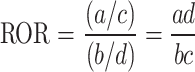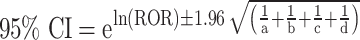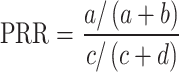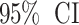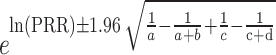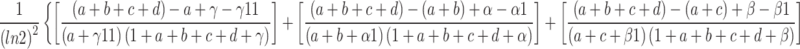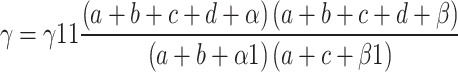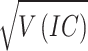Abstract
This study aims to explore potential adverse events (AEs) related to Dupilumab using data from the US FDA Adverse Event Reporting System (FAERS) database. The FAERS database from Q2 2017 to Q4 2023 was mined for AEs related to Dupilumab. The types of AEs reported, along with gender, age distribution, and severity, were evaluated. Signal detection methods including Reporting Odds Ratio, Proportional Reporting Ratio, Bayesian Confidence Propagation Neural Network, and Empirical Bayesian Geometric Mean were used. A total of 11,547,571 AE reports were collected, with 5335 reports suspected of being related to Dupilumab, identifying 307 Preferred Terms involving 27 System Organ Classes. Reports from female patients outnumbered males (56.08% vs. 34.65%). Patients aged 45–65 years reported the most events (21.34%). The number of reports increased significantly in 2023 (34.25%) compared to 2017 (0.42%), with the highest reporting rate from the US (98.07%). Common AEs included Pruritus, Product use in unapproved indication, and Rash, with Product dose omission issue indicating widespread misuse of Dupilumab. High signal strength AEs included Rebound atopic dermatitis, Rebound eczema, Dermatitis atopic, and Dry skin; injection site AEs like Injection site dryness and eczema; new potential AEs such as Dry eye, Eye pruritus, Ocular hyperaemia, Eye irritation, Conjunctivitis, Vision blurred, and Sleep disorder. This study reveals various potential AEs associated with Dupilumab, including newly identified risks. Future research needs to delve deeper into the safety of Dupilumab to better guide its clinical application.
Keywords: Dupilumab, Adverse events, FAERS database, Safety, Data mining
Subject terms: Risk factors, Infectious diseases
Introduction
Dupilumab (brand name: Dupixent) is the world’s first fully human monoclonal antibody (IgG4 type) that targets and recognizes the interleukin-4 receptor alpha subunit (IL-4Rα). By inhibiting the endogenous binding of key type 2 inflammation factors IL-4 and IL-13 to IL-4Rα, it blocks downstream signal transduction, reduces airway inflammation, and decreases eosinophils1. The drug was first approved for the treatment of moderate-to-severe atopic dermatitis (AD) in adults in the United States in March 2017. Its indications have expanded in recent years to include nodular prurigo, chronic rhinosinusitis with nasal polyposis (CRSwNP), eosinophilic esophagitis, and asthma. However, Dupilumab was only approved in China in June 2020, and its indication for asthma was approved in September 2023. Due to its relatively late approval in China, clinical experience with its use is limited, and understanding of its adverse reactions is also relatively lacking. This limits the ability of clinical practitioners to access relevant information and conduct rational drug use. Therefore, this study aims to conduct an in-depth analysis of Dupilumab-related adverse event (AE) data from the FAERS database to better understand the safety profile of this drug and provide important reference information for its rational clinical use.
Bronchial asthma is a chronic inflammatory airway disease characterized by symptoms such as wheezing, shortness of breath, chest tightness, and/or coughing. It is the most prevalent chronic respiratory disease globally. Studies indicate that the global prevalence of bronchial asthma has reached 300 million, and it is expected to rise to 400 million by 2025, causing 461,000 deaths2,3. Although current asthma treatments, including inhaled corticosteroids (ICS) and long-acting beta-agonists (LABA), can alleviate asthma symptoms and reduce acute exacerbations, a significant number of patients remain inadequately controlled, particularly those with severe asthma. These patients may face serious consequences such as airway narrowing and respiratory failure, severely impacting their quality of life and consuming substantial medical resources, making asthma a major cause of disability and death. Therefore, novel biological targeted therapies like Dupilumab have become a research focus in the field of asthma, especially for patients with moderate-to-severe asthma who remain inadequately controlled despite standard treatment.
The ERS/ATS guidelines for severe asthma4 recommend anti-IL-4R monoclonal antibodies for patients with severe asthma with blood eosinophils (EOS) ≥ 150/μl or fractional exhaled nitric oxide (FeNO) > 25 ppb. Castr et al.3 found that in patients aged 12 years and older with inadequately controlled moderate-to-severe asthma, Dupilumab treatment for 52 weeks significantly reduced the frequency of severe asthma exacerbations, improved lung function, and enhanced asthma control, especially in patients with type 2 inflammation-predominant asthma. Additionally, Dupilumab significantly reduced the use of oral corticosteroids (OCS) in OCS-dependent severe asthma patients while also lowering the incidence of asthma exacerbations4. Corren J et al.5 found that the more frequent the previous asthma exacerbations, the more significant the improvement in asthma symptoms with Dupilumab treatment. A real-world study from Japan on severe asthma also showed that patients with a history of biological therapy failure benefited significantly from switching to Dupilumab6. Recent studies7–10 have investigated the AEs of JAK inhibitors used in atopic dermatitis. These studies have shed light on their safety profiles, which provides a new perspective for comparing different systemic therapies in atopic dermatitis.
Despite the new treatment option Dupilumab offers for asthma, information on its potential AEs and safety issues during long-term use remains relatively limited. The FDA’s Adverse Event Reporting System (FAERS) database provides crucial information for monitoring the safety of drugs post-market11–22. To comprehensively assess the safety of Dupilumab, this study will delve into and analyze AE data related to Dupilumab from the FAERS database to further understand the drug’s safety and potential risks, thereby providing valuable reference information for clinical treatment.
Methods
Data sources
The data for this study were sourced from the FAERS database, covering all AE reports from the second quarter of 2016 to the fourth quarter of 2023. These reports were imported into the Statistical Analysis System (SAS) database for organization and analysis. The data included personal information records (DEMO), drug usage records (DRUG), treatment outcome information records (OUTC), AE information records (REAC), report source information records (RPSR), and treatment duration information records (THER).
