Abstract
1. Ruthenium Red (RR) blocks sarcoplasmic reticulum (SR) Ca2+ release in disrupted muscle preparations and this block has been used as a marker for the physiological Ca2+ release pathway. To investigate whether RR can also affect SR Ca2+ release in living muscle, optical signals reflecting Ca2+ release have been measured in intact single frog twitch fibres microinjected with RR. 2. The total myoplasmic concentration of RR, [RRT], was obtained from measurements of RR-related absorbance and apparent diffusion constant, Dapp, of RR in myoplasm was estimated. The value of Dapp was about 1/30 of that expected and can be explained if the majority of RR (approximately 97%) was bound in myoplasm and free [RR] was only 1/30 of [RRT]. 3. Sarcoplasmic reticulum Ca2+ release following action potential stimulation was assessed from a Ca2+-related change in intrinsic birefringence. The birefringence signal was blocked in the presence of RR and the degree of block was clearly dependent upon [RRT]. At 16 degrees C the estimated [RRT] for half-block of the birefringence signal was 23 +/- 4 microM (+/- S.E. of mean; n = 3), and for half-block of the Ca2+ release process itself was 72 +/- 14 microM. The estimated free [RR] for half-block is then 0.8 +/- 0.1 and 2.4 +/- 0.5 microM, respectively. In the cold (6-8 degrees C), the half-blocking concentration of RR, referred to [RRT], appeared to be about 3-fold smaller than that observed at 16 degrees C. 4. The values estimated for the free [RR] which caused half-block of Ca2+ release in intact muscle fibres are in the range reported for RR's action in disrupted preparations, thus supporting the conclusion that the RR-blockable channel observed in disrupted muscle is the physiologically important Ca2+ release channel. 