Abstract
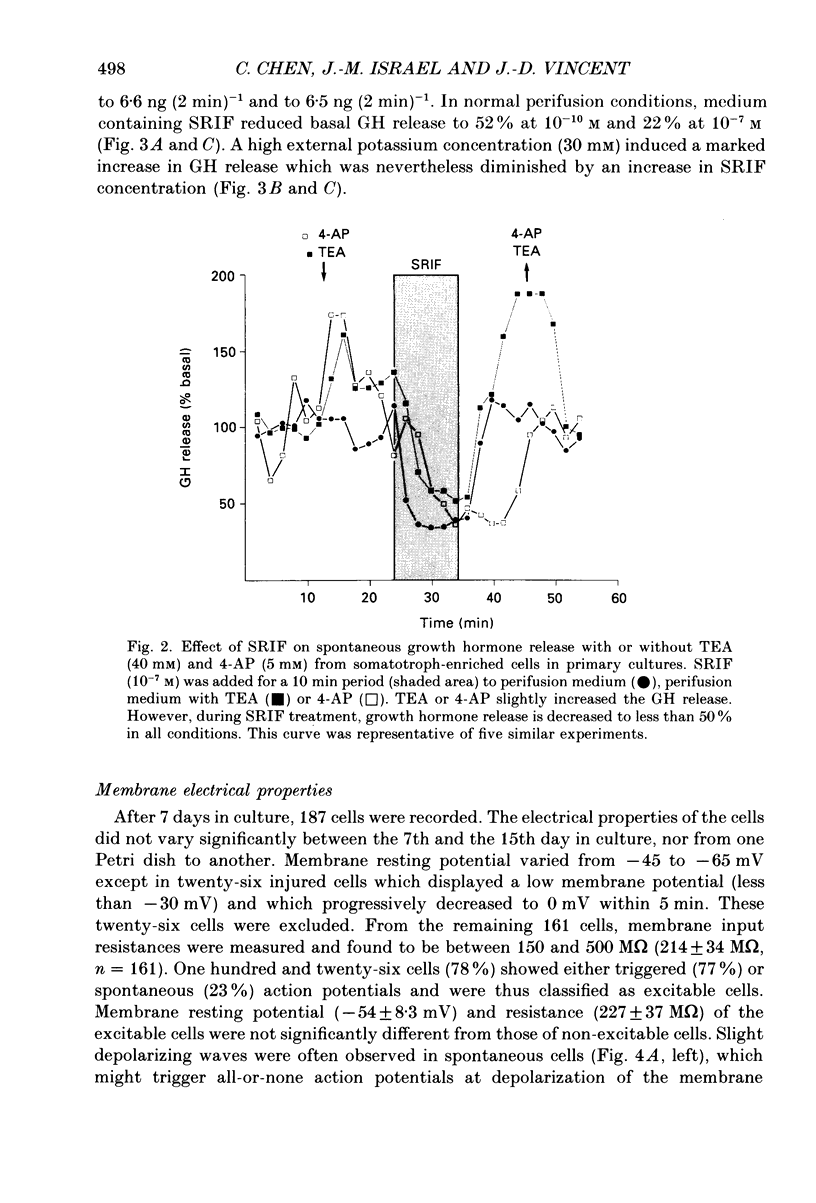
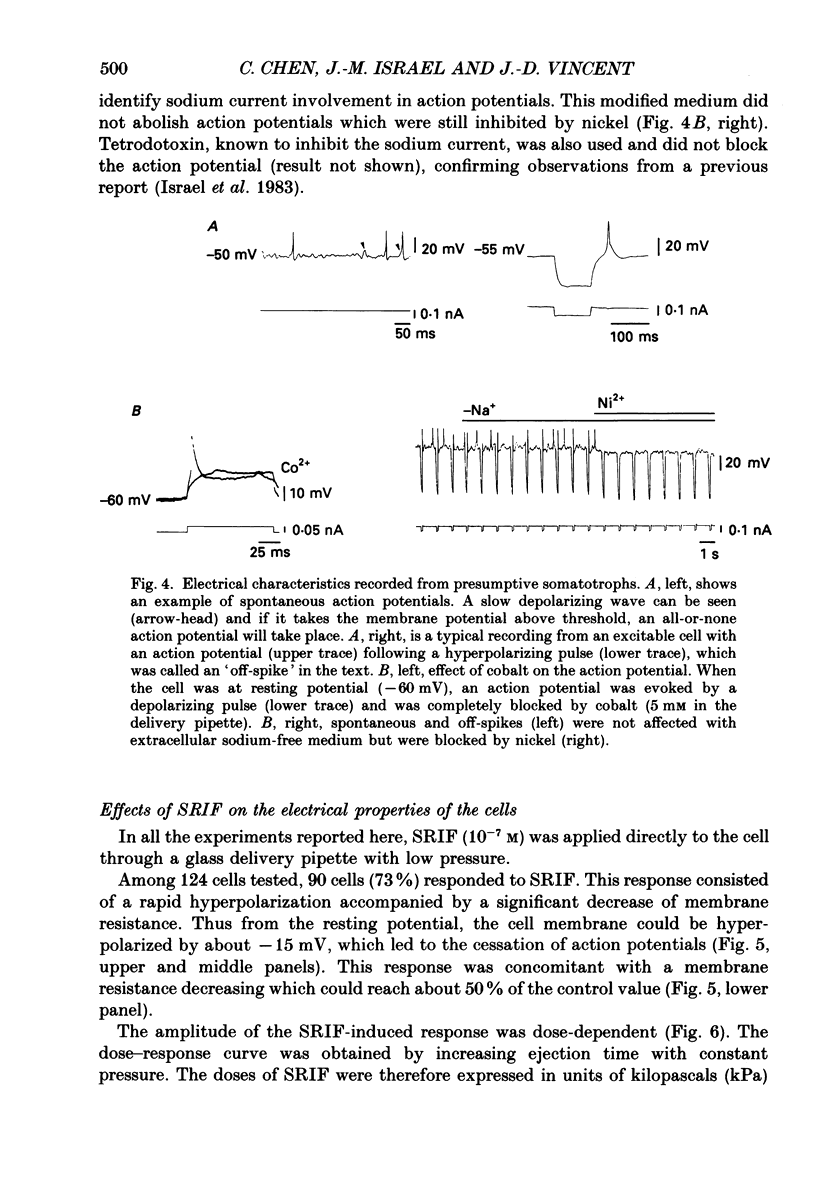
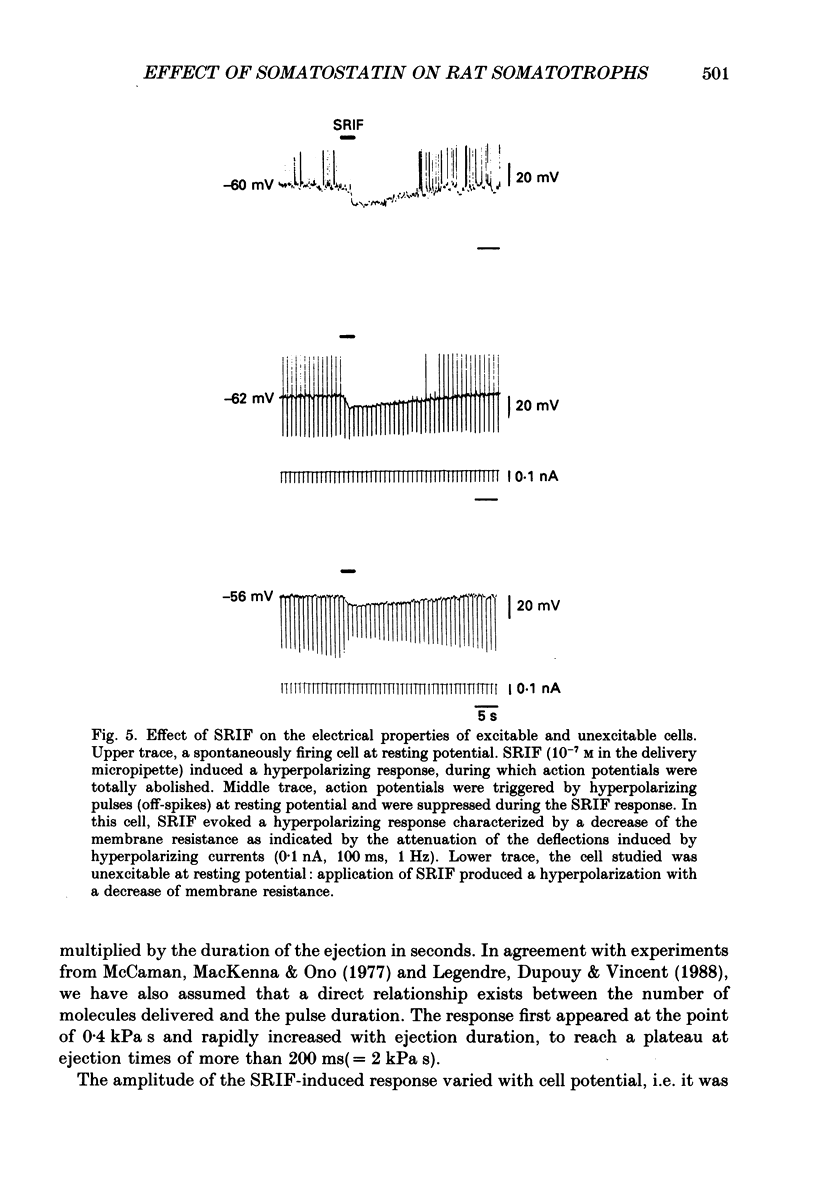
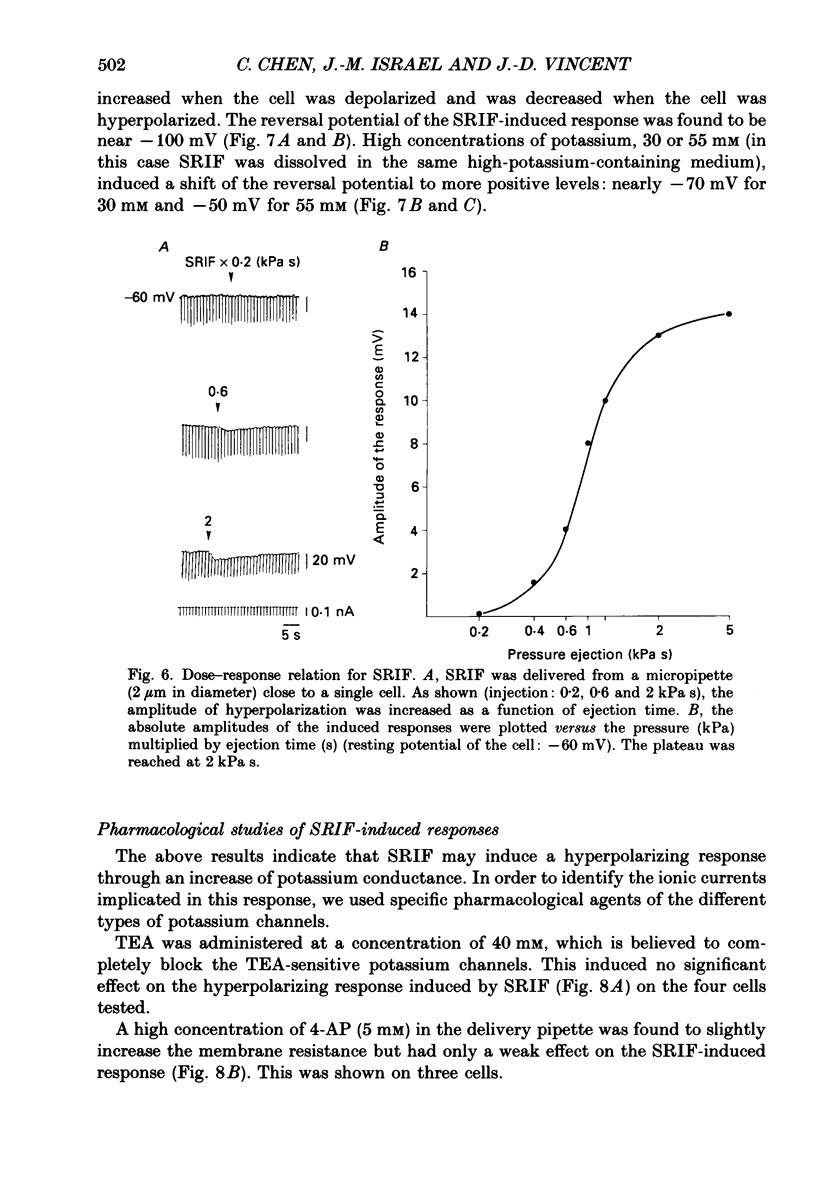
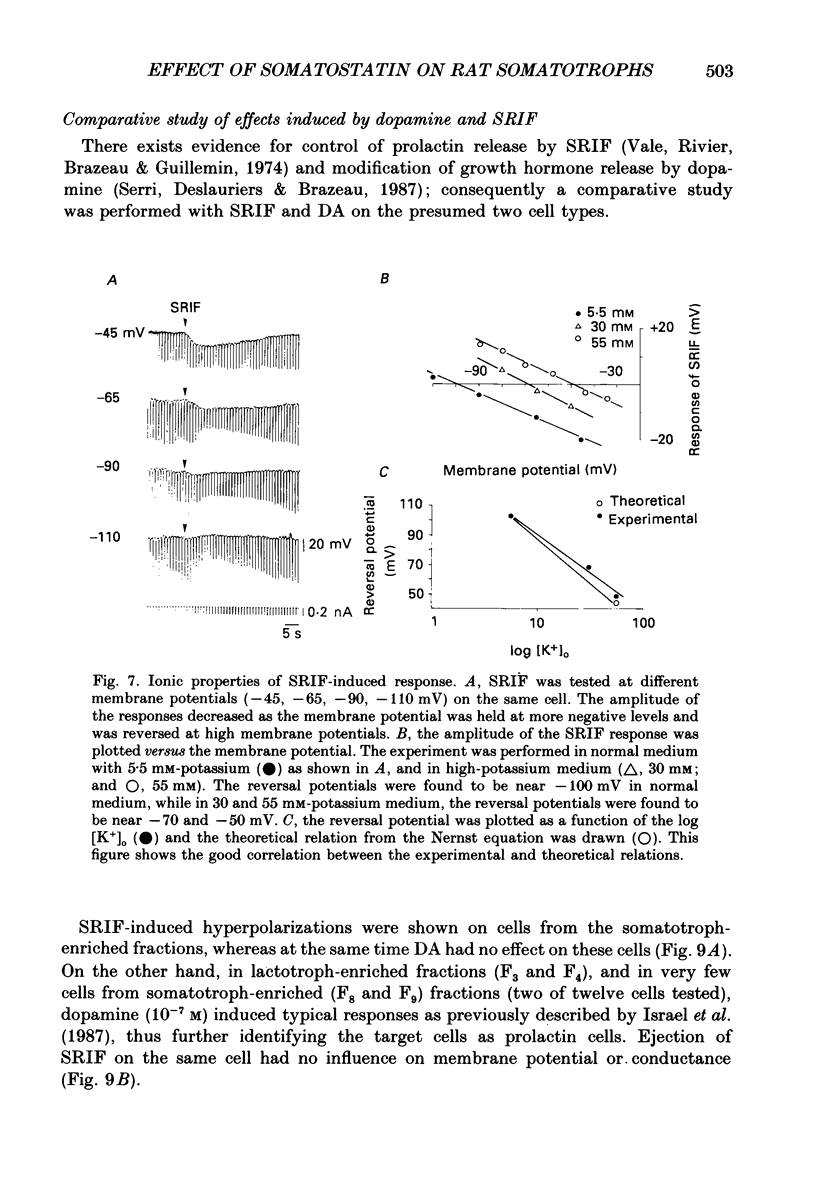
1. Somatotroph cells were obtained from pituitaries of adult male rats by dissociation, separation and enrichment on a continuous gradient of bovine serum albumin at unit gravity. They were kept in culture for 7-15 days before electrophysiological experiments. 2. Immunofluorescent staining of the resulting gradient fractions (numbered F2 to F9) indicated that the majority of somatotrophs (75-85%) were located in the heavy fractions (F8 and F9). However, a small percentage (15-20%) of cells in these fractions were identified as lactotrophs. 3. Perifusion experiments indicated that on the one hand release of growth hormone from somatotroph-enriched fractions was stable at the level of 6 ng (2 min)-1 (10(6) cells)-1 and was markedly inhibited by somatostatin (1.9 ng (2 min)-1 (10(6) cells)-1) but not by dopamine. On the other hand, in the same cell preparations, basal prolactin release (1.6 ng (2 min)-1 (10(6) cells)-1) was significantly reduced by dopamine (0.08 ng (2 min)-1 (10(6) cells)-1) but remained unchanged by somatostatin treatment. 4. The inhibitory effect of somatostatin on growth hormone release was dose dependent. This effect was not abolished by tetraethylammonium (40 mM) or 4-aminopyridine (5 mM), but somatostatin decreased high-potassium-induced release. 