Abstract
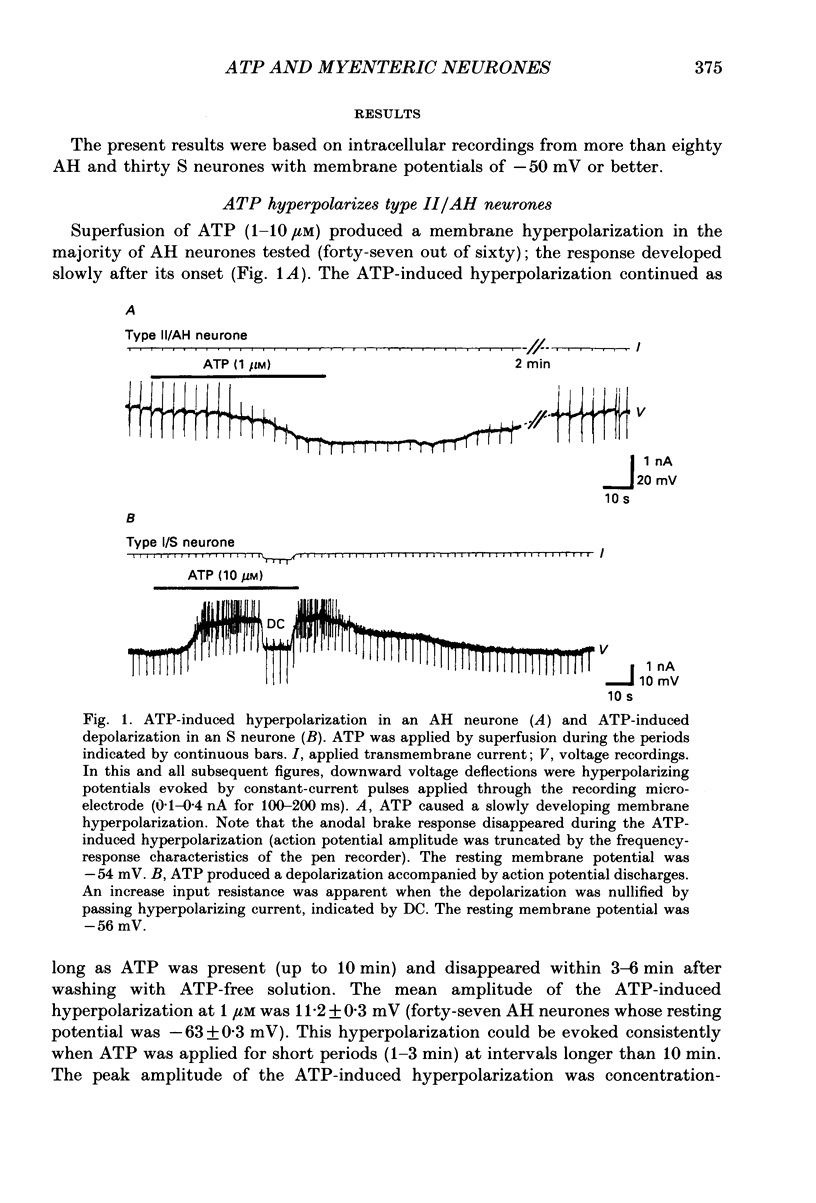
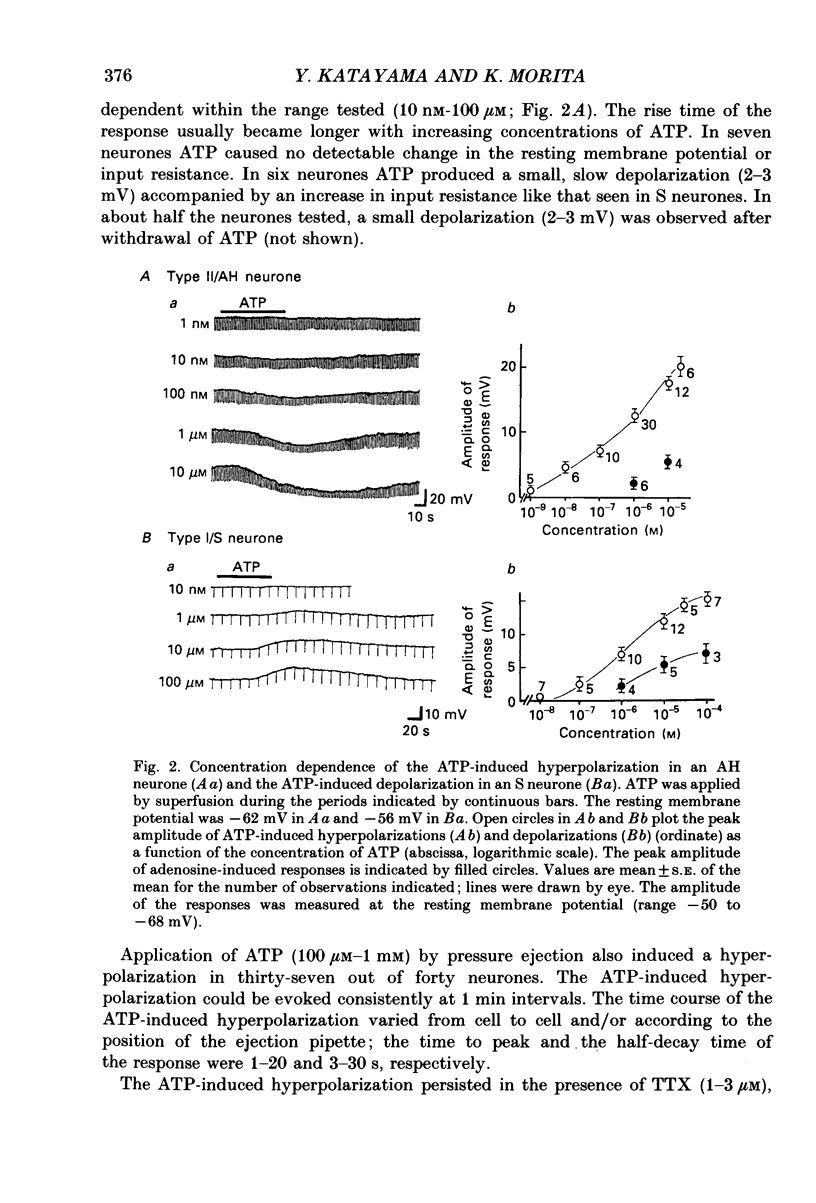
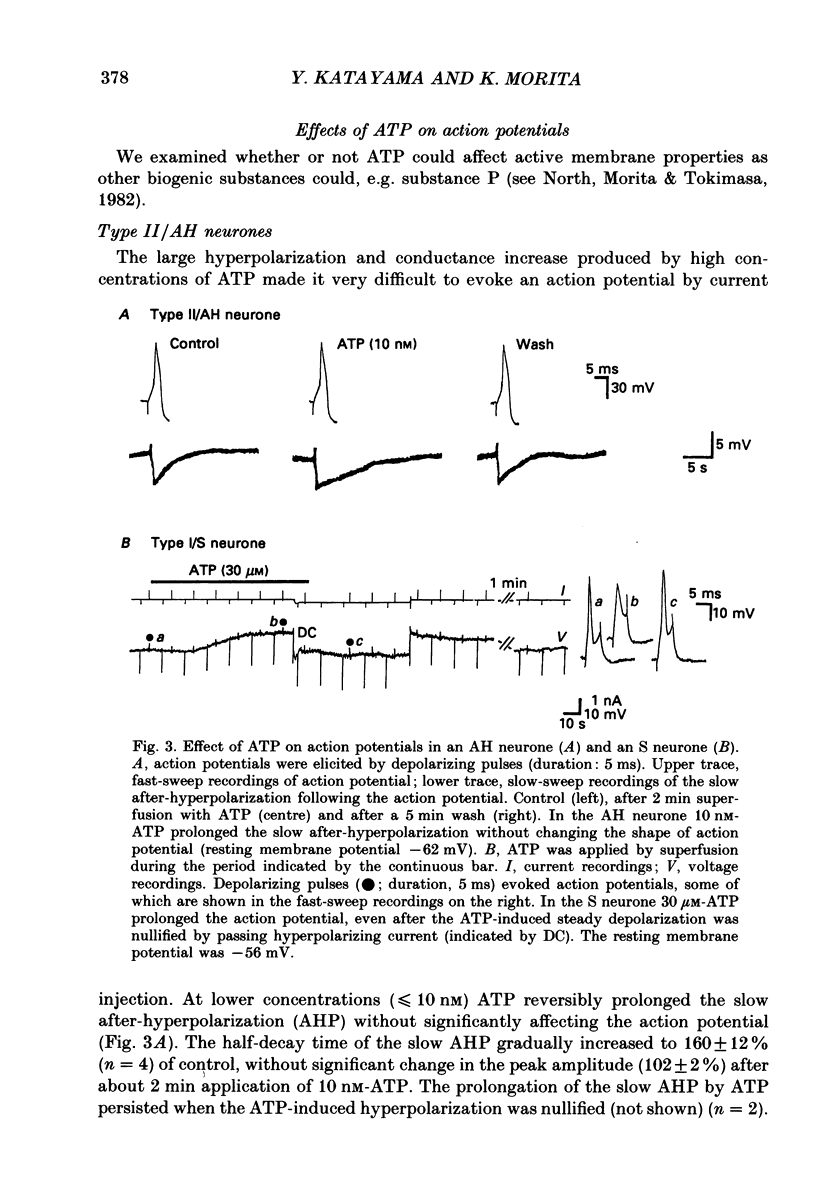
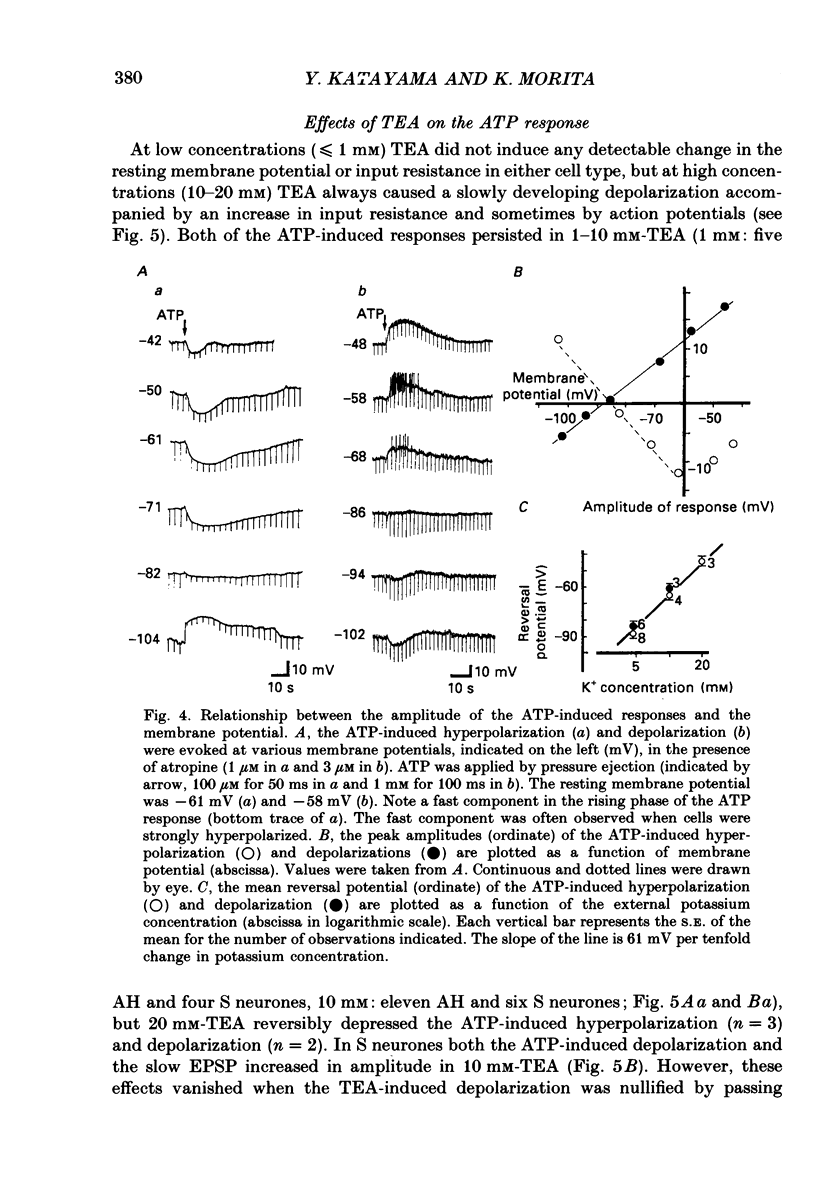
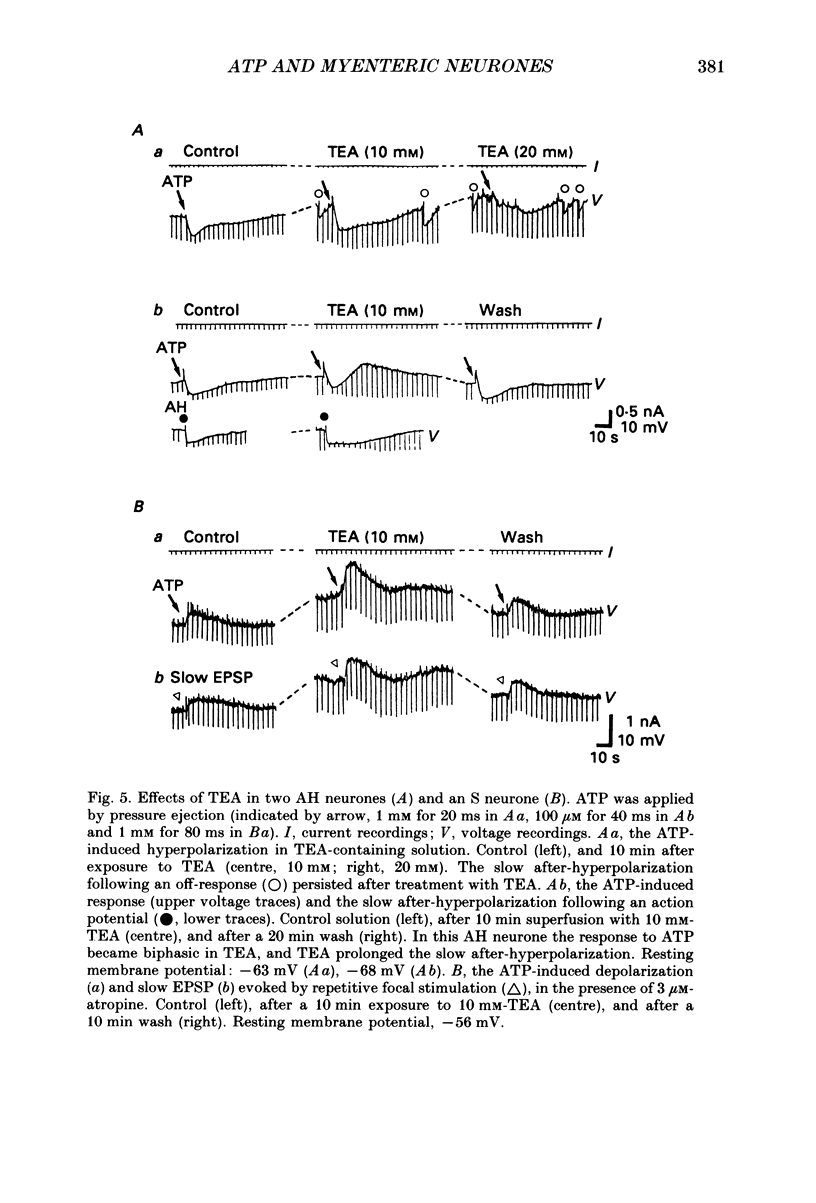
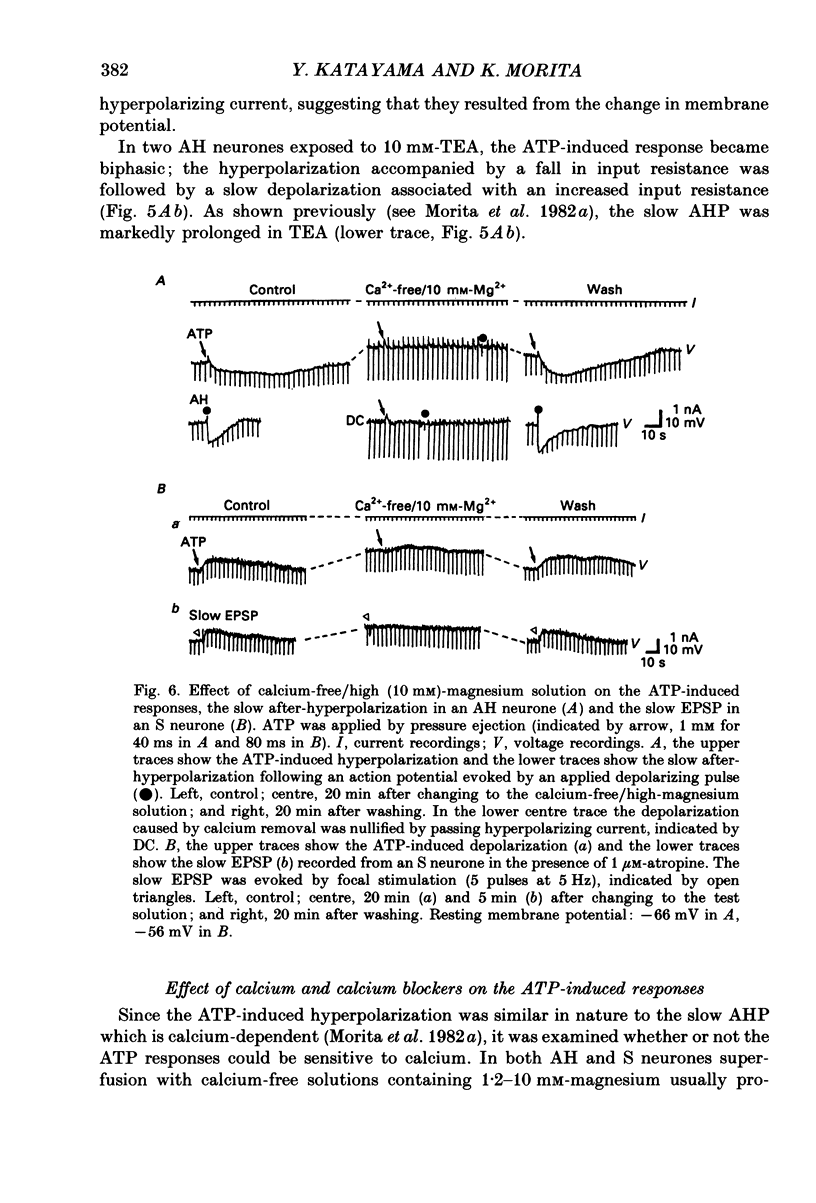
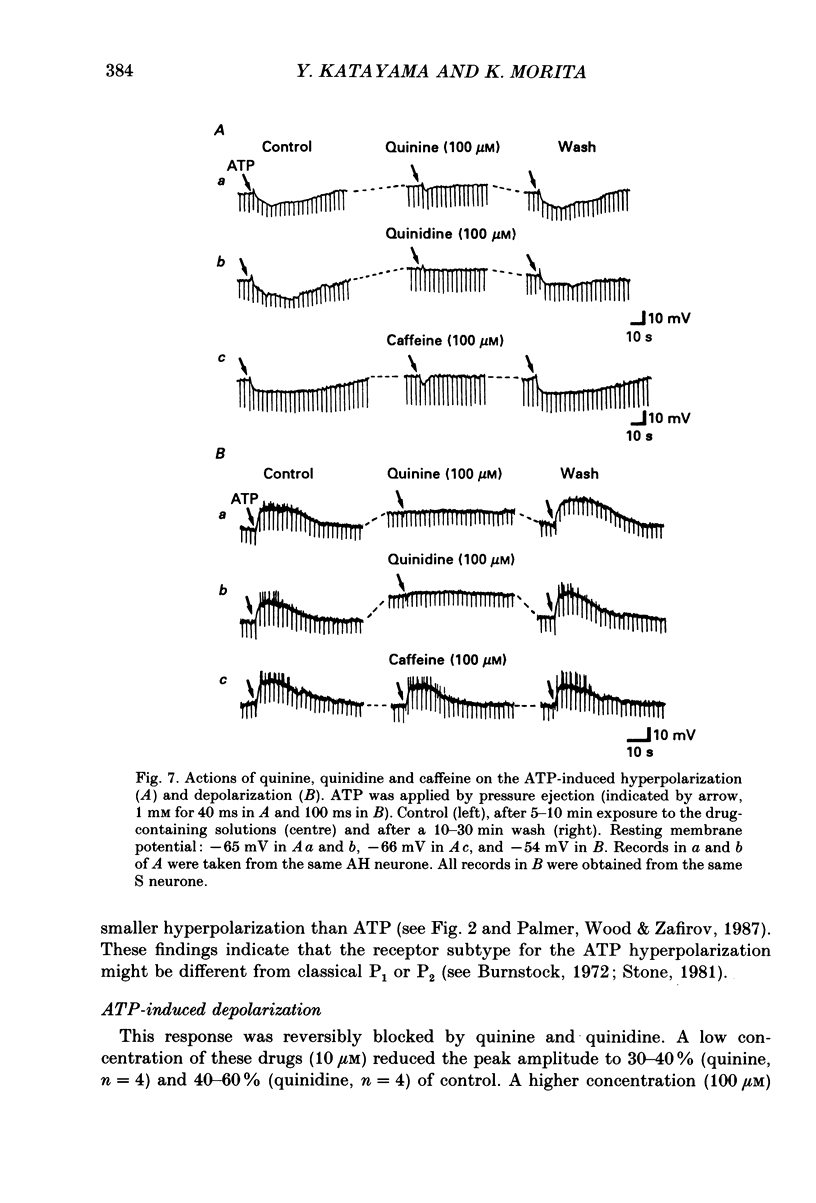
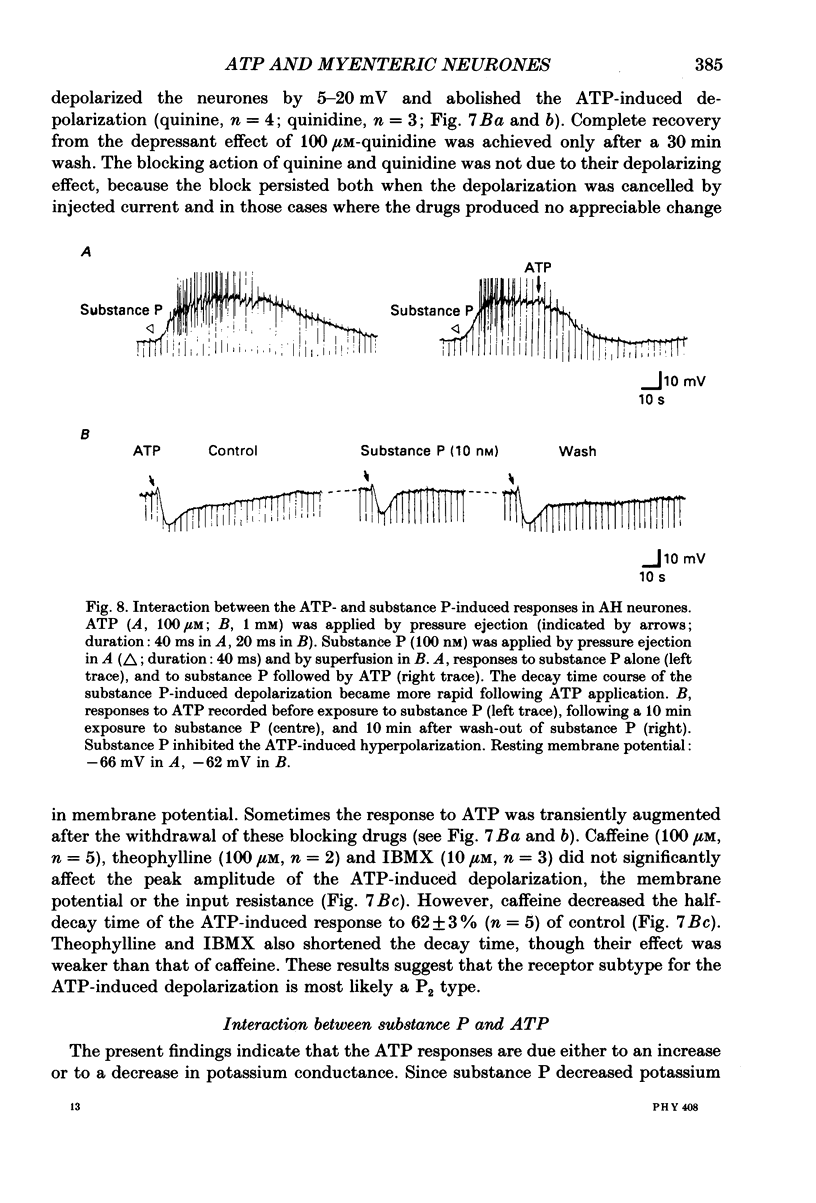
1. Intracellular recordings were made from myenteric neurones isolated from the guinea-pig small intestine to study actions of adenosine 5'-triphosphate (ATP). ATP was applied by superfusion (10 nM-100 microM) or pressure ejection from ATP-containing glass pipettes. 2. Myenteric neurones have been classified into two groups: type I/S neurones and type II/AH neurones. ATP produced a membrane hyperpolarization in 80% of AH neurones and a membrane depolarization in 90% of S neurones in a dose-dependent manner. Adenosine caused responses similar to those induced by ATP in both AH and S neurones, but was less effective than ATP. 3. The ATP-induced hyperpolarization was associated with a fall in input resistance, but the