Abstract
Human language develops in social interactions. In other ape species, the role of social learning in vocal ontogeny can be typically underappreciated, mainly because it has received little empirical attention. Here, we examine the development of pant hoot vocalisations during vocal exchanges in immature wild chimpanzees (Pan troglodytes schweinfurthii) of the Sonso community of the Budongo Forest, Uganda. We investigated how maternal gregariousness, age, sex, and social context are associated with behavioural and vocal responses to other group members’ calls. We show that the older sons of gregarious mothers are more likely to orient their attention, respond vocally to the calls of others, and are overall more exposed to others’ calls compared to other immature individuals. This effect is strongest in the presence of adult males and when their mothers also respond vocally, suggesting that chimpanzee vocal development is enhanced by social and vocal exposure. Our findings are consistent with a more flexible and socially mediated chimpanzee vocal ontogeny than previously assumed and show some parallels with animal vocal learners and children language acquisition.
Subject terms: Biological anthropology, Animal behaviour
Introduction
Language is arguably one of the key features that distinguish humans from other animal species, a fact that continues to foster scientific debate and major research efforts1. Language does not leave direct fossil traces, but comparative research on other species’ communicative systems can provide insights about possible precursor stages and determine whether spoken language has emerged during our evolutionary history as a continuous process2. The vocal communication of non-human primates, our closest living relatives, is of particular research interest and is critical to study precursors shared with other systems of communication in biologically closely related species3. Although this approach has turned out to be very productive, so far it is typically based on research conducted on adult individuals that are already fully-competent communicators4. However, considerably less researched historically and only recently experiencing more attention5,6, but equally important for understanding of the evolution of spoken language, is the evolution of vocal ontogeny in non-human animals, i.e., the learning processes that drive vocal communication from birth to adulthood and the cognitive apparatus responsible for it7.
An effective way of conceptualising vocal development is distinguishing between three processes of learning: (1) how to produce calls (production learning); (2) how to use calls appropriately (usage learning), and (3) how to form appropriate correspondence between calls and their meaning (comprehension or response learning)8,9. Primate vocal development has long been argued to be rather inflexible in these three domains (but see9), especially in terms of production learning10, discouraging research on vocal learning in apes1,11, and leading to the consensus that socially learned vocal communication has likely emerged through convergent evolution in a limited number of biologically distant species or orders (e.g., bats, cetaceans, elephants, and songbirds12).
In humans, social interactions and social feedback are essential for language learning13. Initially, human infants undergo a perceptual learning phase that precedes vocal production learning and during which they selectively attend to auditory or visual communicative signals produced by others14. Greater social experience and exposure to speech provide infants with more communicative opportunities, accelerating their linguistic development15, for example enhancing vocal production and usage learning when exposed to mothers’ responses16 and response learning when infants visually attend to the vocalizations of others14. Socially mediated vocal development has also been documented in animals, though the evidence is mostly from birds, with relatively few studies on mammals and limited evidence in primates12,17. A notable exception is research conducted on marmosets demonstrating that infants learn when to use and how to produce vocalisations through vocal feedback and auditory exposure from their caregivers (common marmosets, Callithrix jacchus18; pygmy marmosets, Cebuella pigmaea19). In contrast, there is a paucity of comparable evidence from great apes, despite increasing evidence for nuanced and flexible adult vocal behaviour20.
Here, we seek to fill in this gap by examining chimpanzee vocal ontogeny for the following reasons. First, among primates, there are fewer studies on great ape vocal ontogeny, which are particularly relevant for studies of language evolution given that they are our closest living relatives21. Second, several key studies on primate vocal development have been conducted in captivity or in artificial settings22,23, where individuals might not express the same social behaviours as in the wild as a result of altered socio-ecological conditions (e.g., restricted spaces, atypical group size and composition)24. Third, many developmental studies have been carried out within experimental and atypical social contexts, including social isolation (squirrel monkeys, Saimiri sciureus25), deafening (squirrel monkeys26), or cross-fostering (rhesus and japanese macaques, Macaca mulatta and M. muscata23). While these studies offered an opportunity to control some potentially influential external factors, they may have introduced limitations on the possibility of detecting social learning and are not ethically permissible on wild great apes.
Regarding vocal ontogeny, a first prerequisite concerns the ability to socially learn vocal production and usage by attending to others’ vocal behaviour, which has been demonstrated in some mammals8,17, including in monkeys18. Infant marmosets who receive more social feedback through parental responses learn to produce calls in the adult form earlier27,28, while vervet monkeys refine the correct use of alarm calls benefitting from exposure to the vocal behaviour of mature group members, who may act as positive reinforcement29,30. Furthermore, vervet infants exposed to intergroup vocal encounters at higher rates learn to produce the appropriate call earlier than those exposed at lower rates31. Although evidence of socially acquired vocalisations in adult great apes has been debated (chimpanzees, Pan troglodytes32–35), a notable exception is a recent study on wild orangutans (Pongo pygmaeus wurmbii and P. abelii) showing that the degree of an individual’s gregariousness predicts their vocal output and structure36. For comprehension or response learning, a prerequisite in both human infants37 and songbirds38 is a preference for attending to conspecific vocalisations. Across several primate species, infants’ responses to others’ vocalizations differ markedly from adults’ responses9. Young vervet monkeys and sooty mangabeys (Cercocebus atys) have the opportunity to learn the referential meaning of alarm calls by attending to the responses of adult conspecifics30,39. There is good evidence that adult primates can extract relevant social information from attending to calls, such as the arrival of a social partner40, which is arguably learned from experience of interacting with others. Critically, how immature chimpanzees acquire this capacity is not known.
Given a long period of dependency on their mother that continues after weaning, the early socialization and learning opportunities of immature chimpanzees depend almost entirely on their mothers’ social associations41,42, and there is rich potential for social factors to play a role in the ontogeny of vocal behaviour. For example, the appropriate use of alarm calls is acquired progressively in young chimpanzees, potentially facilitated by exposure to gaze alternations with their mothers43. High fission-fusion societies, such as those of chimpanzees, are characterised by a dynamic social system in which mature individuals navigate a complex network of kin and non-kin social relationships. Long-distance pant hoot calls and exchanges between parties that are out of visual contact are critical to learn to maintain spatial and social cohesion44,45. When compared to human infants, infant chimpanzees produce spontaneous vocalizations less frequently46 and very rarely produce long-distance calls47,48. Apart from early qualitative studies49,50, a limited amount of systematic research on vocal development in immature chimpanzees is available43,51.
In this study, we focus on the pant hoot vocalisation, a structurally complex sequence of four acoustically distinct phases typically produced in an orderly way and the most frequently uttered vocalisation by adults47. Pant hoots are produced spontaneously (i.e., not in response to others’ calls), as part of vocal choruses, or in response to others’ pant hoots during vocal exchanges45,47,49,52,53. Individuals use these long-distance contact calls to maintain social cohesion, helping to coordinate with and recruit conspecifics across a wide range of contexts, including feeding, travelling, displaying, and inter-community encounters44,45,54. Adult females pant hoot less frequently than adult males overall and are more likely to join others’ calls than to call spontaneously55,56, while immature individuals of both sexes are very rarely observed pant hooting47. Despite a long research tradition of this field57, little is known about the development of pant hoots ontogeny. Recently Soldati et al.58 showed that chimpanzees are capable of producing spontaneous but rudimentary pant hoot-like calls from birth, which likely undergo further developmental changes including the production of build-up and let-down phases. Bründl et al.59 showed that greater maternal gregariousness is positively associated with the offspring’s use of pant-hoots, but only in the first two years of life. We extend this work to examine how immature chimpanzees develop communicative competence as both signallers and receivers during pant hoot exchanges until they become sexually mature and socially independent. We initially compared the spontaneous call rate (usage learning) and response patterns (response learning) during vocal exchanges of mature and immature individuals to establish their developmental changes. Then, we examined the role of mother’s vocal and social behaviors on the immature responsiveness towards pant hoots from group members. First, we predicted that the offspring of more gregarious mothers, exposed to more social interactions, would show greater responsiveness to others’ pant hoot calls. Second, we predicted greater responsiveness with increasing age. Third, since pant hoots are most frequently used by adult males for spatial coordination and during social interactions, we predicted that immature males would show greater responsiveness than immature females. Fourth, given the importance of pant hoot chorusing between closely bonded male social partners52, we predicted that immature individuals would vocally respond more often when their mother vocally responded as well. Fifth, due to the effects of nearby individuals on pant hoot usage54, we predicted that responses would vary depending on the number of males and females in the audience.
