Abstract
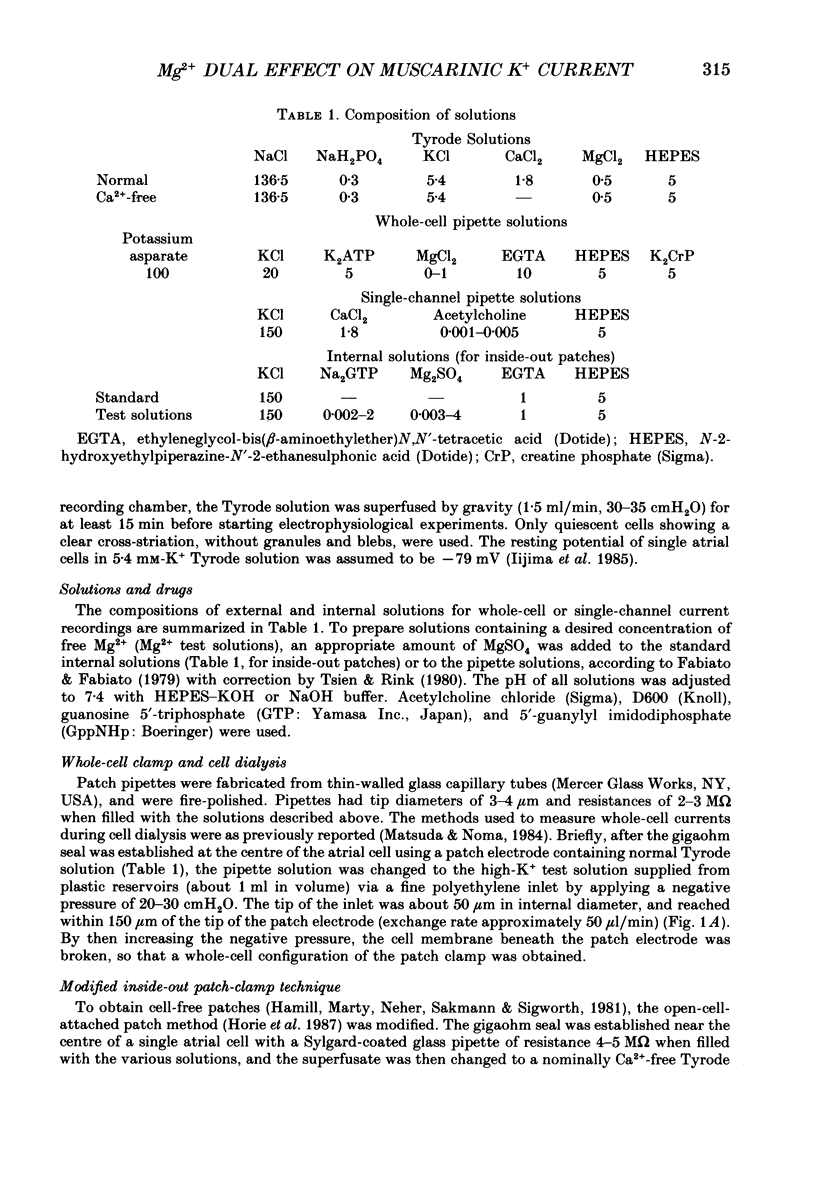
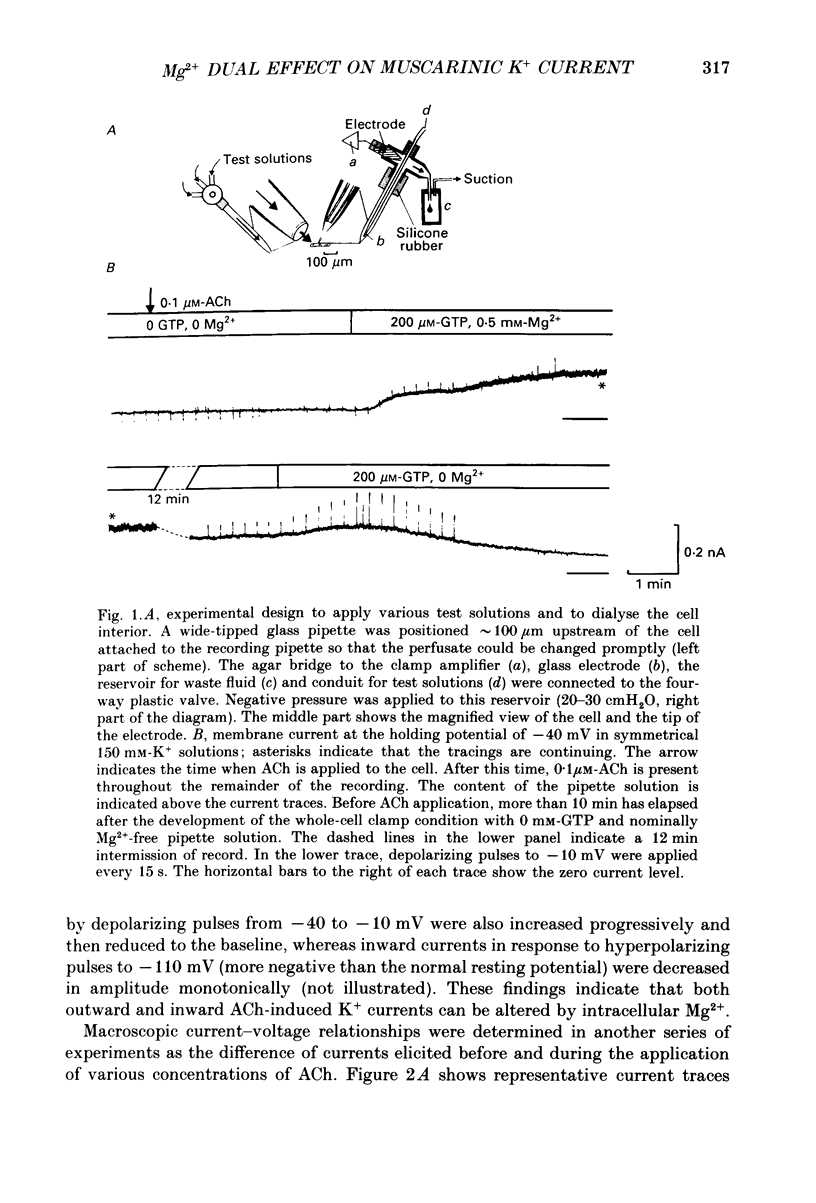
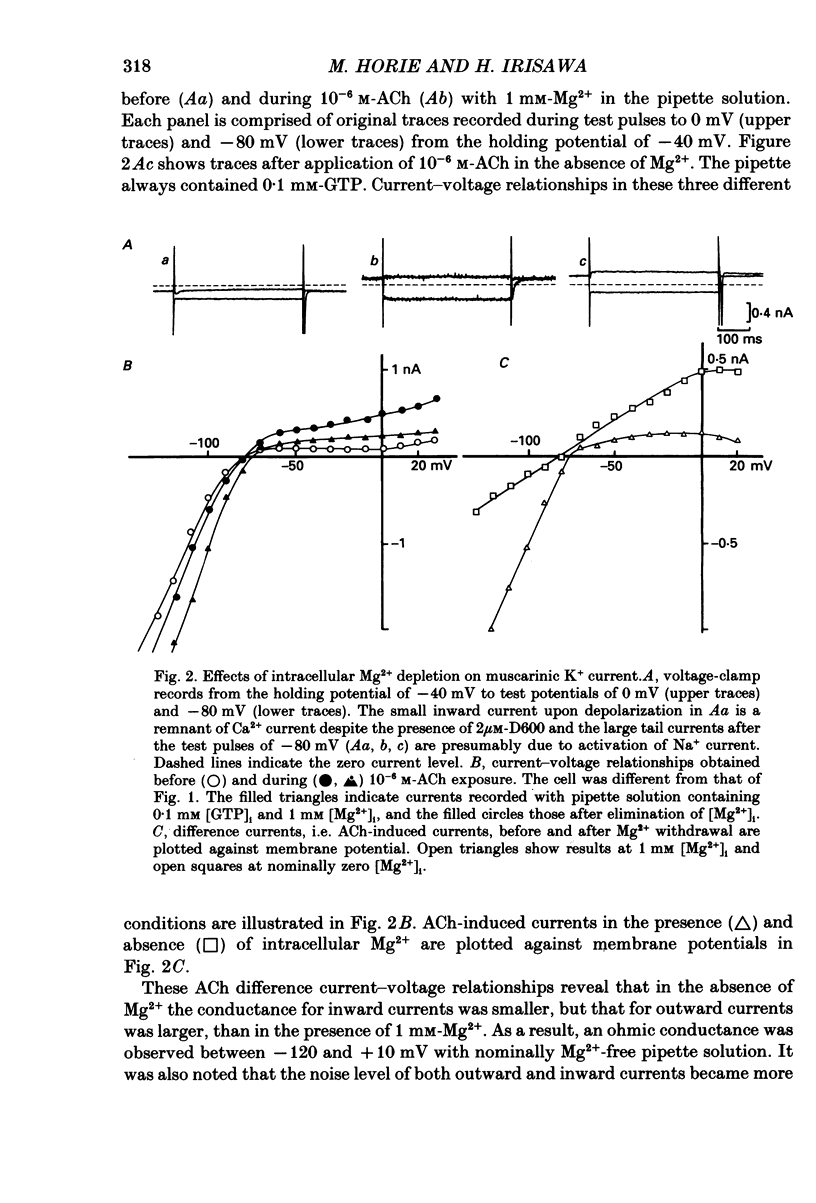
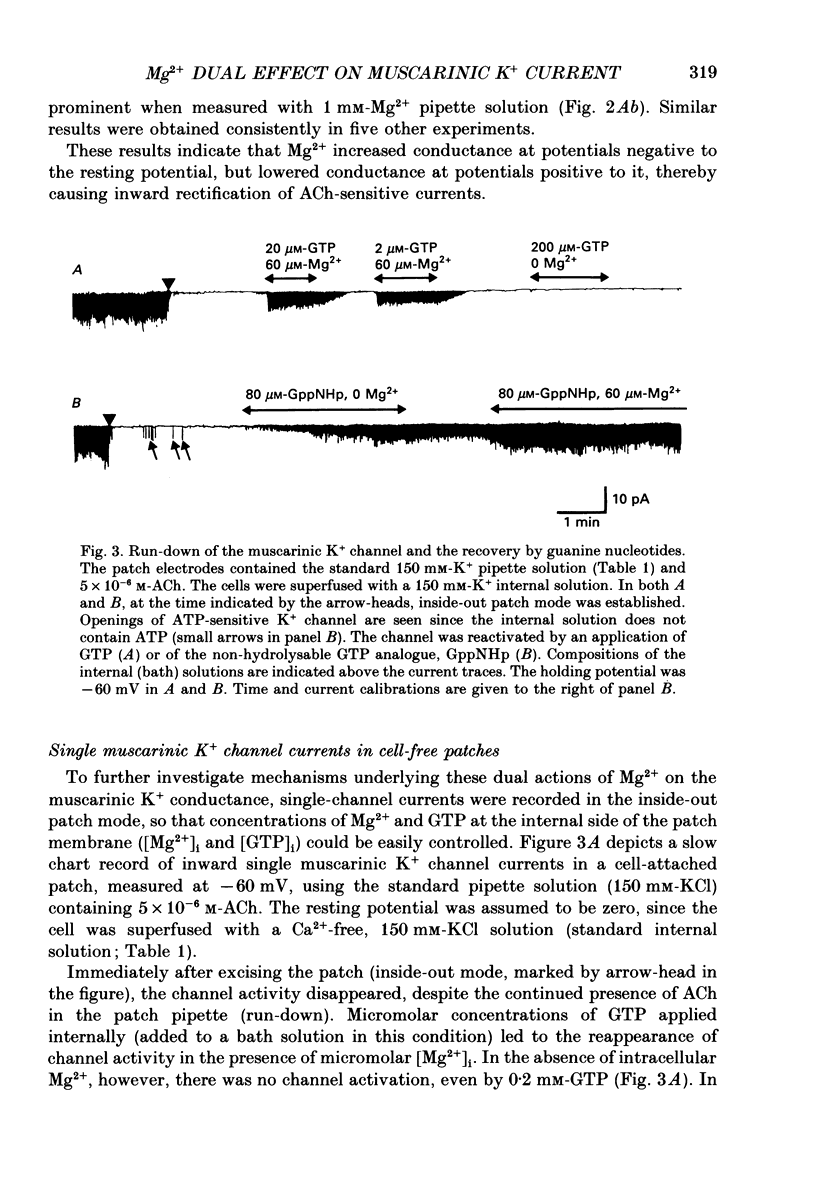
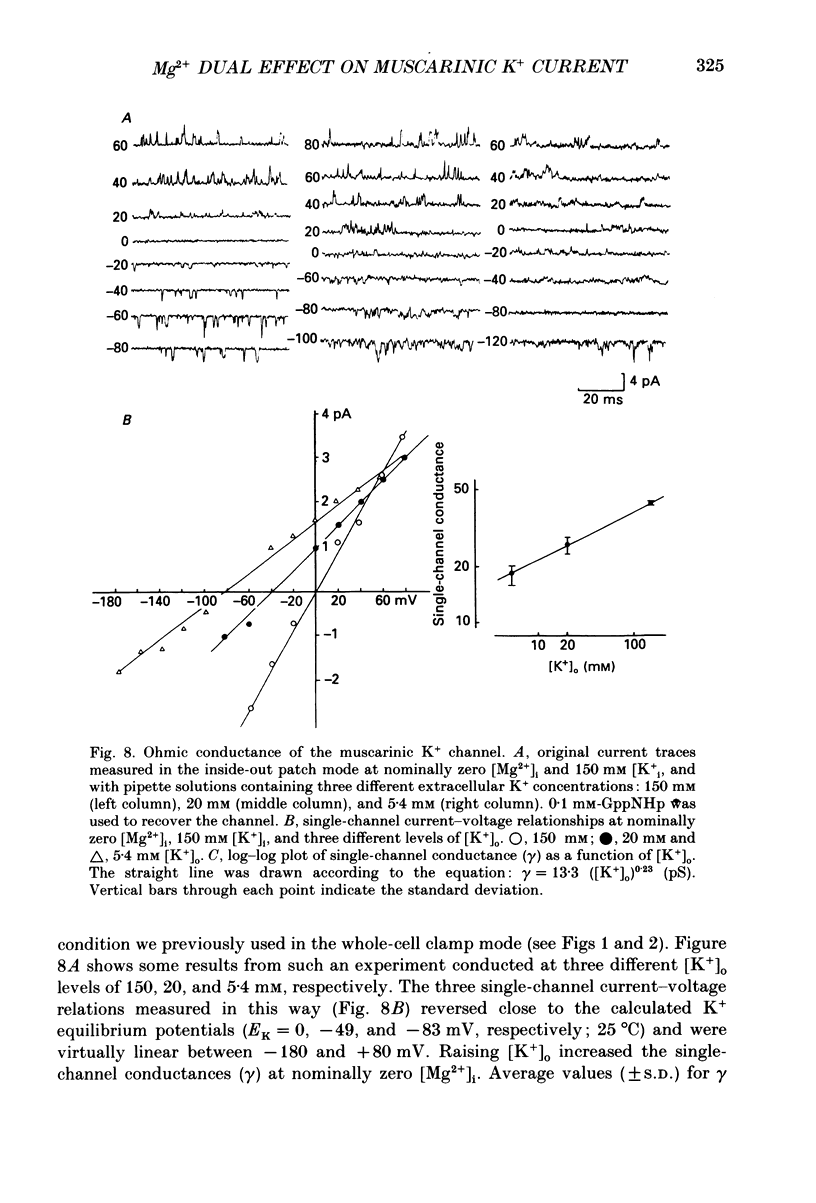
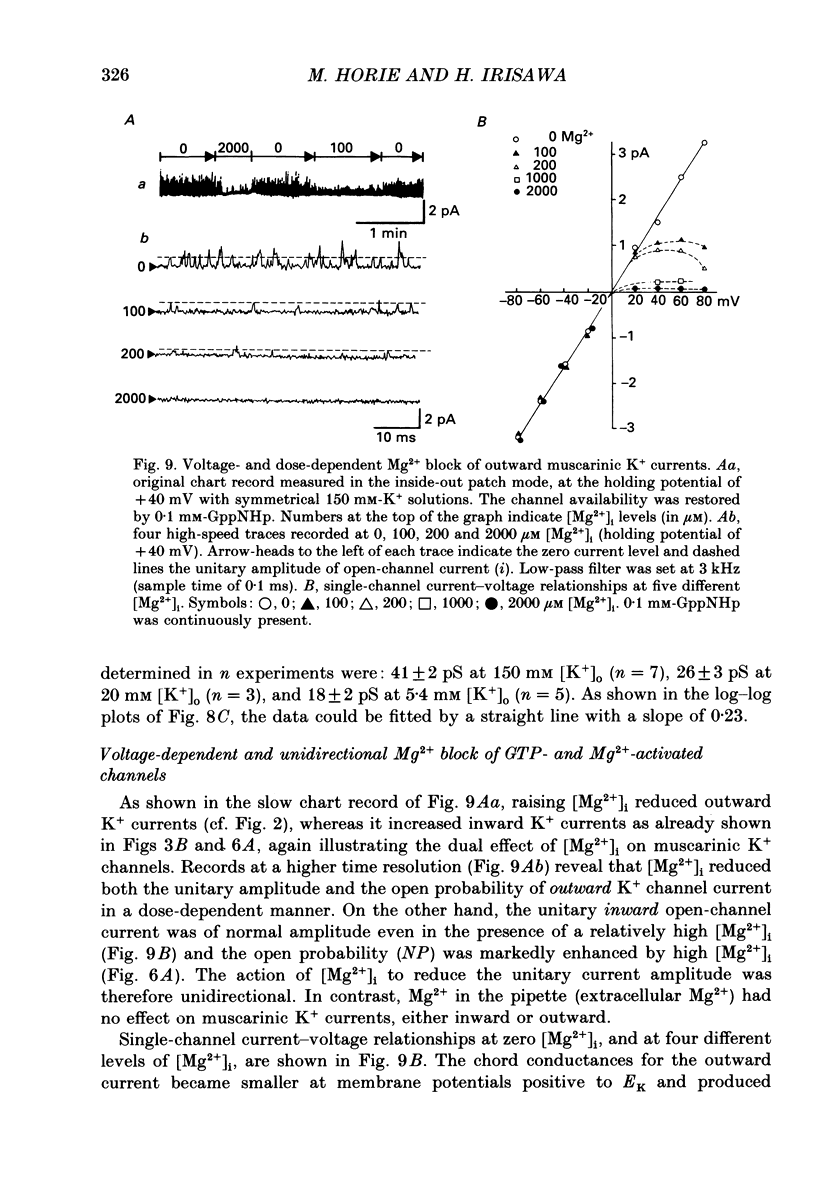
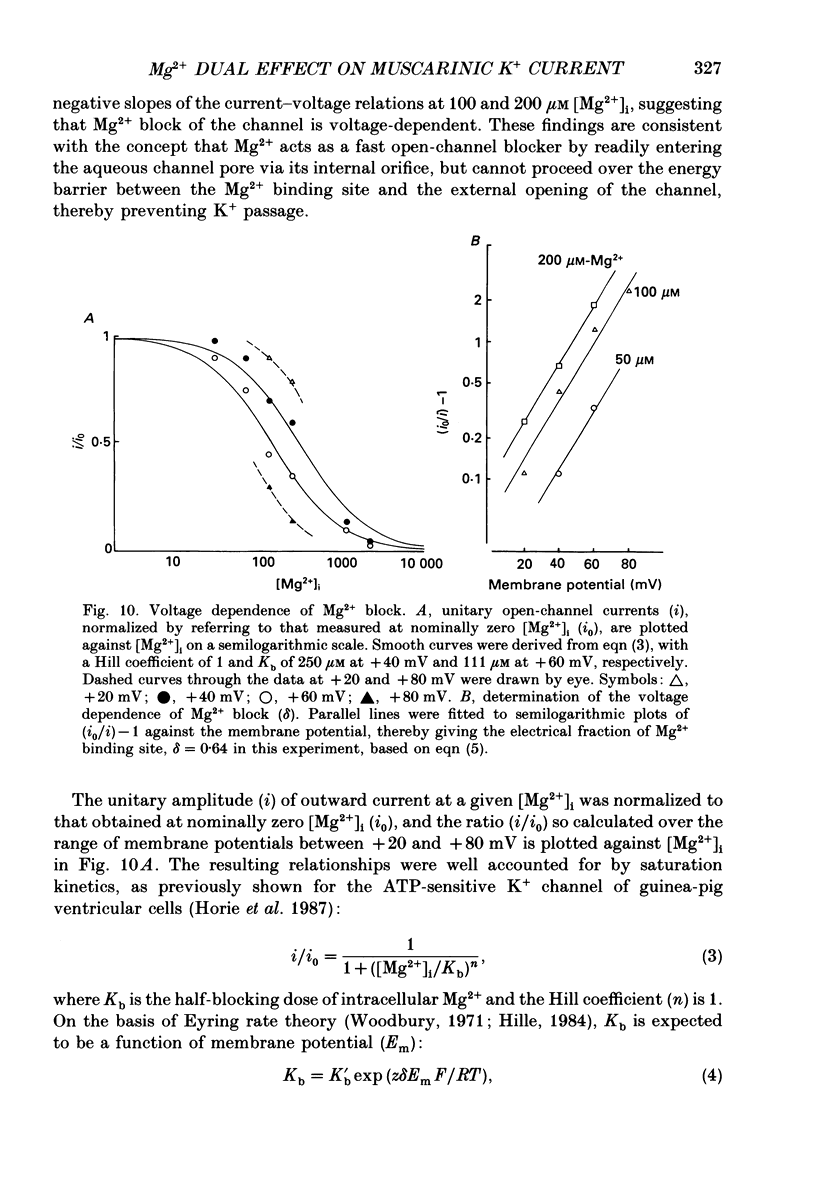
1. The effects of internal Mg2+ ions on the muscarinic acetylcholine (ACh) receptor-mediated K+ currents were investigated in single atrial cells of guinea-pigs, using the whole-cell and inside-out modes of the patch-clamp technique. 2. During cell dialysis in the whole-cell-clamp condition, the depletion of internal Mg2+ increased outward muscarinic K+ currents but decreased inward currents, thereby reducing the inwardly rectifying property of the channels. 3. When inside-out patches were prepared, channel availability was abolished and was reactivated by internal application of guanosine 5'-triphosphate (GTP) or its non-hydrolysable analogue, 5'-guanylyl imidodiphosphate (GppNHp), in the presence of Mg2+. GppNHp led to a recovery of the channels also in the nominal absence of Mg2+ (0[Mg2+]i). 