Abstract
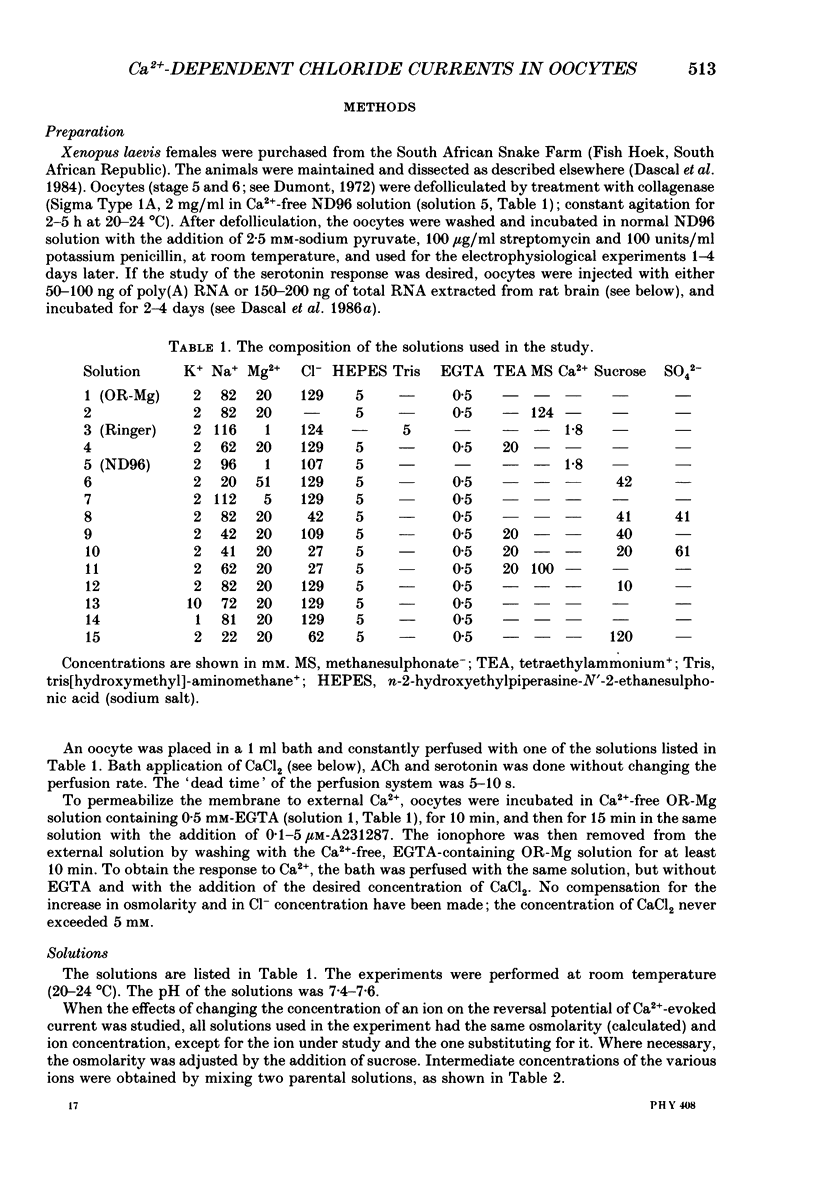
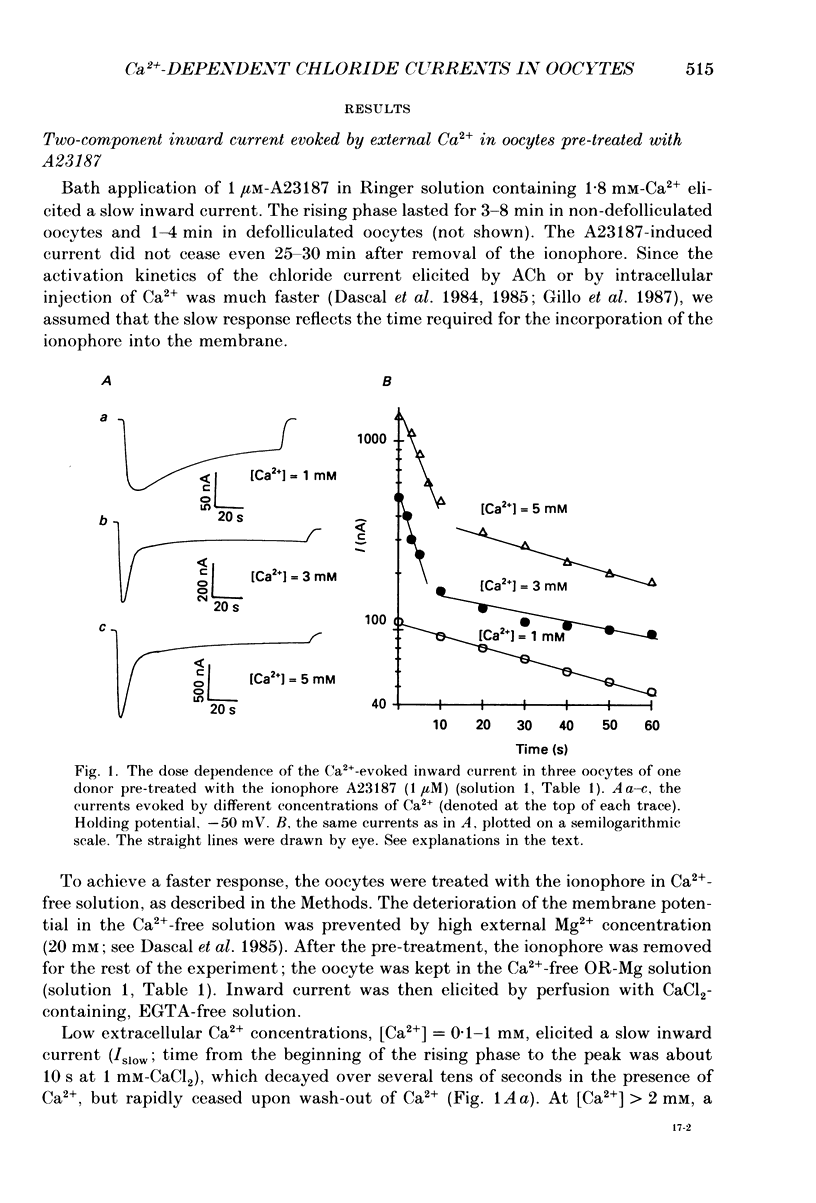
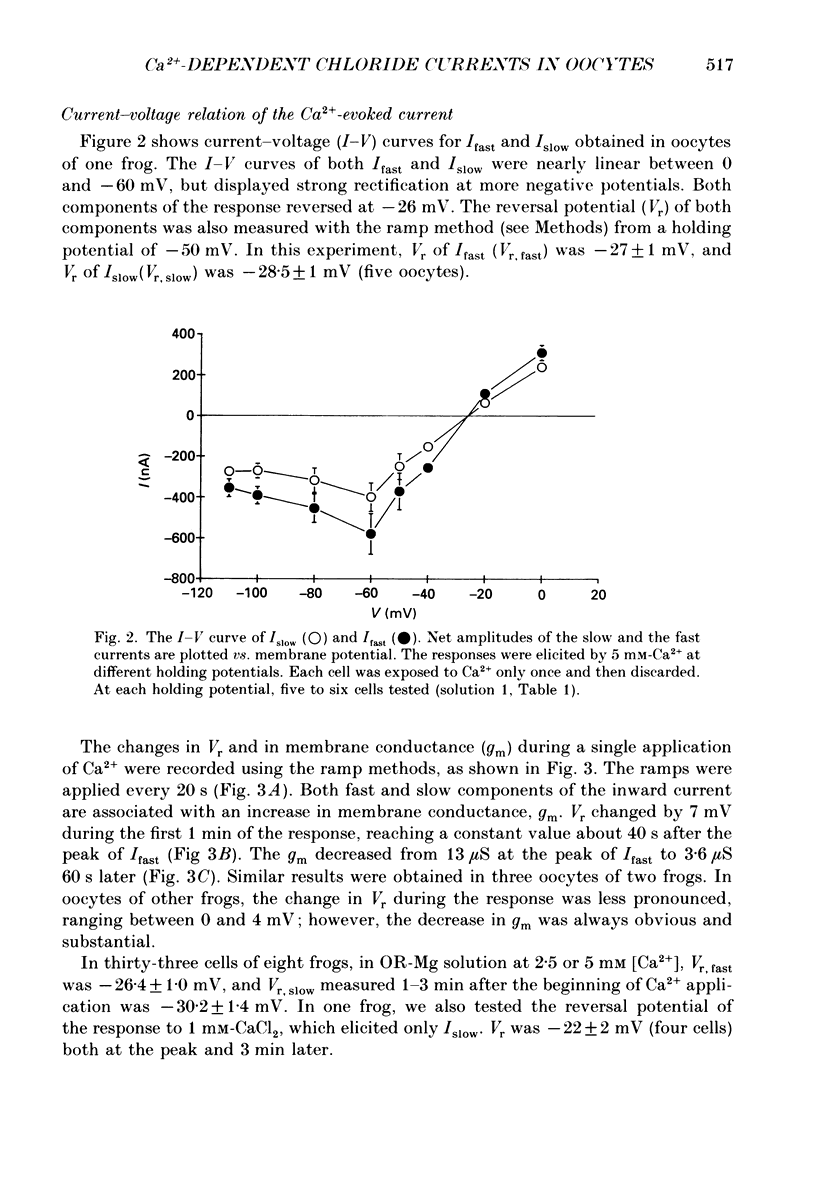
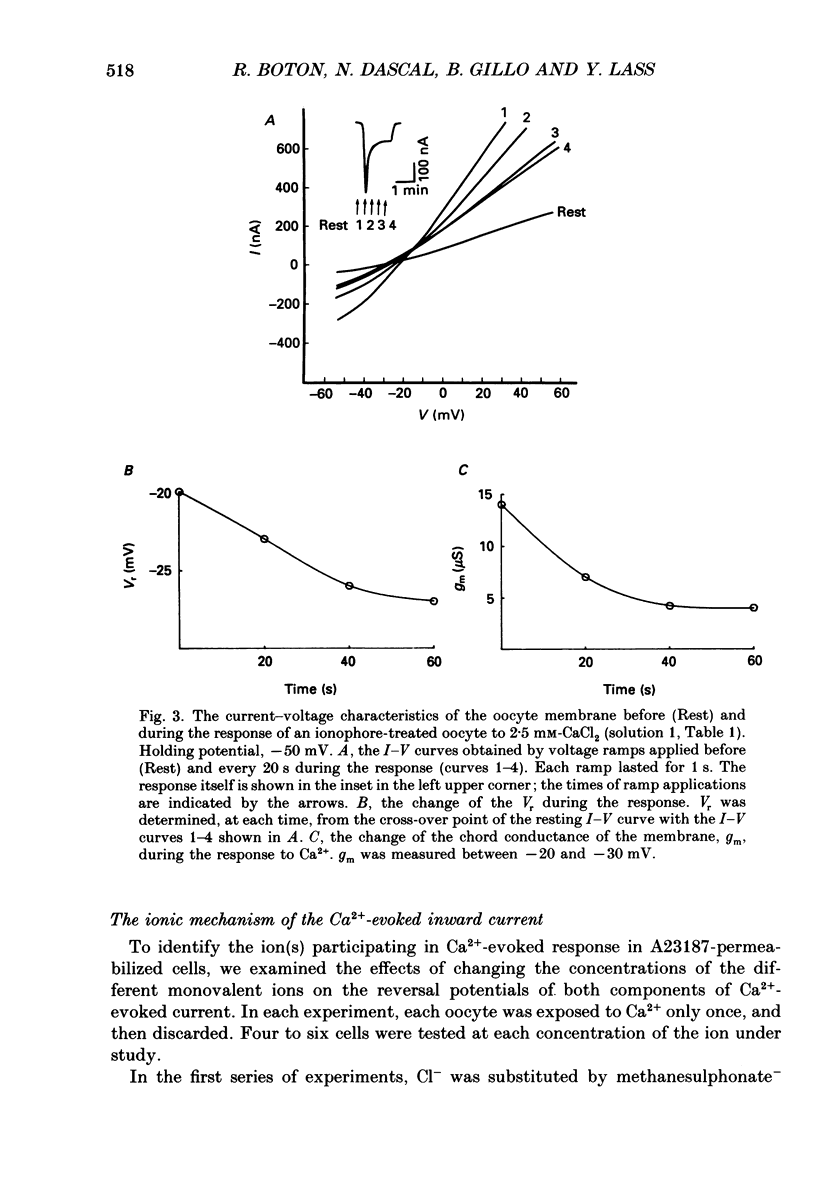
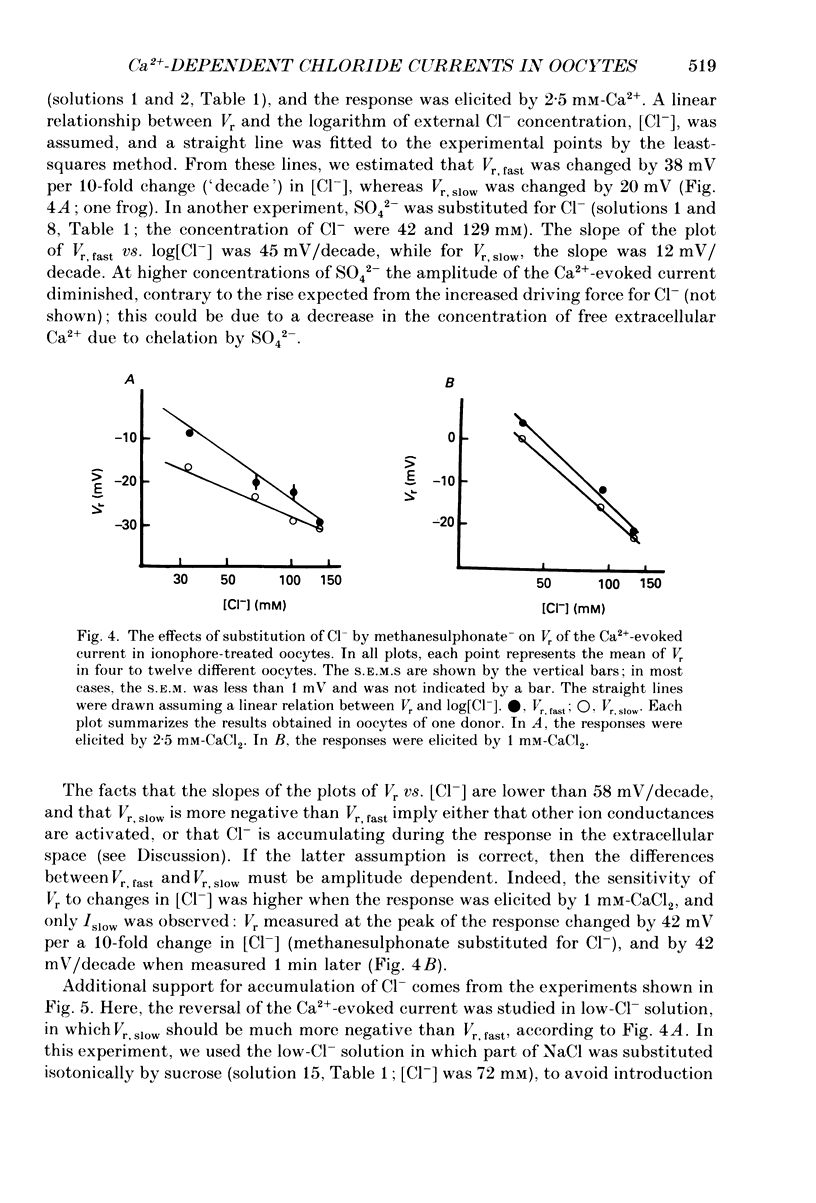
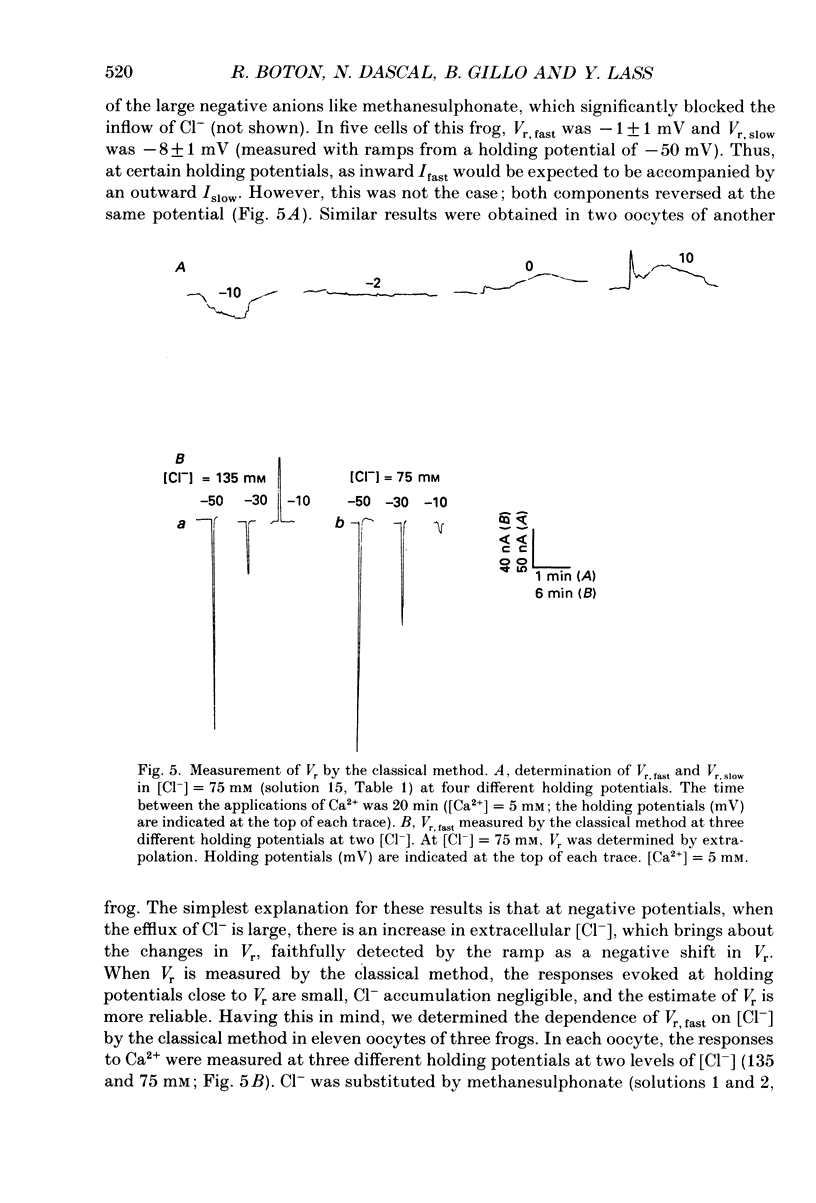
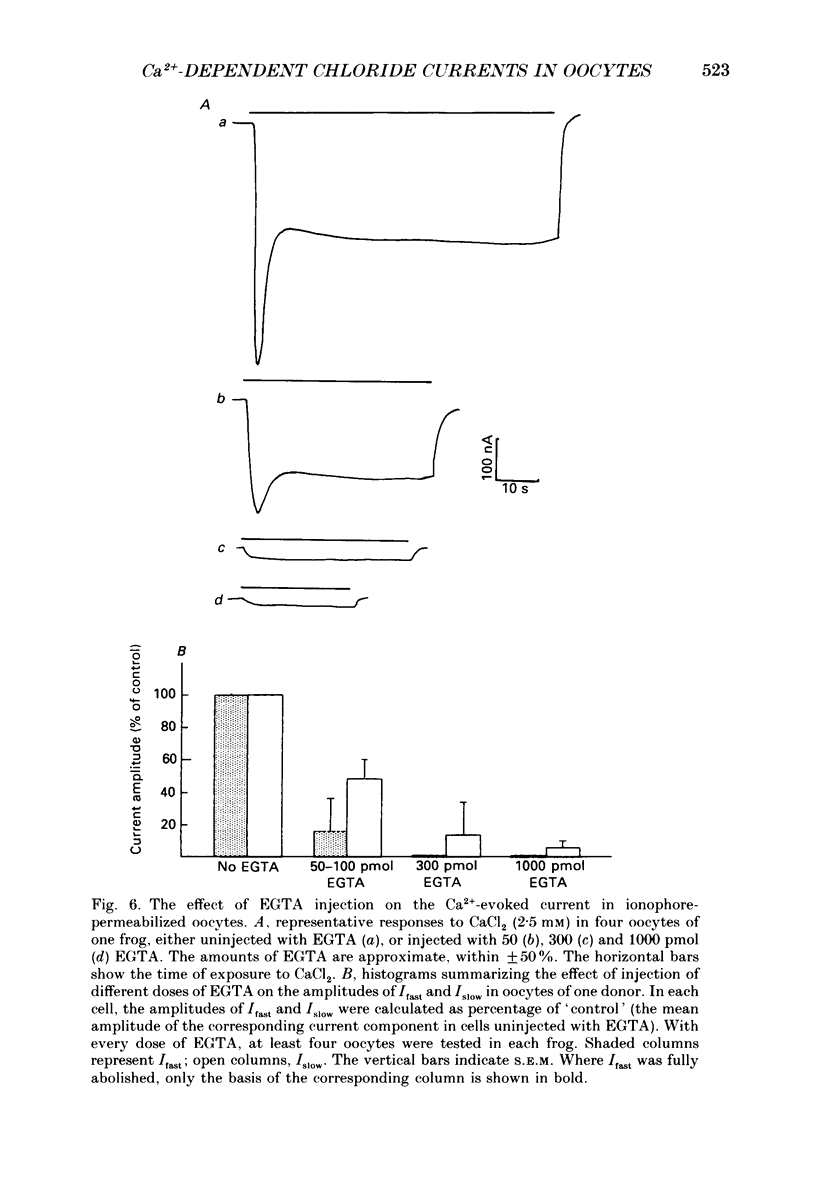
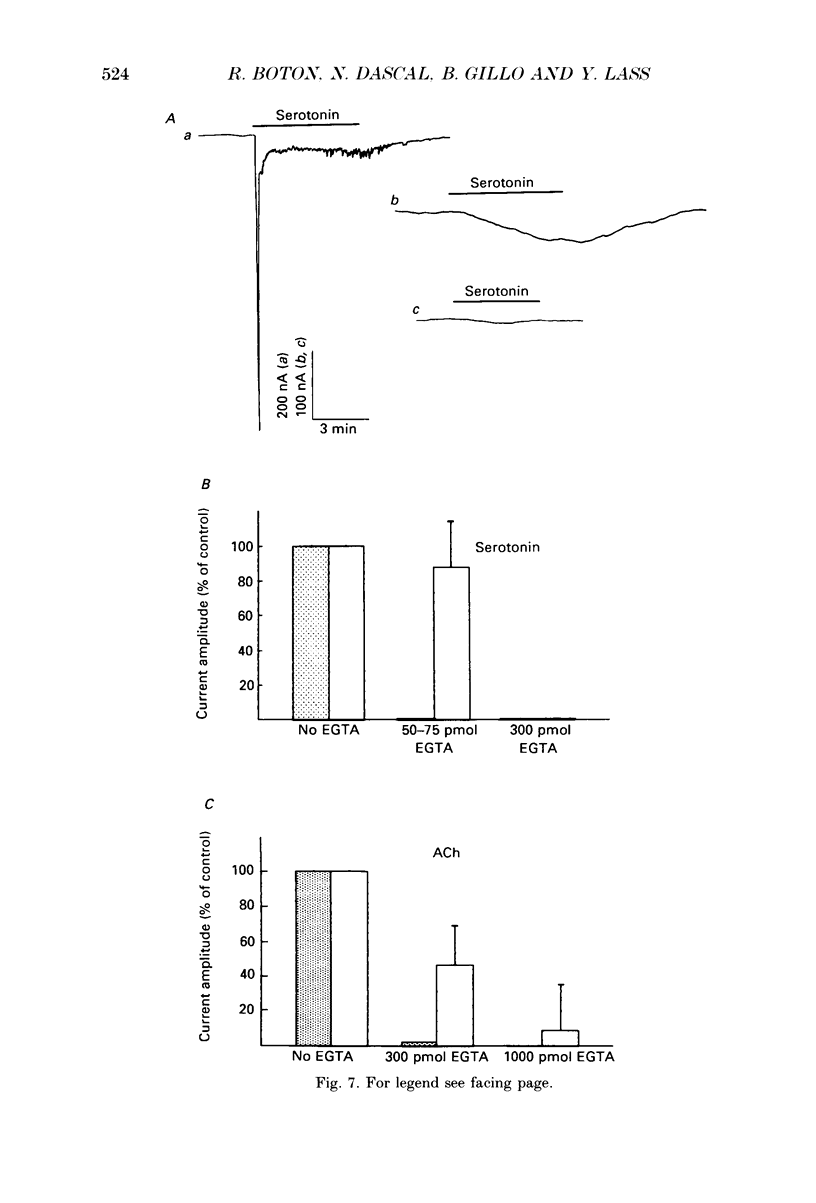
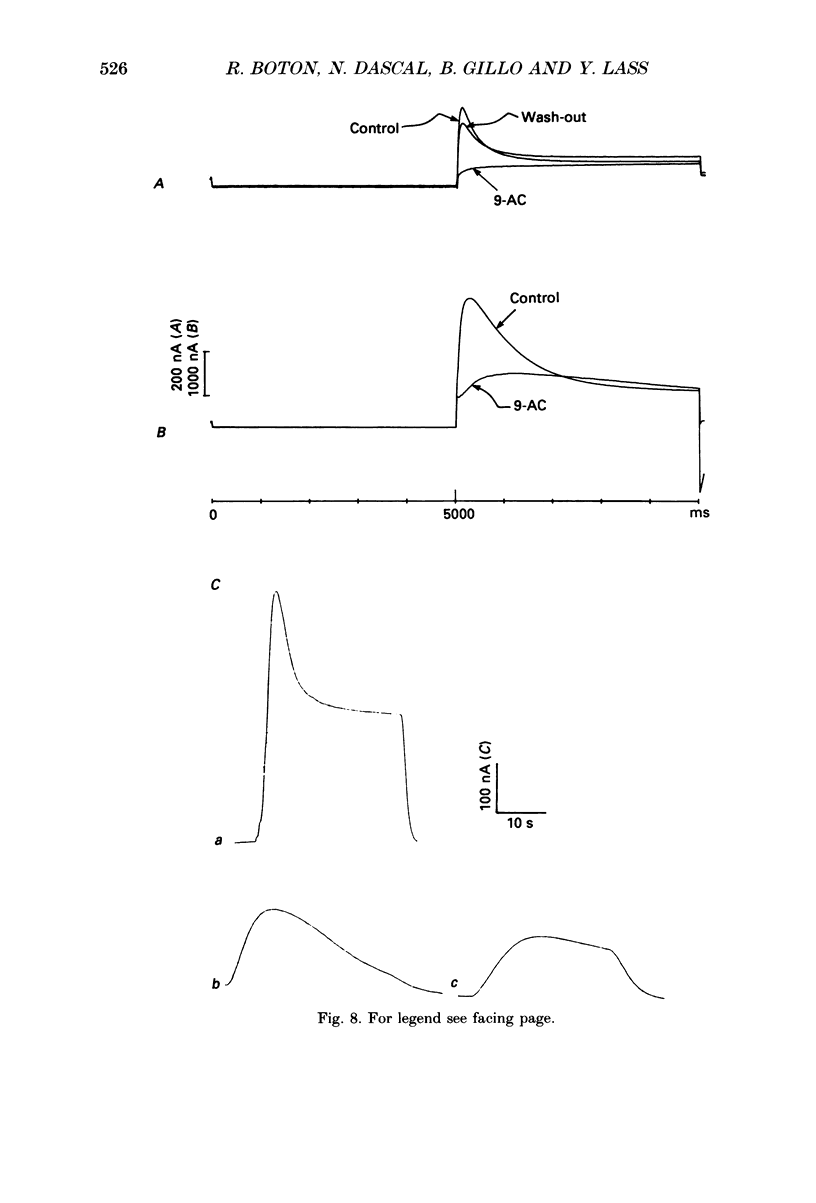
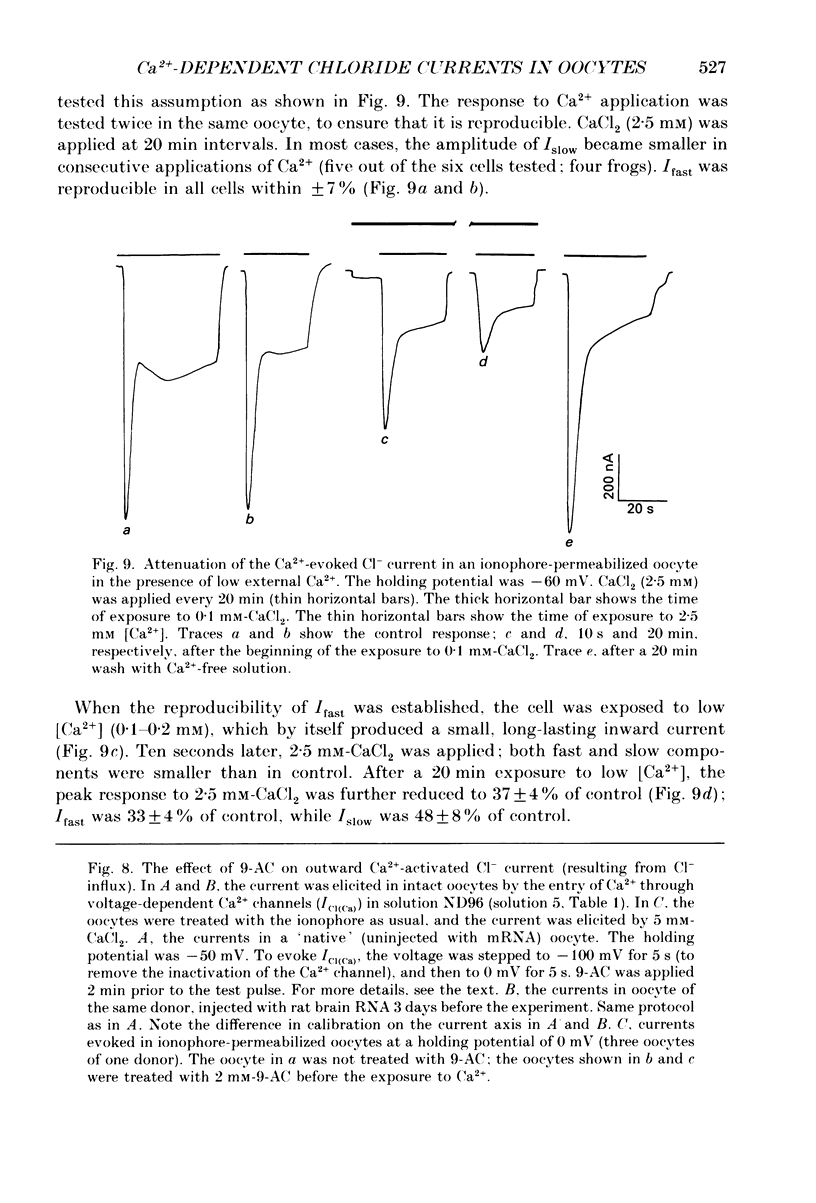
1. Currents evoked by elevated intracellular free Ca2+ in Xenopus laevis oocytes were studied using the two-electrode voltage clamp technique. The elevation in Ca2+ concentration was achieved in three ways: by the use of the divalent cation ionophore A23187; by application of Ca2+-mobilizing neurotransmitters serotonin and acetylcholine (ACh); by the entry of Ca2+ through voltage-dependent channels. 2. In most experiments, the membrane was permeabilized to Ca2+ by a 15 min pretreatment with A23187 in a Ca2+-free solution. Exposure of the ionophore-treated oocytes to external Ca2+ elicited an inward current (at holding potentials of -40 to -60 mV). At external Ca2+ concentrations ([Ca2+]) between 0.1 and 1 mM, the current had a time-to-peak of at least 10 s, and slowly decayed over tens of seconds. At [Ca2+] greater than 2 mM, the inward current had two distinct kinetic components, a fast and transient one (Ifast) and a slow one (Islow). 3. The main carrier of the Ca2+-evoked inward current was Cl-. Several data indicate the existence of a tetraethylammonium (TEA)-sensitive K+ conductance. No evidence for a Na+ current was found. 4. The two components of the Ca2+-evoked inward current in ionophore-permeabilized oocytes, and the two components of the current evoked by ACh and serotonin (the latter in oocytes injected with rat brain RNA but untreated with A23187), were blocked by intracellular injection of the Ca2+ chelator, ethyleneglycolbis-(beta-aminoethyl ether)-N,N,N'N'-tetraacetic acid (EGTA). The two components of these currents displayed different sensitivity to Ca2+ buffering; higher doses of EGTA were necessary to inhibit the slow component than the fast one. 5. One to two minutes of treatment with 2 mM-9-anthracene carboxylic acid (9-AC) fully blocked Ca2+-dependent Cl- current evoked by Ca2+ influx through voltage- dependent Ca2+ channels in intact (untreated with A23187) oocytes. In ionophore-treated oocytes, block of Ifast was observed at holding potentials at which the current was outward (i.e. due to Cl- influx); Islow was inhibited only partially. The block of Ca2+-evoked Cl- efflux by 9-AC developed much more slowly and was less potent. to explain these results, the existence of two sites of 9-AC action is proposed. 6. Exposure of the ionophore-permeabilized oocytes to 0.1-0.2 mM [Ca2+] strongly reduced the response to higher concentrations of Ca2+. Ifast displayed stronger Ca2+-dependent inactivation than Islow.