Results
Comparison with mature chimpanzee spontaneous call rates
Spontaneous pant hoot production rates of mature individuals (mean = 0.48 ± 0.36 per hour) were approximately 10 times greater than those of immatures (mean = 0.05 ± 0.08 per hour) (W = 30.5, p < 0.001). Mature males produced pant hoots spontaneously at a higher rate (mean 0.74 = 0.35 ± per hour) than mature females did (mean = 0.23 ± 0.20 per hour). Immatures rarely produced pant hoots that were not in response to others’ pant hoots (n = 9 instances) and we only observed calls produced spontaneously by immature males (N = 4 individuals). Immature chimpanzees produced pant hoots in response (n = 36) four times more frequently than they produced pant hoots spontaneously (n = 9) (Table S1), while mature chimpanzees produced pant hoots spontaneously (n = 258) and in response (n = 278) with similar rates (Table S2).
Responsiveness of immatures to others’ Pant hoots
We recorded a total of 554 behavioural responses to others’ pant hoots produced by 13 immature chimpanzees (Supplementary Tables S1 & S2). The most frequently recorded response was a head movement towards a pant hoot (35.7%, n = 198) and the second was a vocal response (9.2%, n = 51). All immatures responded with a head movement or a vocalisation at least once. The youngest individuals who responded with a head movement and vocalisation were observed at 16 months and 19 months of age respectively. Of all the vocal responses recorded, 70.6% were pant hoots (n = 36). After hearing a pant hoot from others, immatures either responded with a pant hoot alone (n = 24) or mothers and immatures joined each other’s pant hoot response as part of a chorus (n = 12), including the pant-hoot response of the youngest individual. On occasions where the mother and offspring chorused with each other, the mother initiated the call on five occasions, and the offspring on seven occasions.
Comparison with mature chimpanzee responses
When considering the rate of head movement in response to others’ pant hoots, the difference between the full and null models was significant (LRT: χ21 = 7.59, p = 0.006). Mature individuals were more likely to move their head towards a pant hoot than immatures (Table 1, Supplementary Figure S1). Individuals were also more likely to move their head when a pant hoot originated from outside the party than when it originated from within the focal individual’s party, when it was a group call than when it was a single call, in parties with a smaller number of females, and when resting than when engaged in other behavioural activities (Table 1, Supplementary Figure S1). Regarding the rate of vocal responses to others’ pant hoots, the difference between the full and null models was significant (LRT: χ21 = 8.98, p = 0.003). Mature individuals were more likely to vocally respond after hearing a pant hoot than immatures (Table 2, Supplementary Figure S2). Individuals were also more likely to respond vocally when the number of females in the party was smaller, and when engaged in other behavioural activities than when resting (Table 2, Supplementary Figure S2). Pant hoot response rates of mature individuals (mean = 0.62 ± 0.32 per hour) were three times greater than those of immatures (mean = 0.21 ± 0.25 per hour). Mature males (mean = 0.63 ± 0.26 per hour) and females (mean = 0.59 ± 0.39 per hour) produced response pant hoots at similar rates.
Table 1.
Relationship between whether or not mature or immature individuals moved their head towards a pant hoot and the investigated independent variables. Reference levels are in brackets (reference for ‘activity’ is resting).
| Term | Estimate | SE | Lower CI | Upper CI | χ2 | Z-value | P |
|---|---|---|---|---|---|---|---|
| Intercept | 0.524 | 0.246 | 0.212 | 1.010 | |||
| Age (mature) | 0.619 | 0.204 | -0.080 | 0.633 | 7.585 | 0.006 | |
| Gregariousness | -0.120 | 0.092 | -0.567 | -0.076 | 1.618 | 0.203 | |
| Sex (male) | 0.279 | 0.178 | -0.305 | 0.061 | 2.365 | 0.124 | |
| Call from within party | -0.326 | 0.124 | -0.610 | -0.212 | 6.766 | 0.009 | |
| Solo call | -0.412 | 0.099 | 0.212 | 1.010 | 17.180 | < 0.001 | |
| Number of females | -0.208 | 0.060 | -0.324 | -0.088 | 12.154 | < 0.001 | |
| Number of males | 0.058 | 0.067 | -0.081 | 0.190 | 0.748 | 0.387 | |
| Activity: feeding | -0.317 | 0.120 | -0.554 | -0.079 | -2.651 | 0.008 | |
| Activity: other | -0.511 | 0.157 | -0.829 | -0.210 | -3.249 | 0.001 | |
| Activity: social | -1.257 | 0.165 | -1.572 | -0.921 | -7.622 | < 0.001 |
CI: confidence interval. Control variables are in italic. Significant results are depicted in bold.
Table 2.
Relationship between whether or not mature or immature individuals produced a vocal response to a pant hoot and the investigated independent variables. Reference levels are in brackets (reference for ‘activity’ is resting).
| Term | Estimate | SE | Lower CI | Upper CI | χ2 | Z-value | P |
|---|---|---|---|---|---|---|---|
| Intercept | -2.821 | 0.319 | -3.408 | -2.127 | |||
| Age (mature) | 0.798 | 0.270 | 0.196 | 1.314 | 8.978 | 0.003 | |
| Gregariousness | -0.082 | 0.116 | -0.308 | 0.148 | 0.504 | 0.478 | |
| Sex (male) | 0.269 | 0.225 | -0.188 | 0.716 | 1.410 | 0.235 | |
| Call from within party | -0.242 | 0.163 | -0.546 | 0.093 | 2.258 | 0.133 | |
| Solo call | -0.167 | 0.122 | -0.405 | 0.073 | 1.876 | 0.171 | |
| Number of females | -0.266 | 0.084 | -0.423 | -0.091 | 10.756 | 0.001 | |
| Number of males | 0.076 | 0.084 | -0.086 | 0.247 | 0.814 | 0.367 | |
| Activity: feeding | 0.144 | 0.195 | -0.230 | 0.547 | 0.736 | 0.462 | |
| Activity: other | 0.291 | 0.148 | -0.004 | 0.576 | 1.966 | 0.049 | |
| Activity: social | -0.219 | 0.251 | -0.701 | 0.325 | -0.875 | 0.381 |
CI: confidence interval. Control variables are in italic. Significant results are depicted in bold.
Factors affecting head movement in immatures
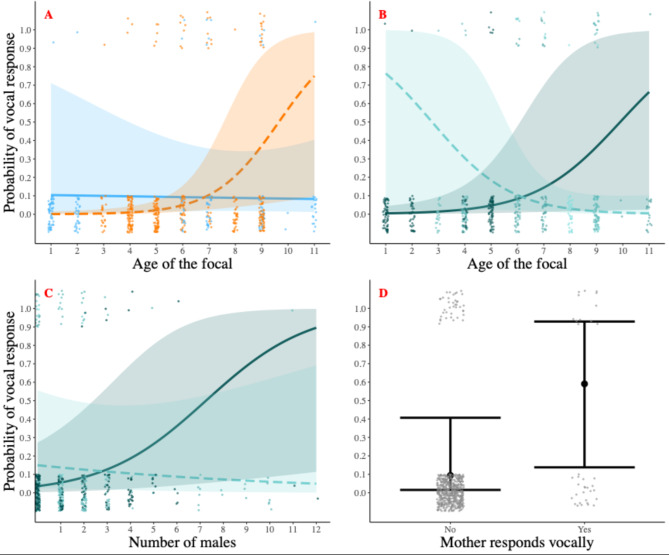
The difference between the full and null models was significant (LRT: χ24 = 11.14, p = 0.025). We found that the immature offspring of more gregarious mothers were more likely to move their head towards the caller than the immature offspring of less gregarious mothers (Table 3; Fig. 1a). There was a positive relationship between the age of immatures and the likelihood of the focal moving their head towards a pant hoot (Table 3; Fig. 1b). Males were more likely to do this than females (Table 3; Fig. 1c). Immatures were also more likely to move their head during social behavioural activities (LRT: χ24 = 20.93, p < 0.001) and after hearing a group call (Table 3, Supplementary Figure S3).