Data processing
The search targeted “Dupilumab” as the specific drug. According to the FAERS database guidelines, duplicate AE reports were identified and removed. Specifically, reports were considered duplicates if they had identical values for key identifiers such as the patient’s unique identifier (when available), the date of the AE, the reported drug, and the nature of the adverse event description. In cases where multiple reports from the same patient with the same drug—related AE were found, only the most recent record was retained. This was done to ensure that each unique AE instance was counted only once, minimizing the potential for over—representation of certain events in the analysis. When identifying reports suspecting Dupilumab as the primary agent, reports were selected if Dupilumab was clearly listed as the suspected drug in the ‘DRUG’ field of the FAERS database records. Additionally, reports where the adverse event description strongly suggested a relationship with Dupilumab use, even if not explicitly stated as the sole suspect, were also included. For example, if the patient’s medical history showed no other recent medications that could plausibly cause the reported AE, and the onset of the AE occurred within a reasonable time frame after starting Dupilumab treatment, such reports were considered suspect for Dupilumab—related AEs. However, in cases of concurrent use of multiple drugs where it was unclear which agent was responsible for the AE, a more conservative approach was taken, and the report was included only if there were additional supporting details implicating Dupilumab. This study utilized the Medical Dictionary for Regulatory Activities (MedDRA) AE terminology set. AE reports were classified and described using MedDRA’s system organ class (SOC) and preferred terms (PT), where SOC represents the category of the AE and PT is the standard term for the AE.
Data mining
During the data mining process, this study employed methods from disproportionality analysis, including Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Empirical Bayesian Geometric Mean (EBGM). Disproportionality analysis is based on the classical four-fold table (Table 1) and calculates ROR, PRR, BCPNN, and EBGM values using specific formulas (Table 2). Higher values indicate stronger signals of AEs associated with Dupilumab, suggesting a higher statistical correlation between Dupilumab and specific AEs.
Table 1.
Four grid table.
| Target AEs | Non-target AEs | Total | |
|---|---|---|---|
| Dupilumab | a | b | a + b |
| Non- Dupilumab | c | d | c + d |
| Total | a + c | b + d | N = a + b + c + d |
Table 2.
ROR, PRR, BCPNN, and EBGM methods, formulas, and thresholds.
| Method | Formula | Threshold |
|---|---|---|
| ROR |
|
a ≥ 3 and 95% CI (lower limit) > 1 |
| PRR |
SE(lnPRR) =
|
a ≥ 3 and 95% CI (lower limit) > 1 |
| BCPNN |
IC =
V(IC) =
IC-2SD = E(IC)−2
|
IC025 > 0 |
| EBGM |
|
EBGM05 > 2 |
Results
Overview of AE reports
In the FAERS database, from Q2 2017 to Q4 2023, a total of 11,547,571 AE reports were collected, with 205,359 reports primarily suspecting Dupilumab. Among these reports, the proportion of male patients (71,160, 34.65%) was lower than that of female patients (111,517, 56.08%). The age group with the most reports was 45–65 years (43,819, 21.34%), followed by 18–45 years (39,215, 19.10%). Over time, the number of reports increased annually, with a significant rise in 2023 (70,329) compared to 2017 (866). Reports mainly came from physicians (57.47%), followed by consumers (34.77%) and pharmacists (6.82%). Nearly all reports originated from the United States, accounting for 98.07%. In terms of AE severity, the majority were classified as Non-Serious (89.16%), while Life-Threatening (0.12%), Hospitalization—Initial or Prolonged (4.56%), Disability (0.44%), and Death (0.39%) were relatively low. Regarding the timing of events, 85.64% of the reports did not provide clear information on the onset of AEs or drug usage dates. Among the provided data, the highest rate of AEs occurred within 0 to 30 days of drug use (8.32%), indicating that AEs may occur shortly after starting the medication. Additionally, AEs reported over 360 days post-medication accounted for 1.82%, suggesting that AEs may still occur after long-term use (Table 3).
Table 3.
Basic information on AEs related to Dupilumab.
| Factors | Number of events (%) |
|---|---|
| Gender | |
| Female | 115,170 (56.08) |
| Male | 71,160 (34.65) |
| Unknown | 19,029 (9.27) |
| Age | |
| < 18 | 17,785 (8.66) |
| 18–45 | 39,215 (19.10) |
| 45–65 | 43,819 (21.34) |
| 65–75 | 14,411 (7.02) |
| ≥ 75 | 8663 (4.22) |
| Unknown | 81,466 (39.67) |
| Reporter | |
| Pharmacist | 14,009 (6.82) |
| Consumer | 71,404 (34.77) |
| Physician | 118,010 (57.47) |
| Other health professionals | 1771 (0.86) |
| Lawyer | 9 (0.00) |
| Unknown | 156 (0.08) |
| Reported countries | |
| United States | 200,438 (97.60) |
| Not specified | 959 (0.47) |
| Japan | 764 (0.37) |
| Germany | 517 (0.25) |
| France | 435 (0.21) |
| UK | 401 (0.20) |
| Canada | 209 (0.10) |
| Australia | 187 (0.09) |
| Report year | |
| 2017 | 866 (0.42) |
| 2018 | 5894 (2.87) |
| 2019 | 12,635 (6.15) |
| 2020 | 20,229 (9.85) |
| 2021 | 38,021 (18.51) |
| 2022 | 57,385 (27.94) |
| 2023 | 70,329 (34.25) |
| Serious outcomes | |
| Death | 792 (0.39) |
| Disability | 904 (0.44) |
| Hospitalization—Initial or Prolonged | 9365 (4.56) |
| Life-Threatening | 247 (0.12) |
| AE occurrence time—medication date (days) | |
| 0–30 | 17,088 (8.32) |
| 31–60 | 2028 (0.99) |
| 61–90 | 1439 (0.70) |
| 91–120 | 1110 (0.54) |
| 121–150 | 808 (0.39) |
| 151–180 | 737 (0.36) |
| 181–360 | 2545 (1.24) |
| > 360 | 3742 (1.82) |
Signal mining results
Using signal mining methods, this study identified 307 PTs involving 27 SOCs. Table 4 lists the SOCs by the number of reports. The highest incidence SOCs were Skin and subcutaneous tissue disorders, General disorders and administration site conditions, Injury, poisoning and procedural complications, Eye disorders, Infections and infestations, Respiratory, thoracic and mediastinal disorders, and Musculoskeletal and connective tissue disorders, consistent with the information recorded in Dupilumab’s package insert. However, AEs such as Psychiatric disorders, Investigations, Metabolism and nutrition disorders, and Reproductive system and breast disorders were not mentioned in the package insert.
Table 4.
