Abstract
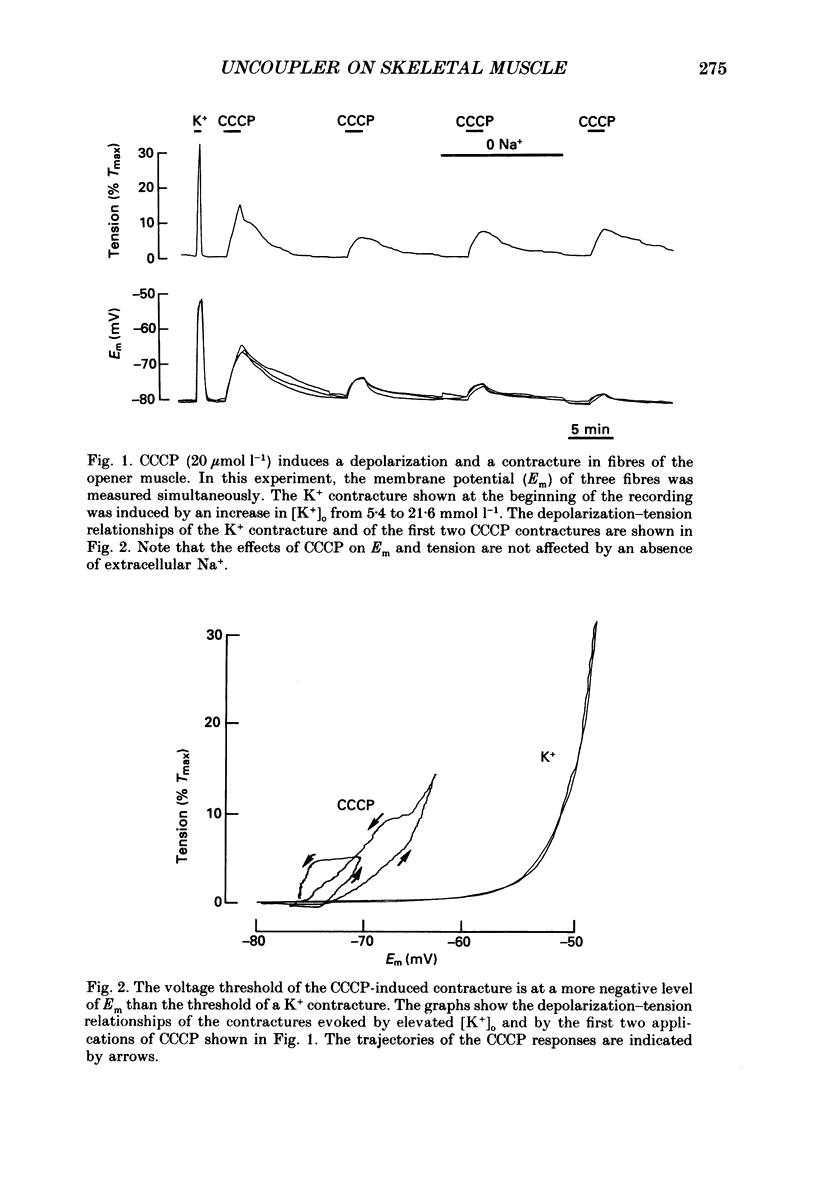
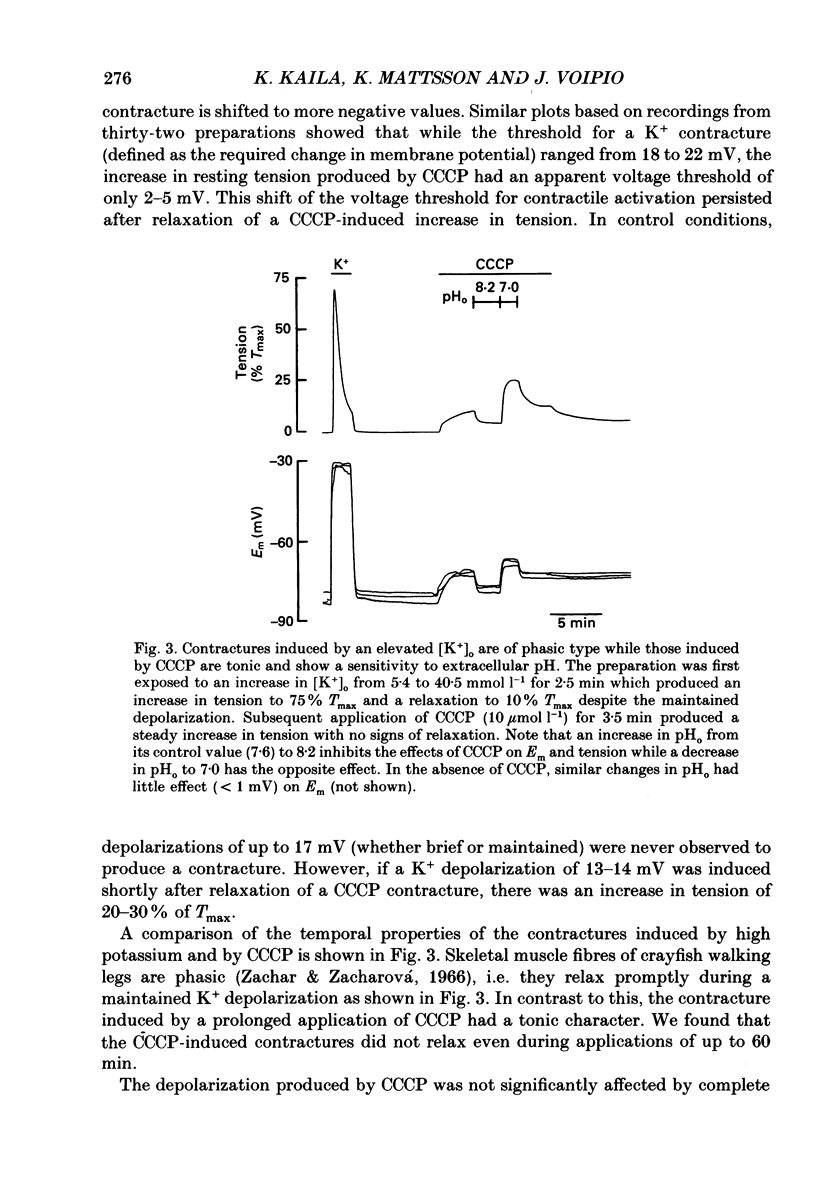
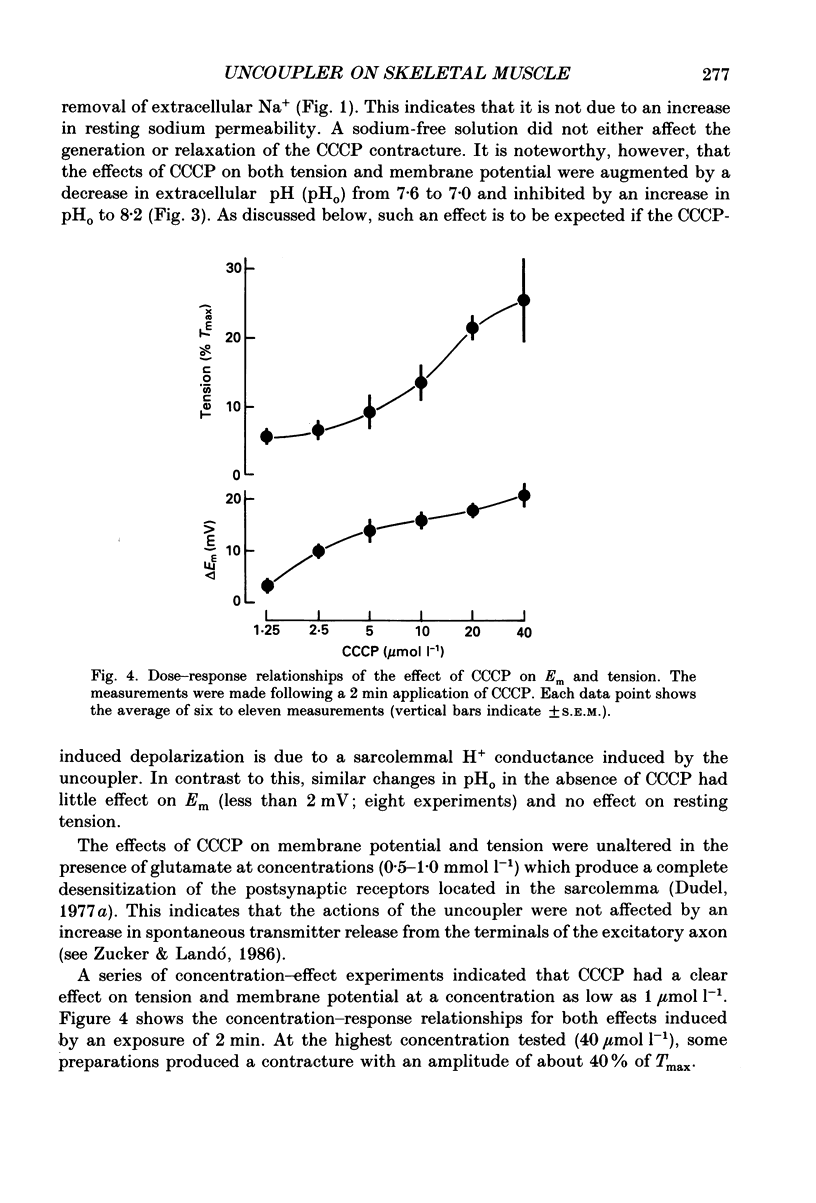
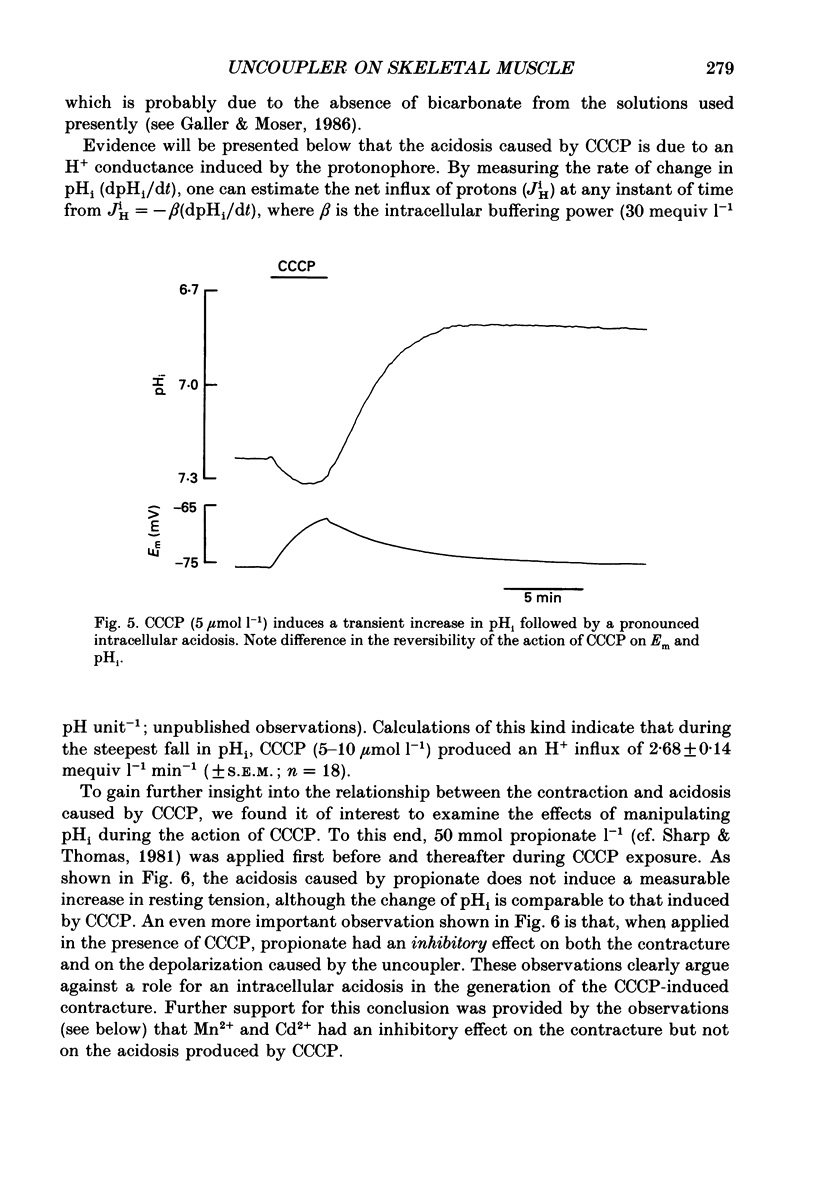
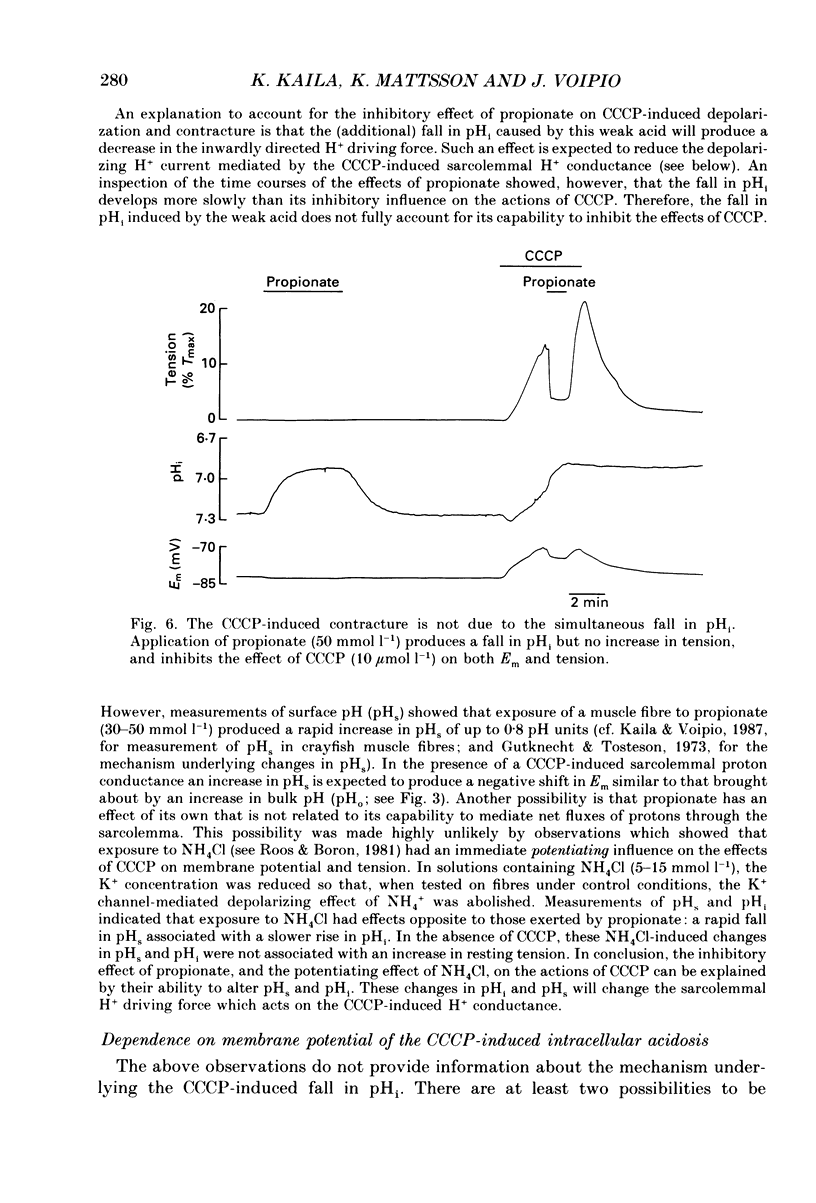
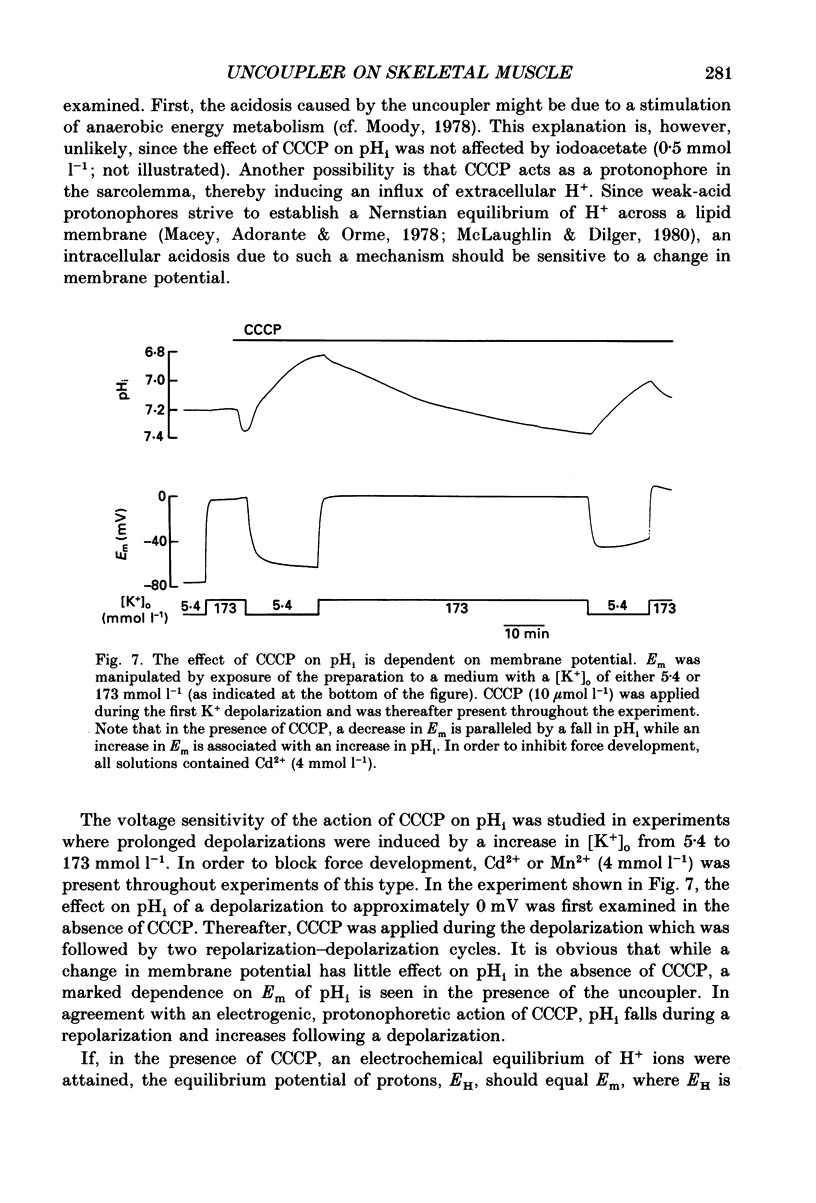
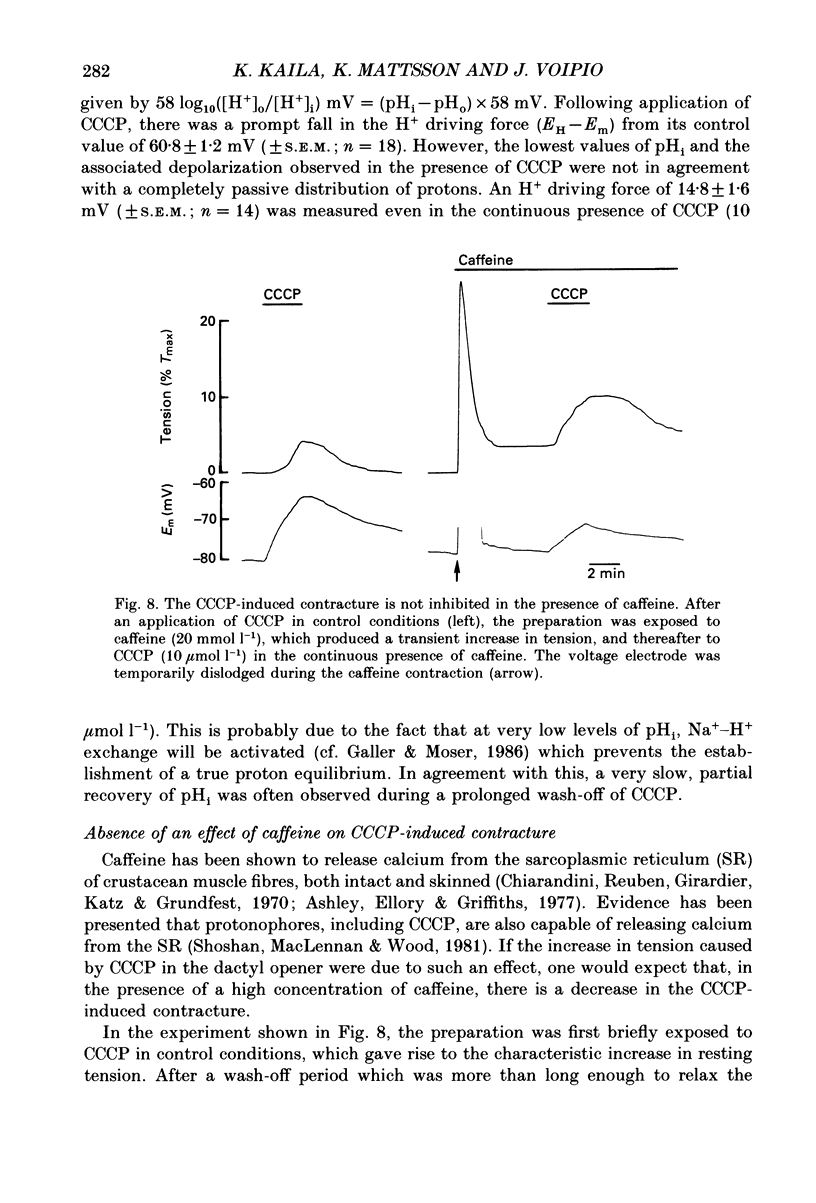
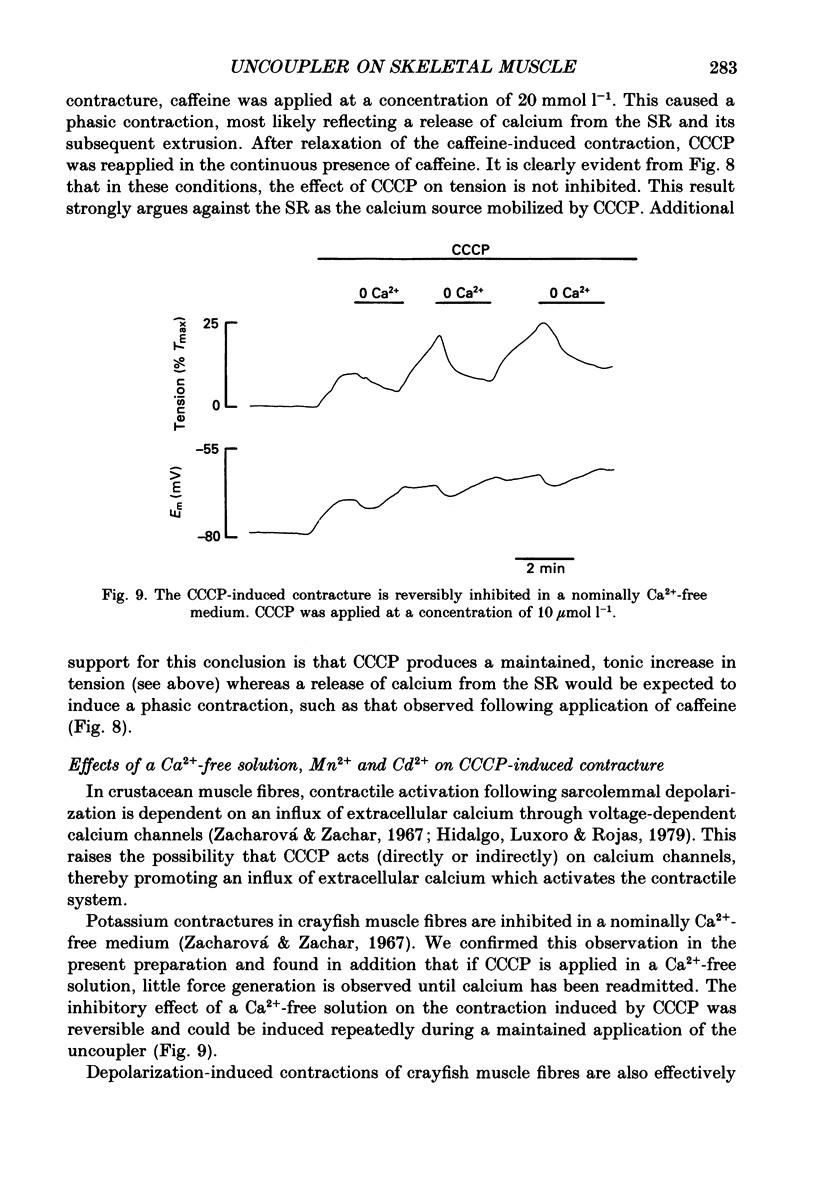
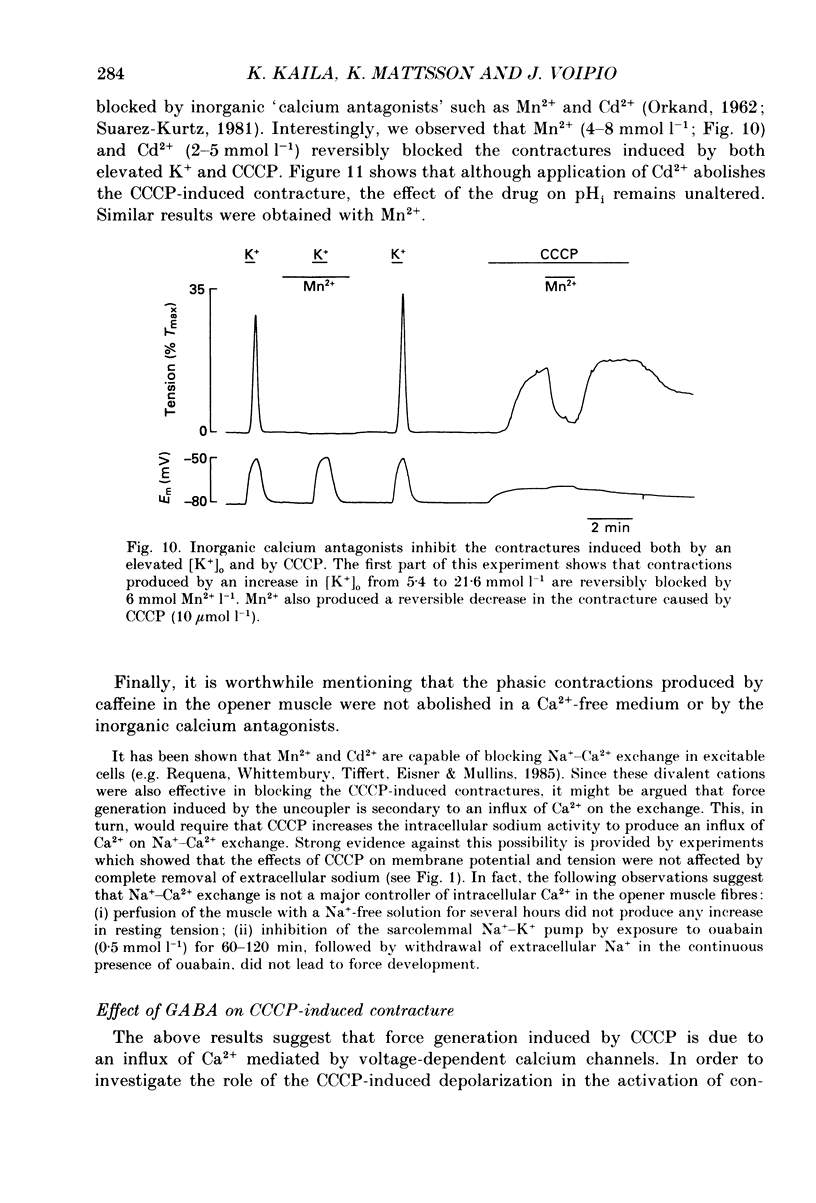
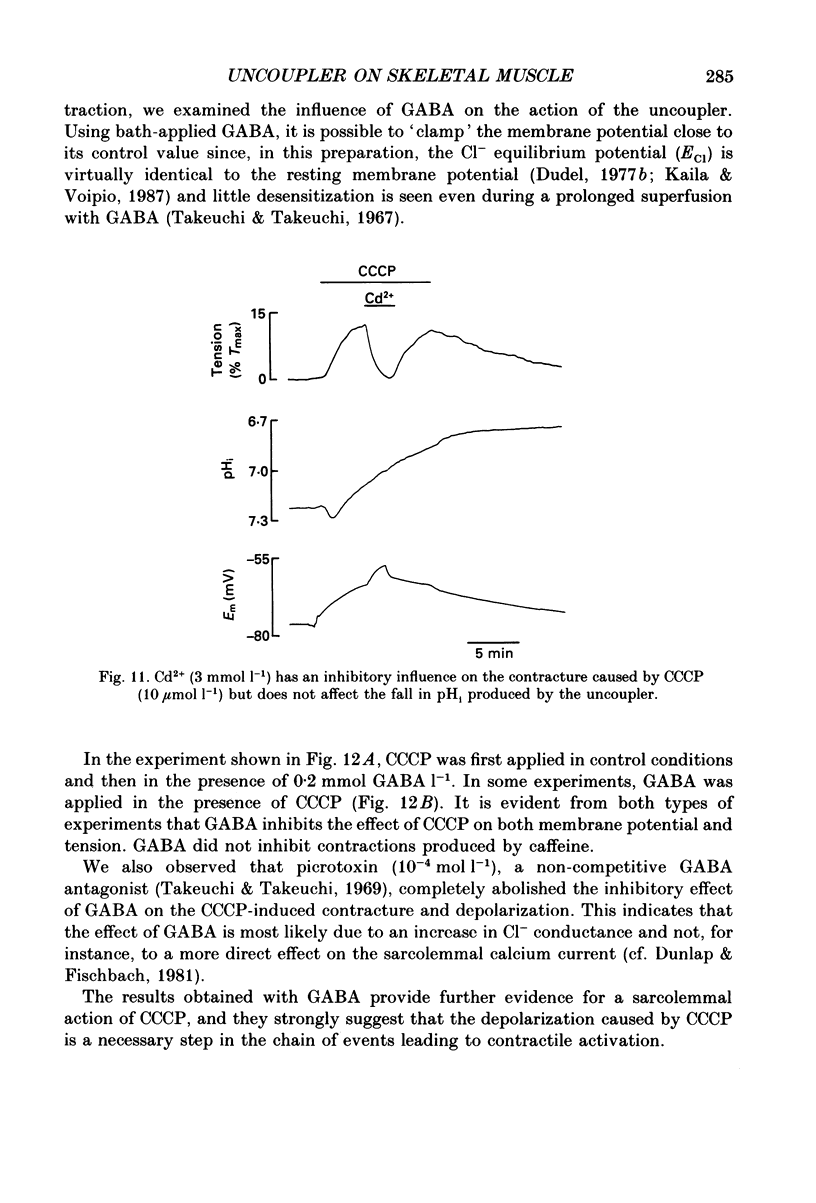
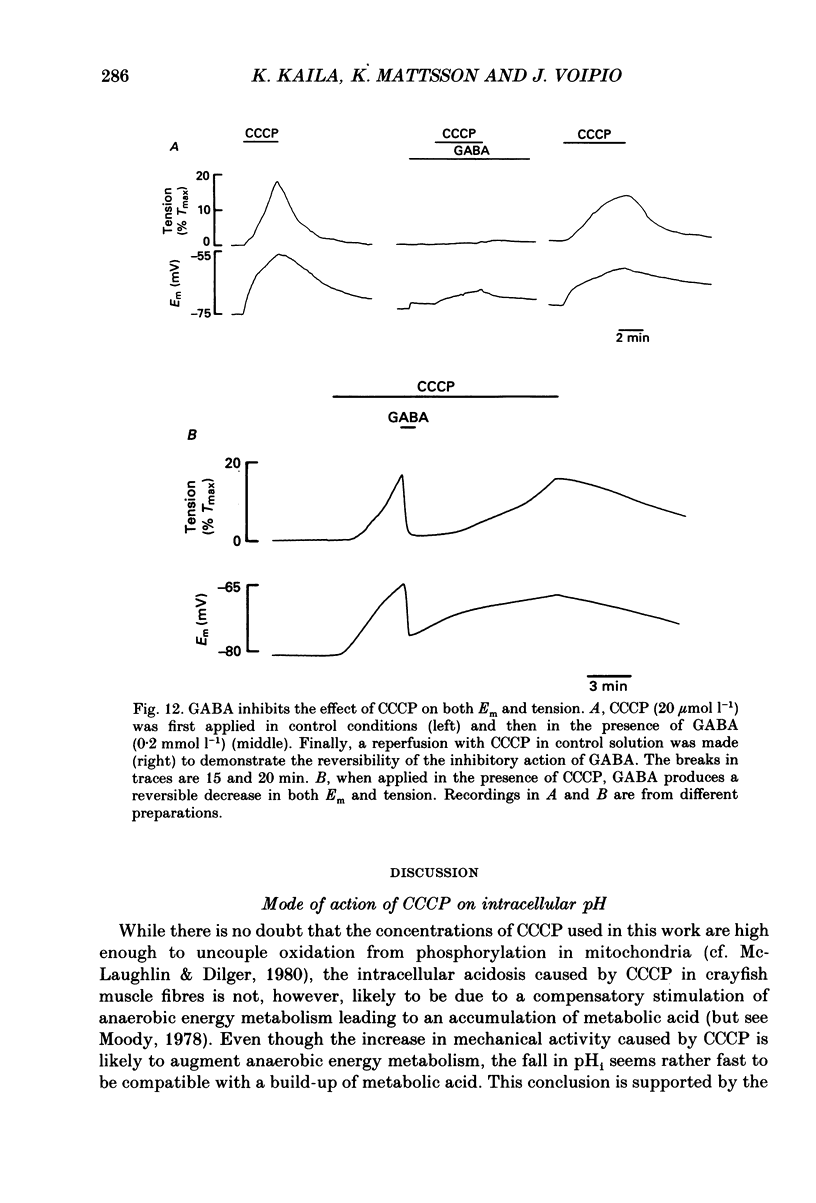
1. The influence of the mitochondrial uncoupling agent carbonylcyanide-m-chlorophenylhydrazone (CCCP) upon resting tension and intracellular pH (pHi) was studied in the dactyl opener muscle of the crayfish. pHi was measured with liquid sensor H+-selective microelectrodes. 2. CCCP (10(-6)-10(-5) mol l-1) induced a reversible, tonic contracture which was associated with a depolarization of the membrane potential. Both effects were augmented by a fall and inhibited by a rise in extracellular pH. The action of CCCP on tension was not mimicked by cyanide + oligomycin or by cyanide + dicyclohexylcarbodiimide nor was it inhibited by pre-exposure to these agents. 3. CCCP produced an initial alkalosis of less than 0.1 units and thereafter a fall in pHi of 0.4-0.6 units during which the sarcolemmal H+ driving force decreased from 61 to 15 mV. The apparent influx of H+ due to CCCP had a maximum of 2.7 mequiv l-1 min-1. The CCCP-induced acidosis was unaffected by iodacetate (0.5 mmol l-1) but it was inhibited by a depolarization of the membrane potential. 