Table 3.
Relationship between whether or not the focal individual moved their head towards the pant hoot produced by another individual and the investigated independent variables. Reference levels are in brackets (reference for ‘activity’ is resting).
| Term | Estimate | SE | Lower CI | Upper CI | χ2 | z-value | P |
|---|---|---|---|---|---|---|---|
| Intercept | -1.097 | 0.386 | -1.816 | -0.233 | |||
| Gregariousness | 0.411 | 0.150 | 0.075 | 0.690 | 5.758 | 0.016 | |
| Age | 0.226 | 0.053 | 0.104 | 0.322 | 9.486 | 0.002 | |
| Sex (male) | 0.613 | 0.251 | 0.080 | 1.117 | 5.373 | 0.020 | |
| Mother chorus | 0.299 | 0.601 | -1.134 | 1.499 | 0.250 | 0.617 | |
| Call from within party | 0.284 | 0.321 | -0.382 | 0.948 | 0.784 | 0.376 | |
| Solo call | -0.479 | 0.222 | -0.917 | -0.003 | 4.713 | 0.030 | |
| Number of females | 0.047 | 0.121 | -0.205 | 0.292 | 0.152 | 0.697 | |
| Number of males | 0.078 | 0.131 | -0.209 | 0.343 | 0.361 | 0.548 | |
| Activity: feeding | -0.101 | 0.286 | -0.677 | 0.489 | -0.354 | 0.723 | |
| Activity: other | -0.785 | 0.420 | -1.586 | 0.154 | -1.868 | 0.062 | |
| Activity: social | -1.205 | 0.333 | -1.826 | -0.461 | -3.623 | < 0.001 |
CI: confidence interval. Control variables are in italic. Significant results are depicted in bold.
Fig. 1.
Likelihood of moving the head towards the source of the pant hoot depending on (A) maternal gregariousness (numerical), (B) offspring’s age (numerical), and (C) offspring’s sex (categorical). Gregariousness values were z-standardized. Confidence bands and bars illustrate the standard errors (95%). Note that raw data, represented here with dots spread around the dependent variable values of either 0 or 1, do not express the influence of other factors included in the model.
Factors affecting vocal response in immatures
The difference between the full and null models was significant (LRT: χ27 = 31.52, p < 0.001). Immatures were more likely to vocally respond to pant hoots when their mothers participated in a chorus (Table 4; Fig. 2d). The confidence intervals indicate that the following significant results should be interpreted with more caution, even if additional tests showed that no individual was responsible for the model output, the model was not overfitted, and it had explanatory power (See Supplementary Information). We found an interaction effect between the age and sex of immatures, meaning that male individuals were more likely to vocally respond when older (Table 4; Fig. 2a). We found an interaction effect between the age of immatures and maternal gregariousness, meaning that the offspring of more gregarious mothers were more likely to vocally respond when older, and the offspring of less gregarious mothers were more likely to vocally respond when younger (Table 4; Fig. 2b). We also found an interaction effect between the maternal gregariousness and the number of males in the party: the offspring of more gregarious mothers were more likely to respond vocally as the number of males increased while the offspring of less gregarious mothers were more likely to vocally respond as the number of males decreased (Table 4; Fig. 2c).
Table 4.
Relationship between whether or not the focal individual produced a vocal response to the pant hoot produced by another individual and the investigated independent variables. Reference levels are in brackets (reference for ‘activity’ is resting).
| Term | Estimate | SE | Lower CI | Upper CI | χ2 | z-value | P |
|---|---|---|---|---|---|---|---|
| Intercept | -2.269 | 0.967 | -4.032 | 0.416 | |||
| Gregariousness | -0.386 | 0.410 | -1.195 | 0.714 | |||
| Age | -0.061 | 0.517 | -1.453 | 1.160 | |||
| Sex (male) | -1.024 | 0.860 | -3.128 | 0.840 | |||
| Mother chorus | 2.630 | 0.659 | 0.787 | 3.792 | 17.123 | < 0.001 | |
| Call from within party | 0.436 | 0.548 | -0.694 | 1.723 | 0.621 | 0.430 | |
| Solo call | -0.218 | 0.375 | -0.976 | 0.622 | 0.341 | 0.562 | |
| Number of females | -0.480 | 0.256 | -0.933 | 0.181 | 3.696 | 0.055 | |
| Number of males | 0.353 | 0.243 | -0.208 | 1.048 | |||
| Activity: feeding | 0.634 | 0.481 | -0.642 | 1.553 | 1.318 | 0.186 | |
| Activity: other | 0.614 | 0.592 | -0.797 | 1.899 | 1.037 | 0.298 | |
| Activity: social | -0.200 | 0.624 | -1.619 | 1.306 | -0.320 | 0.751 | |
| Age*Sex (male) | 2.286 | 1.050 | -0.322 | 4.458 | 4.411 | 0.035 | |
| Age*Gregariousness | 1.759 | 0.791 | -0.435 | 3.237 | 4.655 | 0.030 | |
|
Gregariousness* Number of males |
0.501 | 0.235 | -0.228 | 1.066 | 4.554 | 0.024 |
CI: confidence interval. Interactions are represented by an asterisk between variables. Control variables are in italic. Significant results are depicted in bold.
Fig. 2.
Likelihood of vocally responding to a pant hoot depending on (A) the interaction between age (numerical) and sex (categorical) of the offspring (orange: male; blue: female), (B) the interaction between the age of the offspring (numerical) and maternal gregariousness (numerical; solid sea green: +1 SD above the mean; dashed sea green: -1 SD below the mean), (C) the interaction between the number of males in the party (numerical) and maternal gregariousness (numerical; solid sea green: +1 SD above the mean; dashed sea green: -1 SD below the mean), and (D) whether the mother joined the vocal response (categorical). Confidence bands and bars illustrate the standard errors (95%). Note that raw data, represented here with dots spread around the dependent variable values of either 0 or 1, do not express the influence of other factors included in the model.
Maternal gregariousness and vocal exposure
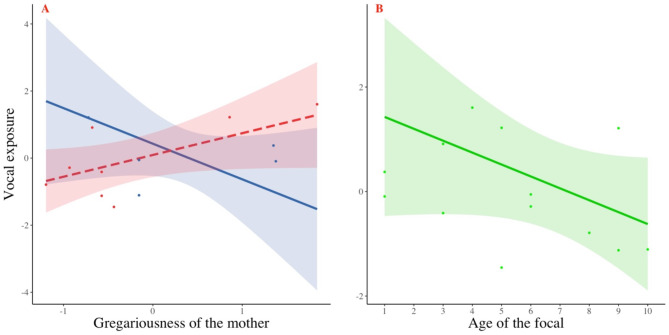
The difference between the full and null models was significant (LRT: χ22 = 10.06, p = 0.007, R2m = 0.59, R2c = 0.38). We found an interaction effect between the sex of the offspring and maternal gregariousness, meaning that the male offspring of more gregarious mothers were more exposed to pant hoots than other immatures (Table 5; Fig. 3a). We also found that younger individuals were more exposed to pant hoots than older individuals (Table 5; Fig. 3b), although the confidence intervals indicate that this effect may not be reliable.
Table 5.