ATP-induced depolarization was accompanied by an increase in input resistance. Both responses reversed in polarity near the potassium equilibrium potential (-84 to -87 mV) and the reversal potential varied with extracellular potassium concentration, as predicted by the Nernst equation. These results indicate that the hyperpolarization is due to an increase, while the depolarization is due to a decrease in potassium conductance. 4. Both the hyperpolarization and the depolarization induced by ATP persisted in calcium-free solution containing 1.2 mM-magnesium, but were markedly reduced or abolished in calcium-free solutions containing 3.7-10 mM-magnesium and by 1 mM-nickel or cobalt. Both responses to ATP persisted in tetraethylammonium (1-10 mM) or tetrodotoxin (1-3 microM)-containing solutions. 5. Quinine and quinidine (1-100 microM) reversibly depressed both the ATP-induced responses. Caffeine (100 microM), theophylline (100 microM) and 3-isobutyl-1-methylxanthine (1-10 microM) did not significantly affect the ATP-induced depolarization but did reversibly depress the ATP-induced hyperpolarization. 6. These results suggest that the ATP-induced hyperpolarization may be due to activation, and the ATP-induced depolarization to inactivation, of a calcium-sensitive potassium conductance.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Bornstein J. C., North R. A., Costa M., Furness J. B. Excitatory synaptic potentials due to activation of neurons with short projections in the myenteric plexus. Neuroscience. 1984 Mar;11(3):723–731. doi: 10.1016/0306-4522(84)90055-1. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Rand M. J. The inhibitory innervation of the taenia of the guinea-pig caecum. J Physiol. 1966 Feb;182(3):504–526. doi: 10.1113/jphysiol.1966.sp007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. The inhibitory nerve fibres in the vagal supply to the guinea-pig stomach. J Physiol. 1966 Aug;185(3):600–612. doi: 10.1113/jphysiol.1966.sp008004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E., Morita K., North R. A. Morphine augments calcium-dependent potassium conductance in guinea-pig myenteric neurones. Br J Pharmacol. 