4. The contraction caused by CCCP was not due to the simultaneous fall in pHi since an intracellular acidosis of equal magnitude, produced by propionate (50 mmol l-1), did not lead to force generation. In addition, propionate had an inhibitory effect on the depolarization and contracture caused by CCCP. 5. Both the depolarization and the contracture caused by CCCP were inhibited by gamma-aminobutyric acid (GABA). The contracture was blocked by Cd2+, Mn2+ and by a nominally Ca2+ -free medium but not by a pre-exposure to caffeine (20 mmol l-1). Cd2+ and Mn2+ had no influence on the fall of pHi caused by CCCP. 6. It is concluded that CCCP induces a sarcolemmal H+ conductance which leads to a fall in pHi and to a depolarization of the membrane potential. This depolarization activates sarcolemmal, voltage-dependent calcium channels and thereby induces an increase in tension. The initial alkalosis produced by CCCP may be due to a transient uptake of H+ by mitochondria.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Orchard C. H. Factors influencing free intracellular calcium concentration in quiescent ferret ventricular muscle. J Physiol. 1984 May;350:615–630. doi: 10.1113/jphysiol.1984.sp015221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. The effects of changes of pH on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1983 Feb;335:555–567. doi: 10.1113/jphysiol.1983.sp014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Ellory J. C., Griffiths P. J. Caffeine and the contractility of single muscle fibres from the barnacle Balanus nubilus. J Physiol. 1977 Jul;269(2):421–439. doi: 10.1113/jphysiol.1977.sp011910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Moisescu D. G. Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils. J Physiol. 