Relationship between the offspring’s exposure to pant hoots and the investigated independent variables. Reference levels are in brackets (reference for ‘activity’ is resting).
| Term | Estimate | SE | Lower CI | Upper CI | T-value | P |
|---|---|---|---|---|---|---|
| Intercept | 0.429 | 0.417 | -0.534 | 1.391 | ||
| Gregariousness | -1.062 | 0.663 | -2.590 | 0.467 | ||
| Age | -0.681 | 0.376 | -1.547 | 0.186 | -1.811 | 0.034 |
| Sex (male) | -0.335 | 0.513 | -1.519 | 0.848 | ||
| Gregariousness*Sex (male) | 1.711 | 0.659 | 0.191 | 3.229 | 2.597 | 0.005 |
CI: confidence interval. Interaction is represented by an asterisk between variables. Control variables are in italic. Significant results are depicted in bold.
Fig. 3.
Vocal exposure to pant hoots of immature individuals depending on (A) the gregariousness value of their mother (numerical; red: males; blue: females), and (B) the age of the focal (numerical). Gregariousness and vocal exposure values were z-standardized. Confidence bands illustrate the standard errors (95%). Note that raw data, represented here with dots spread around the dependent variable values of either 0 or 1, do not express the influence of other factors included in the model.
Discussion
We found that immature chimpanzees rarely produce pant hoots spontaneously, and considerably less often than mature individuals. Instead, they usually respond to the pant hoots of others using different call types, the majority being pant hoots, as part of vocal exchanges. Immatures also respond by moving their heads in the direction of the caller, although less often than mature individuals. The youngest individual we observed producing a pant hoot spontaneously was three years old, while the youngest individual who produced a pant hoot response was 19 months old. We found that more gregarious, older, and male immatures are more likely to move their head towards the calls of others compared to less gregarious, younger, and female immatures. Furthermore, more gregarious and older immatures, older male immatures, and more gregarious immatures in the presence of larger male audiences are more likely to respond vocally compared to younger and female immatures, and in the presence of smaller male audiences. Immatures are more likely to respond vocally when their mother also respond vocally to the same call. Finally, exposure to others’ vocalisations is higher in the male offspring of more gregarious mothers.
Overall our observations suggest that the vocal development of chimpanzees is progressive, consistent with the idea that the acquisition of social calls is less hard-wired and likely slower than that of alarm calls19 and that the period of acquisition of communicative skills is extended in great apes60. We observed that spontaneous pant hoot use was very rare in both infants and juveniles, only becoming frequent in mature individuals where spontaneous and response pant hoots were produced at comparable rates. One possibility is that the increase in spontaneous calling rate coincides with individuals engaging independently from their mother in social contexts mediated by pant hoots, where spontaneous pant hoots could be used to elicit responses from others to coordinate joint movement or as a form of social bonding. We also found that there was a positive effect of the age of individuals on head movements and vocal responses (males and gregarious individuals only). Immature chimpanzees were capable of producing both behavioural responses at the youngest age recorded but reached adult levels of responsiveness as late juveniles. Chimpanzees produce rudimentary pant hoots from birth but only develop adult-like phase structures upon reaching full maturity58. Together with our observation of an increase in spontaneous and response pant hoots when transitioning to maturity, one possibility is that chimpanzees require longer practice or exposure to others’ calls to develop complex vocal sequences, similarly to the development of complex song sequences in songbirds61 and of complex social calls in mice62 and lemurs63. While physical maturation contributes to the ontogeny of vocal behaviours64, our observations indicate that the vocal ontogeny of great apes is less constrained and genetically fixed than previously assumed. Nevertheless, longitudinal, as opposed to cross-sectional, studies using observations from birth until adult-hood are necessary to fully determine the developmental trajectory of these vocal behaviours. While our findings included a relatively broad sample of inviduals, the level of inter-individual variation observed suggests that longitudinal designs may be particularly well suited to investigating. Furthermore, the ontogeny of socially used, long and short distance calls such as pant hoots may also depend on the development of cognitive constructs, such as social awareness44,54. We observed that the responses of immatures varied depending on the audience composition, the type of call, and the context, providing further evidence that primate responses are highly flexible65. As non-focal callers were typically out of sight, we were not able to explore the effects of the caller’s identity or their affiliative relationship on the responses of immatures. Future studies are necessary to investigate how additional fine social factors may impact the ontogeny of responses.
Our study supports the idea that immature chimpanzees develop vocal behaviours by socially interacting with their conspecifics. By spending more time in groups, gregarious mothers increase the exposure of their offspring to social and vocal interactions. Mature individuals and particularly males produce pant hoots most often55,56 and can act as ‘models’ for younger individuals. The fact that the offspring of more gregarious mothers responded vocally more often in the presence of males further corroborates the idea that younger individuals have more opportunities to reply by calling when spending time with males. Greater social opportunities to learn associations between vocalisations and appropriate usage and responses may help to increase the speed of vocal development. The need for experience of others’ vocalisations is not widespread among animals and an important role of auditory feedback has been demonstrated in the development of a few non-human animals regarded as vocal learners, including songbirds, dolphins and whales, bats, and elephants8,12,17,61. Our findings support the idea that the vocal ontogeny of chimpanzees should not be regarded as qualitatively different from that of songbirds and human for instance, despite chimpanzees possessing considerably less sophisticated vocal production learning abilities17,38. Furthermore, with growing evidence of diverse range of vocal learning behaviours across several animal species, we also believe that the dichotomous view of “haves and have-nots” should be abandoned in favour of a more nuanced and continuous approach to the study of vocal learning capacities12.
In addition to providing more exposure and opportunities to learn from others through association patterns, mothers positively affected the vocal responses of their offspring by joining in chorused calls. Offspring and mother pairs might call together to signal their bond strength, similarly to what has been observed in adult males52. Chorusing might also facilitate the learning process, similarly to how parents’ vocal feedback improves appropriate usage learning in marmosets18 and in gibbons66. Because we were not able to investigate the temporal order of callers during chorused calls due to the low number of chorused responses, further studies are needed to clarify the role of chorusing during the vocal development of chimpanzees. Nevertheless, the fact that we did not observe individuals directing vocalisations at each other is potentially at odds with evidence from human caregivers, who typically use vocalisations accompanied by head orientation and directed toward young receivers to facilitate language acquisition16. Similar evidence in great apes is so far lacking6, but might be more easily detected in a short-distance interactions54 as opposed to long-distance exchanges. Determining whether chorusing represents an intermediate developmental stage between directed and broadcasted vocalisations requires further investigation.
Our observations are in line with the idea that mothers and other mature individuals in the group do not actively or directly ‘teach’ immature chimpanzees their vocal behaviour (sensu67). Instead, our findings are more consistent with the ‘master-apprenticeship’ hypothesis, which posits that young chimpanzees acquire behaviours through repeated exposure and observation of a tolerant model in close proximity68. In Western chimpanzees of the Taï Forest, the offspring of mothers who had been highly gregarious use pant hoots more frequently, but this was only observed during the first 2-years of life59. Because we found that greater maternal gregariousness increases the probability of vocal responses in juvenile individuals up to 11-years old, it is possible that these differences are related to greater variation in Eastern female chimpanzees’ association patterns and lower social cohesion than their Western counterparts69. Because pant hoots are used to mediate fission-fusion events and Eastern communities are larger in size than Western ones69, it is likely that young Eastern chimpanzees experience greater variation in social and vocal exposure. Thus, data collected from groups that are particularly small and cohesive may not generalise across the species as a whole. Our findings show the importance of incorporating a wide range of the diverse social structures and patterns of association that characterise chimpanzee fission-fusion social system, particularly when they are used in a comparative framework. Furthermore, we show that vocal ontogeny is an ongoing process that persists well into the juvenile phase and that attending to and interacting with mothers and male group members during vocal exchanges are also key drivers. Early social interactions with conspecifics also play an important role in the acquisition of gestural communication in chimpanzees70, which, similar to their vocal repertoire, some authors argue is based on a largely innate, species-typical repertoire of signals that is then used flexibly71. Overall, greater social inputs provide young chimpanzees with a head start for their communicative skills.
