Abstract
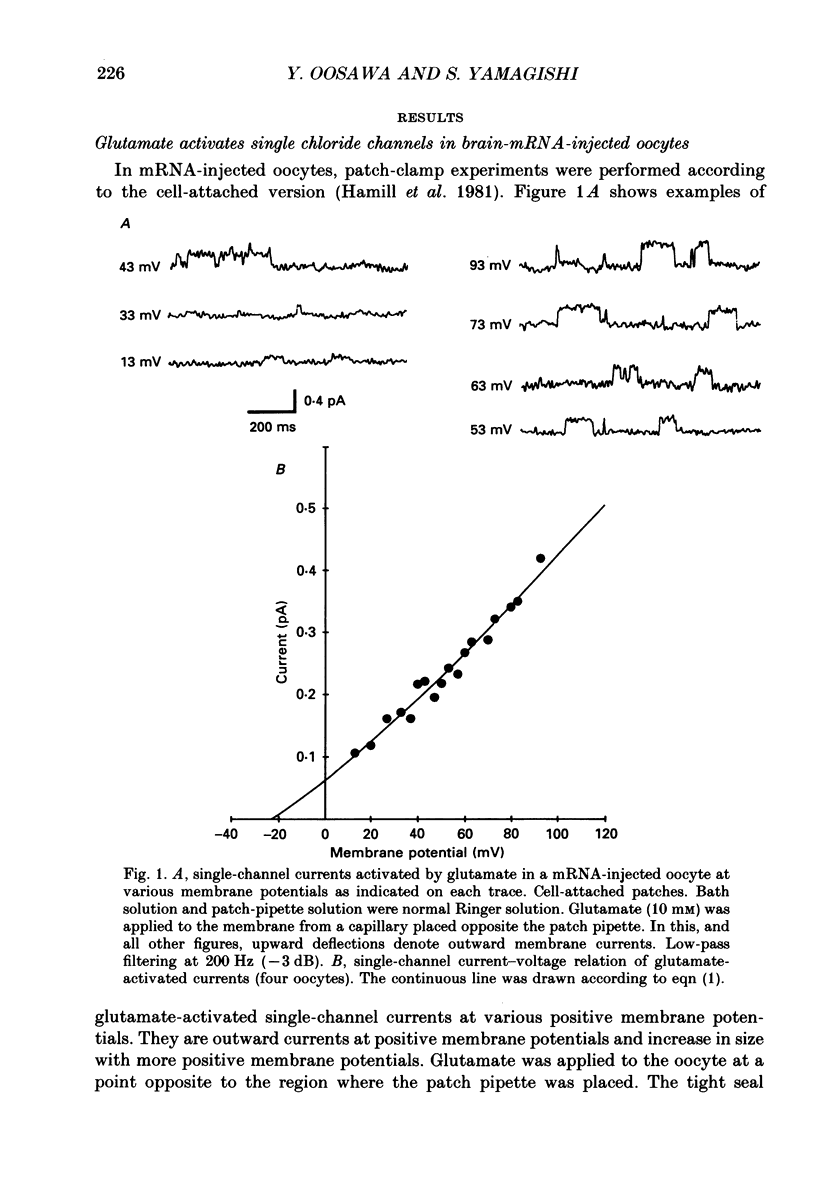
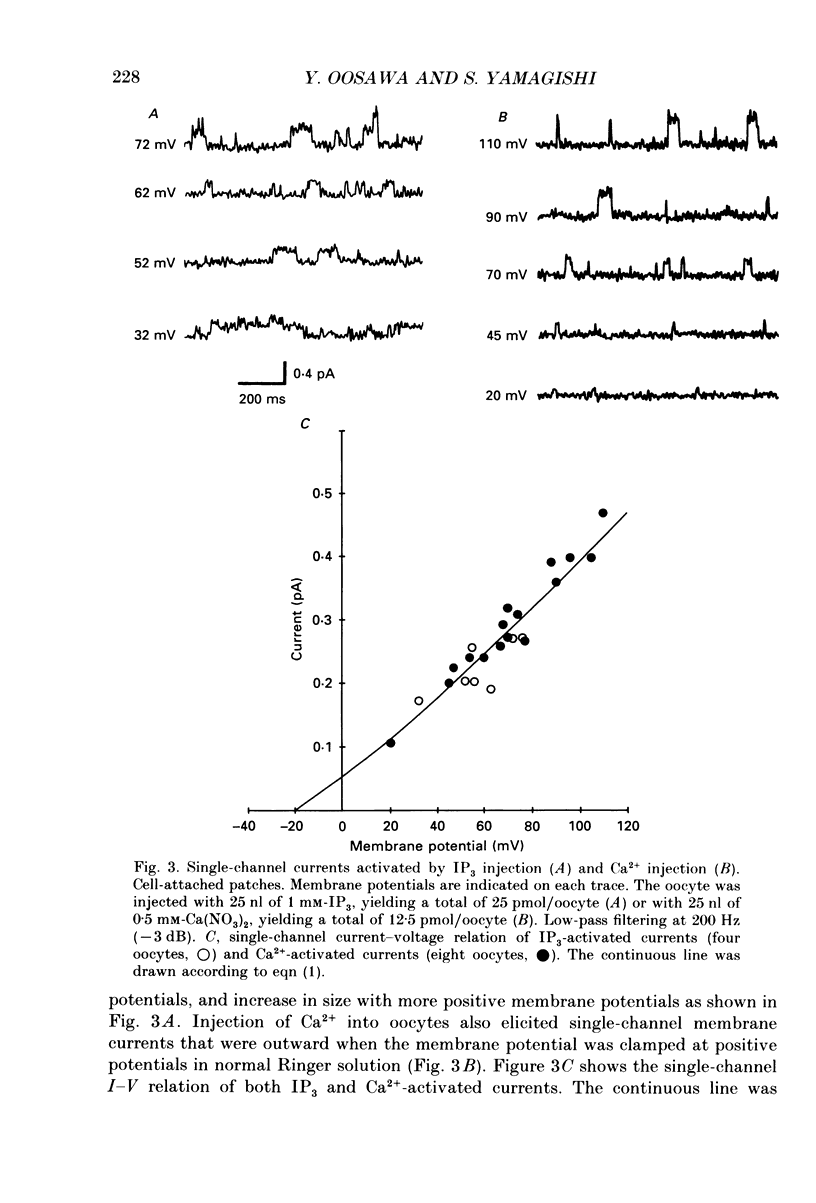
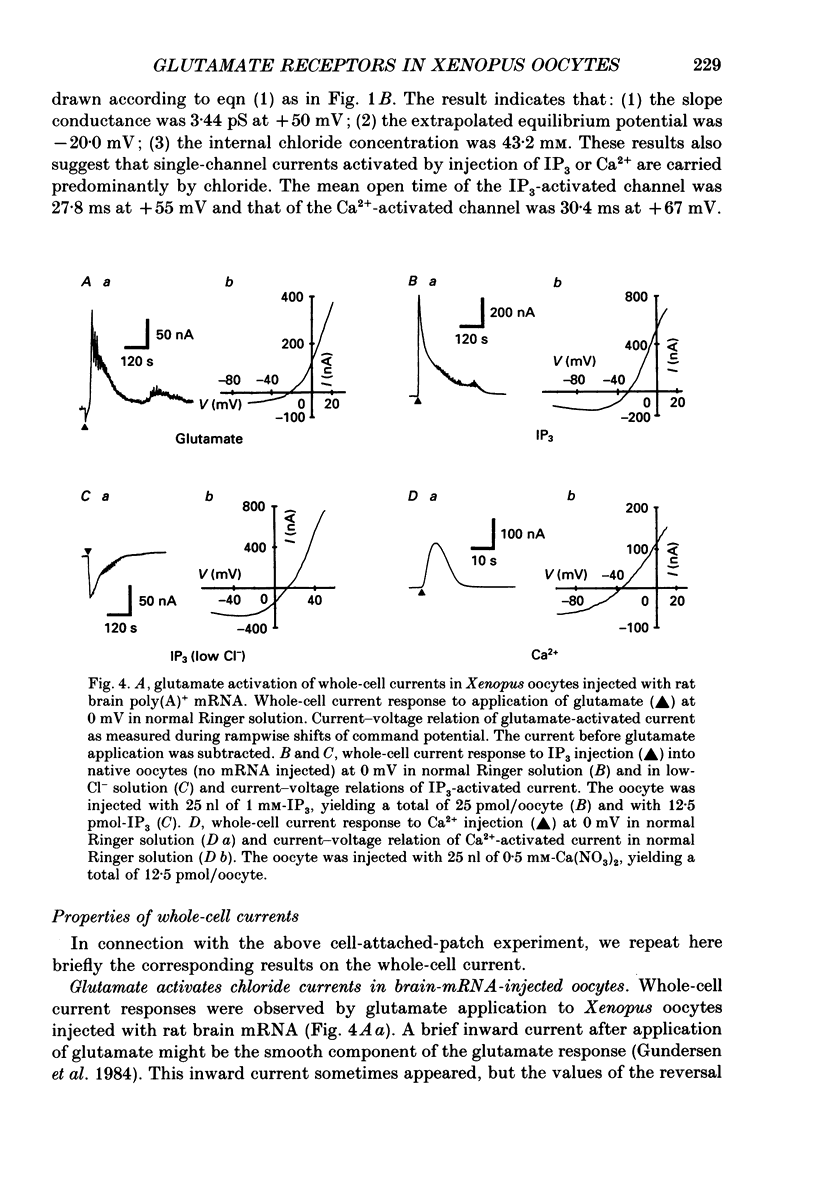
1. Ionic currents were studied in Xenopus laevis oocytes using the patch-clamp and the whole-cell voltage-clamp techniques. 2. Single-channel currents were recorded from the cell-attached patches in oocytes injected with rat brain mRNA when glutamate was applied locally outside the patch. The single-channel conductance was 3.66 pS, and the extrapolated equilibrium potential was -23.0 mV, indicating that the channels were chloride selective. 3. Single-channel currents with similar characteristics were observed in cell-attached patches in native oocytes in response to injection of inositol 1,4,5-trisphosphate (IP3) or Ca2+. 4. Whole-cell currents were evoked by glutamate in oocytes injected with rat brain mRNA. They usually showed an oscillatory component, and reversed direction at about the chloride equilibrium potential. Injection of IP3 or Ca2+ into a native oocyte evoked a transient whole-cell current. The reversal potential was near the chloride equilibrium potential, and it changed from negative to positive in low-chloride solution. 5. The results suggest that the glutamate receptors are not directly coupled with the endogenous chloride channels but indirectly activate these via the messenger system IP3-Ca2+.