1977 Sep;270(3):627–652. doi: 10.1113/jphysiol.1977.sp011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Umbach J. A. Calcium buffering in axons and axoplasm of Loligo. J Physiol. 1987 Feb;383:369–394. doi: 10.1113/jphysiol.1987.sp016414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982 Apr;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- Bittner G. D. Differentiation of nerve terminals in the crayfish opener muscle and its functional significance. J Gen Physiol. 1968 Jun;51(6):731–758. doi: 10.1085/jgp.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Kaila K., Vaughan-Jones R. D. Mechanism of rate-dependent pH changes in the sheep cardiac Purkinje fibre. J Physiol. 1988 Dec;406:483–501. doi: 10.1113/jphysiol.1988.sp017392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarandini D. J., Reuben J. P., Girardier L., Katz G. M., Grundfest H. Effects of caffeine on crayfish muscle fibers. II. Refractoriness and factors influencing recovery (repriming) of contractile responses. J Gen Physiol. 1970 May;55(5):665–687. doi: 10.1085/jgp.55.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold P. H., Bourne P. K. Aequorin measurements of free calcium in single heart cells. 1984 Nov 29-Dec 5Nature. 312(5993):444–446. doi: 10.1038/312444a0. [DOI] [PubMed] [Google Scholar]
- Cohen I., Van Der Kloot W. The effects of pH changes on the frequency of miniature end-plate potentials at the frog neuromuscular junction. J Physiol. 