1984 Apr;81(4):617–622. doi: 10.1111/j.1476-5381.1984.tb16126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E., North R. A., Surprenant A. Quinine blocks a calcium-activated potassium conductance in mammalian enteric neurones. Br J Pharmacol. 1984 Sep;83(1):3–5. doi: 10.1111/j.1476-5381.1984.tb10112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. S., Haylett D. G. Effects of apamin, quinine and neuromuscular blockers on calcium-activated potassium channels in guinea-pig hepatocytes. J Physiol. 1985 Jan;358:373–394. doi: 10.1113/jphysiol.1985.sp015556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A. Calcium and the action of adrenaline, adenosine triphosphate and carbachol on guinea-pig taenia caeci. J Physiol. 1982 Apr;325:423–439. doi: 10.1113/jphysiol.1982.sp014160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. M., North R. A. Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. 1986 Jan 30-Feb 5Nature. 319(6052):405–407. doi: 10.1038/319405a0. [DOI] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Johnson S. M., van Helden D. F. The slow calcium-dependent potassium current in a myenteric neurone of the guinea-pig ileum. J Physiol. 1985 Apr;361:315–337. doi: 10.1113/jphysiol.1985.sp015648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkiss J. P., Lees G. M. Morphological studies of electrophysiologically-identified myenteric plexus neurons of the guinea-pig ileum. Neuroscience. 1983 Mar;8(3):593–608. doi: 10.1016/0306-4522(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., Morita K., North R. A. Mediators of slow synaptic potentials in the myenteric plexus of the guinea-pig ileum. J Physiol. 1981 Nov;320:175–186. doi: 10.1113/jphysiol.1981.sp013942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., North R. A. Slow synaptic potentials in neurones of the myenteric plexus. J Physiol. 1980 Apr;301:505–516. doi: 10.1113/jphysiol.1980.sp013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., North R. A., Williams J. T. The action of substance P on neurons of the myenteric plexus of the guinea-pig small intestine. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1163):191–208. doi: 10.1098/rspb.1979.0101. [DOI] [PubMed] [Google Scholar]