5. In all the cells recorded (n = 187), 14% (n = 26) displayed a low resting potential (less than -30 mV) and poor membrane resistance (less than 50 M omega). The recording was unstable and resting potentials decreased regularly to 0 mV in less than 5 min. The other 86% of the cells displayed resting potentials varying from -45 to -65 mV and had a membrane resistance of more than 150 M omega. Only cells which displayed these membrane characteristics showed clear responses to somatostatin or dopamine, and were therefore chosen for experiments. 6. In all the cells selected for the experiments (n = 161), 78% (n = 126) showed either triggered or spontaneous action potentials. The action potentials remained insensitive to sodium-free bath solution, but were reversibly blocked by the calcium channel blockers cobalt (5 mM) or nickel (5 mM). 7. When the cells were at resting potential, somatostatin induced a hyperpolarizing response associated with a decrease of membrane resistance. During this response, spontaneous or triggered action potentials were inhibited. The hyperpolarizing response induced by somatostatin was dose-dependent.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
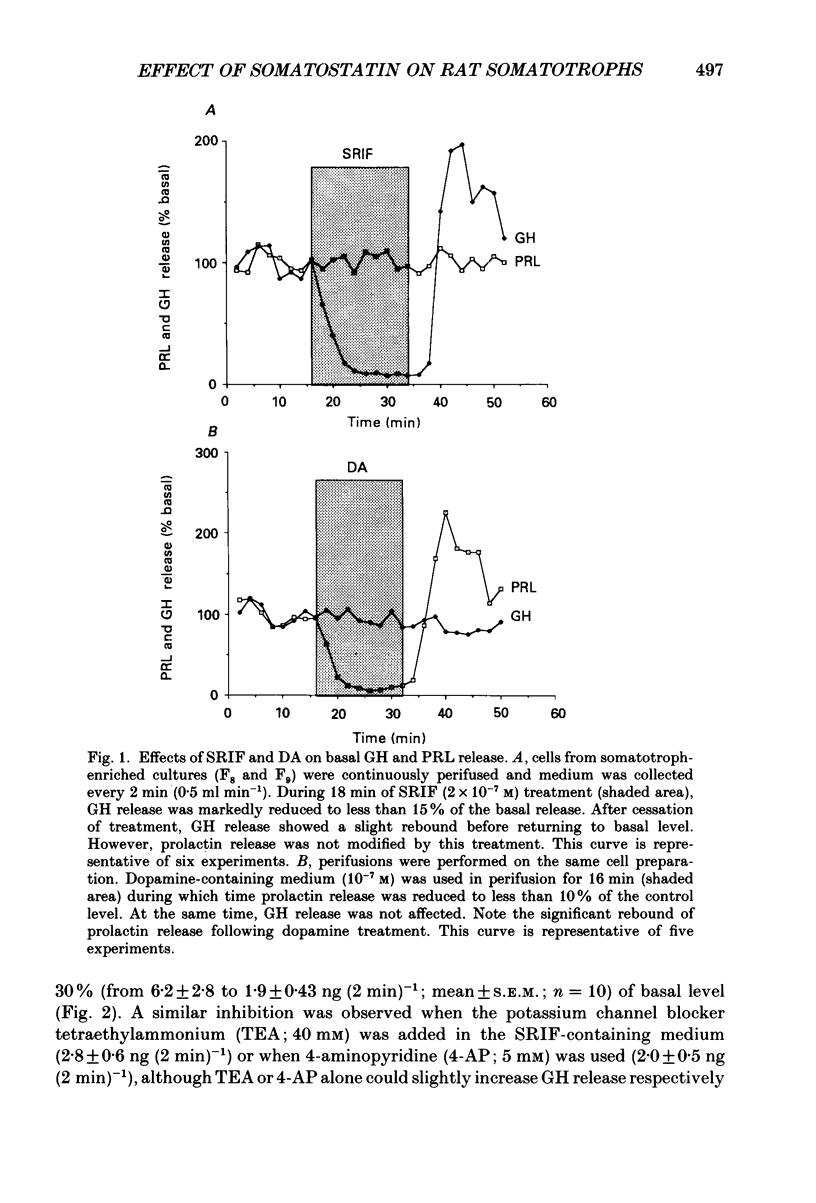
PDF



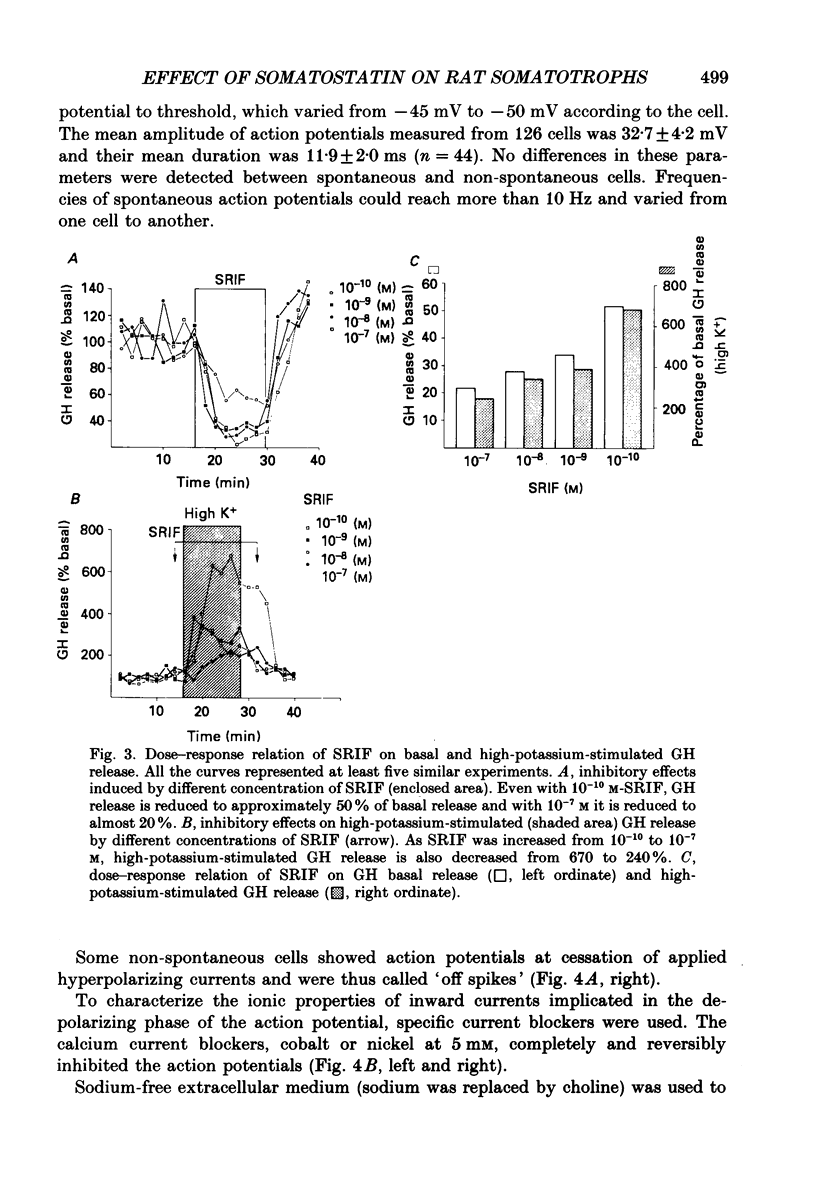






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
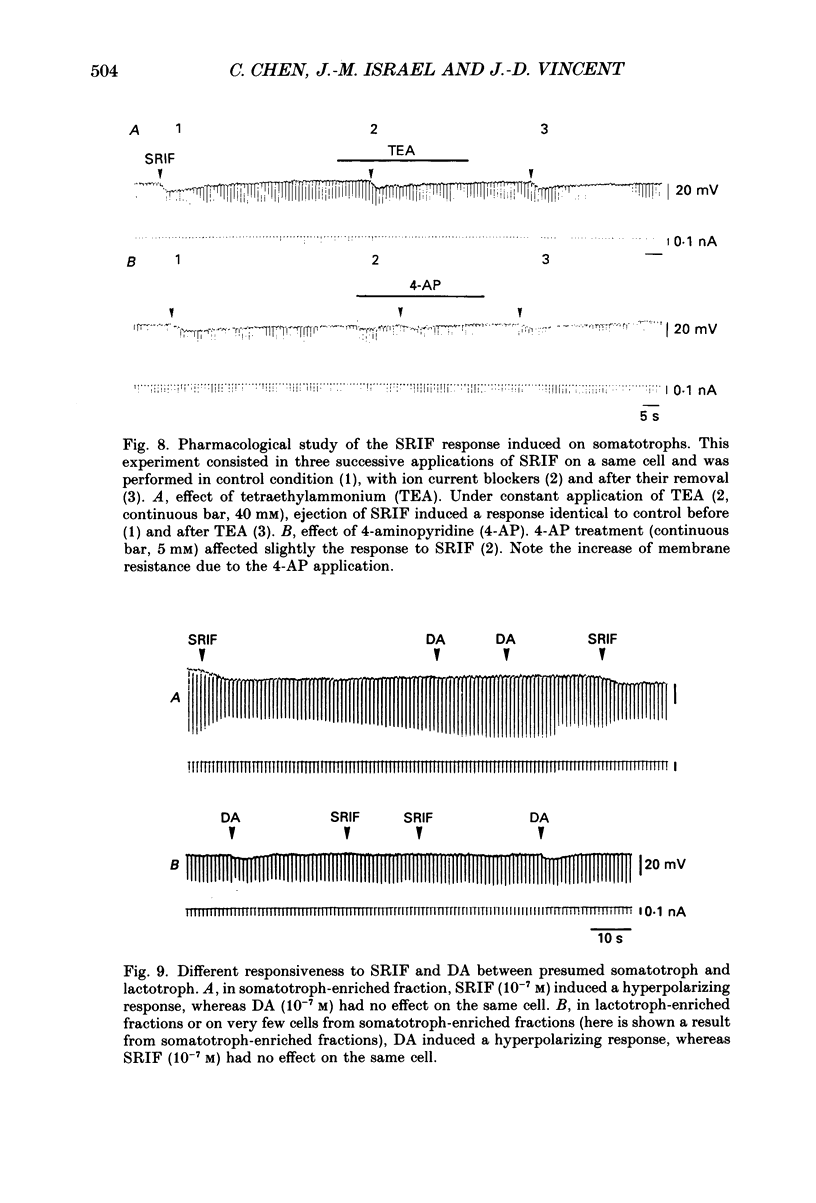