1976 Nov;262(2):401–414. doi: 10.1113/jphysiol.1976.sp011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. The quantal nature of transmission and spontaneous miniature potentials at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:514–529. doi: 10.1113/jphysiol.1961.sp006644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G., Isenberg G. Decoupling of heart muscle cells: correlation with increased cytoplasmic calcium activity and with changes of nexus ultrastructure. J Membr Biol. 1980 Mar 31;53(1):63–75. doi: 10.1007/BF01871173. [DOI] [PubMed] [Google Scholar]
- Dipolo R., Beaugé L. The effect of pH on Ca2+ extrusion mechanisms in dialyzed squid axons. Biochim Biophys Acta. 1982 May 21;688(1):237–245. doi: 10.1016/0005-2736(82)90599-5. [DOI] [PubMed] [Google Scholar]
- Dudel J. Dose-response curve of glutamate applied by superfusion to crayfish muscle synapses. Pflugers Arch. 1977 Mar 11;368(1-2):49–54. doi: 10.1007/BF01063454. [DOI] [PubMed] [Google Scholar]
- Dudel J. Voltage dependence of amplitude and time course of inhibitory synaptic current in crayfish muscle. Pflugers Arch. 1977 Oct 19;371(1-2):167–174. doi: 10.1007/BF00580786. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Use of aequorin for the appraisal of the hypothesis of the release of calcium from the sarcoplasmic reticulum induced by a change of pH in skinned cardiac cells. Cell Calcium. 1985 Apr;6(1-2):95–108. doi: 10.1016/0143-4160(85)90037-5. [DOI] [PubMed] [Google Scholar]
- Galler S., Moser H. The ionic mechanism of intracellular pH regulation in crayfish muscle fibres. J Physiol. 1986 May;374:137–151. doi: 10.1113/jphysiol.1986.sp016071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glagoleva I. M., Liberman E. A., Khashaev Z. Kh. Vliianie razobshchitelei okislitel'nogo fosforilirovaniia na vykhod atsetilkholina iz nervnykh okonchanii. Biofizika. 1970 Jan-Feb;15(1):76–83. [PubMed] [Google Scholar]
