Abstract
Recently, there has been increased interest in colored foods because their unique pigments in fruits and vegetables originate from phytochemicals with antioxidant, anticancer, and antibacterial properties. Consequently, various colored cherry tomatoes have been developed. This study analyzed the lycopene, ascorbic acid, polyphenol, and antioxidant content and activity of 12 cultivars of colored cherry tomatoes harvested in Korea. The red colored cherry tomato cultivars ‘DOTORI RED TY’, ‘KT RED TY’, and ‘BLACK JOY200’ showed significantly higher levels of lycopene, a major antioxidant compound in tomato. Total flavonoid analysis showed that ‘DOTORI RED TY’, ‘TY Item’, and ‘TY Sispen’ had significantly higher concentrations, and a similar trend was observed in total phenolics. The major polyphenols in cherry tomatoes were identified as chlorogenic acid, rutin, and (-)-epigallocatechin gallate, with ‘TY Sispen’ showing significantly higher contents of chlorogenic acid and rutin, while ‘DOTORI RED TY’ exhibited higher levels of (-)-epigallocatechin gallate.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-024-01691-0.
Keywords: Colored cherry tomato, Antioxidant, Polyphenol, Lycopene, Ascorbic acid
Introduction
Reactive oxygen species (ROS) are unstable and highly reactive molecules that are generated during metabolic processes in organisms, such as mitochondrial activity, as well as due to environmental factors, including hormones, stress, and smoking (Zhang et al., 2021). Elevated levels of these reactive oxygen species within cells lead to damage to biological molecules such as DNA, RNA, proteins, and lipids, resulting in chronic diseases like cardiovascular diseases and cancer (Chaudhary et al., 2018). Antioxidants have been demonstrated to neutralize these reactive oxygen species, delaying or preventing oxidative damage. Synthetic antioxidants such as butylated hydroxy anisole (BHA) and butylated hydroxy toluene (BHT) have been developed for this purpose. However, compared to plant-derived phytochemicals, synthetic antioxidants may have long-term side effects when consumed. As a result, there is ongoing research aimed at finding natural antioxidants that are both safe and efficient for people to consume (Agarwal and Rao, 2000; Kang et al., 2002).
Fruits and vegetables are rich in vitamins, minerals, and phytochemicals, which are essential for maintaining health by keeping the blood neutral and preventing chronic diseases (Surh, 2003). Tomatoes contain various flavonoids (quercetin, naringenin, and rutin) and phenolic acids (chlorogenic acid and caffeic acid) (García-Valverde et al., 2013). Phenolic compounds are plant secondary metabolites characterized by one or more hydroxyl groups attached to benzene rings (Chaudhary et al., 2018). Phenolic compounds are known to reduce oxidative stress by scavenging reactive oxygen species, thereby preventing cancer and cardiovascular diseases. They are also known to have effects such as antioxidant, antimicrobial, anti-inflammatory, and anticancer properties (Surh, 2003).
Lycopene, the most prominent phytochemical in tomatoes, not only gives tomatoes their red color, determining their commercial value, but also exhibits antioxidant, anticancer, anti-inflammatory, and anti-mutagenic effects. It is particularly effective in preventing certain types of cancers such as colorectal cancer, prostate cancer, and breast cancer (Li et al., 2021). Lycopene has more than twice the antioxidant activity of β-carotene, and its bioavailability is higher when consumed with β-carotene. Furthermore, the combination of lycopene and vitamin E shows a synergistic effect in reducing the oxidation of low-density lipoprotein (LDL) cholesterol (Przybylska, 2020). About 90% of lycopene is found in the all-trans form, but in human tissues, it primarily exists in the cis isomer form. Cis-isomer lycopene is also generated during digestion in the human body and is produced during typical heating and processing of tomato products. Cis-isomer lycopene is known to be more soluble and more readily absorbed in the body than the all-trans form, resulting in higher bioavailability (Arballo et al., 2021).
Furthermore, tomatoes are rich in both vitamin C and vitamin E. Vitamin C not only prevents scurvy but is also effective in preventing various oxidative stress-related diseases such as aging, cardiovascular diseases, and cancer while vitamin E exhibits lipid radical scavenging activity in cell membranes and plasma (Ali et al., 2021). This research aims to emphasize the comprehensive analysis of bioactive compounds in cherry tomatoes of various colors grown in Korea, highlighting the innovation in assessing their health benefits. By analyzing lycopene, total flavonoid, total phenolic, ascorbic acid, and polyphenol profiles, along with total antioxidant activity using DPPH and ABTS radicals, the study provides a detailed comparison of the bioactive qualities of these tomatoes. This approach offers valuable insights into the nutritional and functional attributes of cherry tomatoes, promoting their potential health benefits and encouraging the consumption of diverse tomato varieties.
Materials and methods
Materials for experiment
A total of 12 cultivars of colored cherry tomatoes were divided into six colors such as red, orange, white, yellow, green, and black, and used for this experiment (Fig. 1). The cultivars ‘DOTORI RED TY’, ‘DOTORI NORANG TY’, ‘Green Joy’, ‘BLACK JOY200’ were harvested from Jayeonteo Co. Ltd. (37°42′03′′N 126°49′38′′E), in Ilsan, Gyeonggi-do, ‘KT RED TY’, ‘KT ORANGE TY’, ‘WHITEJOY TY’, ‘KT NORANG TY’, ‘BLACKLIN’ were harvested from Haemi Tomato Farm (36°42′43′′N 126°31′20′′E) located in Seosan-si, Chungcheongnam-do, and ‘TY Item’, ‘TY Sispen’, ‘YELLOW TINNY’ were harvested from a farm (35°56′06′′N 127°08′52′′E) located in Wanju, Jeollabuk-do. Lycopene analysis was performed on colored cherry tomatoes on the day of harvest. The tomatoes were then quickly frozen using liquid nitrogen at − 196 °C and stored in a freezer at − 20 °C for subsequent extraction.