- Adams D. J., Smith S. J., Thompson S. H. Ionic currents in molluscan soma. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- Bilezikjian L. M., Vale W. W. Stimulation of adenosine 3',5'-monophosphate production by growth hormone-releasing factor and its inhibition by somatostatin in anterior pituitary cells in vitro. Endocrinology. 1983 Nov;113(5):1726–1731. doi: 10.1210/endo-113-5-1726. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Rivier J., Vale W., Guillemin R. Inhibition of growth hormone secretion in the rat by synthetic somatostatin. Endocrinology. 1974 Jan;94(1):184–187. doi: 10.1210/endo-94-1-184. [DOI] [PubMed] [Google Scholar]
- Cobbett P., Ingram C. D., Mason W. T. Sodium and potassium currents involved in action potential propagation in normal bovine lactotrophs. J Physiol. 1987 Nov;392:273–299. doi: 10.1113/jphysiol.1987.sp016780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin M. J., Rogol A. D., Myers G. A., Hewlett E. L. Pertussis toxin blocks the somatostatin-induced inhibition of growth hormone release and adenosine 3',5'-monophosphate accumulation. Endocrinology. 1983 Jul;113(1):209–215. doi: 10.1210/endo-113-1-209. [DOI] [PubMed] [Google Scholar]
- Denef C., Hautekeete E., De Wolf A., Vanderschueren B. Pituitary basophils from immature male and female rats: distribution of gonadotrophs and thyrotrophs as studied by unit gravity sedimentation. Endocrinology. 1978 Sep;103(3):724–735. doi: 10.1210/endo-103-3-724. [DOI] [PubMed] [Google Scholar]