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Ferguson J. E., Joseph S. K., Williamson J. R., Nuccitelli R. Activation of frog (Xenopus laevis) eggs by inositol trisphosphate. I. Characterization of Ca2+ release from intracellular stores. J Cell Biol. 1985 Aug;101(2):677–682. doi: 10.1083/jcb.101.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Ogden D. C. Ion channels activated by L-glutamate and GABA in cultured cerebellar neurons of the rat. Proc R Soc Lond B Biol Sci. 1985 May 22;224(1236):367–373. doi: 10.1098/rspb.1985.0038. [DOI] [PubMed] [Google Scholar]
- Dascal N., Gillo B., Lass Y. Role of calcium mobilization in mediation of acetylcholine-evoked chloride currents in Xenopus laevis oocytes. J Physiol. 1985 Sep;366:299–313. doi: 10.1113/jphysiol.1985.sp015799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Serotonin receptors induced by exogenous messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Aug 22;219(1214):103–109. doi: 10.1098/rspb.1983.0062. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirono C., Yamagishi S., Ohara R., Hisanaga Y., Nakayama T., Sugiyama H. Characterization of mRNA responsible for induction of functional sodium channels in Xenopus oocytes. Brain Res. 1985 Dec 16;359(1-2):57–64. doi: 10.1016/0006-8993(85)91412-x. [DOI] [PubMed] [Google Scholar]