The predominant role of pant hoots in the social lives of adult male chimpanzees is reflected in the greater responsiveness and spontaneous usage we observed in both mature and immature males, particularly in older immature individuals. The importance of social calls in the relationships and interactions between male chimpanzees likely explains the sex differences during its ontogeny. As compared to immature females, males experience more social exposure and opportunities to learn from others due their mothers typically associating and interacting more with group members and particularly with adult males41. Similarly, immature males may develop their pant hoots earlier due to greater exposure to the vocal behaviour of group members. Our findings are consistent with the idea that the development of social skills, including vocal behaviour59, occurs ontogenetically earlier in male chimpanzees72, and that these differences between sexes are in-part explained by the different adaptive sex-dependent pressures in adulthood. It is important to note that sex differences might be differently expressed in populations where females are more gregarious69, restricted to specific developmental phases, or have a combined effect with other factors, as seen during human speech development73. Investigating the effects of other social factors (e.g. centrality in a social network or dominance) in future studies could provide further insights into how sociality shapes chimpanzee vocal ontogeny.
In conclusion, we found that the ontogeny of chimpanzee vocal usage and comprehension are mediated by social and individual factors, in line with the hypothesis that some form of social learning enhances the development of vocal communicative skills, even in species with apparently limited vocal production learning17. Extending this research across chimpanzee communities and great ape species would further clarify the ways in which variation in social dynamics and group structures can drive differences in hominid vocal development. In addition, given new findings that suggest greater flexibility in great ape vocal production than previously assumed20, there is substantial promise for studies exploring how vocal ontogeny is socially-mediated and the potential development of dialects34. Finally, whether the development of the structure and order of pant hoot components is also socially mediated remains an interesting avenue for future research, particularly given the parallels between the combinatorial structures used by primates and those present in human language21. In sum, our findings do not support theories of primate vocal behaviours as fixed and impervious to social influence and are instead consistent with the emerging view that the origin of human language was a continuous evolutionary process built upon precursors, an increasing number of which can be found in the vocal communication of modern ape species.
Methods
Ethics & inclusion statement
One of the co-authors of this study is a local researcher and field assistant with extensive expertise in the study site and species. The project adhered to the ASAB guidelines for the treatment of animals during behavioural studies. It was approved by the Uganda Wildlife Authority (UWA/COD/96/5), the Uganda National Council for Science and Technology (NS 637) and the research ethics committees of the Universities of St Andrews and Neuchâtel (38/2019-B; No 171). Data collection was terminated on 17 March 2020, due to the Covid-19 pandemic (UWA ref: EDO/73/01), to avoid putting the health of the animals at risk.
Study site
The study was conducted with Eastern chimpanzees of the Sonso community (Pan troglodytes schweinfurthii) of the Budongo Forest in western Uganda. At the beginning of the study (September 2018) the community was composed of 74 individuals (11 adult males, 25 adult females, 15 sub-adults, 8 juveniles, and 15 infants). At the end of the study (March 2020) the community was composed of 68 individuals (9 adult males, 26 adult females, 15 sub-adults, 9 juveniles, and 8 infants) (Supplementary Table S3).
Study subjects
We used age categories following Reynolds74 of infants (0–4 years), juveniles (5–9 years), sub-adults (males: 10–15 years, females: 10–14 years), and adults (males: >16 years, females: >15 years). Study subjects were selected to obtain balanced samples of each infant and juvenile age category in the community: 2 of 4 available female infants, 4 of 7 male infants, 2 of 4 female juveniles, 4 of 4 male juveniles, all of which were dependent from their mother (i.e., mother and offspring were always in the same association party) and are collectively referred to as ‘immatures’ hereafter (Supplementary Table S1). In addition, one female aged 10 at the beginning of the study was considered immature as during data collection she was dependent on her mother (always travelled with and engaged in activities in the presence of her mother). Instead, we considered individuals who already spent time with other group members in parties independently of the presence of their mother as mature and refer to them collectively as ‘matures’ hereafter75. We collected data on 25 mature individuals (13 males, 12 females) (Supplementary Table S2). Because collecting data longitudinally was not possible, we opted for a cross-sectional study design. Data were collected between September 2018 and March 2020 and we assigned the age of the focal (in years) to each data point, where for the same individual we may have data across the observation period (Table S1).
Definition of Pant hoot vocalisations
Pant hoots are long-distance vocal sequences composed of up to four acoustically distinct phases, typically produced during traveling and feeding events47,57 (Fig. 4). Each phase contains one or more acoustically similar voiced elements produced in the following order: Introduction, Build-up, Climax, and Let-down. Because pant hoot phases can be omitted or produced in isolation54, we considered a pant hoot when we heard a caller produce at least one of the four phases. Another reason for adopting this criterion is that, although the climax is never produced in isolation54,55, for most distant pant hoots we were only able to hear the climax phase, the highest amplitude phase.
Fig. 4.
Spectrographic representations of pant hoots produced by an infant, a juvenile, a subadult, and an adult chimpanzee from the Sonso community.
Data collection
Data were collected between September and December 2018, February and July 2019, and November 2019 and March 2020, for a total of 15 months. We used focal animal sampling as the main method of data collection, following a different study subject each day from 0700 to 1630 h, approximately 5.5 days a week. We avoided following the same individual on consecutive days while balancing sampling time across individuals. Each focal animal was followed for at least three separate days. We calculated the duration of focal follows by taking into consideration periods of time when the face of the focal animal was visible to ensure reliable observation of behavioural changes. Consequently, focal durations reported here may be more conservative. We collected a total of 170.7 h of observations with a mean of 13.1 h per immature individual (SD = 4.4, range 6.5–21.2; Supplementary Table S1) and a total of 451.9 h of observations with a mean of 18.1 h per mature individual (SD = 8.7, range 8.7–34.6; Supplementary Table S2). We recorded all the observational data on a portable device (Samsung Xplorer 4) using a custom-built CyberTracker database (version 3.496), which automatically paired it with the time of occurrence. We recorded whenever we heard a pant hoot and, if no other calls were produced by other individuals during at least 15 s after the pant hoot we heard, when the focal individual produced a pant hoot we later coded it as a ‘spontaneous’ call. When another individual produced a pant hoot, we recorded the behavioural changes of the focal after the call (see Behavioural responses below). When the pant hoot heard by the focal was a call that was subsequently joined by one or more individuals to form a chorus (hereafter ‘group’ call), we noted the behavioural responses that followed the first call heard.
Behavioural responses
We recorded ‘head movement’ responses when the focal individual moved the head in the direction of a pant hoot for ≥ 1 s within five seconds after the termination of the pant hoot heard, and the movement was at least a 45 degree change from the head position before the call. We excluded instances where the head of the focal individual was already oriented within a 45 degrees angle from the call source. We recorded ‘vocal responses’ when within five seconds after hearing a pant hoot the focal individual produced a vocalisation53. We recorded the type of vocalisation produced following the repertoire by Slocombe and Zuberbühler76. Because in some instances it was possible to assess whether the focal produced a vocal response but not the head orientation with certainty, the number of vocal responses is larger than the number of head movements (see Statistical analyses). As in a previous study on chimpanzee responses to calls53, we used a five-second threshold to maximize the inclusion of responses that may require processing time by receivers engaged in different behavioural activities. To minimize the chances of recording responses to other signals, we did not record behavioural responses if we heard other stimuli between the pant hoot and the response.
We noted whether the call heard by the focal individual was produced by a single individual or multiple individuals (solo vs. chorus), whether the mother of the focal produced a vocal response (if the mother was not visible we excluded the data), and whether the call was uttered from within the party of the focal individual. Party was defined as all mature individuals present in the visual range, which corresponds to a radius of approximately 35 m77. We excluded calls estimated to be produced by neighbouring chimpanzee communities (on the basis of distance, location, and the chimpanzees’ reaction). We continuously scored the behavioural activity of the focal using four categories: (1) resting, which included self-directed grooming; (2) feeding; (3) social, which included grooming and social play; (4) other activity, which included traveling, aggression, and solitary play. We provide raw data of the recorded behaviours in the Supplementary Information (Table S1, S2).