Fig. 1.
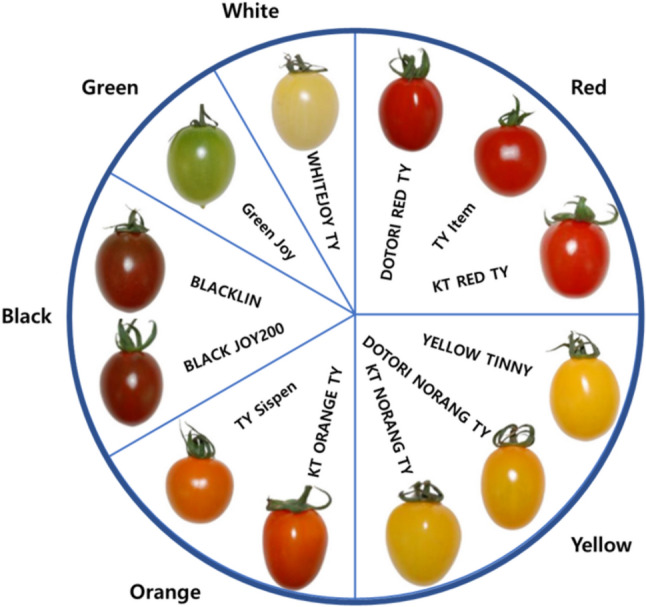
Color, cultivar and appearance of twelve cherry tomatoes (Adapted and modified from Joung et al., 2024)
Reagents and chemicals
The chemicals for antioxidant compounds analysis (lycopene, total flavonoids, total phenols, polyphenols, and ascorbic acid), and antioxidants activities analysis (DPPH and ABTS radical scavenging), methanol was purchased from Fisher Scientific (Pittsburgh, PA, USA), water was obtained from J.T. Baker Chemical Co. (Radnor, PA, USA). Sodium nitrite (NaNO2), benzene, aluminium chloride (AlCl3), Folin–Ciocalteu’s phenol reagent, sodium hydroxide solution (NaOH), sodium carbonate (NaCO3), acetic acid, potassium phosphate monobasic, dinitrophenylhydrazine, meta-phosphoric acid, 2,6-Dichloroindophenol sodium salt hydrate, sulfuric acid(H2SO4), 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2’-Azobis(2-amidinopropane) dihydrochloride (AAPH) were obtained from Sigma–Aldrich (St Louis, MO, USA). Phosphoric acid was obtained from Wako Pure Chemical Industries (Osaka, Japan).
The standard chemicals for antioxidant compounds analysis, gallic acid (CAS: 149-91-7), protocatechuic acid (CAS: 99-50-3), catechol (CAS: 120-80-9), catechin (CAS: 154-23-4), chlorogenic acid (CAS: 327-97-9), (-)-epigallocatechin gallate (CAS: 989-51-5), caffeic acid (CAS: 331-39-5), epicatechin (CAS: 490-46-0), syringic acid (CAS: 530-57-4), 4-methylcatechol (CAS: 452-86-8), epicatechin gallate (CAS: 1257-08-5), p-coumaric acid (CAS: 501-98-4), ferulic acid (CAS: 537-98-4), rutin hydrate (CAS: 207671-50-9), quercetin (CAS: 117-39-5), and ascorbic acid (CAS: 50-81-7) were purchased from Sigma–Aldrich.
Sample extraction
For the extraction of colored cherry tomatoes, a frozen sample of 30 g, stored at – 20 °C, was mixed with 300 mL of 80% methanol and homogenized twice for 3 min and once for 2 min using a blender (JB 3060, Braun Co., Kronberg, Germany). The homogenized solution was then filtered using Whatman #2 filter paper (Whatman International Ltd., Kent, England) and a vacuum filtration device, and concentrated using a rotary evaporator (N-1300, Eyela, Tokyo, Japan). The sample was adjusted to 30 mL using distilled water, divided into several aliquots, and stored at -20℃ for analysis of antioxidant compounds and antioxidant activities (Yang et al., 2019).
Lycopene content
The lycopene contents in the samples were assessed following Bicanic’s methods with slight modifications (Bicanic et al., 2005). Each 1 g of sample was combined with a small amount of deionized water and methanol, and the mixture was agitated following the addition of Celite powder (No. 545). Subsequently, a glass filter (3G4) was placed into a glass vessel, and aspiration-filtration was carried out using an aspirator (AAA71015, Jeio Tech Co. Ltd., Daejeon, Korea) while methanol was added continuously until the yellow color of the sample solution turned a transparent color. This filtrate was then transferred to a 100 mL flask, and mass up with benzene. The absorbance at 487 nm was determined using a spectrophotometer (Optizen POP, Mecasys, Daejeon, Korea), and the lycopene content was quantified as mg/100 g of fresh weight (FW).
C: Concentration of lycopene (µg/mL), estimated from the calibration curve, D: Dilution factor of the test solution, S: Amount of sample (g).
Total flavonoid content
The total flavonoid content in colored cherry tomatoes was measured using a colorimetric assay method (Yang et al., 2019). For the procedure, 1 mL of sample extract was mixed with 4 mL of distilled water and 0.3 mL of 5% NaNO2, and the reaction was allowed to proceed at room temperature for 5 min. Subsequently, 0.3 mL of 10% AlCl3 was added to the mixture, and the reaction continued at room temperature for 6 min. Then, 2 mL of 1N NaOH and 2.4 mL of distilled water were added to bring the total volume to 10 mL. The absorbance of the test solution was determined at 510 nm with a spectrophotometer. A standard calibration curve was prepared using rutin hydrate, and the total flavonoid content was expressed as mg rutin hydrate equivalents (RE)/100 g FW.