- Houamed K. M., Bilbe G., Smart T. G., Constanti A., Brown D. A., Barnard E. A., Richards B. M. Expression of functional GABA, glycine and glutamate receptors in Xenopus oocytes injected with rat brain mRNA. 1984 Jul 26-Aug 1Nature. 310(5975):318–321. doi: 10.1038/310318a0. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Ogura A. Glutamate-induced increase in intracellular Ca2+ concentration in isolated hippocampal neurones. Br J Pharmacol. 1986 Sep;89(1):191–198. doi: 10.1111/j.1476-5381.1986.tb11135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Meek J. L., Iadarola M. J., Chuang D. M., Roth B. L., Costa E. Coupling of inositol phospholipid metabolism with excitatory amino acid recognition sites in rat hippocampus. J Neurochem. 1986 Jan;46(1):40–46. doi: 10.1111/j.1471-4159.1986.tb12922.x. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. Intracellular Ca2+-dependent and Ca2+-independent responses of rat brain serotonin receptors transplanted to Xenopus oocytes. Neurosci Res. 1985 Aug;2(6):491–496. doi: 10.1016/0168-0102(85)90021-5. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Pin J. P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985 Oct 24;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Neher E., Sakmann B. Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5063–5067. doi: 10.1073/pnas.84.14.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]