Gregariousness
We defined “gregariousness” as the probability of finding an individual in a party with other chimpanzees78. Four BCFS field assistants collected data on party composition every 15-minute intervals during the period December 2017 – June 2020 while conducting focal follows on a different mature individual each day as part of long-term data collection. To establish gregariousness, we calculated the number of 15-minute scans in which a mature individual was recorded in a party with other mature individuals. We then calculated the total number of occurrences across all mature study subjects. In Eastern chimpanzees, fission-fusion events occur more frequently than in Western chimpanzees and less gregarious (i.e., peripheral) females are most often recorded by observers when present in large parties69,78. Therefore, we excluded focal data from our calculations and only included instances where an individual was observed with others while not being the focal animal to avoid overestimating the gregariousness values of less gregarious mature females as well as to control for unbalanced sampling efforts across individuals78. Then, we divided the total number of occurrences by the number of times they were observed while not being the focal animal and multiplied by 100 to obtain the probability of finding an individual in a party with other chimpanzees. Values varied between 0 (always alone) and 100 (always with others), with lower values indicating individuals who are less likely to spend time in a group, while higher values indicate individuals who are more likely to spend time in a group. Because all immature chimpanzees considered in this study did not associate with other group members independently of their mother, their gregariousness value was the same as that of their mother. The maternal gregariousness of immature individuals had a mean value of 11.5 (SD = 4.9, range 5.6–20.7; Supplementary Table S1) and the gregariousness of mature individuals had a mean value of 17.7 (SD = 6.2, range 5.8– 29.2; Supplementary Table S2). Because females are typically more peripheral than males, and peripheral females tend to isolate more69,78, we included data from a longer period of time that is largely overlapping with the period during which we conducted observations, which allowed us to calculate gregariousness values reliably for all individuals. During this period, the dominance hierarchy of the Sonso community remained relatively stable54.
General procedure for linear models
We z-transformed the distribution of the quantitative variables into a distribution with a mean of 0 and standard deviation (SD) of 1 to improve the accuracy of parameter estimates. Before fitting the models, we removed observations that contained missing values (NAs) in one or more of the predictor or response variables. To assess the significance of the test predictors, we compared each model with a ‘null’ model comprising only the intercept, control variables, and random effects using a likelihood-ratio test (LRT)79. We assessed the variance inflation factor of the variables (VIF) to measure collinearity and accepted it when < 4.0 80 using the ‘performance’ package81 (version 0.5.1). We calculated p-values using LRT comparing each model with the respective null model using the ‘drop1’ function of the package ‘stats’82 (version 4.0.2). In the linear models, we checked whether the residuals were normally distributed and homogenous by inspecting a scatterplot and quantile-quantile plot of the residuals as a function of the fitted values. In the generalised linear mixed models (GLMM) we included the identity of the focal as a random effect to control for replicated observations and an unbalanced dataset. To detect the presence of influential observations (i.e., outliers), we measured the Cook’s distance using the package ‘performance’81 (version 0.10.2.4). All analyses were carried out using R82 (version 4.1.2). Figures were created using the R package ‘jtools’83 (version 2.2.1). The complete model structures and figures of additional variables can be found in the Supplementary Information. The dataset and code used in the analyses are available online (https://osf.io/ze82d/?view_only=c58e1e88758a4a2bb7801c89991bc92b).
Comparison with mature chimpanzee spontaneous call rate
To examine differences in the production of spontaneous pant hoots, we compared the call rates of mature and immature individuals (Supplementary Tables S1 & S2). We calculated call rates for each individual dividing the number of spontaneously produced pant hoots by the number of focal hours. Given the small sample size, we performed a Shapiro-Wilk test to check whether the distribution of the data departed significantly from normality. The data from mature chimpanzees did not significantly depart from normal distribution (W = 0.935, p-value = 0.115). Because the data from immatures did so (W = 0.653, p-value < 0.001), we used a non-parametric Wilcoxon rank-sum test.
Comparison with mature chimpanzee responses
To examine differences in the behavioural responses of mature and immature chimpanzees, we created two GLMMs with a binomial error structure using the R package ‘lme4’84 (version 3.6.3). In the first model, as the dependent variable we included whether or not (0/1) the focal individual moved their head towards the pant hoot they heard (n = 1995 pant hoots, 1269 of which had a head movement). In the second model, as the dependent variable we included whether or not (0/1) the focal individual vocally responded to the pant hoot they heard (n = 2502 pant hoots, 325 of which had a vocal response). In both models, we included whether the individuals were mature or immature (categorical with two levels; reference level: mature), the gregariousness (numerical), and sex (categorical with two levels; reference level: male) of the focal as independent variables. Because pant hoots are flexibly used depending on the social context and affected by audience composition54, we included as control variables whether the pant hoot was produced within or outside the focal’s party (categorical with two levels), whether the call was a solo or group call (categorical with two levels), the behavioural activity of the focal (categorical with four levels; reference level: resting), and the number of females and males in the party (numerical). There was no collinearity between the examined independent variables (maximum VIF: 1.55 and 1.59) and the dependent variables were not over-dispersed (dispersion ratio: 0.97 and 0.92).
Behavioural responses of immature individuals
To investigate which factors impact the behavioural responses of immatures to others’ pant hoots, we created two GLMMs with a binomial error structure. In the ‘head movement model’ we considered as the dependent variable whether or not (0/1) the focal individual moved their head towards the pant hoot (n = 401 pant hoots, 198 of which had a head movement). Second, we created a ‘vocal response’ model where the dependent variable was whether or not (0/1) the focal individual produced a vocalisation in response (n = 549 pant hoots, 51 of which had a vocal response). In both models, we included as independent variables the maternal gregariousness (numerical), age of the focal (numerical in years), sex of the focal (categorical), and whether the mother joined or not the vocal response (categorical; ‘mother chorus’ hereafter). We included the same control variables as in the previous models. We initially included a three-way interaction between maternal gregariousness, age, and sex, to control for confounding factors given that 1) mothers with male offspring are more gregarious41, 2) the association of offspring with adult group members changes during ontogeny42, and 3) behavioural differences between the sexes can appear at different ontogenetic stages75. We also included an interaction between both the number of males and females with the maternal gregariousness because more gregarious mothers tend to spend more time in larger parties. We then removed non-significant interactions (estimates with P > 0.05) one at a time. We did not interpret the main effects that were part of an interaction when the interaction was statistically significant because the effect on the response of one variable was conditional on the state/value of the other and would thus be unreliable85. There was no collinearity between the examined independent variables (maximum VIF: 1.95 and 1.71) and the dependent variables were not over-dispersed (dispersion ratio: 1.04 and 0.66).
Maternal gregariousness and vocal exposure
We tested whether maternal gregariousness was related to the offspring’s rate of exposure to others’ pant hoots. We created a linear model in which we used the number of pant hoots heard by the immatures per hour of focal following (‘vocal exposure’ hereafter; mean 3.36 ± 0.91) as the dependent variable and the same independent variables as in the previous models. There was no collinearity between the examined independent variables (maximum VIF: 1.72).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Swiss National Science Foundation grant (501100001711-166458) awarded to K.Z., the St Leonard College Inter-University scholarship awarded to J.C. and K.Z., and individual mobility grants from Swissuniversities and St Leonard College awarded to A.S. We are grateful to the management, staff, field assistants and researchers of the Budongo Conservation Field Station (BCFS) for their support and discussions. We thank the Uganda Wildlife Authority and the Uganda National Council for Science and Technology for permission to conduct the study. We are thankful to Charles Paxton for statistical advice.
Author contributions
A.S. conceived the research; A.S. and G.M. collected the data during fieldwork; A.S., P.F., and G.D. developed methodology; A.S. and C.H. performed the data curation; A.S. analysed the data; A.S., P.F., G.D., and K.Z. wrote the manuscript; All authors reviewed the manuscript; K.Z. and J.C. supervised the study and provided resources; A.S., K.Z., and J.C. acquired financial support. All authors gave final approval for publication.
Data availability
Additional information has been uploaded as part of the Supplementary Information. The data and code used in the analyses are available using the following link: https://osf.io/ze82d/?view_only=c58e1e88758a4a2bb7801c89991bc92b.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-93207-x.