- Denef C., Hautekeete E., Rubin L. A specific population of gonadotrophs purified from immature female rat pituitary. Science. 1976 Nov 19;194(4267):848–851. doi: 10.1126/science.790569. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Aspects of the calcium hypothesis of stimulus-secretion coupling: electrical activity in adenohypophyseal cells, and membrane retrieval after exocytosis. Methods Cell Biol. 1981;23:483–501. doi: 10.1016/s0091-679x(08)61515-0. [DOI] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Dual modulation of K channels by thyrotropin-releasing hormone in clonal pituitary cells. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4282–4286. doi: 10.1073/pnas.82.12.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufy B., Vincent J. D., Fleury H., Du Pasquier P., Gourdji D., Tixier-Vidal A. Dopamine inhibition of action potentials in a prolactin secreting cell line is modulated by oestrogen. Nature. 1979 Dec 20;282(5741):855–857. doi: 10.1038/282855a0. [DOI] [PubMed] [Google Scholar]
- Epelbaum J., Enjalbert A., Krantic S., Musset F., Bertrand P., Rasolonjanahary R., Shu C., Kordon C. Somatostatin receptors on pituitary somatotrophs, thyrotrophs, and lactotrophs: pharmacological evidence for loose coupling to adenylate cyclase. Endocrinology. 1987 Dec;121(6):2177–2185. doi: 10.1210/endo-121-6-2177. [DOI] [PubMed] [Google Scholar]
