zeitgeber compared to temperature and therefore the observed temperature variations under LD conditions can be neglected. Under constant lighting conditions temperature needs to be constant, because environmental temperature cycles can sustain peripheral circadian clocks (20). In our isolation cabinets this is the case and temperature remains constant after lights off (around 22.5°C).
Fluorescent light bulbs and lighting regimen
The choice of the correct fluorescent light bulb is a critical factor for wheel-running experiments. Usually, a light intensity of 300-400 lux at the level of the cage is chosen. Working with albino animals, one should keep in mind that they do not have any eye pigments. In this case, lower light intensities should be chosen in order not to damage their eyes.
Besides the mere intensity the color temperature of the bulb is also important. Color temperature is a measure of the visual “whiteness” of the light and its unit is degrees Kelvin (K). Light sources described as warm have a low color temperature and range from red to yellow. Cold ones on the other hand display a high color temperature and range towards the blue end of the spectrum. Natural daylight has a temperature between 6000 and 7000 K. The bulb described above has a luminous flux of 1000 lumen and a color temperature of 6500 K.
Entrainment to LD 12:12 and subsequent release into constant darkness
For standard wheel-running experiments mice should be between 2 and 6 months of age. Mice being younger than 2 months could freeze to death in the well ventilated cabinets while older mice often show low performance and other age related effects both of which can influence the analysis of running-wheel experiments (21, 22).
Additionally, it is normally preferable to use only male animals because in females the estrous cycle may influence general activity and wheel-running performance, which adds an additional rhythmic component that may complicate the evaluation.
Before any experiment can take place, it is essential that the mice are fully adapted to the isolation cabinets, to the cage and the wheel. For this, animals are entrained for 2 weeks to a standard LD cycle (e.g. LD 12:12 for mice, or LD 14:10 for hamsters) (Fig. 7A). After this time the experiments can be started.
In an entrained situation, the following parameters can be determined: Onset phase angle, onset variation, duration of the activity phase (α) and the rest phase (ρ), the daily overall activity and the percentage of light phase activity. To determine τ, animals are transferred to constant darkness (DD) by switching off the lights in the isolation cabinets at ZT12 and not turning them on again the next day. In DD, animals begin to free-run with an internal period close to 24 hours. On the first 2-3 days, animals often display unstable period lengths. These transients should be excluded from the evaluation.
Animals should be kept in constant darkness for at least 2 weeks. Once a stable free-running rhythm is established, one can determine the onset error, the length of the alpha and rho phases, the internal period length, and the overall activity (revolutions/day). The onset error is the average difference (in hours or minutes) of the true activity onsets compared to the theoretical onsets predicted by a least square fit regression line drawn through all onsets of the analyzed time span. Alpha and rho phase can be calculated after determination of onset and offset times. The batch analysis function of the ClockLab data analysis software (Actimetrics) allows the automatic determination of most of these values for a given set of animals.
Calculation of CTs
For many circadian experiments it is necessary to calculate the subjective phases (CT, or circadian time) of an animal’s rhythm. A specific CT value is calculated upon the individual organism’s free-running rhythm. For this, one needs to determine the internal period length (τ) and the onset of activity on the day prior to the day of the experiment (Day A).
It is important that the organism tested displays a stable rhythm during the time used to calculate the period length. Activity onsets should be determined for at least 6 consecutive days and a least square fit should be used to calculate τ. The last onset before the day of the experiment is determined as CT12 (Day A). CT12 on the following day (Day B) is calculated as follows:
CT12 Day B = CT12 Day A + τ - 24 hrs
To calculate circadian hours (or units), one divides the internal period length by 24 (see definitions section):
1 circadian hour = τ / 24
To define a circadian time earlier than CT12 (CT0 to CT12), one has to subtract X times one circadian hour from the predicted CT12 Day B .