- Gutknecht J., Tosteson D. C. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science. 1973 Dec 21;182(4118):1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- Heinonen E., Akerman K. E., Kaila K. Depolarization of the mitochondrial membrane potential increases free cytosolic calcium in synaptosomes. Neurosci Lett. 1984 Aug 24;49(1-2):33–37. doi: 10.1016/0304-3940(84)90132-0. [DOI] [PubMed] [Google Scholar]
- Hidalgo J., Luxoro M., Rojas E. On the role of extracellular calcium in triggering contraction in muscle fibres from barnacle under membrane potential control. J Physiol. 1979 Mar;288:313–330. [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Coll K. E., Cooper R. H., Marks J. S., Williamson J. R. Mechanisms underlying calcium homeostasis in isolated hepatocytes. J Biol Chem. 1983 Jan 25;258(2):731–741. [PubMed] [Google Scholar]
- Kaila K., Vaughan-Jones R. D. Influence of sodium-hydrogen exchange on intracellular pH, sodium and tension in sheep cardiac Purkinje fibres. J Physiol. 1987 Sep;390:93–118. doi: 10.1113/jphysiol.1987.sp016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Voipio J., Akerman K. E. Free extracellular [Ca2+] at photoreceptor level equals that in vitreous in frog and carp eyes. Invest Ophthalmol Vis Sci. 1984 Dec;25(12):1395–1401. [PubMed] [Google Scholar]
- Kaila K., Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987 Nov 12;330(6144):163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea T. J. A comparison of the abilities of CO2/HCO3-., protonophores and changes in solution pH to release Ca2+ from the SR of barnacle myofibrillar bundles. Pflugers Arch. 1986 Mar;406(3):315–322. doi: 10.1007/BF00640921. [DOI] [PubMed] [Google Scholar]