- Hayasaki-Kimura N., Takahashi K. Studies on action of somatostatin on growth hormone release in relation to calcium and cAMP. Proc Soc Exp Biol Med. 1979 Jul;161(3):312–318. doi: 10.3181/00379727-161-40543. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Farquhar M. G. Hormone secretion by cells dissociated from rat anterior pituitaries. J Cell Biol. 1973 Nov;59(2 Pt 1):276–303. doi: 10.1083/jcb.59.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram C. D., Bicknell R. J., Mason W. T. Intracellular recordings from bovine anterior pituitary cells: modulation of spontaneous activity by regulators of prolactin secretion. Endocrinology. 1986 Dec;119(6):2508–2518. doi: 10.1210/endo-119-6-2508. [DOI] [PubMed] [Google Scholar]
- Israel J. M., Denef C., Vincent J. D. Electrophysiological properties of normal somatotrophs in culture. An intracellular study. Neuroendocrinology. 1983 Sep;37(3):193–199. doi: 10.1159/000123542. [DOI] [PubMed] [Google Scholar]
- Israel J. M., Jaquet P., Vincent J. D. The electrical properties of isolated human prolactin-secreting adenoma cells and their modification by dopamine. Endocrinology. 1985 Oct;117(4):1448–1455. doi: 10.1210/endo-117-4-1448. [DOI] [PubMed] [Google Scholar]
- Israel J. M., Kirk C., Vincent J. D. Electrophysiological responses to dopamine of rat hypophysial cells in lactotroph-enriched primary cultures. J Physiol. 1987 Sep;390:1–22. doi: 10.1113/jphysiol.1987.sp016682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraicer J., Chow A. E. Release of growth hormone from purified somatotrophs: use of perifusion system to elucidate interrelations among Ca++, adenosine 3',5'-monophosphate, and somatostatin. Endocrinology. 1982 Oct;111(4):1173–1180. doi: 10.1210/endo-111-4-1173. [DOI] [PubMed] [Google Scholar]