CTX earlier than CT12 = CT12 Day B – X * 1 circadian hour
For CTX values later than CT12 (CT13 to CT24), one has to add X times 1 circadian hour to CT12 Day B .
CTX later than CT12 = CT12 Day B + X * 1 circadian hour
Some scientists do not use circadian time but divide the subjective circadian day into 360 degrees. In this case 0° corresponds to CT0, 180°C to CT12 and 360° to CT24 (Fig. 7B).
Fig. 7. Adaptation time and CT diagram. (A).
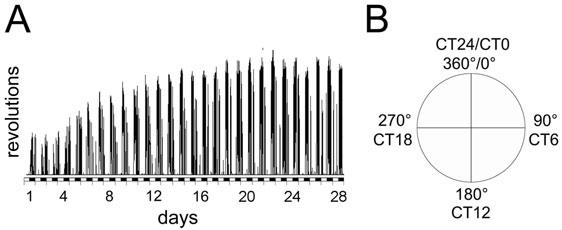
Linear recording of wheel-running activity of a male wild type mouse (C57BL/6 x 129SV) under LD 12:12 conditions. Without prior wheel-running experience mice need two to three weeks to fully develop their wheel-running capacity. Only after this initial training (and entraining) phase experimental manipulations should be applied (X-axis: days of experiment; Y-axis wheel revolutions; black and white bars indicate dark and light phases, respectively; bin size for activity counts is 6 min). (B) Diagram comparing circadian time (CT) in degrees versus CT units.
Phase resetting by brief light pulses
Aschoff type I
Resetting experiments according to Aschoff type I protocol (23) are done with mice that display a stable free-running rhythm in constant darkness and do not lose circadian rhythmicity (24). To determine a full light phase response curve (PRC), pulses have to be applied subsequently at CTs throughout the circadian cycle. Because of the long dead zone of the rodent light PRC exposure times can normally be restricted to the subjective night. However, one or two time points during the subjective day should be considered to exclude unexpected light responsiveness during this time (for example in a transgenic mouse strain). To get a rough overview, light pulses are normally given only at cardinal wild-type PRC time points of the circadian cycle like CT10 (the end of the dead zone), CT14 (maximum phase delay), and CT22 (maximum phase advance).
In this setup the circadian time has to be determined individually for each mouse. When kept in the described 12 cage isolation chambers, individual animals have to be removed from the chamber in their cages and be placed under an illumination screen for the time of the light exposure.
It is crucial, that the mice are not unnecessarily disturbed by changing cages or by supplying food or water for at least four days before and after the light pulse. As a standard, pulse durations of 15 min are used. After a light pulse mice are returned to constant darkness for about ten days before the next light pulse can be administered.
For quantification of the phase shift, regression lines are drawn through six to ten activity onsets prior to the light pulse and a minimum of six onsets after the light pulse (Fig. 5A and B). The activity onsets of the two to three days following the stimulus are normally not included in the regression lines because the oscillator is still in transition. To determine the extent of the phase shift, the distance between the two lines is calculated on the first day after the light pulse. The period length of the free-running rhythm can change slightly after a light pulse (see aftereffects). In this case, the regression lines drawn through the onsets are not in parallel.
Phase advances are noted as positive values while phase delays result in negative phase shifts. The value of the phase shift depends on the species/strain and the experimental setup. For 129SvEvBrd/129 Ola mice one can expect phase delays of up to 90 min (CT14) and phase advances of up to 40 min (CT22) after 15 min light exposure (25). By pulsing all animals sitting in the same isolation cabinet at once, a PRC can be created (Fig. 5D). Retrospectively, the CT when the light pulse was administered is calculated for each animal. Doing so with many animals, one gets easily the phase shifts for each CT and thus can establish a PRC.
Aschoff type II
When working with animals that display unstable circadian rhythmicity in constant darkness, the determination of circadian times may cause problems and testing phase shifts with an Aschoff type I protocol is not possible. In this case we suggest using the Aschoff type II protocol as follows (23, 26).