Total phenolic content
The total phenolic content of colored cherry tomatoes was determined using the Folin-Ciocalteu colorimetric assay method (Yang et al., 2019). A mixture of 0.2 mL of sample dilution, 2.6 mL of distilled water, and 0.2 mL of Folin-Ciocalteu’s phenol reagent was left to react at room temperature for 6 min. Then, 2 mL of 7% Na2CO3 was added to the mixture, and the reaction continued at room temperature for 90 min. The absorbance of the test solution was measured at 750 nm using a spectrophotometer. A calibration curve was created with gallic acid as the standard. The total phenolic content was reported as mg gallic acid equivalents (GAE)/100 g FW.
Polyphenol content
The individual polyphenols in the extract of colored cherry tomatoes were analyzed using HPLC according to the method described by Chen et al. (2001). The extract was diluted with a solution (0.2 M KH2PO4 buffer with the pH adjusted to 3.0 using phosphoric acid: MeOH: D.W = 2: 3: 15) and filtered through a 0.45 µm syringe filter. The filtrate was then injected into an eclipse XDBC-18 column (150 × 4.6 mm, 5 µm, Agilent, USA, 40 °C), and separation was achieved using HPLC (Agilent 1100 series, Agilent Technol., Palo Alto, CA, USA). Acetic acid (3%) and methanol were used as the mobile phase under gradient conditions, with detection at 280 nm and a flow rate of 1.0 mL/min. Analysis was conducted by injecting 10 µL of the sample. Standard calibration curves were prepared using gallic acid, protocatechuic acid, catechol, catechin, chlorogenic acid, (-)-epigallocatechin gallate, caffeic acid, epicatechin, syringic acid, 4-methylcatechol, epicatechin gallate, p-coumaric acid, ferulic acid, and rutin, which were purchased from Sigma (St. Louis, MO, USA). The polyphenol content was expressed as mg/100 g FW.
Total ascorbic acid content
The total ascorbic acid content in the samples was quantified using the dinitrophenylhydrazine (DNPH) method as described by Terada et al. (1978) and Kim et al. (2024). The samples (5 g) were homogenized and thoroughly mixed with 100 mL of 6% metaphosphoric acid buffer and subsequently centrifuged at 15,000 rpm for 20 min. The supernatant was subsequently filtered using Whatman #1 filter paper. Then, 1 mL of the supernatant was mixed with 0.05 mL of 2% DCIP (2,6-Dichlorophenolindophenol) and allowed to react at room temperature for 1 h. Afterward, 1 mL of 2% thiourea was introduced, followed by the addition of 0.5 mL of 2% DNPH. The mixture was vortexed and incubated for 3 h at 60 °C. After cooling, 2.5 mL of 90% H2SO4 was gradually added to eliminate the osazone. The absorbance was then measured at 540 nm with a spectrophotometer, and a standard calibration curve was created using ascorbic acid as the reference. The total ascorbic acid content was quantified and expressed as mg/100 g FW.
DPPH radical scavenging activity
The antioxidant activity analysis of colored cherry tomato extract using the DPPH radical scavenging assay was conducted with modifications to the method described by Yang et al. (2019) and Hwang et al. (2020). A mixture of 50 µL of sample dilution and 2950 µL of 0.2 mM DPPH solution was allowed to react at room temperature for 30 min. Then, the absorbance was assessed at 517 nm using a spectrophotometer. A calibration curve was created with ascorbic acid, and the DPPH radical scavenging activity was quantified as mg vitamin C equivalents (VCE)/100 g FW.
ABTS radical scavenging activity
The antioxidant activity of the samples was evaluated using a modified ABTS assay method, based on the procedures detailed by Yang et al. (2019) and Hwang et al. (2020). A solution was prepared with 1 mM 2,2’-Azobis(2-amidinopropane) dihydrochloride (AAPH), 2.5 mM ABTS, and PBS buffer, then allowed to react at 70 °C for 40 min in a water bath. This solution was subsequently diluted with PBS to achieve an absorbance between 0.63 and 0.67 at 734 nm. Next, 20 μL of the sample extract was mixed with 980 μL of the ABTS solution and incubated at 37 °C for 10 min. The absorbance of this mixture was measured at 734 nm using a spectrophotometer. A calibration curve was created using ascorbic acid as the standard. The ABTS radical scavenging activity was expressed as mg VCE/100 g FW.
Statistical analysis
The statistical analysis was carried out using SPSS version 20 (SPSS Inc., Chicago, IL, USA). An analysis of variance (ANOVA) was conducted, followed by Duncan’s multiple range test to evaluate the significance of differences between samples (p < 0.05). The results were reported as the mean ± standard deviation from three independent measurements.