- Lea T. J., Ashley C. C. Carbon dioxide or bicarbonate ions release Ca2+ from internal stores in crustacean myofibrillar bundles. J Membr Biol. 1981;61(2):115–125. doi: 10.1007/BF02007638. [DOI] [PubMed] [Google Scholar]
- Lea T. J., Ashley C. C. Increase in free Ca2+ in muscle after exposure to CO2. Nature. 1978 Sep 21;275(5677):236–238. doi: 10.1038/275236a0. [DOI] [PubMed] [Google Scholar]
- Macey R. I., Adorante J. S., Orme F. W. Erythrocyte membrane potentials determined by hydrogen ion distribution. Biochim Biophys Acta. 1978 Sep 22;512(2):284–295. doi: 10.1016/0005-2736(78)90253-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Dilger J. P. Transport of protons across membranes by weak acids. Physiol Rev. 1980 Jul;60(3):825–863. doi: 10.1152/physrev.1980.60.3.825. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. J Physiol. 1980 Jan;298:111–129. doi: 10.1113/jphysiol.1980.sp013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta C. V., Williamson J. R. Spermine. A regulator of mitochondrial calcium cycling. J Biol Chem. 1984 Nov 10;259(21):12978–12983. [PubMed] [Google Scholar]
- Nosek T. M., Fender K. Y., Godt R. E. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science. 1987 Apr 10;236(4798):191–193. doi: 10.1126/science.3563496. [DOI] [PubMed] [Google Scholar]