After 2 weeks of entrainment to LD 12:12 conditions, mice are released into a first period of constant darkness during 2-3 weeks. Thereafter, mice are re-entrained to a LD 12:12 cycle (15 days) before release into DD for at least ten days. In the first day of DD (with the nocturnal time points starting immediately after the last “lights off”), the light pulse is administered at the desired time points. Advantages of this protocol are that the light pulses can be administered simultaneously to all the mice without any disturbance like manipulating the cage (see Aschoff type I protocol).
For the determination of the phase shift in this protocol, regression lines are fitted through 6 consecutive activity onsets before (LD) and after the light pulse (DD). For the DD regressions the first day is disregarded because of possible transition effects. The phase shift is calculated as the difference between the two regression lines on the first day after the light pulse. Some species/strains show an apparent phase-shift after release into DD even without prior light administration. Therefore it is mandatory to monitor LD-DD transitions without light pulse and adjust the experimental data accordingly (Fig. 5C).
Jet lag
In the lab, delaying the LD cycle (simulating westward flights) or advancing it (simulating eastward flights) can simulate jet lag very easily. After 10 days in the same LD cycle (e.g. LD 12:12, lights on from 6 am to 6 pm) the LD cycle is delayed (e.g. by 8 hours; LD 12:12, lights on from 10 pm to 10 am) for 2 to 3 weeks. Since the animals cannot adjust their rhythm immediately to such a shift, some transients will be observed. After a stable entrainment is established, the LD cycle can again be advanced to the initial LD cycle (e.g. LD 12:12, lights on from 6 am to 6 pm), which simulates a transmeridian flight to the east. Sporadic jet lags have only minor impact on the general health state while regular jet lags have been shown to influence physiology, the immune system and may even promote cancer (27). Chronic jet lags can be observed in persons working predominantly at night, often changing their work shift, or frequently traveling by transmeridian flights.
To assess chronic jet lag in the laboratory, mice are entrained to LD 12:12 for around 10 days with lights on at 6 am and lights off at 6 pm (Fig. 5E). After full entrainment is accomplished, the animals are confronted to chronic jet lag by advancing the LD 12:12 cycle in series of 8 hours every 2 days for 10 days. This means that the first shift changes lights on from 6 am to 2 pm, the second from 2 pm to 10 pm, the third from 10 pm to 6 am, and so forth. After the 6th shift, the animals are released into DD for 2 days before sacrificing them at the desired CTs (28). Alternatively, mice can also be subjected to alternating advance and delay shifts to study chronic jet lag. Such a protocol is a more realistic simulation, because usually one travels to another continent and back home.
Constant light conditions
A second type of free-run condition (as opposed to DD) is constant light (LL). In LL, behavior in response to different light intensities can be tested. After a standard training phase in LD, one normally starts with a period of DD before gradually increasing the light intensity. Standard paradigms use 14 to 20 days per lighting condition. It should, however, be ensured that the rhythm is stabilized in a particular condition before applying the next level of intensity. Like in DD, onset error, the length of the alpha- and rho-phases, the internal period length, and the overall activity (revolutions/day) can be determined for any given light intensity.
In wild type mice, the robustness of the activity rhythm is decreased with increasing light intensities. Besides the activity depressing effect of bright light exposure, the tonic light signaling to the SCN hampers the coupling of the single cell oscillators in this area and eventually renders the pacemaker output arrhythmic (29). In addition, constant light modulates the period length of the circadian system with τ increasing roughly in proportion with the logarithm of the applied light intensity (“Aschoff's rule”) (30).
Appendix
Does constant darkness have an impact on the health of mice?