- Morita K., Katayama Y., Koketsu K., Akasu T. Actions of ATP on the soma of bullfrog primary afferent neurons and its modulating action on the GABA-induced response. Brain Res. 1984 Feb 20;293(2):360–363. doi: 10.1016/0006-8993(84)91243-5. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A. Significance of slow synaptic potentials for transmission of excitation in guinea-pig myenteric plexus. Neuroscience. 1985 Feb;14(2):661–672. doi: 10.1016/0306-4522(85)90317-3. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. Muscarinic agonists inactivate potassium conductance of guinea-pig myenteric neurones. J Physiol. 1982 Dec;333:125–139. doi: 10.1113/jphysiol.1982.sp014443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. J Physiol. 1982 Aug;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., North R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973 Jun;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Persistent calcium-sensitive potassium current and the resting properties of guinea-pig myenteric neurones. J Physiol. 1987 May;386:333–353. doi: 10.1113/jphysiol.1987.sp016537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M., Wood J. D., Zafirov D. H. Elevation of adenosine 3',5'-phosphate mimics slow synaptic excitation in myenteric neurones of the guinea-pig. J Physiol. 1986 Jul;376:451–460. doi: 10.1113/jphysiol.1986.sp016163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M., Wood J. D., Zafirov D. H. Purinergic inhibition in the small intestinal myenteric plexus of the guinea-pig. J Physiol. 1987 Jun;387:357–369. doi: 10.1113/jphysiol.1987.sp016577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuba M. F., Vladimirova I. A. Effect of apamin on the electrical responses of smooth muscle to adenosine 5'-triphosphate and to non-adrenergic, non-cholinergic nerve stimulation. Neuroscience. 1980;5(5):853–859. doi: 10.1016/0306-4522(80)90154-2. [DOI] [PubMed] [Google Scholar]
- Siggins G. R., Gruol D. L., Padjen A. L., Formans D. S. Purine and pyrimidine mononucleotides depolarise neurones of explanted amphibian sympathetic ganglia. Nature. 1977 Nov 17;270(5634):263–265. doi: 10.1038/270263a0. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol. 1984 Jan;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W. Physiological roles for adenosine and adenosine 5'-triphosphate in the nervous system. Neuroscience. 1981;6(4):523–555. doi: 10.1016/0306-4522(81)90145-7. [DOI] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. A comparison of the effects of adenosine triphosphate with noradrenaline and with the inhibitory potential of the guinea-pig taenia coli. J Physiol. 1973 May;231(1):167–177. doi: 10.1113/jphysiol.1973.sp010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. D., Leslie R. A. Depolarization-induced release of adenosine 5'-triphosphate from isolated varicosities derived from the myenteric plexus of the guinea pig small intestine. J Neurosci. 1982 Feb;2(2):206–215. doi: 10.1523/JNEUROSCI.02-02-00206.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