- ORKAND R. K. The relation between membrane potential and contraction in single crayfish muscle fibres. J Physiol. 1962 Apr;161:143–159. doi: 10.1113/jphysiol.1962.sp006878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C. H. The role of the sarcoplasmic reticulum in the response of ferret and rat heart muscle to acidosis. J Physiol. 1987 Mar;384:431–449. doi: 10.1113/jphysiol.1987.sp016462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I., Parnas H., Dudel J. Neurotransmitter release and its facilitation in crayfish muscle. V. Basis for synapse differentiation of the fast and slow type in one axon. Pflugers Arch. 1982 Dec;395(4):261–270. doi: 10.1007/BF00580788. [DOI] [PubMed] [Google Scholar]
- Peracchia C., Dulhunty A. F. Low resistance junctions in crayfish. Structural changes with functional uncoupling. J Cell Biol. 1976 Aug;70(2 Pt 1):419–439. doi: 10.1083/jcb.70.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena J., DiPolo R., Brinley F. J., Jr, Mullins L. J. The control of ionized calcium in squid axons. J Gen Physiol. 1977 Sep;70(3):329–353. doi: 10.1085/jgp.70.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena J., Whittembury J., Tiffert T., Eisner D. A., Mullins L. J. The influence of chemical agents on the level of ionized [Ca2+] in squid axons. J Gen Physiol. 1985 Jun;85(6):789–804. doi: 10.1085/jgp.85.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sharp A. P., Thomas R. C. The effects of chloride substitution on intracellular pH in crab muscle. J Physiol. 1981 Mar;312:71–80. doi: 10.1113/jphysiol.1981.sp013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan V., MacLennan D. H., Wood D. S. A proton gradient controls a calcium-release channel in sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4828–4832. doi: 10.1073/pnas.78.8.