To demonstrate that wheel-running in constant darkness is not harmful for mice, their state of health was daily monitored by gently checking them with night vision goggles (Rigel 3200) during their active phase as it is demanded and approved by the Swiss Federal Veterinay Office (FVO). Additionally, a small-scale study has been done by their request to further demonstrate this. For this, four females and four males with a mixed C75BL/6 x 129S5/SvEvBrd background together with four males carrying a 129SvEvBrd/129 Ola background were held in constant darkness in an isolation cabinet as described above. Body weight was determined every two to three days for three weeks with a balance (WEDO Digi 2000, 2000g/1g). Night vision goggles possessing an infrared beam were used for all manipulations because of the insensitivity of the rodent visual system to these longer wavelengths. Infrared light may act as a Zeitgeber in reptiles whereas it does not in rodents.
Our results show that constant darkness does not have any negative effects on the health of mice during wheel-running experiments. Both females and males (C75BL/6 x 129S5/SvEvBrd) did not lose any significant weight during monitoring. The same is true for males carrying a 129SvEvBrd/129 Ola background (Fig. 8A and B). A single male animal that lost weight within this group was overweight in the beginning of the experiment. It steadily lost weight during the first twelve days and stabilized it then on the same extent than his companions. Weighing mice is only one of many possible parameters to assess health status. In conclusion, this implicates that the exposure to constant darkness for a period of some weeks has no negative consequences on the health of mice.
Fig. 8. Impact of constant darkness on weight and overall activity of wild-type mice. (A).
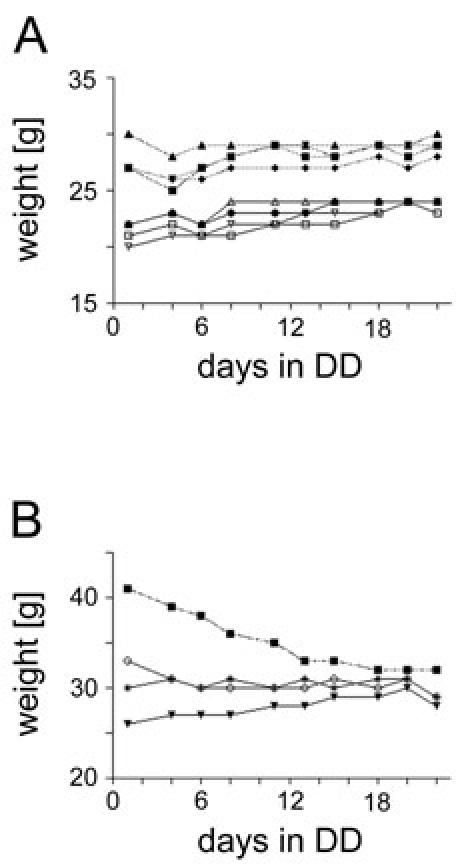
Weight curves of 8 littermates (males: solid lines / females: hatched lines) with a mixed C75BL/6 x 129S5/SvEvBrd background. Mice were reared in a normal LD 12:12 cycle and then transferred to constant darkness (DD). They were housed individually in wheel-running cages where food and water were accessible ad libitum. During 22 days, they were weighed regularly using night vision goggles. The x-axis displays the days spent in constant darkness whereas the y-axis displays the weight [g]. (B) Weight curves of 4 male mice with a 129SvEvBrd/129Ola background. Only one mouse (black square) that was overweight in the beginning looses weight during monitoring.
Supplemental Information
Web resources
Acknowledgments
We would like to thank April Bezdek and Gurudutt Pendyala for critically reading the manuscript. Financial support from the Swiss National Science Foundation, the BrainTime project (EC 5th framework Grant QLRT-2001-01829) and the State of Fribourg is gratefully acknowledged.