- Kraicer J., Spence J. W. Release of growth hormone from purified somatotrophs: use of high K+ and the ionophore A23187 to elucidate interrelations among Ca++, adenosine 3',5'-monophosphate, and somatostatin. Endocrinology. 1981 Feb;108(2):651–657. doi: 10.1210/endo-108-2-651. [DOI] [PubMed] [Google Scholar]
- Legendre P., Dupouy B., Vincent J. D. Excitatory effect of noradrenaline on pacemaker cells in spinal cord primary cultures. Neuroscience. 1988 Feb;24(2):647–658. doi: 10.1016/0306-4522(88)90358-2. [DOI] [PubMed] [Google Scholar]
- McCaman R. E., McKenna D. G., Ono J. K. A pressure system for intracellular and extracellular ejections of picoliter volumes. Brain Res. 1977 Nov 4;136(1):141–147. doi: 10.1016/0006-8993(77)90138-x. [DOI] [PubMed] [Google Scholar]
- Mihara S., North R. A., Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. J Physiol. 1987 Sep;390:335–355. doi: 10.1113/jphysiol.1987.sp016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. D., Madamba S. G., Joëls M., Siggins G. R. Somatostatin augments the M-current in hippocampal neurons. Science. 1988 Jan 15;239(4837):278–280. doi: 10.1126/science.2892268. [DOI] [PubMed] [Google Scholar]