Results and discussion
Lycopene content
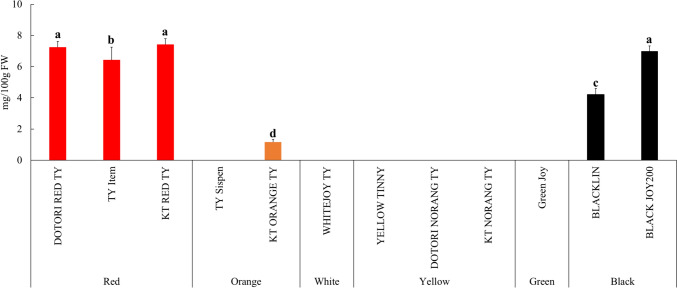
The lycopene content of colored cherry tomatoes is shown in Fig. 2. The lycopene levels of the ‘DOTORI RED TY’, ‘KT RED TY’, and ‘BLACK JOY200’ varieties were significantly higher, at 7.25 ± 0.14, 7.42 ± 0.20, and 6.99 ± 0.13 mg/100 g FW, respectively. Conversely, ‘KT ORANGE TY’ demonstrated significantly lower levels at 1.16 ± 0.18 mg/100 g FW, and lycopene was not detected in other cultivars. The lycopene content in tomatoes varies considerably depending on their stage of ripeness, with more mature tomatoes having higher levels of lycopene. Approximately half of the total lycopene is produced and accumulated during the deep red phase of maturation (Helyes et al., 2006). Besides maturity stage, environmental factors including can also influence the lycopene content in the fruit. Sunlight intensity impacts the temperature surrounding the plant (Dumas et al., 2003).
Fig. 2.
Lycopene contents of colored cherry tomatoes. Vertical bars indicate standard deviation. Different letters are significant differences by Duncan’s multiple range test (p < 0.05)
In general, lycopene can be obtained from foods such as tomatoes, watermelon, grapefruit, apricot, and guava. Notably, tomatoes and products derived from them play a major role, providing about 50% of the daily lycopene intake. Furthermore, tomatoes, when subjected to heat processing, disrupt cell walls, leading to the release of lycopene from chromoplasts. Thus, processed tomato products have a higher bioavailability compared to fresh tomatoes (Agarwal and Rao, 2000). The research by Choi et al. (2014) found that the lycopene levels in orange, red, and black cherry tomatoes were consistent with the findings of our study, and also lycopene was not detected in green cherry tomatoes. The Antioxidant levels in tomatoes differ based on their types or varieties. Typically, red tomato varieties have higher lycopene concentrations, while orange tomatoes tend to have more beta-carotene. The fruit’s color is influenced by the relative amounts of lycopene and beta-carotene (Rosati et al., 2000). Additionally, Coyago-Cruz et al. (2019) found that the lycopene content of red cherry tomatoes ranged from 4.7 to 69.2 mg/100 g dry weight (DW) depending on the cultivars, while lycopene was not detected in orange and yellow cherry tomatoes. Even within red cherry tomatoes, different cultivars exhibited various levels of lycopene, and this result confirmed that lycopene was not detected in yellow and green cultivars in our study. According to the study by Oluk et al. (2019), cherry tomatoes cultivated in Turkey showed lycopene contents of 43.08 ± 0.35 mg/kg FW for red, 2.01 ± 0.01 mg/kg FW for orange, 0.02 ± 0.01 mg/kg FW for yellow, and 8.45 ± 0.13 mg/kg FW for black, indicating that the cultivars we experimented with contained higher lycopene levels. Several studies have shown that greenhouse-grown tomatoes typically exhibit higher lycopene content than those cultivated in open fields. This difference is attributed to the difficulty in controlling climatic factors such as rainfall and temperature in field conditions. Increased solar radiation exposure results in elevated fruit temperatures and lighter fruit coloration, factors directly influencing lycopene levels (Brandt et al., 2006; Pék et al., 2011). Therefore, lycopene content is closely related not only to the variety but also to the cultivation environment.
Total flavonoid content
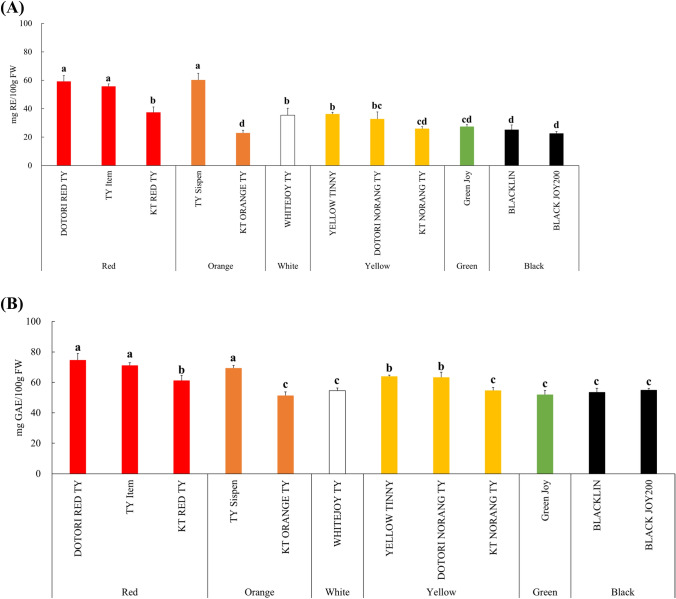
The total flavonoid content of colored cherry tomatoes is shown in Fig. 3(A). The highest levels were observed in ‘DOTORI RED TY,’ ‘TY Item,’ and ‘TY Sispen,’ with contents of 59.26 ± 3.96, 55.74 ± 1.68, and 60.26 ± 4.55 mg RE/100 g FW, respectively. Conversely, ‘KT ORANGE TY,’ ‘BLACKLIN,’ and ‘BLACK JOY200’ exhibited the lowest levels, measuring 22.89 ± 1.87, 25.22 ± 3.38, and 22.58 ± 1.43 mg RE/100 g FW, respectively. Tomatoes contain various flavonoids. Rutin, in particular, accounts for over 94% of the total flavonols in tomatoes and is a flavonoid known for its high antioxidant activity and its bioavailability (Slimestad and Verheul, 2009). Furthermore, according to the study by Choi et al. (2014), flavonoid compounds in various colored cherry tomatoes were predominantly represented by naringenin chalcone and rutin. Generally, cherry tomatoes contain about 3 to 4 times more total flavonoids than regular tomatoes. Flavonoids in tomatoes are predominantly synthesized in the fruit peel, and it is believed that cherry tomatoes have a larger surface area per unit weight compared to regular tomatoes (Kavitha et al., 2014; Slimestad and Verheul, 2009). According to Lenucci et al. (2006), the total flavonoid content of 14 cherry tomato cultivars ranged from 13.4 to 62.2 mg RE/100 g FW. Additionally, according to Noor et al. (2014), the total flavonoid content of red and yellow cherry tomatoes was 171 mg CE/g FW and 188 mg CE/100 g FW, respectively, showing no significant difference based on color. In our research, the red cherry tomato ‘DOTORI RED TY’ exhibited approximately 2.3 times higher levels of total flavonoids (59.26 mg RE/100 g FW) compared to the yellow cherry tomato ‘KT NORANG TY’ (25.97 mg RE/100 g FW).