Appendix
Protocols
Equipment
Wheel-running facility
Isolation cabinets:
Light bulb: Mazdafluor, Symphony AZURA 965, 18 W
Light bulb mounting: Mazda, type: Mx204-118 (230V, 50Hz, 0.37A)
Fan: accessories by Monacor, Article No. 03.1670 (CF-1212, 12V=/500mA)
Wheel-running cages:
Cage: Tecniplast 1155M (280 mm long x 105 mm wide x 125 mm high)
Stainless steel wire lid: Tecniplast 1155M115
Stainless running wheel: Trixie GmbH, Article No. 6083 (diameter 115 mm)
Magnet: Fehrenkemper Magnetsysteme, Article No. 34.601300702
Magnetic switch: Reed-Relais 60, Conrad Electronic AG, No. 503835-22
Mouse housing:
Water bottles: Tecniplast ACBTO262 (260 ml, 55 x 128 mm, polycarbonate, with silicone ring)
Bottle caps: Tecniplast ACCP2521
Nestlets (5 x 5 cm): EBECO
Animal bedding: Schill AG Bedding type 3-4
Food (γ irradiated): Provimi Kliba, No. 3432
Computer Hardware and software [4]:
Microsoft Windows PC (e.g. Dell, Intel Pentium III running Windows 2000 or better)
Data acquisition board: National Instruments AMUX 64-T (fitted with 10-kΩ resistors)
RJ45 socket
PCI 6503 card National Instruments
National Instruments NI-DAQ software
ClockLab software package, Actimetrics
All components listed above can be purchased in a ready to use package from Actimetrics
Methods
Abbreviations
LD - light dark cycle (usually LD 12:12 cycles)
DD - constant darkness
LL - constant light
ZT - Zeitgeber time (ZT0 corresponds to lights on)
CT - circadian time
Experimental set-up
6 mutant mice and 6 wild-type controls (2-6 months old)
Mutant and wild-type mice need to have the same genetic background and should be of similar age (most suitable are littermates)
Table 1.
Aschoff type I
| Lighting condition | Duration | Measurements |
| LD 12:12 | 10-15 days | |
| DD | 10-15 days | Period length of free-running rhythm (τ), onset error, length of activity period (alpha-period) |
| Light pulse at CT14 | 15 min | Phase shift |
| DD | 10-15 days | |
| Light pulse at CT22 | 15 min | Phase shift |
| DD | 10-15 days | |
| Light pulse at CT10 | 15 min | Phase shift |
| DD | 10-15 days | |
| LD 12:12 | 10-15 days | Onset phase angle, onset error, rel. light phase activity, length of activity period |
Table 2.
Aschoff type II
| Lighting condition | Duration | Measurements |
| LD 12:12 | 10-15 days | |
| DD | 10-15 days | Period length, onset error, length of activity period |
| LD 12:12 | 10-15 days | Onset phase angle, onset error, rel. light phase activity, length of activity period |
| Light pulse at ZT14 | 15 min | Phase shift (compare to LD → DD without light pulse) |
| DD | 10-15 days | |
| LD 12:12 | 10-15 days | Onset phase angle, onset error, rel. light phase activity, length of activity period → compare to previous LD 12:12 |
| Light pulse at ZT22 | 15 min | Phase shift (compare to LD → DD without light pulse) |
| DD | 10-15 days |
Table 3.
Optional for Aschoff type I and Aschoff type II
| Lighting condition | Duration | Measurements |
| LL (~5 lux) | 10-15 days | Period length, onset error, length of activity period |
| LL (~50 lux) | 10-15 days | Period length, onset error, length of activity period |
| LL (~500 lux) | 10-15 days | Period length, onset error, length of activity period |
| Overall activity (revolutions/day) has to be monitored for every lighting condition. Above experiments should be done with at least 10 to 12 mice before performing statistical analysis. | ||
Formulas for CT calculations
1CT = internal period length / 24
CTX earlier than CT12 = CT12 Day B – X * 1CT
CTY earlier than CT12 = CTX + 24
CTX later than CT12 = CT12 Day B + X * 1CT
CTY later than CT12 = CTX – 24
Relative time = absolute time / 60
Jet lag: An example of a typical experimental setup
Table 4.