- Nikitovitch-Winer M. B., Atkin J., Maley B. E. Colocalization of prolactin and growth hormone within specific adenohypophyseal cells in male, female, and lactating female rats. Endocrinology. 1987 Aug;121(2):625–630. doi: 10.1210/endo-121-2-625. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Zyzek E. Calcium efflux from the rat neurohypophysis. J Physiol. 1982 Apr;325:281–299. doi: 10.1113/jphysiol.1982.sp014150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace C. S., Tarvin J. T. Somatostatin: mechanism of action in pancreatic islet beta-cells. Diabetes. 1981 Oct;30(10):836–842. doi: 10.2337/diab.30.10.836. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Palmer M. R., Wuerthele S. M., Hoffer B. J. Physical and physiological characteristics of micropressure ejection of drugs from multibarreled pipettes. Neuropharmacology. 1980 Oct;19(10):931–938. doi: 10.1016/0028-3908(80)90001-5. [DOI] [PubMed] [Google Scholar]
- Richardson U. I., Schonbrunn A. Inhibition of adrenocorticotropin secretion by somatostatin in pituitary cells in culture. Endocrinology. 1981 Jan;108(1):281–290. doi: 10.1210/endo-108-1-281. [DOI] [PubMed] [Google Scholar]
- Schofield J. G., Bicknell R. J. Effects of somatostatin and verapamil on growth hormone release and 45Ca fluxes. Mol Cell Endocrinol. 1978 Jan;9(3):255–268. doi: 10.1016/0303-7207(78)90068-0. [DOI] [PubMed] [Google Scholar]
- Serri O., Deslauriers N., Brazeau P. Dual action of dopamine on growth hormone release in vitro. Neuroendocrinology. 1987 May;45(5):363–367. doi: 10.1159/000124760. [DOI] [PubMed] [Google Scholar]