References
- 1.Hauser, M. D. et al. The mystery of Language evolution. Front. Psychol.5, (2014). [DOI] [PMC free article] [PubMed]
- 2.Hauser, M. D., Chomsky, N. & Fitch, W. T. The faculty of Language: what is it, who has it, and how did it evolve? Science298, 1569–1579 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Fedurek, P. & Slocombe, K. E. Primate vocal communication: A useful tool for Understanding human speech and Language evolution?? Hum. Biol.83, 153–173 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Eaton, T. et al. Bottoms-up! Rejecting Top-down Human-centered approaches in comparative psychology. Int. J. Comp. Psychol.31, (2018).
- 5.ter Haar, S. M. et al. Cross-species parallels in babbling: animals and algorithms. Phil Trans. R Soc. B. 376, 20200239 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schick, J. et al. The function and evolution of child-directed communication. PLoS Biol.20, e3001630 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oller, D. K., Griebel, U. & Warlaumont, A. S. Vocal development as a guide to modeling the evolution of Language. Top. Cogn. Sci.8, 382–392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janik, V. M. & Slater, P. J. B. The different roles of social learning in vocal communication. Anim. Behav.60, 1–11 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Seyfarth, R. & Cheney, D. Some general features of vocal development in nonhuman primates. in Social Influences on Vocal Development (eds Snowdon, C. T. & Hausberger, M.) 249–273 (Cambridge University Press, doi:10.1017/CBO9780511758843.013. (1997).
- 10.Egnor, S. E. R. & Hauser, M. D. A paradox in the evolution of primate vocal learning. Trends Neurosci.27, 649–654 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Bolhuis, J. J., Tattersall, I., Chomsky, N. & Berwick, R. C. How could Language have evolved?? PLoS Biol.12, e1001934 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janik, V. M. & Knörnschild, M. Vocal production learning in mammals revisited. Phil Trans. R Soc. B. 376, 20200244 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhl, P. K. Early Language acquisition: cracking the speech code. Nat. Rev. Neurosci.5, 831–843 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Vouloumanos, A. & Werker, J. F. Listening to Language at birth: evidence for a bias for speech in neonates. Dev. Sci.10, 159–164 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Goldstein, M. H., King, A. P. & West, M. J. Social interaction shapes babbling: Testing parallels between birdsong and speech. Proceedings of the National Academy of Sciences 100, 8030–8035 (2003). [DOI] [PMC free article] [PubMed]
- 16.Hoff, E. How social contexts support and shape Language development. Dev. Rev.26, 55–88 (2006). [Google Scholar]
- 17.Vernes, S. C. et al. The multi-dimensional nature of vocal learning. Phil Trans. R Soc. B. 376, 20200236 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi, D. Y., Liao, D. A. & Ghazanfar, A. A. Vocal learning via social reinforcement by infant marmoset monkeys. Curr. Biol.27, 1844–1852e6 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Snowdon, C. T., Elowson, M. A. & Roush, R. S. Social influences on vocal development in new world primates. in Social Influences on Vocal Development (eds Snowdon, C. T. & Hausberger, M.) 234–248 (Cambridge University Press, doi:10.1017/CBO9780511758843.012. (1997).
- 20.Slocombe, K. E., Lahiff, N. J., Wilke, C. & Townsend, S. W. Chimpanzee vocal communication: what we know from the wild. Curr. Opin. Behav. Sci.46, 101171 (2022). [Google Scholar]
- 21.Fitch, W. T. & Zuberbühler, K. Primate precursors to human Language: beyond discontinuity. in Evolution of Emotional Communication (eds Altenmüller, E., Schmidt, S. & Zimmermann, E.) 26–48 (Oxford University Press, doi:10.1093/acprof:oso/9780199583560.003.0002. (2013).
- 22.Hammerschmidt, K., Newman, J. D., Champoux, M. & Suomi, S. J. Changes in Rhesus macaque ‘coo’ vocalizations during early development. Ethology106, 873–886 (2000). [Google Scholar]
- 23.Owren, M. J., Dieter, J. A., Seyfarth, R. & Cheney, D. Vocalizations of rhesus (Macaca mulatta) and Japanese (M. Fuscata) macaques cross-fostered between species show evidence of only limited modification. Dev. Psychobiol.26, 389–406 (1993). [DOI] [PubMed] [Google Scholar]
- 24.Harrison, R. A. & van de Waal, E. The unique potential of field research to understand primate social learning and cognition. Curr. Opin. Behav. Sci.45, 101132 (2022). [Google Scholar]
- 25.Winter, P., Schott, D., Ploog, D. & Handley, P. Ontogeny of squirrel monkey calls under normal conditions and under acoustic isolation. Behav47, 230–239 (1973). [DOI] [PubMed] [Google Scholar]
- 26.Talmage-Riggs, G., Winter, P., Ploog, D. & Mayer, W. Effect of deafening on the vocal behavior of the squirrel monkey (Saimiri sciureus). Folia Primatol.17, 404–420 (1972). [DOI] [PubMed] [Google Scholar]
- 27.Gultekin, Y. B. & Hage, S. R. Limiting parental feedback disrupts vocal development in marmoset monkeys. Nat. Commun.8, 14046 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi, D. Y. et al. The developmental dynamics of marmoset monkey vocal production. Science349, 734–738 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Cheney, D. & Seyfarth, R. Attending to behaviour versus attending to knowledge: examining monkeys’ attribution of mental States. Anim. Behav.40, 742–753 (1990). [Google Scholar]
- 30.Seyfarth, R. & Cheney, D. Vocal development in Vervet monkeys. Anim. Behav.34, 1640–1658 (1986). [Google Scholar]
- 31.Hauser, M. D. Ontogenetic changes in the comprehension and production of Vervet monkey (Cercopithecus aethiops) vocalizations. J. Comp. Psychol.103, 149–158 (1989). [Google Scholar]
- 32.Fischer, J., Wheeler, B. C. & Higham, J. P. Is there any evidence for vocal learning in chimpanzee food calls? Curr. Biol.25, R1028–R1029 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Watson, S. K. et al. Vocal learning in the functionally referential food grunts of chimpanzees. Curr. Biol.25, 495–499 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Crockford, C., Herbinger, I., Vigilant, L. & Boesch, C. Wild chimpanzees produce Group-Specific calls: a case for vocal learning?? Ethology110, 221–243 (2004). [Google Scholar]
- 35.Desai, N. P., Fedurek, P., Slocombe, K. E. & Wilson, M. L. Chimpanzee pant-hoots encode individual information more reliably than group differences. Am. J. Primatol.1–2310.1002/ajp.23430 (2022). [DOI] [PMC free article] [PubMed]
- 36.Lameira, A. R. et al. Sociality predicts orangutan vocal phenotype. Nat. Ecol. Evol.6, 644–652 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vouloumanos, A. & Werker, J. F. Tuned to the signal: the privileged status of speech for young infants. Dev. Sci.7, 270–276 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Doupe, A. J. & Kuhl, P. K. Birdsong and human speech: common themes and mechanisms. Annu. Rev. Neurosci.22, 567–631 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Zuberbühler, K., León, J., Deshpande, A. & Quintero, F. Socially scripted vocal learning in primates. Curr. Opin. Behav. Sci.46, 101153 (2022). [Google Scholar]
- 40.Schel, A. M., Machanda, Z., Townsend, S. W., Zuberbühler, K. & Slocombe, K. E. Chimpanzee food calls are directed at specific individuals. Anim. Behav.86, 955–965 (2013). [Google Scholar]
- 41.Murray, C. M. et al. Early social exposure in wild chimpanzees: mothers with sons are more gregarious than mothers with daughters. Proc. Natl. Acad. Sci. USA. 111, 18189–18194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pusey, A. E. Mother-offspring relationships in chimpanzees after weaning. Anim. Behav.31, 363–377 (1983). [Google Scholar]
- 43.Dezecache, G., Crockford, C. & Zuberbühler, K. The development of communication in alarm contexts in wild chimpanzees. Behav. Ecol. Sociobiol.73, 104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fedurek, P., Donnellan, E. & Slocombe, K. E. Social and ecological correlates of long-distance Pant hoot calls in male chimpanzees. Behav. Ecol. Sociobiol.68, 1345–1355 (2014). [Google Scholar]
- 45.Mitani, J. C. & Nishida, T. Contexts and social correlates of long-distance calling by male chimpanzees. Anim. Behav.45, 735–746 (1993). [Google Scholar]
- 46.Oller, D. K. et al. Language origins viewed in spontaneous and interactive vocal rates of human and Bonobo infants. Front. Psychol.10, (2019). [DOI] [PMC free article] [PubMed]
- 47.Marler, P. & Tenaza, R. Signalling behaviour of apes with special reference to vocalisations. in How Animals Communicate (ed Sebeok, T. A.) 965–1033 (Indiana University Press, Bloomington, Indiana USA, (1977). [Google Scholar]
- 48.Taylor, D., Dezecache, G. & Davila-Ross, M. Filling in the gaps: acoustic gradation increases in the vocal ontogeny of chimpanzees (Pan troglodytes). Am. J. Primatol.83, (2021). [DOI] [PubMed]
- 49.Goodall, J. The Chimpanzees of Gombe: Patterns of Behavior (Harvard University Press, 1986).