The signal strength of AEs of Dupilumab at the SOC level.
| System organ class | SOC code | Case reports | ROR (95% CI) | PRR (95% CI) | χ2 | IC (IC025) | EBGM (EBGM05) |
|---|---|---|---|---|---|---|---|
| Skin and subcutaneous tissue disorders | 10,040,785 | 112,752 | 4.91 (4.88–4.95) | 4.03 (4.01–4.05) | 255,001.46 | 1.94 (1.93) | 3.83 (3.80) |
| General disorders and administration site conditions | 10,018,065 | 103,596 | 1.22 (1.21–1.23) | 1.18 (1.17–1.18) | 3258.52 | 0.23 (0.22) | 1.17 (1.16) |
| Injury, poisoning and procedural complications | 10,022,117 | 86,606 | 1.57 (1.55–1.58) | 1.47 (1.46–1.48) | 14,264.29 | 0.54 (0.53) | 1.46 (1.44) |
| Eye disorders | 10,015,919 | 42,589 | 5.04 (4.99–5.10) | 4.70 (4.65–4.74) | 116,716.59 | 2.14 (2.13) | 4.41 (4.37) |
| Infections and infestations | 10,021,881 | 28,442 | 1.05 (1.04–1.06) | 1.05 (1.03–1.06) | 59.23 | 0.06 (0.05) | 1.04 (1.03) |
| Respiratory, thoracic and mediastinal disorders | 10,038,738 | 24,477 | 1.08 (1.07–1.10) | 1.08 (1.07–1.09) | 149.56 | 0.11 (0.09) | 1.08 (1.06) |
| Musculoskeletal and connective tissue disorders | 10,028,395 | 18,963 | 0.74 (0.73–0.75) | 0.75 (0.74–0.76) | 1631.36 | − 0.41 (− 0.43) | 0.75 (0.74) |
| Nervous system disorders | 10,029,205 | 17,112 | 0.43 (0.42–0.43) | 0.44 (0.44–0.45) | 12,741.77 | − 1.15 (− 1.18) | 0.45 (0.44) |
| Gastrointestinal disorders | 10,017,947 | 14,099 | 0.32 (0.32–0.33) | 0.34 (0.34–0.35) | 19,139.29 | − 1.53 (− 1.55) | 0.35 (0.34) |
| Psychiatric disorders | 10,037,175 | 13,594 | 0.48 (0.47–0.49) | 0.50 (0.49–0.51) | 7253.64 | − 1.00 (− 1.02) | 0.50 (0.49) |
| Investigations | 10,022,891 | 8132 | 0.26 (0.26–0.27) | 0.28 (0.27–0.28) | 16,297.73 | − 1.84 (− 1.87) | 0.28 (0.27) |
| Surgical and medical procedures | 10,042,613 | 6822 | 0.98 (0.95–1.00) | 0.98 (0.95–1.00) | 3.97 | − 0.03 (− 0.07) | 0.98 (0.95) |
| Immune system disorders | 10,021,428 | 4644 | 0.75 (0.73–0.78) | 0.76 (0.74–0.78) | 362.94 | − 0.40 (− 0.44) | 0.76 (0.74) |
| Social circumstances | 10,041,244 | 3257 | 1.44 (1.39–1.49) | 1.43 (1.38–1.48) | 416.12 | 0.51 (0.46) | 1.42 (1.37) |
| Vascular disorders | 10,047,065 | 2570 | 0.26 (0.25–0.27) | 0.27 (0.26–0.28) | 5238.09 | − 1.88 (− 1.94) | 0.27 (0.26) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 10,029,104 | 1919 | 0.11 (0.10–0.11) | 0.11 (0.11–0.12) | 14,129.79 | − 3.15 (− 3.22) | 0.11 (0.11) |
| Cardiac disorders | 10,007,541 | 1909 | 0.18 (0.17–0.19) | 0.18 (0.18–0.19) | 7068.95 | − 2.43 (− 2.49) | 0.19 (0.18) |
| Product issues | 10,077,536 | 1837 | 0.20 (0.19–0.21) | 0.20 (0.19–0.21) | 5784.26 | − 2.27 (− 2.34) | 0.21 (0.20) |
| Metabolism and nutrition disorders | 10,027,433 | 1768 | 0.17 (0.16–0.18) | 0.17 (0.17–0.18) | 7059.33 | − 2.50 (− 2.57) | 0.18 (0.17) |
| Blood and lymphatic system disorders | 10,005,329 | 1348 | 0.16 (0.15–0.17) | 0.16 (0.15–0.17) | 6025.48 | − 2.62 (− 2.70) | 0.16 (0.15) |
| Ear and labyrinth disorders | 10,013,993 | 1299 | 0.61 (0.57–0.64) | 0.61 (0.57–0.64) | 330.07 | − 0.71 (− 0.79) | 0.61 (0.58) |
| Renal and urinary disorders | 10,038,359 | 1161 | 0.11 (0.10–0.12) | 0.11 (0.11–0.12) | 8378.42 | − 3.14 (− 3.23) | 0.11 (0.11) |
| Reproductive system and breast disorders | 10,038,604 | 1036 | 0.28 (0.26–0.30) | 0.28 (0.27–0.30) | 1897.31 | − 1.81 (− 1.90) | 0.29 (0.27) |
| Hepatobiliary disorders | 10,019,805 | 369 | 0.09 (0.08–0.10) | 0.09 (0.08–0.10) | 3511.37 | − 3.48 (− 3.63) | 0.09 (0.08) |
| Endocrine disorders | 10,014,698 | 266 | 0.20 (0.17–0.22) | 0.20 (0.18–0.22) | 864.99 | − 2.31 (− 2.49) | 0.20 (0.18) |
| Pregnancy, puerperium and perinatal conditions | 10,036,585 | 215 | 0.11 (0.10–0.13) | 0.11 (0.10–0.13) | 1536.45 | − 3.15 (− 3.34) | 0.11 (0.10) |
| Congenital, familial and genetic disorders | 10,010,331 | 147 | 0.11 (0.09–0.13) | 0.11 (0.09–0.13) | 1070.82 | − 3.17 (− 3.40) | 0.11 (0.09) |
Based on the frequency of PT occurrences and EBGM values, the top 30 ranks were listed in Tables 5 and 6, respectively. By occurrence frequency (Table 5), the top 10 PTs included Pruritus, Product use in unapproved indication, Rash, Product dose omission issue, Dermatitis atopic, Injection site pain, Product use issue, Eczema, Dry skin, and Condition aggravated. High incidence rates of Product use in unapproved indication, Product dose omission issue, Incorrect dose administered, Product dose omission in error, and Accidental exposure to product suggest serious issues with product indications and improper use and handling, which should be clinically emphasized. From the EBGM value ranking in Table 6, although AEs like Conjunctival papillae, Periorbital dermatitis, and Cutaneous T-cell lymphoma stage III were not highly frequent, their signal strength was significant, indicating they may be new potential AE signals.
Table 5.