4. The activation of single-channel currents by intracellular GTP and Mg2+ was dose-dependent. Both concentration-response curves were fitted by saturation kinetics with Hill coefficients of 1, and the half-maximum doses were 24 +/- 8 microM for GTP and 67 +/- 14 microM for Mg2+. The effects of Mg2+ on activation of K+ currents were additive with those of GTP, suggesting the presence of two independent binding sites for GTP and Mg2+. 5. The single-channel conductance became virtually ohmic when measured at nominally zero [Mg2+]i while GppNHp was used to recover the channel activity. Micromolar [Mg2+]i reduced the unitary amplitude of single open-channel currents in a dose- and voltage-dependent manner, showing half-blocking doses of 293 microM at +40 mV and 115 microM at +60 mV. 6. Voltage-dependent kinetics of Mg2+ block were described using equations based on Eyring rate theory (Woodbury, 1971; Hille, 1984), where the coefficient for voltage dependence (delta) was 0.63. 7. Intracellular Mg2+, at a physiological concentration, has a dual action on the muscarinic K+ channel: first Mg2+ activates the channel in the presence of GTP through GTP-binding proteins (G proteins), and secondly it blocks outward currents through the channel, thereby causing the inwardly rectifying property.
Full text
PDF


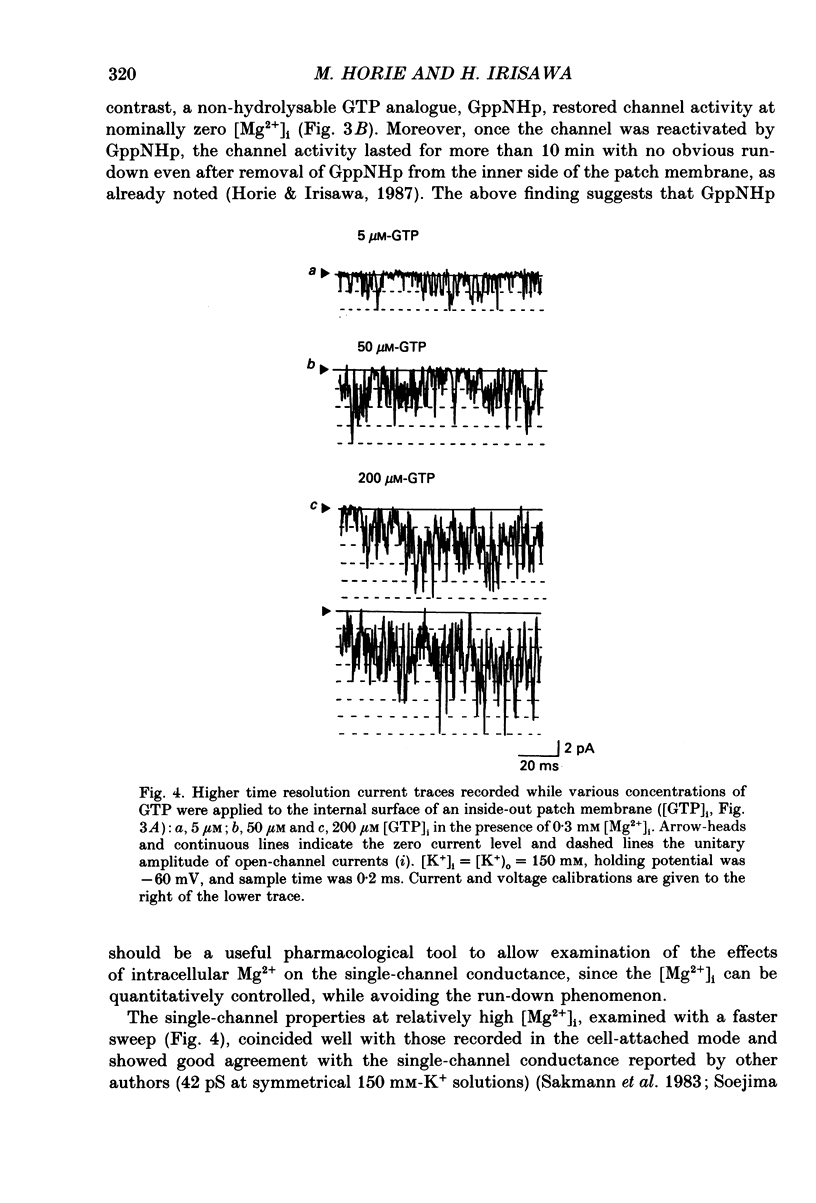

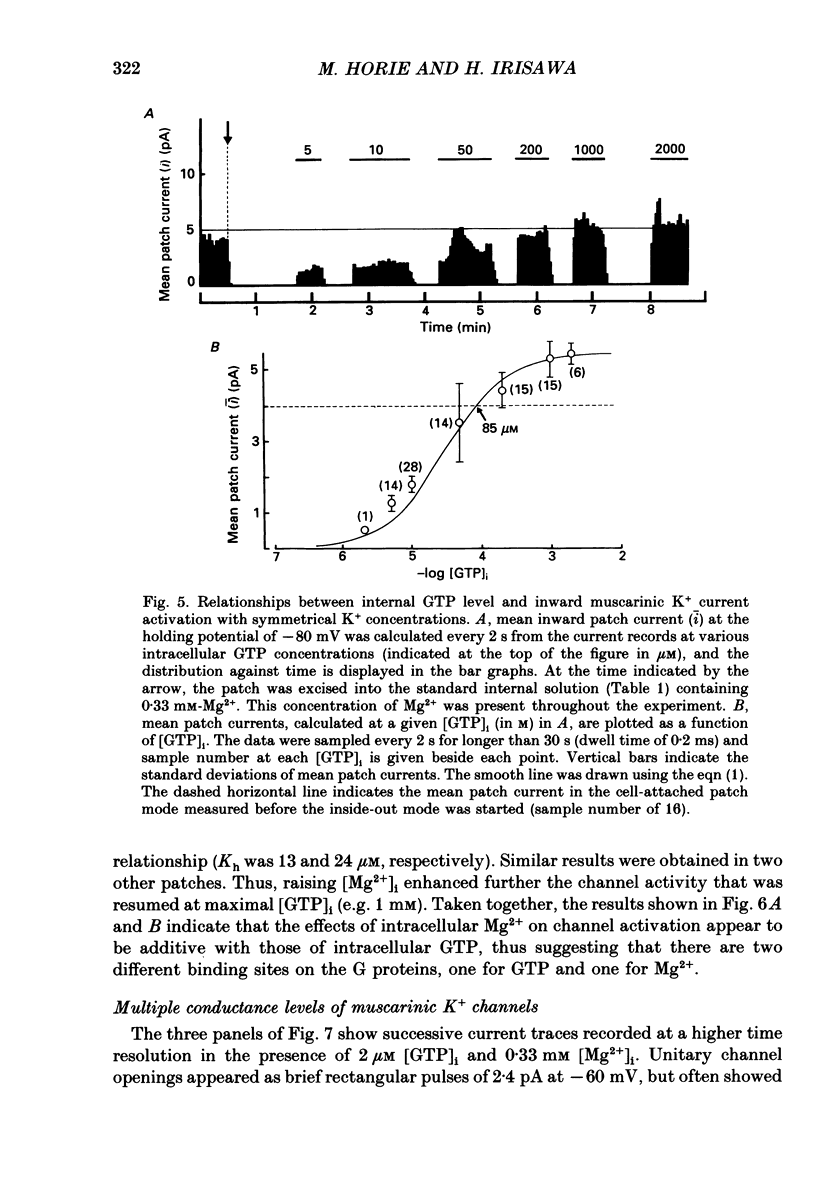
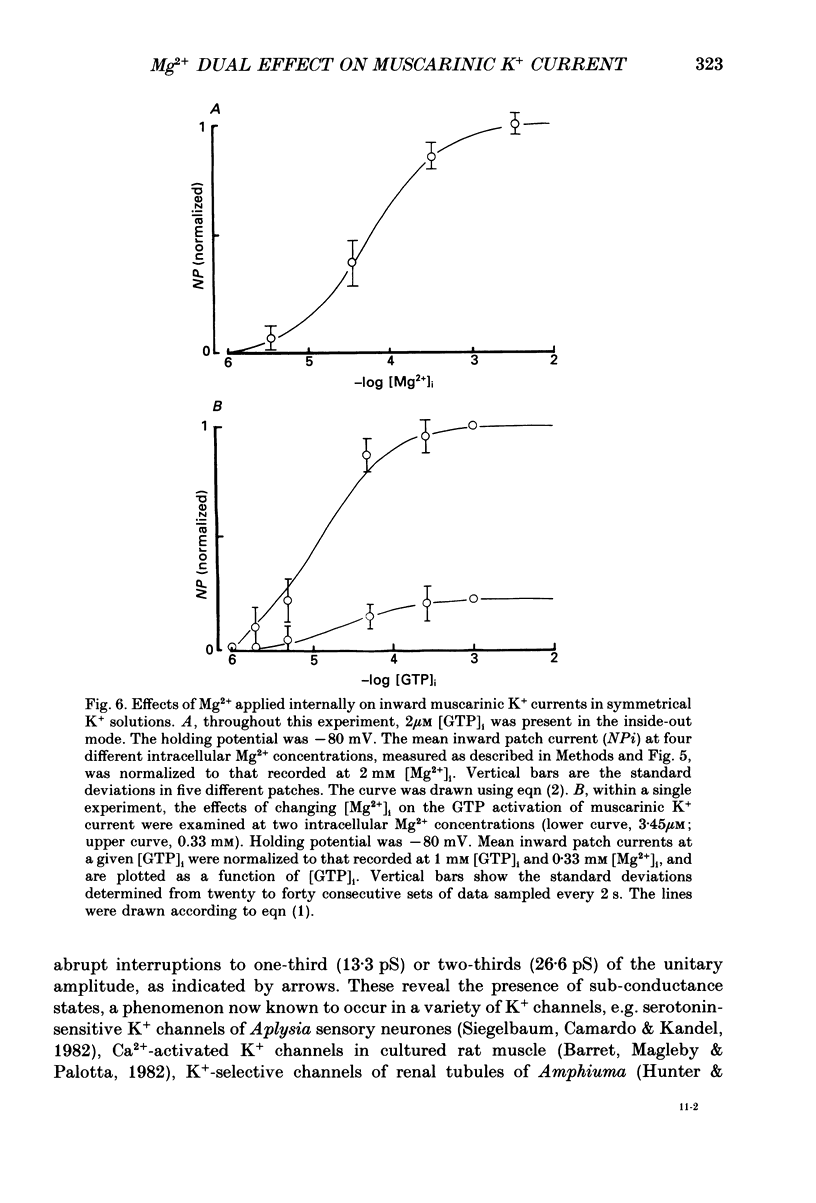
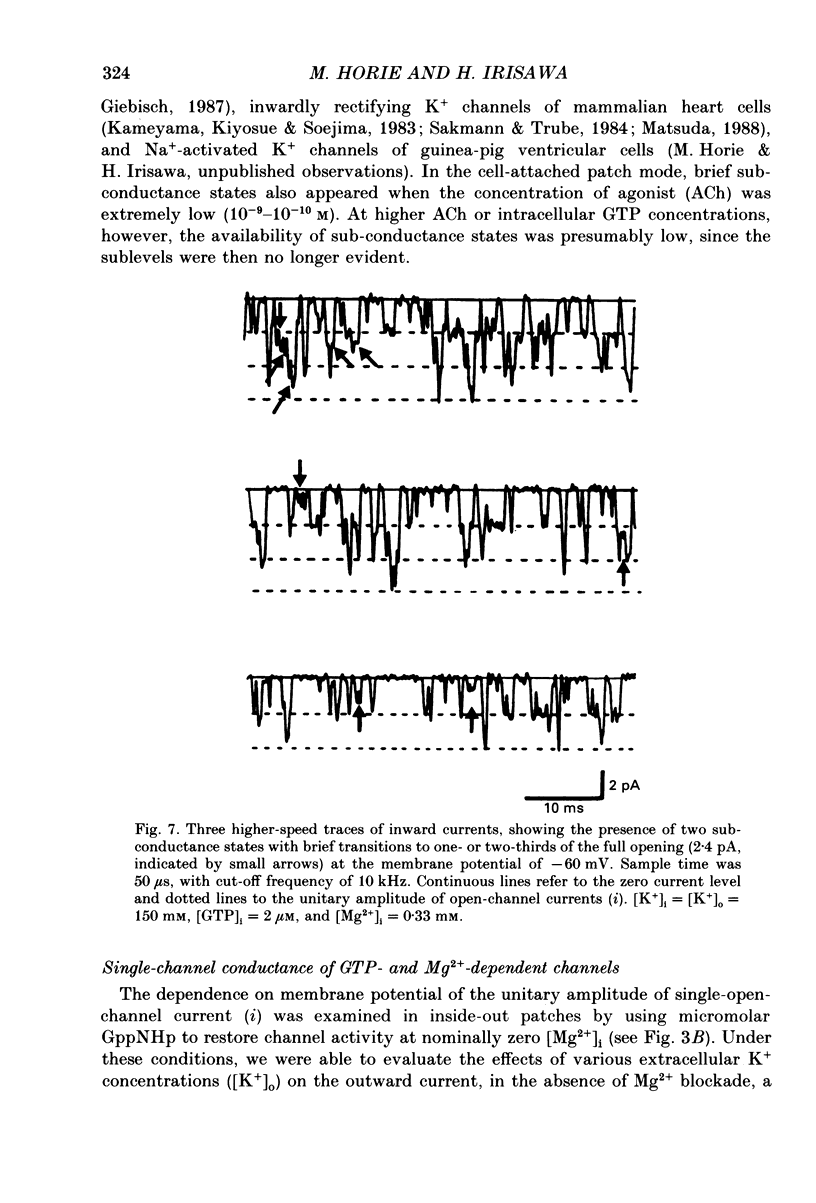





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., Gamiño S. M., Giraldez F., González-Serratos H. Intracellular free magnesium in frog skeletal muscle fibres measured with ion-selective micro-electrodes. J Physiol. 1986 Sep;378:461–483. doi: 10.1113/jphysiol.1986.sp016230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter L. A., McGuigan J. A. Free intracellular magnesium concentration in ferret ventricular muscle measured with ion selective micro-electrodes. Q J Exp Physiol. 1986 Jul;71(3):467–473. doi: 10.1113/expphysiol.1986.sp003005. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Changes by acetylcholine of membrane currents in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:201–217. doi: 10.1113/jphysiol.1986.sp015969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Characterization of the acetylcholine-induced potassium current in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:219–237. doi: 10.1113/jphysiol.1986.sp015970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Yatani A., Grenet D., Brown A. M., Birnbaumer L. The alpha subunit of the GTP binding protein Gk opens atrial potassium channels. Science. 1987 Apr 24;236(4800):442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. GTP and GDP activation of K+ channels that can be inhibited by ATP. Pflugers Arch. 1986 Nov;407(5):564–565. doi: 10.1007/BF00657518. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Findlay I. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 1987 Oct;410(3):313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Findlay I. The effects of magnesium upon adenosine triphosphate-sensitive potassium channels in a rat insulin-secreting cell line. J Physiol. 1987 Oct;391:611–629. doi: 10.1113/jphysiol.1987.sp016759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier D., Nargeot J., Ojeda C., Rougier O. The action of acetylcholine on background conductance in frog atrial trabeculae. J Physiol. 1978 Jan;274:381–396. doi: 10.1113/jphysiol.1978.sp012154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Gupta P., Moore R. D. NMR studies of intracellular metal ions in intact cells and tissues. Annu Rev Biophys Bioeng. 1984;13:221–246. doi: 10.1146/annurev.bb.13.060184.001253. [DOI] [PubMed] [Google Scholar]
- HUTTER O. F., TRAUTWEIN W. Vagal and sympathetic effects on the pacemaker fibers in the sinus venosus of the heart. J Gen Physiol. 1956 May 20;39(5):715–733. doi: 10.1085/jgp.39.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Metzger P., Weingart R. Free magnesium in sheep, ferret and frog striated muscle at rest measured with ion-selective micro-electrodes. J Physiol. 