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretag A. H. Muscle chloride channels. Physiol Rev. 1987 Apr;67(2):618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Dascal N., Gillo B., Lass Y. Role of calcium mobilization in mediation of acetylcholine-evoked chloride currents in Xenopus laevis oocytes. J Physiol. 1985 Sep;366:299–313. doi: 10.1113/jphysiol.1985.sp015799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Landau E. M. Cyclic GMP mimics the muscarinic response in Xenopus oocytes: identity of ionic mechanisms. Proc Natl Acad Sci U S A. 1982 May;79(9):3052–3056. doi: 10.1073/pnas.79.9.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Landau E. M., Lass Y. Xenopus oocyte resting potential, muscarinic responses and the role of calcium and guanosine 3',5'-cyclic monophosphate. J Physiol. 1984 Jul;352:551–574. doi: 10.1113/jphysiol.1984.sp015310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Snutch T. P., Lübbert H., Davidson N., Lester H. A. Expression and modulation of voltage-gated calcium channels after RNA injection in Xenopus oocytes. Science. 1986 Mar 7;231(4742):1147–1150. doi: 10.1126/science.2418503. [DOI] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Mantei N., Weissmann C. DNA sequences preceding the rabbit beta-globin gene are required for formation in mouse L cells of beta-globin RNA with the correct 5' terminus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1411–1415. doi: 10.1073/pnas.78.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I., Petersen O. H. Acetylcholine stimulates a Ca2+-dependent C1- conductance in mouse lacrimal acinar cells. Pflugers Arch. 1985 Mar;403(3):328–330. doi: 10.1007/BF00583609. [DOI] [PubMed] [Google Scholar]
- Gillo B., Lass Y., Nadler E., Oron Y. The involvement of inositol 1,4,5-trisphosphate and calcium in the two-component response to acetylcholine in Xenopus oocytes. J Physiol. 1987 Nov;392:349–361. doi: 10.1113/jphysiol.1987.sp016784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A. L., Snutch T., Lübbert H., Dowsett A., Marshall J., Auld V., Downey W., Fritz L. C., Lester H. A., Dunn R. Messenger RNA coding for only the alpha subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7503–7507. doi: 10.1073/pnas.83.19.