The top 50 AEs of Dupilumab ranked by case reports.
| SOC | PTs | Case Reports | ROR (95% CI) | PRR (95% CI) | χ2 | IC (IC025) | EBGM (EBGM05) |
|---|---|---|---|---|---|---|---|
| Skin and subcutaneous tissue disorders | Pruritus | 19,061 | 7.13 (7.02–7.24) | 6.90 (6.80–7.00) | 86,178.74 | 2.64 (2.62) | 6.26 (6.16) |
| Injury, poisoning and procedural complications | Product use in unapproved indication | 18,805 | 7.07 (6.96–7.17) | 6.84 (6.74–6.94) | 84,079.06 | 2.63 (2.61) | 6.21 (6.11) |
| Skin and subcutaneous tissue disorders | Rash | 17,302 | 5.17 (5.09–5.25) | 5.02 (4.95–5.10) | 51,582.41 | 2.23 (2.21) | 4.69 (4.62) |
| Injury, poisoning and procedural complications | Product dose omission issue | 16,199 | 3.51 (3.45–3.57) | 3.43 (3.38–3.48) | 26,531.41 | 1.72 (1.69) | 3.29 (3.24) |
| Skin and subcutaneous tissue disorders | Dermatitis atopic | 14,885 | 557.25 (528.81–587.22) | 540.72 (513.20–569.72) | 756,704.62 | 5.69 (5.66) | 51.90 (49.25) |
| General disorders and administration site conditions | Injection site pain | 12,531 | 6.96 (6.83–7.09) | 6.81 (6.68–6.93) | 55,609.42 | 2.63 (2.60) | 6.18 (6.07) |
| Injury, poisoning and procedural complications | Product use issue | 10,575 | 7.38 (7.23–7.53) | 7.25 (7.10–7.39) | 50,616.65 | 2.71 (2.68) | 6.53 (6.40) |
| Skin and subcutaneous tissue disorders | Eczema | 8653 | 44.04 (42.81–45.29) | 43.29 (42.10–44.52) | 202,249.76 | 4.63 (4.60) | 24.91 (24.22) |
| Skin and subcutaneous tissue disorders | Dry skin | 8442 | 6.62 (6.47–6.77) | 6.53 (6.38–6.67) | 35,502.60 | 2.57 (2.54) | 5.95 (5.82) |
| General disorders and administration site conditions | Condition aggravated | 7653 | 2.95 (2.88–3.02) | 2.92 (2.85–2.99) | 9234.73 | 1.50 (1.46) | 2.83 (2.76) |
| Musculoskeletal and connective tissue disorders | Arthralgia | 7391 | 2.25 (2.20–2.31) | 2.24 (2.18–2.29) | 4888.89 | 1.13 (1.10) | 2.19 (2.14) |
| Eye disorders | Dry eye | 6125 | 21.29 (20.67–21.93) | 21.04 (20.44–21.67) | 85,186.01 | 3.96 (3.92) | 15.59 (15.14) |
| Injury, poisoning and procedural complications | Inappropriate schedule of product administration | 5887 | 2.49 (2.43–2.56) | 2.47 (2.41–2.54) | 4972.40 | 1.27 (1.23) | 2.41 (2.35) |
| Skin and subcutaneous tissue disorders | Skin exfoliation | 5494 | 7.30 (7.09–7.51) | 7.23 (7.03–7.43) | 26,176.67 | 2.70 (2.66) | 6.52 (6.34) |
| Skin and subcutaneous tissue disorders | Erythema | 5443 | 2.97 (2.89–3.05) | 2.95 (2.87–3.03) | 6679.33 | 1.51 (1.47) | 2.85 (2.77) |
| Eye disorders | Eye pruritus | 5389 | 30.11 (29.13–31.13) | 29.80 (28.84–30.80) | 98,140.13 | 4.30 (4.26) | 19.83 (19.19) |
| Respiratory, thoracic and mediastinal disorders | Asthma | 5297 | 6.34 (6.16–6.52) | 6.28 (6.11–6.46) | 21,199.14 | 2.52 (2.48) | 5.75 (5.59) |
| General disorders and administration site conditions | Injection site erythema | 4833 | 8.22 (7.97–8.47) | 8.15 (7.91–8.39) | 26,505.27 | 2.85 (2.81) | 7.24 (7.03) |
| General disorders and administration site conditions | Injection site swelling | 4823 | 12.06 (11.69–12.44) | 11.95 (11.59–12.33) | 39,965.84 | 3.32 (3.28) | 10.03 (9.73) |
| Psychiatric disorders | Sleep disorder | 4624 | 9.20 (8.91–9.49) | 9.12 (8.84–9.41) | 28,801.40 | 3.00 (2.95) | 7.99 (7.74) |
| Eye disorders | Ocular hyperaemia | 4486 | 15.53 (15.02–16.05) | 15.40 (14.90–15.92) | 47,468.25 | 3.62 (3.57) | 12.31 (11.91) |
| Injury, poisoning and procedural complications | Incorrect dose administered | 4309 | 2.43 (2.36–2.51) | 2.42 (2.35–2.50) | 3458.08 | 1.24 (1.19) | 2.36 (2.29) |
| General disorders and administration site conditions | Therapeutic response decreased | 4109 | 11.31 (10.94–11.70) | 11.23 (10.86–11.61) | 31,938.24 | 3.25 (3.20) | 9.53 (9.21) |
| Eye disorders | Eye irritation | 3843 | 10.50 (10.14–10.87) | 10.43 (10.08–10.79) | 27,660.10 | 3.16 (3.11) | 8.95 (8.65) |
| Infections and infestations | Conjunctivitis | 3791 | 38.59 (37.02–40.22) | 38.30 (36.76–39.91) | 81,991.11 | 4.53 (4.47) | 23.20 (22.26) |
| Eye disorders | Vision blurred | 3182 | 3.41 (3.29–3.54) | 3.40 (3.28–3.52) | 5082.17 | 1.70 (1.65) | 3.26 (3.14) |
| Injury, poisoning and procedural complications | Product dose omission in error | 3025 | 18.66 (17.91–19.44) | 18.55 (17.81–19.33) | 37,799.99 | 3.82 (3.76) | 14.20 (13.63) |
| Injury, poisoning and procedural complications | Accidental exposure to product | 2859 | 3.06 (2.95–3.18) | 3.05 (2.94–3.16) | 3739.16 | 1.56 (1.50) | 2.94 (2.83) |
| General disorders and administration site conditions | Discomfort | 2751 | 5.74 (5.51–5.97) | 5.71 (5.49–5.94) | 9712.91 | 2.40 (2.34) | 5.28 (5.07) |
| General disorders and administration site conditions | Injection site pruritus | 2743 | 7.71 (7.40–8.02) | 7.67 (7.37–7.98) | 14,011.42 | 2.78 (2.72) | 6.87 (6.60) |
Table 6.