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981 Feb 13;211(4483):712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Activation of skinned arthropod muscle fibres by Ca2+ and Sr2+. J Muscle Res Cell Motil. 1980 Mar;1(1):73–87. doi: 10.1007/BF00711926. [DOI] [PubMed] [Google Scholar]
- Suarez-Kurtz G. The role of calcium in excitation--contraction coupling in crustacean muscle fibers. Can J Physiol Pharmacol. 1982 Apr;60(4):446–458. doi: 10.1139/y82-065. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J Physiol. 1969 Nov;205(2):377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion permeability of the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1967 Aug;191(3):575–590. doi: 10.1113/jphysiol.1967.sp008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Influence of surface pH on intracellular pH regulation in cardiac and skeletal muscle. Am J Physiol. 1986 May;250(5 Pt 1):C748–C760. doi: 10.1152/ajpcell.1986.250.5.C748. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Eisner D. A., Lederer W. J. Effects of changes of intracellular pH on contraction in sheep cardiac Purkinje fibers. J Gen Physiol. 1987 Jun;89(6):1015–1032. doi: 10.1085/jgp.89.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachar J., Zacharová D. Potassium contractures in single muscle fibres of the crayfish. J Physiol. 1966 Oct;186(3):596–618. doi: 10.1113/jphysiol.1966.sp008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Landò L. Mechanism of transmitter release: voltage hypothesis and calcium hypothesis. Science. 1986 Feb 7;231(4738):574–579. doi: 10.1126/science.2868525. [DOI] [PubMed] [Google Scholar]