Fig. 3.
(A) Total flavonoids contents of colored cherry tomatoes. Vertical bars indicate standard deviation. Different letters are significant differences by Duncan’s multiple range test (p < 0.05). (B) Total phenolics contents of colored cherry tomatoes. Vertical bars indicate standard deviation. Different letters are significant differences by Duncan’s multiple range test (p < 0.05)
Total phenolic content
The total phenolic content of the colored cherry tomatoes is shown in Fig. 3(B). The total phenolic content of ‘DOTORI RED TY’, ‘TY Item’, and ‘TY Sispen’ was significantly higher at 74.74 ± 4.29, 71.17 ± 1.77, and 69.33 ± 1.84 mg GAE/100 g FW, respectively. Kavitha et al. (2014) found that the total phenolic content in regular tomatoes varied between 20.32 and 42.60 mg GAE/100 g FW, whereas cherry tomatoes ranged from 48.20 to 73.40 mg GAE/100 g FW. The total phenolic content was found to be higher in cherry tomatoes compared to regular tomatoes. According to Oluk et al. (2019), the total phenolic content of red, orange, yellow, and brown cherry tomatoes was 44.01 ± 1.03, 42.38 ± 2.25, 35.45 ± 1.05, and 46.87 ± 1.19 mg GAE/100 g FW, respectively, indicating lower contents compared to our study. Furthermore, Erdinc et al. (2018) found that the total phenolic content of red, yellow, and black cherry tomatoes was 71.85–74.89, 40.39–58.15, and 71.85–74.89 mg GAE/100 g FW, respectively. In comparison to our results, the total phenolic content in cherry tomatoes showed either similar level or minor variations depending on color. These differences can be attributed to factors such as cultivar, ripeness, environmental conditions, and storage practices.
Polyphenol content
The polyphenol content of colored cherry tomatoes is presented in Table 1. The HPLC chromatograms of the blank, polyphenol standard solution (50 mg/L), and sample are shown in Supplementary Fig. 1. The major polyphenols identified in colored cherry tomatoes were rutin (quercetin-3-O-rutinoside), chlorogenic acid, and (-)-epigallocatechin gallate (EGCG). Among these, chlorogenic acid accounted for the highest proportion, ranging from 48.32% to 73.58% of the total polyphenol content, followed by rutin at 9.04% to 34.31%, and (-)-epigallocatechin gallate at 9.60% to 21.17%.
Table 1.
Polyphenol contents of colored cherry tomatoes
| Color | Cultivar | Chlorogenic acid | Rutin | (-)-epigallocatechin gallate |
|---|---|---|---|---|
| Red | DOTORI RED TY | 12.64 ± 2.05d | 4.16 ± 1.00d | 3.05 ± 1.11a |
| TY Item | 14.45 ± 0.10b | 5.36 ± 0.77c | 2.10 ± 1.28def | |
| KT RED TY | 7.98 ± 4.83f | 5.67 ± 1.67b | 2.87 ± 0.94b | |
| Orange | TY Sispen | 16.33 ± 4.03a | 6.36 ± 1.07a | 2.11 ± 1.36def |
| KT ORANGE TY | 7.44 ± 4.52g | 3.85 ± 1.24e | 2.09 ± 1.07ef | |
| White | WHITEJOY TY | 13.29 ± 1.61c | 2.51 ± 1.17g | 2.41 ± 1.01c |
| Yellow | YELLOW TINNY | 6.66 ± 1.36h | 3.93 ± 0.98e | 2.29 ± 1.49cd |
| DOTORI NORANG TY | 9.36 ± 0.95e | 2.80 ± 0.75f | 2.43 ± 1.52c | |
| KT NORANG TY | 8.36 ± 2.44f | 3.80 ± 1.57e | 1.97 ± 0.42f | |
| Green | Green Joy | 9.10 ± 2.21e | 1.12 ± 0.42i | 2.15 ± 0.41def |
| Black | BLACKLIN | 6.31 ± 2.18hi | 2.22 ± 0.76h | 2.25 ± 0.47cde |
| BLACK JOY200 | 6.00 ± 2.65i | 2.09 ± 0.64h | 2.17 ± 0.37de |
Mean separation within columns by Duncan’s multiple range test (p < 0.05) (n = 3)
Chlorogenic acid was significantly highest in ‘TY Sispen’ (16.33 ± 0.33 mg/100 g FW) and significantly lowest in ‘BLACK JOY200’ (6.00 ± 0.22 mg/100 g FW). Chlorogenic acid constitutes 75% of the total phenolic content in immature green cherry tomatoes and 35% in red cherry tomatoes (Slimestad and Verheul, 2009). According to Coyago-Cruz et al. (2019), the chlorogenic acid content in red cherry tomatoes ranged from 3.8 to 4.3 mg/100 g DW, while orange cherry tomatoes had 68.5 mg/100 g DW, yellow cherry tomatoes had 23.1 mg/100 g DW, and green cherry tomatoes had 13.3 mg/100 g DW. The chlorogenic acid content in orange cherry tomatoes is similar to that of ‘KT ORANGE TY’ in our study (7.44 ± 0.37 mg/100 g FW), but the remaining content is lower compared to our study. Rutin, the most important flavonoid in tomatoes, is synthesized in the final step of the phenol biosynthesis pathway (Giuntini et al., 2008; Slimestad and Verheul, 2009). ‘TY Sispen’ exhibited the significantly highest rutin content (6.36 ± 0.06 mg/100 g FW), followed by ‘KT RED TY’ and ‘TY Item’. Ahn (2018) reported that the rutin content in three cherry tomato varieties harvested in Korea ranged from 24.31 to 50.85 mg/100 g DW, which is comparable to or lower than the rutin content found in the red cherry tomatoes used in this study (4.16–5.67 mg/100 g FW).
