Abstract
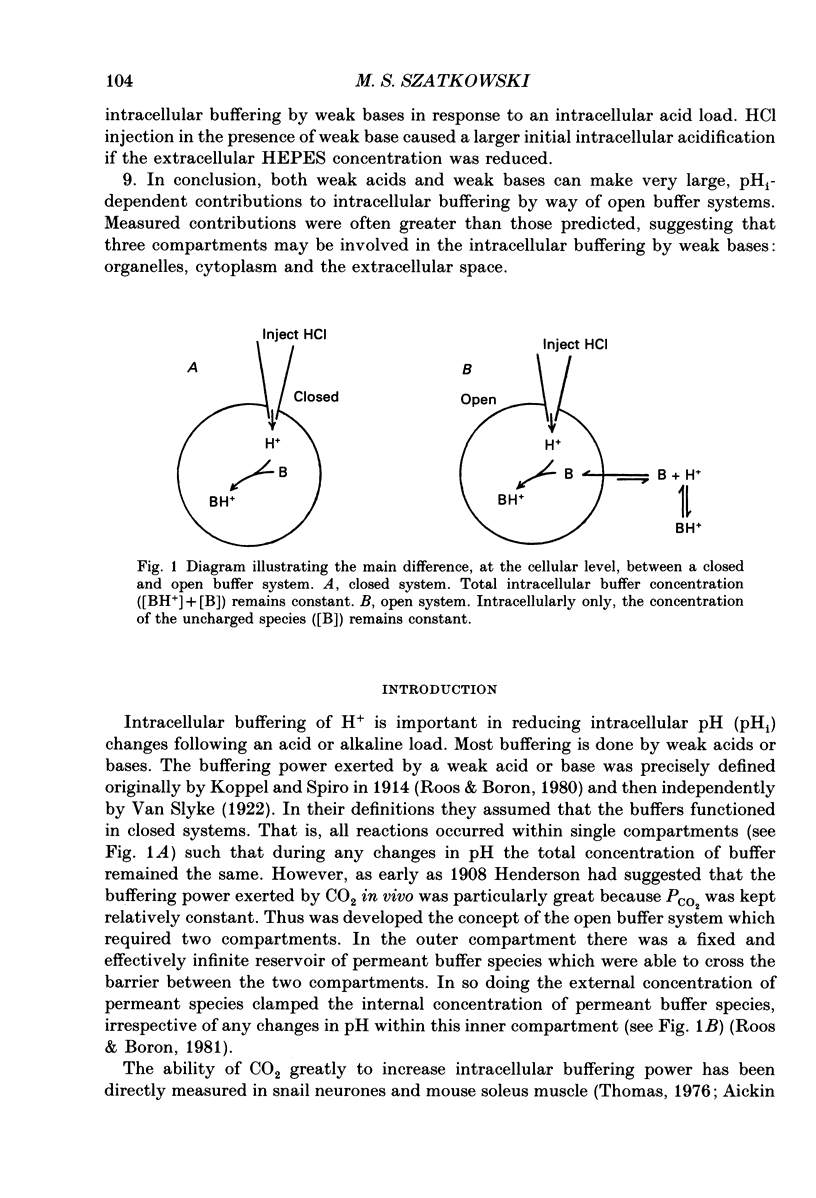
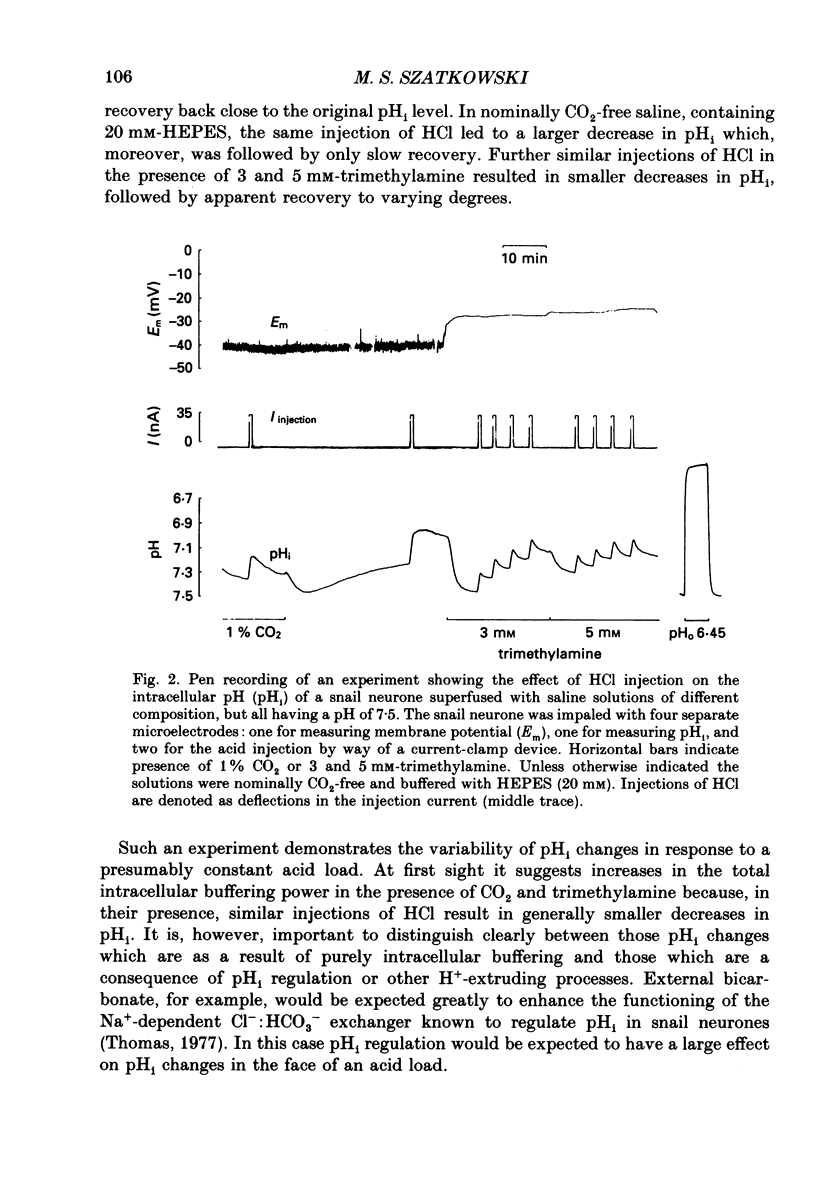
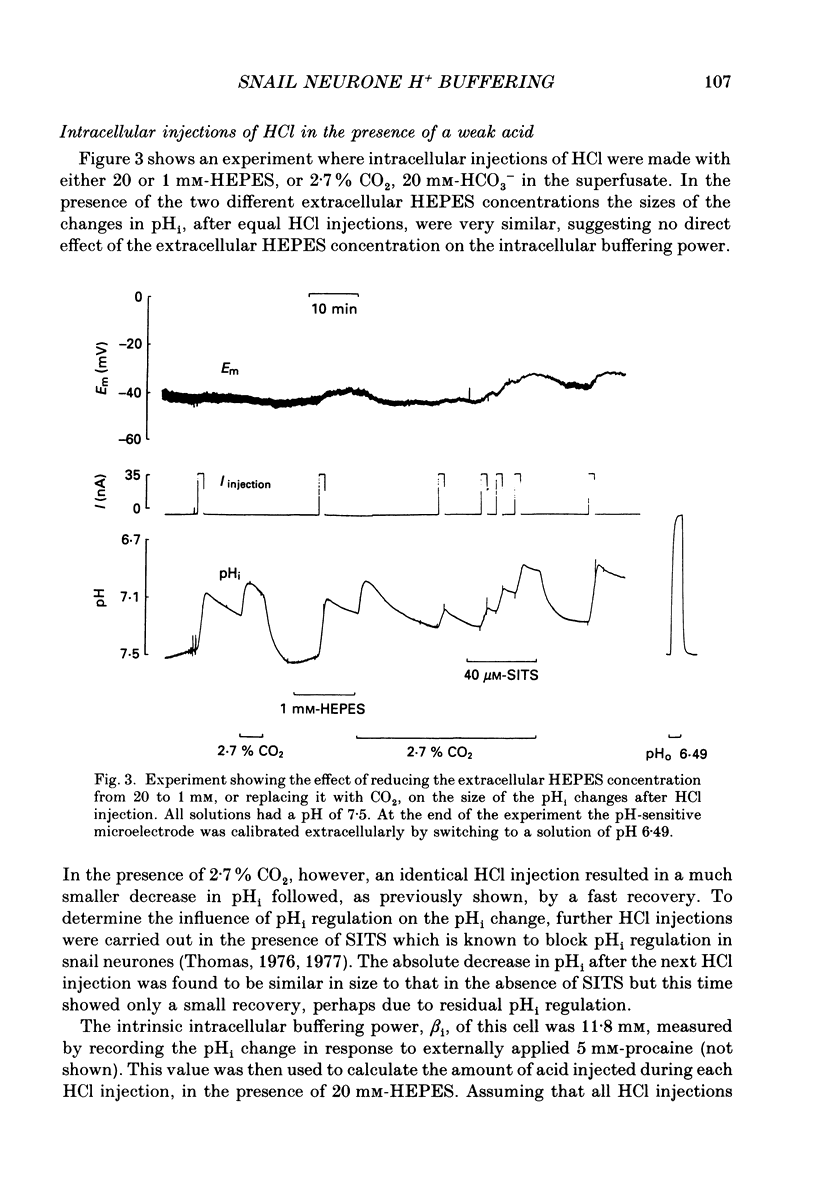
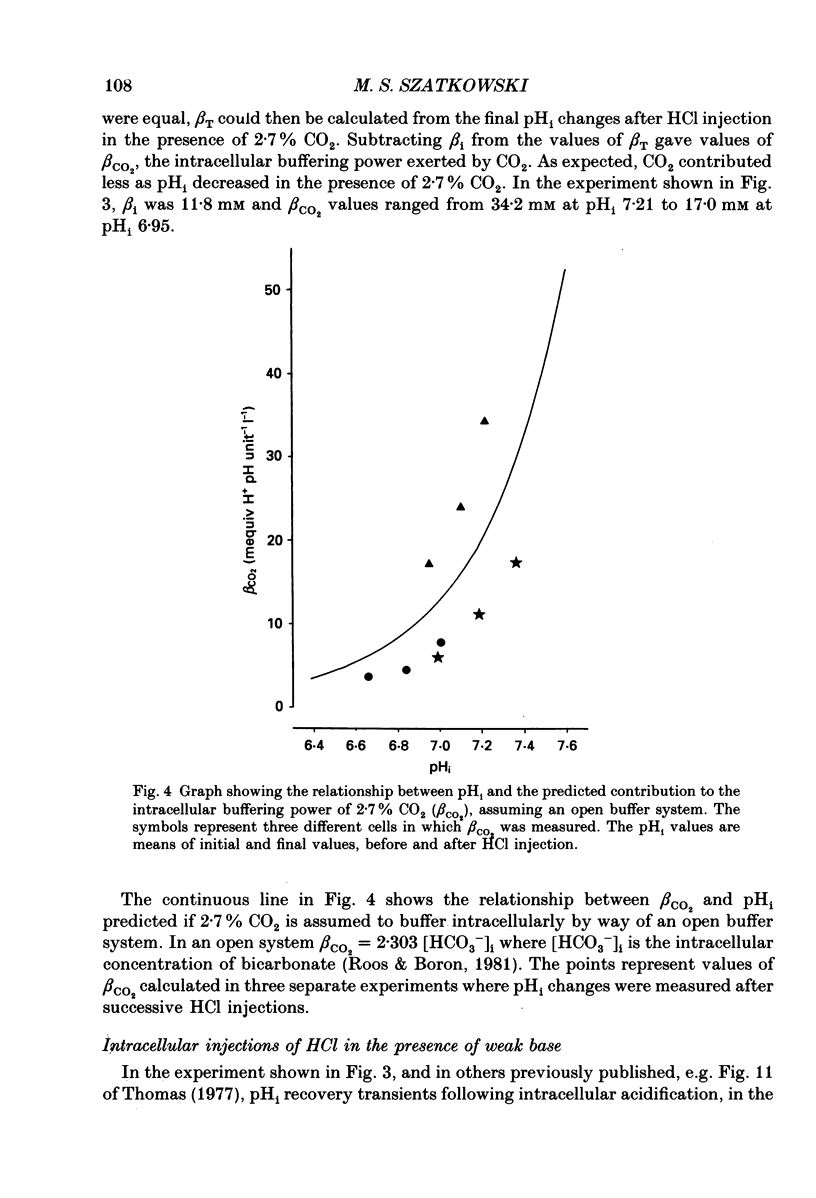
1. Intracellular pH (pHi) was measured in snail neurones using pH-sensitive glass microelectrodes. The influence of externally applied weak acids and bases on the total intracellular buffering power (beta T) was investigated by monitoring the pHi changes caused by the intracellular ionophoretic injection of HCl. 2. In the absence of weak acids or bases a reduction in the extracellular HEPES concentration had no effect on pHi or on beta T. It did, however, reduce slightly the rate of pHi recovery following HCl injection. 3. The presence of CO2 greatly increased beta T. However, as predicted for an open buffer system, the contributions to intracellular buffering by CO2 (beta CO2) decreased as pHi decreased. 