The top signal strength of AEs of Dupilumab ranked by EBGM at the PTs level.
| SOC | PTs | Case reports | ROR (95% CI) | PRR (95% CI) | χ2 | IC (IC025) | EBGM (EBGM05) |
|---|---|---|---|---|---|---|---|
| Skin and subcutaneous tissue disorders | Rebound atopic dermatitis | 250 | 2011.95 (949.32–4264.04) | 2010.94 (948.85–4261.87) | 13,679.69 | 5.51 (5.26) | 55.75 (26.30) |
| Skin and subcutaneous tissue disorders | Rebound eczema | 329 | 772.37 (510.32–1168.99) | 771.87 (510.00–1168.20) | 17,220.82 | 5.52 (5.30) | 53.41 (35.29) |
| Skin and subcutaneous tissue disorders | Dermatitis atopic | 14,885 | 557.25 (528.81–587.22) | 540.72 (513.20–569.72) | 756,704.62 | 5.69 (5.66) | 51.90 (49.25) |
| Eye disorders | Conjunctival papillae | 6 | 168.92 (34.09–836.95) | 168.92 (34.09–836.94) | 250.39 | 2.60 (1.14) | 42.98 (8.67) |
| Eye disorders | Periorbital irritation | 104 | 91.52 (67.03–124.95) | 91.50 (67.02–124.92) | 3546.27 | 4.73 (4.37) | 35.48 (25.98) |
| Eye disorders | Periorbital dermatitis | 10 | 80.44 (30.62–211.33) | 80.44 (30.62–211.32) | 323.03 | 3.07 (1.96) | 33.71 (12.83) |
| General disorders and administration site conditions | Injection site dryness | 261 | 70.02 (58.38–83.97) | 69.98 (58.36–83.92) | 7912.68 | 4.83 (4.60) | 31.76 (26.48) |
| Surgical and medical procedures | Nasal polypectomy | 45 | 66.68 (43.30–102.70) | 66.68 (43.30–102.69) | 1332.85 | 4.22 (3.69) | 31.07 (20.17) |
| General disorders and administration site conditions | Injection site exfoliation | 198 | 65.22 (53.15–80.03) | 65.20 (53.13–80.00) | 5800.18 | 4.74 (4.48) | 30.75 (25.06) |
| General disorders and administration site conditions | Injection site eczema | 54 | 64.70 (43.76–95.66) | 64.69 (43.76–95.64) | 1575.77 | 4.31 (3.82) | 30.64 (20.72) |
| Eye disorders | Eczema eyelids | 123 | 64.14 (49.53–83.06) | 64.13 (49.52–83.04) | 3573.61 | 4.62 (4.30) | 30.51 (23.56) |
| Eye disorders | Eyelid skin dryness | 297 | 51.17 (43.73–59.88) | 51.14 (43.71–59.84) | 7651.43 | 4.65 (4.44) | 27.28 (23.31) |
| Eye disorders | Atopic keratoconjunctivitis | 10 | 46.92 (20.27–108.61) | 46.92 (20.27–108.60) | 245.15 | 2.97 (1.91) | 26.05 (11.25) |
| Skin and subcutaneous tissue disorders | Neurodermatitis | 292 | 45.70 (39.16–53.33) | 45.67 (39.14–53.29) | 7044.59 | 4.56 (4.36) | 25.66 (21.99) |
| Skin and subcutaneous tissue disorders | Eczema | 8653 | 44.04 (42.81–45.29) | 43.29 (42.10–44.52) | 202,249.76 | 4.63 (4.60) | 24.91 (24.22) |
| Infections and infestations | Conjunctivitis | 3791 | 38.59 (37.02–40.22) | 38.30 (36.76–39.91) | 81,991.11 | 4.53 (4.47) | 23.20 (22.26) |
| Eye disorders | Ocular surface disease | 67 | 38.11 (27.95–51.97) | 38.11 (27.95–51.96) | 1443.79 | 4.12 (3.70) | 23.13 (16.96) |
| Eye disorders | Periorbital inflammation | 50 | 37.05 (25.93–52.94) | 37.04 (25.92–52.93) | 1057.69 | 3.99 (3.51) | 22.74 (15.91) |
| Eye disorders | Eyelids pruritus | 514 | 36.90 (33.02–41.25) | 36.87 (32.99–41.20) | 10,839.51 | 4.44 (4.29) | 22.68 (20.29) |
| Respiratory, thoracic and mediastinal disorders | Chronic rhinosinusitis with nasal polyps | 18 | 36.20 (20.02–65.44) | 36.20 (20.02–65.44) | 374.98 | 3.38 (2.60) | 22.42 (12.40) |
| Ear and labyrinth disorders | Ear dryness | 7 | 35.83 (13.89–92.43) | 35.83 (13.89–92.43) | 144.84 | 2.59 (1.37) | 22.29 (8.64) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | Cutaneous T-cell lymphoma stage III | 7 | 35.83 (13.89–92.43) | 35.83 (13.89–92.43) | 144.84 | 2.59 (1.37) | 22.29 (8.64) |
| Eye disorders | Vernal keratoconjunctivitis | 10 | 35.19 (15.97–77.55) | 35.19 (15.97–77.55) | 204.43 | 2.90 (1.87) | 22.04 (10.00) |
| Eye disorders | Giant papillary conjunctivitis | 8 | 34.65 (14.36–83.60) | 34.65 (14.36–83.60) | 161.84 | 2.70 (1.56) | 21.83 (9.05) |
| Skin and subcutaneous tissue disorders | Skin weeping | 540 | 34.20 (30.73–38.06) | 34.16 (30.70–38.02) | 10,819.98 | 4.38 (4.23) | 21.64 (19.45) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | Cutaneous T-cell lymphoma stage IV | 12 | 33.78 (16.52–69.11) | 33.78 (16.52–69.11) | 238.60 | 3.04 (2.10) | 21.49 (10.51) |
| Skin and subcutaneous tissue disorders | Eczema weeping | 18 | 31.67 (17.78–56.43) | 31.67 (17.78–56.42) | 342.19 | 3.33 (2.55) | 20.63 (11.58) |
| Eye disorders | Eye pruritus | 5389 | 30.11 (29.13–31.13) | 29.80 (28.84–30.80) | 98,140.13 | 4.30 (4.26) | 19.83 (19.19) |
| Eye disorders | Eyelid rash | 175 | 28.49 (23.75–34.17) | 28.48 (23.74–34.16) | 3081.45 | 4.12 (3.87) | 19.25 (16.05) |
| Surgical and medical procedures | Lung volume reduction surgery | 3 | 28.15 (7.04–112.57) | 28.15 (7.04–112.57) | 52.38 | 1.77 (0.06) | 19.10 (4.78) |
When comparing with prior clinical trial data23,24, the incidence of common AEs like Pruritus, Rash, and Dermatitis atopic in our FAERS—based analysis was generally in line with what was reported. For example, Castro et al.'s study on Dupilumab in moderate—to—severe asthma patients showed that skin—related AEs were frequently observed. However, the frequency of “Product use in unapproved indication” and “Product dose omission issue” was much higher in our FAERS data, which might be due to the real—world nature of the database, reflecting issues in clinical practice that may not be fully captured in controlled trials. Regarding the newly identified potential AEs such as Dry eye, Eye pruritus, and Ocular hyperaemia, a review of previous post—marketing studies25 found some scattered reports, but the signal strength and frequency in our study were more prominent.
Discussion
Bronchial asthma is a heterogeneous disease characterized by chronic airway inflammation, airway hyperresponsiveness, and variable airflow obstruction. Due to issues such as air pollution, the incidence of asthma has been increasing globally26. It is estimated that there are at least 300 million asthma patients worldwide, with the 2019 China Pulmonary Health (CPA) study showing an overall asthma prevalence of 4.2% in China, equating to approximately 45.7 million patients27. Currently, asthma control levels in China are not ideal, with an overall control rate of only 28.5%28. High doses of inhaled corticosteroids (ICS) and/or oral corticosteroids (OCS) are crucial for controlling severe asthma, yet about 44% of severe asthma patients fail to achieve effective control, leading to increased risks of osteoporosis, cataracts, and other related complications. This situation consumes significant medical resources and expenses, placing a heavy burden on patients’ families and society28.