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Voltage-operated channels induced by foreign messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Nov 22;220(1218):131–140. doi: 10.1098/rspb.1983.0092. [DOI] [PubMed] [Google Scholar]
- Hirono C., Ito I., Sugiyama H. Neurotensin and acetylcholine evoke common responses in frog oocytes injected with rat brain messenger ribonucleic acid. J Physiol. 1987 Jan;382:523–535. doi: 10.1113/jphysiol.1987.sp016382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. A., Cross N. L., Picheral B. Studies of the voltage-dependent polyspermy block using cross-species fertilization of amphibians. Dev Biol. 1983 Aug;98(2):319–326. doi: 10.1016/0012-1606(83)90362-7. [DOI] [PubMed] [Google Scholar]
- Jaffe L. A., Kado R. T., Muncy L. Propagating potassium and chloride conductances during activation and fertilization of the egg of the frog, Rana pipiens. J Physiol. 1985 Nov;368:227–242. doi: 10.1113/jphysiol.1985.sp015855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. A., Schlichter L. C. Fertilization-induced ionic conductances in eggs of the frog, Rana pipiens. J Physiol. 1985 Jan;358:299–319. doi: 10.1113/jphysiol.1985.sp015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Kegel D. R., Wolf B. D., Sheridan R. E., Lester H. A. Software for electrophysiological experiments with a personal computer. J Neurosci Methods. 1985 Feb;12(4):317–330. doi: 10.1016/0165-0270(85)90016-0. [DOI] [PubMed] [Google Scholar]
- Kline D., Nuccitelli R. The wave of activation current in the Xenopus egg. Dev Biol. 1985 Oct;111(2):471–487. doi: 10.1016/0012-1606(85)90499-3. [DOI] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977 Dec 22;270(5639):739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. P., Nargeot J., Snutch T. P., Davidson N., Lester H. A. Ca channels induced in Xenopus oocytes by rat brain mRNA. J Neurosci. 1987 Mar;7(3):875–881. doi: 10.1523/JNEUROSCI.07-03-00875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. A Ca-dependent Cl- conductance in cultured mouse spinal neurones. Nature. 1984 Oct 11;311(5986):567–570. doi: 10.1038/311567a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. A transient inward current elicited by hyperpolarization during serotonin activation in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):279–292. doi: 10.1098/rspb.1985.0002. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Neher E., Sakmann B. Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5063–5067. doi: 10.1073/pnas.84.14.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young G. P., Young J. D., Deshpande A. K., Goldstein M., Koide S. S., Cohn Z. A. A Ca2+-activated channel from Xenopus laevis oocyte membranes reconstituted into planar bilayers. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5155–5159. doi: 10.1073/pnas.81.16.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]