Simulating a westward transmeridian flight
| Lighting condition | Duration | Measurements |
| LD 12:12 (L 6 am to 6 pm) | 10-15 days | Onset phase angle, onset error, rel. light phase activity, length of activity period |
| LD 12:12 (L 10 pm to 10 am) | 2-3 weeks | Onset phase angle, onset error, rel. light phase activity, length of activity period, time in transience |
| LD 12:12 (L 6 am to 6 pm) | 10-15 days | Onset phase angle, onset error, rel. light phase activity, length of activity period |
Table 5.
Simulating an eastward transmeridian flight
| Lighting condition | Duration | Measurements |
| LD 12:12 (L 6 am to 6 pm) | 10-15 days | Onset phase angle, onset error, rel. light phase activity, length of activity period |
| LD 12:12 (L 10 pm to 10 am) | 2-3 weeks | Onset phase angle, onset error, rel. light phase activity, length of activity period, time in transience |
| LD 12:12 (L 6 am to 6 pm) | 10-15 days | Onset phase angle, onset error, rel. light phase activity, length of activity period |
Table 6.
Chronic jet lag: An example of a typical experimental setup (Ref: 28)
| Lighting condition | Duration | Measurements |
| LD 12:12 (L 6 am to 6 pm) | 10 days | Onset phase angle, onset error, rel. light phase activity, length of activity period |
| LD 12:12 (L 2 pm to 2 am) | 2 days | |
| LD 12:12 (L 10 pm to 10 am) | 2 days | |
| LD 12:12 (L 6 am to 6 pm) | 2 days | |
| LD 12:12 (L 2 pm to 2 am) | 2 days | |
| LD 12:12 (L 10 pm to 10 am) | 2 days | |
| LD 12:12 (L 6 am to 6 pm) | 2 days | |
| DD | 2 days | |
| One should always keep in mind that jet lag can be caused by both shifting time backwards and forwards. | ||
Definitions
Overall activity - Average activity bouts (revolutions/day) over the specified period (alpha- and rho-phase).
Period length - Length of displayed overt rhythm. In DD, it represents the endogenous internal rhythm (τ).
Onset error - Difference [h] between the onsets of activity and the constructed regression line. To achieve this, a least
square fit regression line is plotted through the onsets of activity for the specified period.
Onset phase angle - Difference [h] between an external (entraining) and an internal period of an entrained organism
e.g. anticipated or delayed activity onset.
Rel. light phase activity - Activity [%] during light phase (rho-phase in mice) relative to the overall activity.
Length of activity period - Duration [h] of the alpha-phase (often given as relative value compared to the period
length).
References
- Dunlap JC, Loros JL, DeCoursey PJ. CHRONOBIOLOGY – biological timekeeping. Sinauer Associates. 2004;3(24):67–105. [Google Scholar]
- Pittendrigh CS. On the mechanism of the entrainment of a circadian rhythm by light cycles. In: Circadian clocks; edited by: Aschoff J, Amsterdam: Elsevier (1965); 277-297.
- Pittendrigh CS. Circadian systems: entrainment. In: Handbook of behavioral neurobiology, Vol. 4 Biological Rhythms, edited by Aschoff J, New York: Plenum Press (1981); 95-124.
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J Comp Physiol A. 1976;106:291–331. [Google Scholar]
- Aschoff J, Daan S, Honma KI. Zeitgeber, entrainment, and masking: some unsettled questions. In: Vertebrate Circadian System (Structure and Physiology), edited by Aschoff J, Daan S, Gross GA, Berlin: Springer-Verlag (1982); 13-24.
- Takahashi JS, Turek FW, Moore RY. Circadian Clocks. Handbook of Behavioral Neurobiology. 2001;12:7–43. [Google Scholar]
- Fuller CA, Fuller P. Circadian Rhythms. Encyclopedia of the human brain. (2002); 793-812.