Research indicates that IL-4 and IL-13 are key targets driving diseases such as asthma, atopic dermatitis, allergic rhinitis, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, and nodular prurigo29. The interaction of IL-4 and IL-13 with the IL-4Ra receptor subunit can stimulate the production of immunoglobulin E and airway remodeling mediators. Additionally, IL-13 regulates nitric oxide production in the respiratory system, increases mucus production, and enhances smooth muscle contractility30. Dupilumab, a fully human monoclonal antibody (IgG4 type), inhibits IL-4 and IL-13 signaling by specifically binding to the IL-4Rα subunit shared by IL-4 and IL-13 receptor complexes, blocking IL-4Rα and inhibiting the inflammatory response induced by these cytokines, including the release of pro-inflammatory cytokines, chemokines, nitric oxide, and IgE. However, the exact mechanism of Dupilumab is not yet fully understood. Currently, Dupilumab is indicated for maintenance treatment of asthma in adolescents and adults aged 12 years and older, particularly for: (1) patients with asthma characterized by type 2 inflammation (elevated eosinophils and/or FeNO) who remain inadequately controlled despite medium- to high-dose ICS combined with other asthma control medications; (2) OCS-dependent asthma patients. The 2020 GINA guidelines recommend biologics like Dupilumab for step 5 asthma treatment, emphasizing its ability to reduce acute exacerbations and lower ICS doses31.
A phase III randomized, double-blind, placebo-controlled study32 in patients aged 12 years and older with uncontrolled moderate-to-severe asthma used 200 mg Dupilumab (loading dose 400 mg) or 300 mg Dupilumab (loading dose 600 mg) every 2 weeks for 52 weeks. The results showed that Dupilumab significantly reduced the annual exacerbation rate of moderate-to-severe asthma and comorbid perennial allergic rhinitis (PAR), rapidly and persistently improved FEV1, and enhanced asthma control as measured by the Asthma Control Questionnaire (ACQ-5). Additionally, Dupilumab significantly reduced specific IgE concentrations in patients with baseline allergen-specific IgE positivity compared to placebo, supporting its inhibitory effect on IgE-mediated allergic inflammatory responses32.
Dupilumab was only approved for asthma indication in China in 2023, and the number of clinical treatment cases remains limited. Cohort studies on Dupilumab for asthma treatment in China are rare. According to a study by Li et al.33 on 20 severe asthma patients treated with Dupilumab at the First Affiliated Hospital of Guangzhou Medical University from 2019 to 2022, the treatment showed good initial efficacy for type 2 inflammation phenotype severe asthma. Patients showed significant improvements in asthma control levels and lung function compared to before treatment, along with a significant reduction in OCS dosage, consistent with the findings of Castro M et al.3. For patients with a history of biological therapy, whether they responded well or poorly to monoclonal antibodies, switching to Dupilumab for four months resulted in significant improvements in asthma control levels and lung function, along with a significant reduction in OCS dosage, all with statistical significance33. Additionally, the study33 found few adverse reactions to Dupilumab, with observed reactions including significant eosinophilia (2 cases), but no evidence of organ damage due to elevated eosinophils. Other reactions were mainly injection site rashes (3 cases), headaches (1 case), which were alleviated with antihistamines or rest, with no other serious adverse reactions observed.
This study conducted an in-depth analysis of FAERS database data from Q2 2017 to Q4 2023, identifying a series of AE signals related to Dupilumab. The clinical relevance of these identified signals will be discussed in detail, assessing their impact on existing treatment guidelines, and proposing improvements to data quality.
Analysis of Dupilumab report composition
This study found that the number of reports from female patients exceeded those from male patients. This may reflect a higher prevalence of bronchial asthma in females or differences in drug reactions or reporting behavior between genders. The increasing trend in the number of reports over the years could be associated with a rise in drug usage, increased public awareness of reporting, or improvements in the reporting system. Physicians accounted for more than half of the reporting sources, indicating their higher awareness of drug reactions. The significantly higher proportion of reports from the United States may reflect its robust drug monitoring system and high public participation. Most AE reports were classified as non-serious, suggesting a favorable safety profile for Dupilumab in clinical treatment.
Regarding the timing of AEs, a significant proportion occurred within 30 days of medication use, indicating the need for close monitoring of patient reactions during the initial treatment period. Additionally, the possibility of AEs occurring after long-term use underscores the importance of long-term follow-up. However, the lack or abnormality of AE timing in 85.64% of the reports highlights issues with data completeness and accuracy, potentially affecting our comprehensive understanding of Dupilumab’s safety. These findings provide critical information about the safety profile of Dupilumab and emphasize the need to improve drug monitoring systems, particularly in data collection methods and reporting quality.
Known AEs
This study identified 307 PTs across 27 SOCs for Dupilumab through signal mining in the FAERS database. The incidence of AEs in most SOC categories matched the information recorded in Dupilumab’s package insert. In the PT frequency rankings, the high frequency of "Product use in unapproved indication" and "Product dose omission issue" is particularly notable, ranking second and fourth among all AEs, respectively. This indicates a widespread issue of inappropriate use of Dupilumab, highlighting the need for it to be prescribed by healthcare professionals with experience in diagnosing and treating its indications to prevent misuse and avoid missed doses. Additionally, the high frequency of AEs such as pruritus, rash, and atopic dermatitis aligns with common comorbidities in bronchial asthma patients, further validating the accuracy of the data provided by the pharmaceutical manufacturers. When comparing with JAK inhibitors used in atopic dermatitis, although both are systemic therapies, their AE profiles show some differences. For example, JAK inhibitors may have unique AEs related to their mechanism of action, such as an increased risk of infections due to immunosuppression34,35. In contrast, Dupilumab has a different mechanism targeting IL-4Rα, and its AEs, like the newly discovered eye—related AEs, may be related to abnormal immune regulation in specific tissues.
Biological mechanisms and clinical impact of newly discovered potential AEs
Eye-related AEs
Eye-related AEs such as Dry eye, Ocular hyperaemia, Eye irritation, Conjunctivitis, and Vision blurred are common with Dupilumab. The mechanisms are not entirely clear but may include:Abnormal immune regulation of the ocular mucosa, where Th2 pathway inhibition by Dupilumab could lead to increased Th1/Th17 polarization, exacerbating Th1/Th17-dependent immune responses36. This includes an increase in peripheral blood eosinophils, a hallmark of allergic conjunctivitis. Dupilumab blocks IL-4 and IL-13 signaling, increasing the activity of ligands like OX40 ligand associated with allergic ocular surface diseases, which are related to conjunctival papillary inflammatory responses37. A study by Bakker et al. on conjunctival biopsy specimens from patients with Dupilumab-related conjunctivitis revealed a significant decrease in conjunctival epithelial goblet cells and an increase in T cells and eosinophils. This suggests that Dupilumab could reduce goblet cells and mucin, destabilizing the tear film and impairing the mucosal epithelial barrier function38. However, goblet cell density normalized after discontinuing the drug for four months39. Thyssen et al.40 suggested that Dupilumab might exacerbate demodex infestation and IL-17 mediated ocular inflammation, causing meibomian gland dysfunction.These findings indicate a need to monitor ocular reactions during Dupilumab treatment and conduct timely ophthalmologic examinations for new or worsening eye symptoms.