Additionally, Choi et al. (2014) found that the rutin content of red, orange, green, and black varieties ranged from 40.2 to 52.8 mg/100 g DW, 28.2 to 60.0 mg/100 g DW, 11.4 to 17.6 mg/100 g DW, and 23.7 mg/100 g DW, respectively, which is consistent with our study results.
(-)-Epigallocatechin gallate (EGCG) is a substance belonging to flavonoids, specifically flavonols, and in this study, ‘DOTORI RED TY’ exhibited the significantly highest content at 3.05 ± 0.09 mg/100 g FW.
Total ascorbic acid content
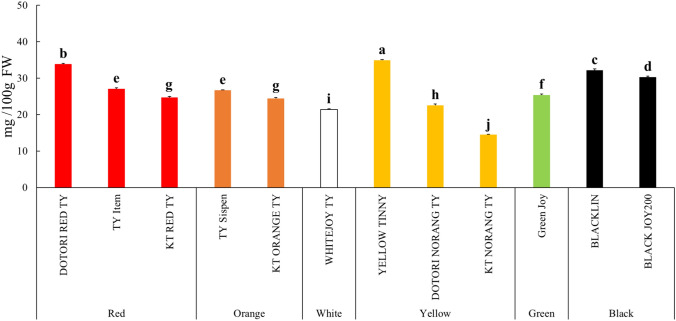
The total ascorbic acid content of colored cherry tomatoes is shown in Fig. 4. ‘YELLOW TINNY’ exhibited the significantly highest total ascorbic acid content at 34.89 ± 0.25 mg/100 g FW, while ‘KT NORANG TY’ showed the significantly lowest content at 14.46 ± 0.18 mg/100 g FW. Raffo et al. (2006) found that the total ascorbic acid content in cherry tomatoes varied between 31 and 71 mg/100 g, depending on the harvest season. Overall, red cherry tomatoes showed higher total ascorbic acid content compared to colored cherry tomatoes. Typically, the total ascorbic acid content in tomatoes is affected by environmental factors such as light and temperature. It has been reported that plants respond to light by increasing the content of ascorbic acid, and exposure to light is beneficial for the accumulation of ascorbic acid in cherry tomatoes as well (Davey et al., 2000; Ntagkas et al., 2019). According to the study by Bhandari et al. (2016), the ascorbic acid content of red cherry tomatoes harvested in Korea ranged from 1.07 to 22.67 mg/100 g FW, which is similar to or lower than the content observed in our study. Oluk et al. (2019) observed ascorbic acid contents of 32.88 mg/100 g FW in red cherry tomatoes, 22.83 mg/100 g FW in orange tomatoes, 19.91 mg/100 g FW in yellow tomatoes, and 15.68 mg/100 g FW in black tomatoes. In our study, the average total ascorbic acid content in black cherry tomatoes was about twice as high, at 31.22 mg/100 g FW, while the levels in the other color varieties were comparable. The differences in ascorbic acid content in tomatoes may be due to both environmental factors, such as temperature and light, and genetic factors (Martí et al., 2018).
Fig. 4.
Total ascorbic acid contents of colored cherry tomatoes. Vertical bars indicate standard deviation. Different letters are significant differences by Duncan’s multiple range test (p < 0.05)
Antioxidant activity
Figure 5(A) shows that ‘TY Item’ and ‘TY Sispen’ exhibited significantly higher DPPH radical scavenging activity than other samples, with values of 51.16 ± 0.46 and 51.31 ± 0.98 mg VCE/100 g FW, respectively. According to the study by Kavitha et al. (2014), the analysis of DPPH radical scavenging activity in regular tomatoes and cherry tomatoes showed values ranging from 22.37 to 37.83 mg VCE/100 g FW and 33.16 to 54.12 mg VCE/100 g FW, respectively, indicating higher values for cherry tomatoes. This is attributed to the larger surface area of cherry tomatoes compared to regular one, leading to higher lycopene content and consequently higher antioxidant activity. The analysis of antioxidant substances required to reduce DPPH radicals by 50% in red and yellow cherry tomatoes showed no significant difference, with values of 46.47 ± 0.38 µg/mL and 46.22 ± 4.51 µg/mL, respectively (Noor et al., 2014).
Fig. 5.