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harbor Symp Quant Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105(5):683–694. doi: 10.1016/S0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- Klante G, Steinlechner S. A short red light pulse during dark phase of LD-cycle perturbs the hamster’s circadian clock. J Comp Physiol. 1995;177(6):775–780. doi: 10.1007/BF00187636. [DOI] [PubMed] [Google Scholar]
- Hofstetter JR, Hofstetter AR, Hughes AM, Mayeda AR. Intermittent long-wavelength red light increases the period of daily locomotor activity in mice. J Circadian Rhythms. 2005;3(1):8–8. doi: 10.1186/1740-3391-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and liability of spontaneous frequency. J Comp Physiol A. 1976;106:223–252. [Google Scholar]
- Daan S, Merrow M, Roenneberg T. External time – internal time. J Biol Rhythms. 2002;17(2):107–109. doi: 10.1177/074873002129002375. [DOI] [PubMed] [Google Scholar]
- Steinlechner S, Jacobmeier B, Scherbarth F, Dernbach H, Kruse F, Albrecht U. Robust circadian rhythmicity of Per1 and Per2 mutant mice in constant light, and dynamics of Per1 and Per2 gene expression under long and short photoperiods. J Biol Rhythms. 2002;17(3):202–209. doi: 10.1177/074873040201700303. [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol A. 1976;106:253–266. [Google Scholar]
- Zivkovic B. Clock tutorial #6: To entrain or not to entrain, that is the question. (2005); http://circadiana.blogspot.com
- Albrecht U, Foster RG. Placing ocular mutants into a functional context: a chronobiological approach. Methods. 2002;28:465–477. doi: 10.1016/s1046-2023(02)00266-9. [DOI] [PubMed] [Google Scholar]
- Banjanin S, Mrosovsky N. Preferences of mice, Mus musculus, for different types of running wheel. Lab Anim. 2000;34(3):313–318. doi: 10.1258/002367700780384681. [DOI] [PubMed] [Google Scholar]
- Deboer T, Tobler I. Running wheel size influences circadian rhythm period and its phase shift in mice. J Comp Phys. 2000;186(10):969–973. doi: 10.1007/s003590000150. [DOI] [PubMed] [Google Scholar]
- Brown SA, Zumbrunn G, Flwury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12:1574–1583. doi: 10.1016/S0960-9822(02)01145-4. [DOI] [PubMed] [Google Scholar]
- Ingram DK, London ED, Reynolds MA, Waller SB, Goodrick CL. Differential effects of age on motor performance in two mouse strains. Neurobiol Aging. 1981;2(3):221–227. doi: 10.1016/0197-4580(81)90025-7. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. American Physiol Soc. 1997. pp. R1957–R1964. [DOI] [PubMed]
- Aschoff J. Response curves in circadian periodicity. In: Circadian Clocks, edited by Aschoff J, North-Holland Amsterdam (1965); 95-111.
- Albrecht U, Oster H. The circadian clock and behavior. Behav Brain Res. 2001;125(1-2):89–91. doi: 10.1016/S0166-4328(01)00288-1. [DOI] [PubMed] [Google Scholar]
- Oster H, Baeriswyl S, Van Der Horst GT, Albrecht U. Loss of circadian rhythmicity in aging mPer1-/-mCry2-/- mutant mice. Genes Dev. 2003;17(11):1366–1379. doi: 10.1101/gad.256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. mPer1 and mPer2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16(2):100–104. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- Knutsson A. Health disorders of shift workers. Occup Med (London) 2003;53(2):103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- Filipski E, Delaunay F, King VM, Wu M, Claustrat B, Gréchez-Cassiau A, Guettier C, Hastings MH, Lévi F. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8(3):267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Changes of frequency of periods of activity of mice in constant light and lasting darkness. Pflugers Arch. 1952;255(3):197–203. doi: 10.1007/BF00363483. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ. Resetting the suprachiasmatic nucleus clock. Front Biosci. 2004;9:56–62. doi: 10.2741/1200. [DOI] [PubMed] [Google Scholar]