1982 Dec;333:173–188. doi: 10.1113/jphysiol.1982.sp014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H. Rectification of muscarinic K+ current by magnesium ion in guinea pig atrial cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H210–H214. doi: 10.1152/ajpheart.1987.253.1.H210. [DOI] [PubMed] [Google Scholar]
- Hunter M., Giebisch G. Multi-barrelled K channels in renal tubules. Nature. 1987 Jun 11;327(6122):522–524. doi: 10.1038/327522a0. [DOI] [PubMed] [Google Scholar]
- Iijima T., Irisawa H., Kameyama M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol. 1985 Feb;359:485–501. doi: 10.1113/jphysiol.1985.sp015598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingwall J. S. Phosphorus nuclear magnetic resonance spectroscopy of cardiac and skeletal muscles. Am J Physiol. 1982 May;242(5):H729–H744. doi: 10.1152/ajpheart.1982.242.5.H729. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kiyosue T., Soejima M. Single channel analysis of the inward rectifier K current in the rabbit ventricular cells. Jpn J Physiol. 1983;33(6):1039–1056. doi: 10.2170/jjphysiol.33.1039. [DOI] [PubMed] [Google Scholar]
- Katada T., Tamura M., Ui M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch Biochem Biophys. 1983 Jul 1;224(1):290–298. doi: 10.1016/0003-9861(83)90212-6. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Role of intracellular Mg2+ in the activation of muscarinic K+ channel in cardiac atrial cell membrane. Pflugers Arch. 1986 Nov;407(5):572–574. doi: 10.1007/BF00657521. [DOI] [PubMed] [Google Scholar]
- Kurose H., Katada T., Haga T., Haga K., Ichiyama A., Ui M. Functional interaction of purified muscarinic receptors with purified inhibitory guanine nucleotide regulatory proteins reconstituted in phospholipid vesicles. J Biol Chem. 1986 May 15;261(14):6423–6428. [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. J Physiol. 1988 Mar;397:237–258. doi: 10.1113/jphysiol.1988.sp016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Mubagwa K., Carmeliet E. Effects of acetylcholine on electrophysiological properties of rabbit cardiac Purkinje fibers. Circ Res. 1983 Dec;53(6):740–751. doi: 10.1161/01.res.53.6.740. [DOI] [PubMed] [Google Scholar]
- Noma A., Peper K., Trautwein W. Acetylcholine-induced potassium current fluctuations in the rabbit sino-atrial node. Pflugers Arch. 1979 Sep;381(3):255–262. doi: 10.1007/BF00583257. [DOI] [PubMed] [Google Scholar]
- Noma A., Trautwein W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1978 Nov 30;377(3):193–200. doi: 10.1007/BF00584272. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Noma A., Trautwein W. On the kinetics of the potassium channel activated by acetylcholine in the S-A node of the rabbit heart. Pflugers Arch. 1980 Jul;386(2):101–109. doi: 10.1007/BF00584196. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Sorota S., Tsuji Y., Tajima T., Pappano A. J. Pertussis toxin treatment blocks hyperpolarization by muscarinic agonists in chick atrium. Circ Res. 1985 Nov;57(5):748–758. doi: 10.1161/01.res.57.5.748. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]