Disease exacerbation and reduced therapeutic response
Like all therapeutic proteins, Dupilumab may be immunogenic, leading to disease exacerbation and reduced therapeutic response. In a study by Castro et al.3, involving 1,264 adults with inadequately controlled moderate-to-severe asthma, 52 reported elevated blood eosinophil levels, and 4 experienced serious AEs such as eosinophilia and chronic eosinophilic pneumonia exacerbation, leading to discontinuation. This may be explained by the persistence of Th2A cell clusters producing eosinophil chemoattractant IL-541.
Drug use-related AEs
High incidences of Product use in unapproved indication and Product dose omission in error indicate that physicians may lack experience with Dupilumab, necessitating further education on its indications and contraindications. Issues like Product dose omission issue, Product use issue, and Inappropriate schedule of product administration suggest a need for better communication with patients about correct, regular, and sufficient drug use. Some issues might stem from patient non-compliance, such as forgetting injections or intentionally reducing doses due to concerns about side effects or misunderstanding the drug’s effects. Educating patients on asthma and Dupilumab can enhance treatment adherence, reduce oral steroid use, lower asthma exacerbation rates, and improve quality of life.
Sleep-related AEs
While Dupilumab was the first biologic approved for atopic dermatitis, showing excellent results in improving pruritus and sleep within two weeks, it may paradoxically affect sleep in some cases. The incidence of sleep-related side effects is currently unknown. A study by Alroobaea et al.42 using data from the WHO VigiBase™ database found that sleep-related AEs were rare, with only general sleep disorders and nighttime breathing difficulties showing statistical significance, suggesting that Dupilumab may not improve sleep quality in all patients, highlighting the need for individualized treatment/management.
Limitations
Despite providing important data for the safety assessment of Dupilumab, this study has limitations. The FAERS database relies on spontaneous reports, which may contain reporting biases, including but not limited to completeness, accuracy, and timeliness, potentially affecting the representativeness and generalizability of conclusions. To reduce reporting bias, we took several approaches. First, we cross—referenced the FAERS data with other available data sources when possible. For example, we compared the AE reports related to Dupilumab in FAERS with data from clinical trials and observational studies in the literature to identify any discrepancies or additional patterns. Second, we developed a standardized review process for the collected reports. A team of researchers with expertise in pharmacovigilance and dermatology reviewed each report to ensure that the information was accurately interpreted and classified. This process helped to minimize misclassification of AEs and improved the reliability of the data. However, it should be noted that these methods have their own limitations, and future studies should focus on more rigorous case verification processes and validation analyses using other databases to enhance the reliability of findings.
Conclusion
This study provides a comprehensive analysis of AEs associated with Dupilumab use, revealing that most related AEs are non-serious and occur mainly within 30 days of drug use. Although Dupilumab shows good efficacy for bronchial asthma in clinical trials, issues such as Pruritus, Product use in unapproved indication, Rash, Product dose omission issue, Dermatitis atopic, Injection site pain, Product use issue, Eczema, Dry skin, and Condition aggravated have been identified. The high incidence of Product use in unapproved indication, Product dose omission issue, Incorrect dose administered, and Product dose omission in error underscores the serious issues of inappropriate use and operation, warranting clinical attention. These findings emphasize the importance of detailed patient monitoring during Dupilumab use and the necessity of individualized treatment plans.
To better manage patients on Dupilumab, several actionable guidance measures are recommended. For monitoring strategies, a baseline ophthalmological examination should be conducted before starting treatment, including assessments of visual acuity, tear film stability, and conjunctival and corneal status. During treatment, patients should be regularly asked about eye symptoms at each follow-up visit, and a comprehensive ophthalmological examination should be arranged promptly if any issues are reported. In addition to eye—related monitoring, vital signs and general conditions should be closely observed during the initial 30—day treatment period and long—term follow—up, with appropriate laboratory tests performed based on the patient’s condition.
Regarding patient education, healthcare providers should ensure patients fully understand the correct injection technique, the importance of adhering to the prescribed dosing schedule, and the approved indications of Dupilumab. Written educational materials can be provided, and patients should be encouraged to ask questions. Regular follow—up appointments are also crucial for reinforcing this education.
For the early identification and management of AEs, patients should be educated to recognize the early signs of potential AEs, especially eye—related symptoms like dryness, itching, or redness, and report them immediately. Once reported, healthcare providers should conduct a timely evaluation. Mild AEs such as mild injection—site reactions or transient skin rashes can be managed with simple supportive measures, while severe AEs like severe allergic reactions or disease exacerbations require immediate medical intervention. These measures can help improve the safety and effectiveness of Dupilumab treatment in clinical practice.
Acknowledgements
This study was performed using the FAERS source that was provided by the FDA. The information, results, or interpretation of the current study do not represent any opinion of the FDA.
Author contributions
Hui Gao and Chengying Liu conceived the study; Hui Gao, Liqiang Cao and Chengying Liu collected the report; Hui Gao and Chengying Liu wrote the manuscript and edited the manuscript. All authors have approved publishment of the manuscript.