5. Intramembrane charge movements in skeletal muscle are thought to underlie the dependence of SR Ca2+ release on transverse tubular membrane potential. Charge movements were measured in RR-injected fibres at 4-6 degrees C using a three-microelectrode, middle-of-the-fibre voltage-clamp technique. Injected fibres did not survive well in solutions made hypertonic to prevent fibre movement and allow measurement of suprathreshold charge; therefore charge movements below contraction threshold were studied in isotonic solution.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
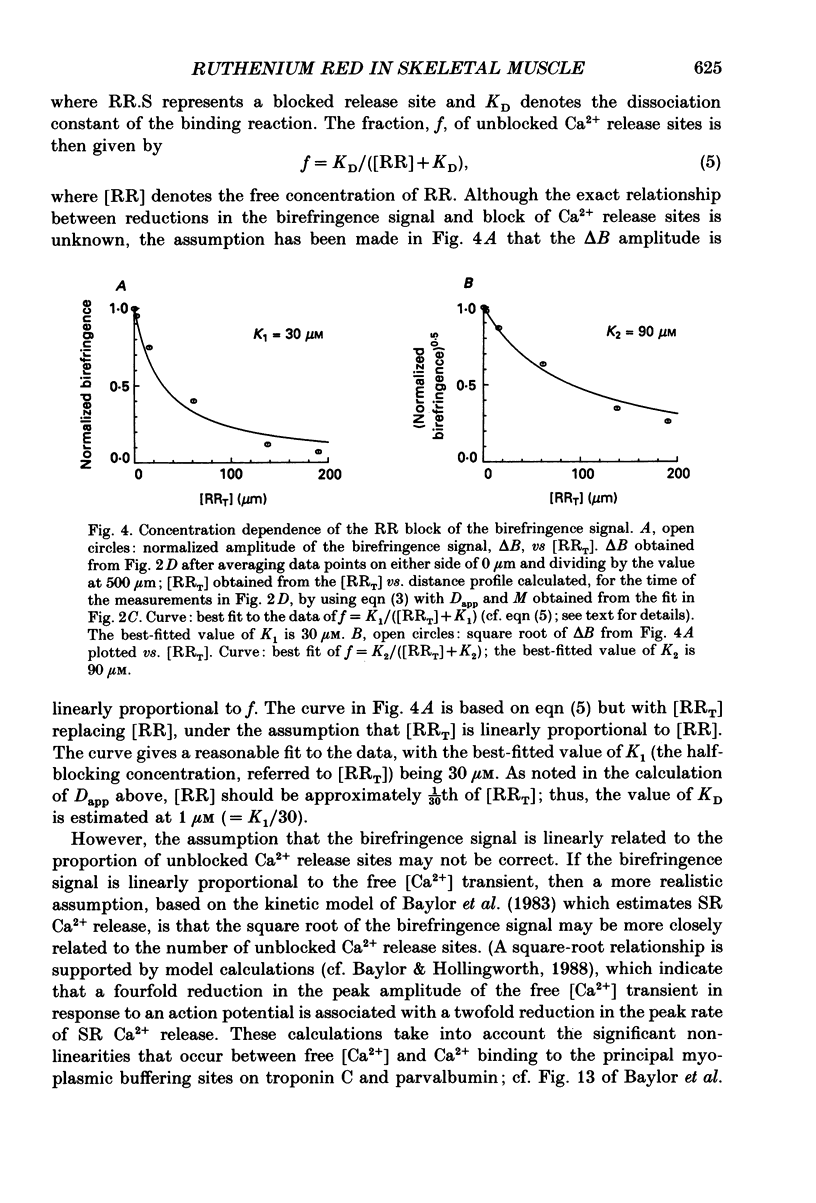
PDF











Selected References
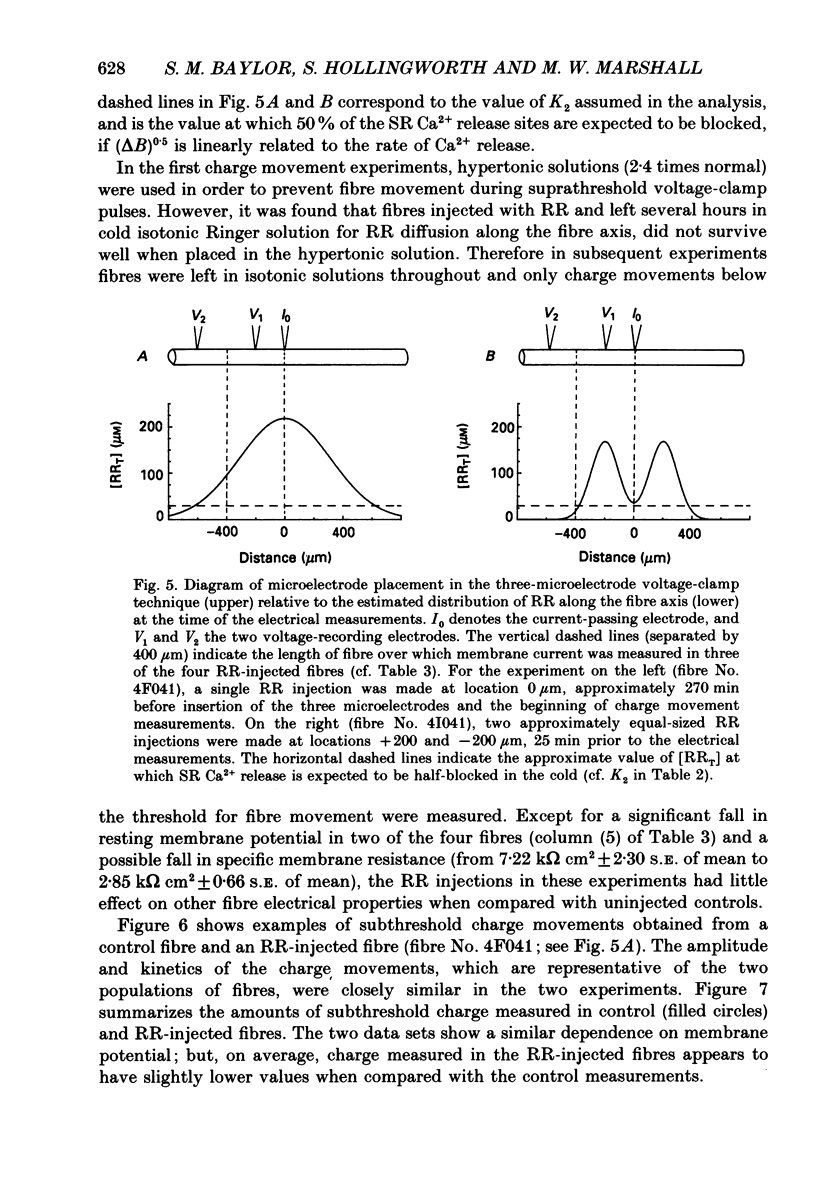
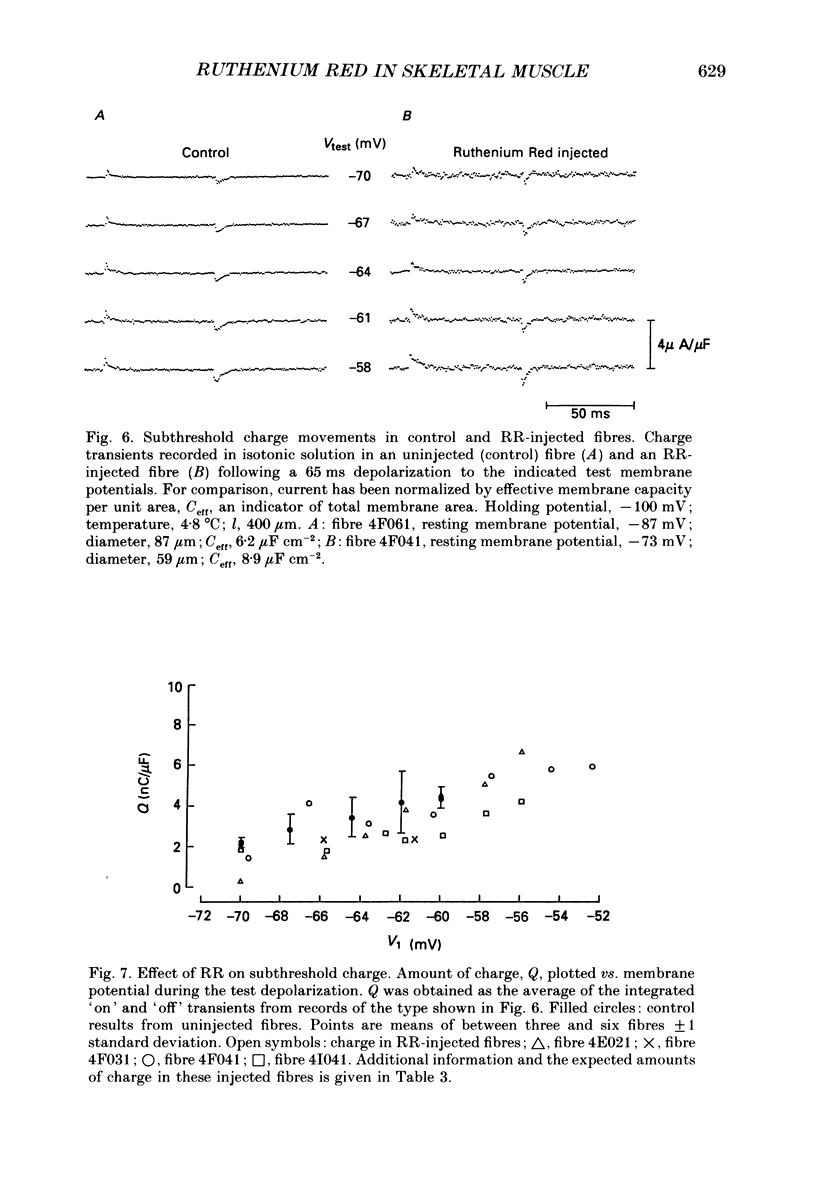
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Charge movement in the membrane of striated muscle. J Physiol. 1976 Jan;254(2):339–360. doi: 10.1113/jphysiol.1976.sp011235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Rakowski R. F. Charge movement and mechanical repriming in skeletal muscle. J Physiol. 1976 Jan;254(2):361–388. doi: 10.1113/jphysiol.1976.sp011236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Marshall M. W. Sodium currents in mammalian muscle. J Physiol. 1977 Jun;268(1):223–250. doi: 10.1113/jphysiol.1977.sp011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniu B., Kim D. H., Morii M., Ikemoto N. Inhibitors of Ca2+ release from the isolated sarcoplasmic reticulum. I. Ca2+ channel blockers. Biochim Biophys Acta. 1985 Jun 11;816(1):9–17. doi: 10.1016/0005-2736(85)90387-6. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Use of metallochromic dyes to measure changes in myoplasmic calcium during activity in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:139–177. doi: 10.1113/jphysiol.1982.sp014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S., Hui C. S., Quinta-Ferreira M. E. Properties of the metallochromic dyes Arsenazo III, Antipyrylazo III and Azo1 in frog skeletal muscle fibres at rest. J Physiol. 1986 Aug;377:89–141. doi: 10.1113/jphysiol.1986.sp016178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. A large birefringence signal preceding contraction in single twitch fibres of the frog. J Physiol. 