(A) DPPH radical scavenging activities of colored cherry tomatoes. Vertical bars indicate standard deviation. Different letters are significant differences by Duncan’s multiple range test (p < 0.05). (B) ABTS radical scavenging activities of colored cherry tomatoes. Vertical bars indicate standard deviation. Different letters are significant differences by Duncan’s multiple range test (p < 0.05)
The ABTS radical scavenging activity of colored cherry tomatoes is shown in Fig. 5(B). ‘TY Sispen’ and ‘DOTORI RED TY’ exhibited significantly higher values at 87.79 ± 1.94 and 87.49 ± 2.87 mg VCE/100 g FW, respectively. On the other hand, ‘KT ORANGE TY’, ‘WHITEJOY TY’, ‘KT NORANG TY’, ‘Green Joy’, ‘BLACKLIN’, and ‘BLACK JOY200’ cultivars exhibited significantly lower activities. However, the activity ranged between 87.79 and 60.37 ± 2.46 mg VCE/100 g FW. Chun et al. (2005) and Floegel et al. (2011) reported the ABTS radical scavenging activity of regular tomatoes as 39.1 ± 3.1 mg VCE/100 g FW and 29.44 ± 1.60 mg VCE/100 g FW, respectively. Our results with colored cherry tomatoes indicate a higher ABTS radical scavenging activity compared to regular tomatoes, attributed to their higher content not only of lycopene but also of total flavonoid and total phenolic contents (Kavitha et al., 2014; Bhandari et al., 2016).
In summary, red cherry tomato cultivars, such as ‘DOTORI RED TY’, ‘KT RED TY’, and ‘BLACK JOY200’, have higher lycopene levels, while ‘DOTORI RED TY’, ‘TY Item’, and ‘TY Sispen’ exhibit increased total flavonoid and phenolic content. The findings highlight the potential of these cultivars to offer enhanced antioxidant and health benefits. Future studies should aim to identify genetic factors linked to high concentrations of beneficial compounds, including lycopene, flavonoids, and phenolics, in cherry tomatoes. This information could facilitate the development of genetically optimized cultivars with enhanced nutritional profiles.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
No funding was received.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Young-Jun Kim, Email: kimyj@seoultech.ac.kr.
Youngjae Shin, Email: ys234@dankook.ac.kr.
References
- Agarwal S, Rao AV. Tomato lycopene and its role in human health and chronic diseases. Canadian Medical Association Journal. 163: 739-744 (2000) [PMC free article] [PubMed] [Google Scholar]
- Ahn JB. Characterization of lycopene, β-carotene, and phenolic compounds of domestic cherry tomato cultivars. Food Engineering Progress. 22: 9-16 (2018) [Google Scholar]
- Ali MY, Sina AAI, Khandker SS, Neesa L, Tanvir EM, Kabir A, Khalil ML, Gan SH. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods. 10: 342 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arballo J, Amengual J, Erdman Jr JW. Lycopene: A critical review of digestion, absorption, metabolism, and excretion. Antioxidants. 10: 342 (2021) [DOI] [PMC free article] [PubMed]
- Bhandari SR, Cho MC, Lee JG. Genotypic variation in carotenoid, ascorbic acid, total phenolic, and flavonoid contents, and antioxidant activity in selected tomato breeding lines. Horticulture Environment and Biotechnology. 57: 440-452 (2016) [Google Scholar]
- Bicanic D, Fogliano V, Luterotti S, Swarts J, Piani G, Graziani G. Quantification of lycopene in tomato products: comparing the performances of a newly proposed direct photothermal method and high-performance liquid chromatography. Journal of the Science of Food and Agriculture. 85: 1149–1153 (2005) [Google Scholar]
- Brandt S, Pék Z, Barna É, Lugasi A, Helyes L. Lycopene content and colour of ripening tomatoes as affected by environmental conditions. Journal of the Science of Food and Agriculture. 86: 568-572 (2006) [Google Scholar]
- Chaudhary P, Sharma A, Singh B, Nagpal AK. Bioactivities of phytochemicals present in tomato. Journal of Food Science and Technology. 55: 2833-2849 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zuo Y, Deng Y. Separation and determination of flavonoids and other phenolic compounds in cranberry juice by high-performance liquid chromatography. Journal of Chromatography A. 913: 387-395 (2001) [DOI] [PubMed] [Google Scholar]
- Choi SH, Kim DS, Kozukue N, Kim HJ, Nishitani Y, Mizuno M, Levin CE, Friedman M. Protein, free amino acid, phenolic, β-carotene, and lycopene content, and antioxidative and cancer cell inhibitory effects of 12 greenhouse-grown commercial cherry tomato varieties. Journal of Food Composition and Analysis. 32: 115-127 (2014) [Google Scholar]
- Chun OK, Kim DO, Smith N, Schroeder D, Han JT, Lee CY. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. Journal of the Science of Food and Agriculture. 85: 1715-1724 (2005) [Google Scholar]
- Coyago-Cruz E, Corell M, Moriana A, Mapelli-Brahm P, Hernanz D, Stinco CM, Beltrán-Sinchiguano E, Meléndez-Martínez AJ. Study of commercial quality parameters, sugars, phenolics, carotenoids and plastids in different tomato varieties. Food Chemistry. 277: 480-489 (2019) [DOI] [PubMed] [Google Scholar]
- Davey MW, Montagu MV, Inze D, Sanmartin M, Kanellis A, Smirnoff N, Fletcher J. Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. Journal of the Science of Food and Agriculture. 80: 825–860 (2000)
- Dumas Y, Dadomo M, Lucca GD, Grolier P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. Journal Science Food Agriculture. 83: 369-382 (2003) [Google Scholar]
- Erdinc C, Ekincialp A, Gundogdu M, Eser F, Sensoy S. Bioactive components and antioxidant capacities of different miniature tomato cultivars grown by altered fertilizer applications. Journal of Food Measurement and Characterization. 12: 1519-1529 (2018) [Google Scholar]