- Sheppard M. S., Spence J. W., Kraicer J. Release of growth hormone from purified somatotrophs: role of adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate. Endocrinology. 1979 Jul;105(1):261–268. doi: 10.1210/endo-105-1-261. [DOI] [PubMed] [Google Scholar]
- Stratmann I. E., Ezrin C., Sellers E. A. Estrogen-induced transformation of somatotrophs into mammotrophs in the rat. Cell Tissue Res. 1974;152(2):229–238. doi: 10.1007/BF00224697. [DOI] [PubMed] [Google Scholar]
- Szabo M., Frohman L. A. Effects of porcine stalk median eminence and prostaglandin E2 on rat growth hormone secretion in vivo and their inhibition by somatostatin. Endocrinology. 1975 Apr;96(4):955–961. doi: 10.1210/endo-96-4-955. [DOI] [PubMed] [Google Scholar]
- Vale W., Brazeau P., Rivier C., Brown M., Boss B., Rivier J., Burgus R., Ling N., Guillemin R. Somatostatin. Recent Prog Horm Res. 1975;31:365–397. doi: 10.1016/b978-0-12-571131-9.50014-1. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier C., Brazeau P., Guillemin R. Effects of somatostatin on the secretion of thyrotropin and prolactin. Endocrinology. 1974 Oct;95(4):968–977. doi: 10.1210/endo-95-4-968. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Shibuya N., Ogata E. Hyperpolarization of the membrane potential caused by somatostatin in dissociated human pituitary adenoma cells that secrete growth hormone. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6198–6202. doi: 10.1073/pnas.83.16.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]