Data availability
The dataset generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matsunaga, K. et al. Dupilumab: Basic aspects and applications to allergic diseases. Allergol Int69(2), 187–196 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Holguin, F., et al. Management of severe asthma: A European respiratory Society/American thoracic society guideline. Eur Respir J. 2020. 55(1). [DOI] [PubMed]
- 3.Castro, M. et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med378(26), 2486–2496 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Rabe, K. F. et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med378(26), 2475–2485 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Corren, J. et al. Dupilumab improves asthma outcomes irrespective of frequency of previous asthma exacerbation history. Ann Allergy Asthma Immunol123(2), 222-224.e1 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Numata, T. et al. Real-world effectiveness of dupilumab for patients with severe asthma: A retrospective study. J Asthma Allergy15, 395–405 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagino, T. et al. Effectiveness of switching from baricitinib 4 mg to upadacitinib 30 mg in patients with moderate-to-severe atopic dermatitis: a real-world clinical practice in Japan. J. Dermatol. Treatment34(1), 2276043 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Hagino, T. et al. Long-term effectiveness and safety of upadacitinib for Japanese patients with moderate-to-severe atopic dermatitis: a real-world clinical study. J. Dermatol. Treatment35(1), 2344591 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Hagino, T., Saeki, H,, Fujimoto, E., et al. Effectiveness and safety of upadacitinib retreatment after withdrawal in moderate-to-severe atopic dermatitis: a retrospective study. Clin. Exp. Dermatol., 2024: llae410. [DOI] [PubMed]
- 10.Hagino, T. et al. Effectiveness of dose increase in upadacitinib from 15 mg to 30 mg for patients with moderate-to-severe atopic dermatitis: A Real-world clinical practice in Japan. Clin. Drug Investig.44(4), 261–269 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Zhu, H. et al. Mining and analysis of adverse event signals of Cariprazine based on the real-world data of FAERS database. J. Affect. Disorders347, 45–50 (2024). [DOI] [PubMed] [Google Scholar]
- 12.Jiang, Y. et al. Analysis of post-market adverse events of istradefylline: a real-world study base on FAERS database. Sci. Rep.14(1), 7659 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang, Y. et al. Unveiling the adverse events of Nusinersen in spinal muscular atrophy management based on FAERS database. Sci. Rep.14(1), 17138 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu, R. et al. Multidimensional assessment of adverse events of bupropion: A large-scale data analysis from the FAERS database. J. Affect. Disorders354, 649–655 (2024). [DOI] [PubMed] [Google Scholar]
- 15.Zhou, Q. et al. Adverse events of epidiolex: a real-world drug safety surveillance study based on the FDA adverse event reporting system (FAERS) database. Asian J. Psychiatry90, 103828 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Jiang, Y., Du, Z., Shen, Y., et al. The correlation of Esketamine with specific adverse events: a deep dive into the FAERS database. Eur. Arch. Psychiatry Clin. Neurosci., 2023: 1–9. [DOI] [PubMed]
- 17.Jiang, Y. et al. Safety assessment of Brexpiprazole: Real-world adverse event analysis from the FAERS database. J. Affect. Disorders346, 223–229 (2024). [DOI] [PubMed] [Google Scholar]
- 18.Li, Z., Gu, J., Du, Z., et al. Characteristics of adverse events and clinical risks of Lecanemab based on FAERS data. J. Affect. Disorders, 2025. [DOI] [PubMed]
- 19.Jiang, Y. et al. Exploring adverse events of Vilazodone: Evidence from the FAERS database. BMC Psychiatry24(1), 371 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, Q. et al. Clinical safety of daridorexant in insomnia treatment: Analysis of FDA adverse event reports. J. Affect. Disorders362, 552–559 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Jiang, Y. et al. Unveiling potential adverse events associated with escitalopram oxalate: A real-world analysis based FDA adverse event reporting system database. J. Psychopharmacol.38(6), 567–578 (2024). [DOI] [PubMed] [Google Scholar]
- 22.Wang, Q., Qu, K., Du, Z., et al. Mining and analysis of security alert signals of valbenazine based on the Food and Drug Administration Adverse Event Reporting System database. J. Psychopharmacol., 2024: 02698811241248391. [DOI] [PubMed]
- 23.Castro, M. et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N. Engl. J. Med.378(26), 2486–2496 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Rabe, K. F. et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N. Engl. J. Med.378(26), 2475–2485 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Alroobaea, R., Rubaiee, S., Hanbazazah, A. S., et al. IL-4/13 Blockade and sleep-related adverse drug reactions in over 37,000 Dupilumab reports from the World Health Organization Individual Case Safety reporting pharmacovigilance database (VigiBase™): A big data and machine learning analysis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26(11). [DOI] [PubMed]
- 26.Global Initiative for asthma. Global Strategy for Asthma Management and Prevention, 2021. www.ginasthma.org.
- 27.Huang, K. et al. Prevalence, risk factors, and management of asthma in China: A national cross-sectional study. Lancet394(10196), 407–418 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Zhong, N. et al. Uncontrolled asthma and its risk factors in adult Chinese asthma patients. Ther Adv Respir Dis10(6), 507–517 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynn, T. A. Type 2 cytokines: Mechanisms and therapeutic strategies. Nat Rev Immunol15(5), 271–282 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Charles, D. et al. Real-world efficacy of treatment with benralizumab, dupilumab, mepolizumab and reslizumab for severe asthma: A systematic review and meta-analysis. Clin Exp Allergy52(5), 616–627 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petsky, H. L., Li, A. and Chang, A. B. Tailored interventions based on sputum eosinophils versus clinical symptoms for asthma in children and adults. Cochrane Database Syst. Rev., 2017. 8(8): CD005603. [DOI] [PMC free article] [PubMed]
- 32.Busse, W. W. et al. Efficacy of dupilumab on clinical outcomes in patients with asthma and perennial allergic rhinitis. Ann Allergy Asthma Immunol125(5), 565-576.e1 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Lin H, Zhang Xn, et al. Preliminary clinical observation on the efficacy of Dupilumab in the treatment of severe asthma. Chin. J. Tuberculosis Respir. Dis. 2023, 46(9): 909–915. [DOI] [PubMed]
- 34.Mansilla-Polo, M., Morgado-Carrasco, D. Biologics Versus JAK Inhibitors. Part II: Risk of Infections. A narrative review. Dermatol. Ther. 2024, 14(8): 1983–2038. [DOI] [PMC free article] [PubMed]
- 35.Alves, C., Penedones, A., Mendes, D., et al. The risk of infections associated with JAK inhibitors in rheumatoid arthritis: a systematic review and network meta-analysis. JCR: J. Clin. Rheumatol. 2022, 28(2): e407–e414. [DOI] [PubMed]
- 36.Jo, C. E. et al. Facial and neck erythema associated with dupilumab treatment: A systematic review. J Am Acad Dermatol84(5), 1339–1347 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Shen, E. et al. Dupilumab-induced follicular conjunctivitis. Ocul Immunol Inflamm27(8), 1339–1341 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Bakker, D. S. et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol180(5), 1248–1249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voorberg, A. N. et al. Recurrence of conjunctival goblet cells after discontinuation of dupilumab in a patient with dupilumab-related conjunctivitis. J Eur Acad Dermatol Venereol34(2), e64–e66 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Thyssen, J. P. et al. Conjunctivitis in atopic dermatitis patients with and without dupilumab therapy-international eczema council survey and opinion. J Eur Acad Dermatol Venereol33(7), 1224–1231 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bangert, C., et al., Persistence of mature dendritic cells, T(H)2A, and Tc2 cells characterize clinically resolved atopic dermatitis under IL-4Ralpha blockade. Sci. Immunol. 2021. 6(55). [DOI] [PubMed]
- 42.Alroobaea, R. et al. IL-4/13 Blockade and sleep-related adverse drug reactions in over 37,000 Dupilumab reports from the World Health Organization Individual Case Safety reporting pharmacovigilance database (VigiBase): A big data and machine learning analysis. Eur Rev Med Pharmacol Sci26(11), 4074–4081 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated during and analyzed during the current study are available from the corresponding author on reasonable request.