1977 Jan;264(1):141–162. doi: 10.1113/jphysiol.1977.sp011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunschwig J. P., Brandt N., Caswell A. H., Lukeman D. S. Ultrastructural observations of isolated intact and fragmented junctions of skeletal muscle by use of tannic acid mordanting. J Cell Biol. 1982 Jun;93(3):533–542. doi: 10.1083/jcb.93.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Membrane particles and transmission at the triad. Fed Proc. 1975 Apr;34(5):1382–1389. [PubMed] [Google Scholar]
- Gilly W. F., Hui C. S. Voltage-dependent charge movement in frog slow muscle fibres. J Physiol. 1980 Apr;301:175–190. doi: 10.1113/jphysiol.1980.sp013197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hollingworth S., Marshall M. W. A comparative study of charge movement in rat and frog skeletal muscle fibres. J Physiol. 1981 Dec;321:583–602. doi: 10.1113/jphysiol.1981.sp014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Jenden D. J., Fairhurst A. S. The pharmacology of ryanodine. Pharmacol Rev. 1969 Mar;21(1):1–25. [PubMed] [Google Scholar]
- Kovács L., Ríos E., Schneider M. F. Calcium transients and intramembrane charge movement in skeletal muscle fibres. Nature. 1979 May 31;279(5712):391–396. doi: 10.1038/279391a0. [DOI] [PubMed] [Google Scholar]
- Kovács L., Schümperli R. A., Szücs G. Comparison of birefringence signals and calcium transients in voltage-clamped cut skeletal muscle fibres of the frog. J Physiol. 1983 Aug;341:579–593. doi: 10.1113/jphysiol.1983.sp014825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Schlatterer R. G., Nicar M., Campbell K. P., Sutko J. L. The effects of ryanodine on passive calcium fluxes across sarcoplasmic reticulum membranes. J Biol Chem. 1987 Feb 25;262(6):2711–2718. [PubMed] [Google Scholar]