- 50.Plooij, F. X. The Behavioral Development of Free-Living Chimpanzee Babies and Infants (Ablex Publishing Corp., 1984).
- 51.Laporte, M. N. C. & Zuberbühler, K. The development of a greeting signal in wild chimpanzees: development of greeting signal in chimpanzees. Dev. Sci.14, 1220–1234 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Fedurek, P., Machanda, Z. P., Schel, A. M. & Slocombe, K. E. Pant hoot chorusing and social bonds in male chimpanzees. Animal Behaviour 86, 189–196 (2013).
- 53.Arcadi, A. C. Vocal responsiveness in male wild chimpanzees: implications for the evolution of Language. J. Hum. Evol.39, 205–224 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Soldati, A., Fedurek, P., Dezecache, G., Call, J. & Zuberbühler, K. Audience sensitivity in chimpanzee display Pant hoots. Anim. Behav.190, 23–40 (2022). [Google Scholar]
- 55.Arcadi, A. C. Phrase structure of wild chimpanzee Pant hoots: patterns of production and interpopulation variability. Am. J. Primatol.39, 159–178 (1996). [DOI] [PubMed] [Google Scholar]
- 56.Crunchant, A. S., Stewart, F. A. & Piel, A. K. Vocal communication in wild chimpanzees: a call rate study. PeerJ9, e12326 (2021). [DOI] [PMC free article] [PubMed]
- 57.Marler, P. & Hobbett, L. Individuality in a Long-Range vocalization of wild chimpanzees. Z. Für Tierpsychologie. 38, 97–109 (1975). [PubMed] [Google Scholar]
- 58.Soldati, A. et al. The ontogeny of vocal sequences: insights from a newborn wild chimpanzee (Pan troglodytes schweinfurthii). Int. J. Primatol. 1–24. 10.1007/s10764-022-00321-y (2022).
- 59.Bründl, A. C. et al. Maternal effects on the development of vocal communication in wild chimpanzees. iScience10515210.1016/j.isci.2022.105152 (2022). [DOI] [PMC free article] [PubMed]
- 60.Bründl, A. C. et al. Systematic mapping of developmental milestones in wild chimpanzees. Dev. Sci.24, e12988 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Baptista, L. F. & Gaunt, S. L. L. Social interaction and vocal development in birds. in Social Influences on Vocal Development (eds Snowdon, C. T. & Hausberger, M.) 23–40 (Cambridge University Press, Cambridge, doi:10.1017/CBO9780511758843.003. (1997). [Google Scholar]
- 62.Grimsley, J. M. S., Monaghan, J. J. M. & Wenstrup, J. J. Development of social vocalizations in mice. PLOS ONE. 6, e17460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langehennig-Peristenidou, A., Romero-Mujalli, D., Bergmann, T. & Scheumann, M. Features of animal babbling in the vocal ontogeny of the Gray mouse lemur (Microcebus murinus). Sci. Rep.13, 21384 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishimura, T., Mikami, A., Suzuki, J. & Matsuzawa, T. Descent of the larynx in chimpanzee infants. Proc. Natl. Acad. Sci.100, 6930–6933 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seyfarth, R. & Cheney, D. Signalers and receivers in animal communication. Annu. Rev. Psychol.54, 145–173 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Koda, H. et al. Possible role of Mother-Daughter vocal interactions on the development of Species-Specific song in Gibbons. PLoS ONE. 8, e71432 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caro, T. M. & Hauser, M. D. Is there teaching in nonhuman animals?? Q. Rev. Biol.67, 151–174 (1992). [DOI] [PubMed] [Google Scholar]
- 68.Matsuzawa, T. et al. Emergence of culture in wild chimpanzees: education by Master-Apprenticeship. in Primate Origins of Human Cognition and Behavior (ed Matsuzawa, T.) 557–574 (Springer Japan, Tokyo, doi:10.1007/978-4-431-09423-4_28. (2001). [Google Scholar]
- 69.Lehmann, J. & Boesch, C. Sexual differences in chimpanzee sociality. Int. J. Primatol.29, 65–81 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fröhlich, M., Müller, G., Zeiträg, C., Wittig, R. M. & Pika, S. Gestural development of chimpanzees in the wild: the impact of interactional experience. Anim. Behav.134, 271–282 (2017). [Google Scholar]
- 71.Byrne, R. W. et al. Great ape gestures: intentional communication with a rich set of innate signals. Anim. Cogn.20, 755–769 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lonsdorf, E. V. et al. Sex differences in wild chimpanzee behavior emerge during infancy. PLoS ONE. 9, e99099 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Etchell, A. et al. A systematic literature review of sex differences in childhood Language and brain development. Neuropsychologia114, 19–31 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reynolds, V. The Chimpanzees of the Budongo Forest: Ecology, Behaviour and Conservation (OUP Oxford, 2005).
- 75.Pusey, A. E. Behavioural changes at adolescence in chimpanzees. Behaviour115, 203–246 (1990). [Google Scholar]
- 76.Slocombe, K. E. & Zuberbühler, K. Vocal communication in chimpanzees. in The Mind of the Chimpanzee (eds Lonsdorf, E. V., Stephen, R. R. & Matsuzawa, T.) 192–207 (University of Chicago Press, doi:10.7208/9780226492810. (2010).
- 77.Newton-Fisher, N. E. Association by male chimpanzees: A social tactic?? Behav136, 705–730 (1999). [Google Scholar]
- 78.Thompson, M. E. & Wrangham, R. W. Comparison of sex differences in gregariousness in Fission-Fusion species. in Primates of Western Uganda (eds Newton-Fisher, N. E., Notman, H., Paterson, J. D. & Reynolds, V.) 209–226 (Springer, New York, NY, doi:10.1007/978-0-387-33505-6_12. (2006). [Google Scholar]
- 79.Faraway, J. J. Linear Models With R (Chapman & Hall/CRC, 2006).
- 80.Quinn, G. P. & Keough, M. J. Experimental Designs and Data Analysis for Biologists (Cambridge University Press, 2002).
- 81.Lüdecke, D., Makowski, D., Waggoner, P. & Patil, P. Performance: an R package for assessment, comparison and testing of statistical models. J. Open. Source Softw.6, 3139 (2021). [Google Scholar]
- 82.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2023).
- 83.Long, J. A. jtools: Analysis and Presentation of Social Scientific Data. (2022).
- 84.Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear Mixed-Effects models using lme4. J. Stat. Softw.67, 1–48 (2015). [Google Scholar]
- 85.Underwood, A. J. Experiments in Ecology: their Logical Design and Interpretation Using Analysis of Variance (Cambridge University Press, 1997).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional information has been uploaded as part of the Supplementary Information. The data and code used in the analyses are available using the following link: https://osf.io/ze82d/?view_only=c58e1e88758a4a2bb7801c89991bc92b.