- Floegel A, Kim DO, Chung SJ, Koo SI, Chun OK. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of Food Composition and Analysis. 24: 1043-1048 (2011) [Google Scholar]
- García-Valverde V, Navarro-González I, García-Alonso J, Periago MJ. Antioxidant bioactive compounds in selected industrial processing and fresh consumption tomato cultivars. Food and Bioprocess Technology. 6: 391-402 (2013) [Google Scholar]
- Giuntini D, Lazzeri V, Calvenzani V, Dall’Asta C, Galaverna G, Tonelli C, Petroni K, Ranieri A. Flavonoid profiling and biosynthetic gene expression in flesh and peel of two tomato genotypes grown under UV-B-depleted conditions during ripening. Journal of Agricultural and Food Chemistry. 56: 5905-5915 (2008) [DOI] [PubMed] [Google Scholar]
- Helyes L, Pék Z, Lugasi A. Tomato fruit quality and content depend on stage of maturity. HortScience. 41: 1400-1401 (2006) [Google Scholar]
- Hwang H, Kim YJ, Shin Y. Assessment of physicochemical quality, antioxidant content and activity, and inhibition of cholinesterase between unripe and ripe blueberry fruit. Foods. 9: 690 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung M, Kim YJ, Yun CI, Shin Y. Comparative evaluation of physicochemical characteristics, organic acid, and sugar compositions in colored cherry tomatoes. Korean Journal of Food Science and Technology. 56: 8-14 (2024) [Google Scholar]
- Kang MH, Choi CS, Kim ZS, Chung HK, Min KS, Park CG, Park HW. Antioxidative activities of ethanol extract prepared from leaves, seed, branch and aerial part of Crotalaria sessiflora L. Korean Journal of Food Science and Technology. 34: 1098-1102 (2002) [Google Scholar]
- Kavitha P, Shivashankara KS, Rao VK, Sadashiva AT, Ravishankar KV, Sathish GJ. Genotypic variability for antioxidant and quality parameters among tomato cultivars, hybrids, cherry tomatoes and wild species. Journal of the Science of Food and Agriculture. 94: 993-999 (2014) [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim YJ, Shin Y. Comparative analysis of polyphenol content and antioxidant activity of different parts of five onion cultivars harvested in Korea. Antioxidants. 13: 197 (2024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenucci MS, Cadinu D, Taurino M, Piro G, Dalessandro G. Antioxidant composition in cherry and high-pigment tomato cultivars. Journal of Agricultural and Food Chemistry. 54: 2606-2613 (2006) [DOI] [PubMed] [Google Scholar]
- Li N, Wu X, Zhuang W, Xia L, Chen Y, Wu C, Rao Z, Du L, Zhao R, Yi M, Wan Q, Zhou Y. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chemistry. 343: 128396 (2021) [DOI] [PubMed] [Google Scholar]
- Martí R, Leiva-Brondo M, Lahoz I, Campillo C, Cebolla-Cornejo J, Roselló S. Polyphenol and L-ascorbic acid content in tomato as influenced by high lycopene genotypes and organic farming at different environments. Food Chemistry. 239: 148-156 (2018) [DOI] [PubMed] [Google Scholar]
- Noor Atiqah AAK, Maisarah AM, Asmah R. Comparison of antioxidant properties of tamarillo (Cyphomandra betacea), cherry tomato (Solanumly copersicum var. cerasiform) and tomato (Lyopersicon esulentum). International Food Research Journal. 21: 2355-2362 (2014) [Google Scholar]
- Ntagkas N, Woltering E, Nicole C, Labrie C, Marcelis LF. Light regulation of vitamin C in tomato fruit is mediated through photosynthesis. Environmental and Experimental Botany. 158: 180-188 (2019) [Google Scholar]
- Oluk AC, Ata A, Ünlü M, Yazici E, Karaşahin Z, Eroǧlu EÇ, Canan I. Biochemical characterisation and sensory evaluation of differently coloured and shaped tomato cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 47: 599-607 (2019) [Google Scholar]
- Pék Z, Szuvandzsiev P, Nemenyi A, Helyes L, Lugasi A. The effect of natural light on changes in antioxidant content and color parameters of vine-ripened tomato (Solanum lycopersicum L.) fruits. HortScience. 46: 583-585 (2011) [Google Scholar]
- Przybylska S. Lycopene – a bioactive carotenoid offering multiple health benefits: a review. International Journal of Food Science & Technology. 55: 11-32 (2020) [Google Scholar]
- Raffo A, La Malfa G, Fogliano V, Maiani G, Quaglia G. Seasonal variations in antioxidant components of cherry tomatoes (Lycopersicon esculentum cv. Naomi F1). Journal of Food Composition and Analysis. 19: 11-19 (2006) [Google Scholar]
- Rosati C, Aquilani R, Dharmapuri S, Pallara P, Marusic C, Tavazza R, Bouvier F, Camara B, Giuliano G. Metabolic engineering of beta-carotene and lycopene content in tomato fruit (short communication). Plant Journal. 24: 413-419 (2000) [DOI] [PubMed] [Google Scholar]
- Slimestad R, Verheul M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill.) cultivars. Journal of the Science of Food and Agriculture. 89: 1255-1270 (2009) [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nature Reviews Cancer. 3: 768-780 (2003) [DOI] [PubMed] [Google Scholar]
- Terada M, Watanabe Y, Kunitomo M, Hayashi E. Differential rapid analysis of ascorbic acid and ascorbic acid 2-sulfate by dinitrophenylhydrazine method. Analytical Biochemistry. 84: 604-608 (1978) [DOI] [PubMed]
- Yang H, Kim YJ, Shin Y. Influence of ripening stage and cultivar on physicochemical properties and antioxidant compositions of aronia grown in south korea. Foods. 8: 598 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Duan D, Song ZL, Liu T, Hou Y, Fang J. Small molecules regulating reactive oxygen species homeostasis for cancer therapy. Medicinal Research Reviews. 41: 342-394 (2021) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.