- Lännergren J., Noth J. Tension in isolated frog muscle fibers induced by hypertonic solutions. J Gen Physiol. 1973 Feb;61(2):158–175. doi: 10.1085/jgp.61.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Comparison of arsenazo III optical signals in intact and cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):41–81. doi: 10.1085/jgp.89.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H., Racker E. Mechanism of calcium release from skeletal sarcoplasmic reticulum. J Membr Biol. 1982;66(3):193–201. doi: 10.1007/BF01868494. [DOI] [PubMed] [Google Scholar]
- Oetliker H. An appraisal of the evidence for a sarcoplasmic reticulum membrane potential and its relation to calcium release in skeletal muscle. J Muscle Res Cell Motil. 1982 Sep;3(3):247–272. doi: 10.1007/BF00713037. [DOI] [PubMed] [Google Scholar]
- Ohnishi S. T. Calcium-induced calcium release from fragmented sarcoplasmic reticulum. J Biochem. 1979 Oct;86(4):1147–1150. doi: 10.1093/oxfordjournals.jbchem.a132609. [DOI] [PubMed] [Google Scholar]
- Pessah I. N., Francini A. O., Scales D. J., Waterhouse A. L., Casida J. E. Calcium-ryanodine receptor complex. Solubilization and partial characterization from skeletal muscle junctional sarcoplasmic reticulum vesicles. J Biol Chem. 1986 Jul 5;261(19):8643–8648. [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985 Aug 1;316(6027):446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- Suarez-Kurtz G., Parker I. Birefringence signals and calcium transients in skeletal muscle. Nature. 1977 Dec 22;270(5639):746–748. doi: 10.1038/270746a0. [DOI] [PubMed] [Google Scholar]
- Volpe P., Salviati G., Chu A. Calcium-gated calcium channels in sarcoplasmic reticulum of rabbit skinned skeletal muscle fibers. J Gen Physiol. 1986 Feb;87(2):289–303. doi: 10.1085/jgp.87.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. The increase in the rate of heat production of frog's skeletal muscle caused by hypertonic solutions. J Physiol. 1970 May;208(1):49–64. doi: 10.1113/jphysiol.1970.sp009105. [DOI] [PMC free article] [PubMed] [Google Scholar]