4. When added to the superfusate, procaine, 4-aminopyridine, trimethylamine and NH4Cl (1-10 mM) all increased steady-state pHi. Procaine was fastest at increasing pHi and 4-aminopyridine the slowest. All four of these weak bases increased beta T. 5. The intracellular buffering action by these weak bases varied. HCl injection in the presence of procaine usually resulted in steady-state pHi changes with no pHi transients. In the presence of the other three weak bases HCl injections resulted in intracellular acidifications which were followed by pHi recovery-like transients. However, these were not blocked by SITS (4-acetamido-4'-isothiocyanatostilbene-2,2'-disulphonic acid) or by CaCl2 and I thus conclude that these transients were as a result of slow or incomplete intracellular buffering by the weak bases. 6. In many cells there was a good correlation between the measured contributions to intracellular buffering by the weak bases (beta base) and those predicted assuming a simple two-compartment open system. In all cases, as predicted, beta base increased as pHi decreased. 7. I found a clear relationship between the concentration of external buffer (HEPES) and the rate at which weak bases, applied to the superfusate, were able to increase pHi. The greater the extracellular buffer concentration the greater was the speed of intracellular alkalinization. 8. Lowering the extracellular buffer concentration reduced the efficiency of intracellular buffering by weak bases in response to an intracellular acid load. HCl injection in the presence of weak base caused a larger initial intracellular acidification if the extracellular HEPES concentration was reduced. 9. In conclusion, both weak acids and weak bases can make very large, pHi-dependent contributions to intracellular buffering by way of open buffer systems.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

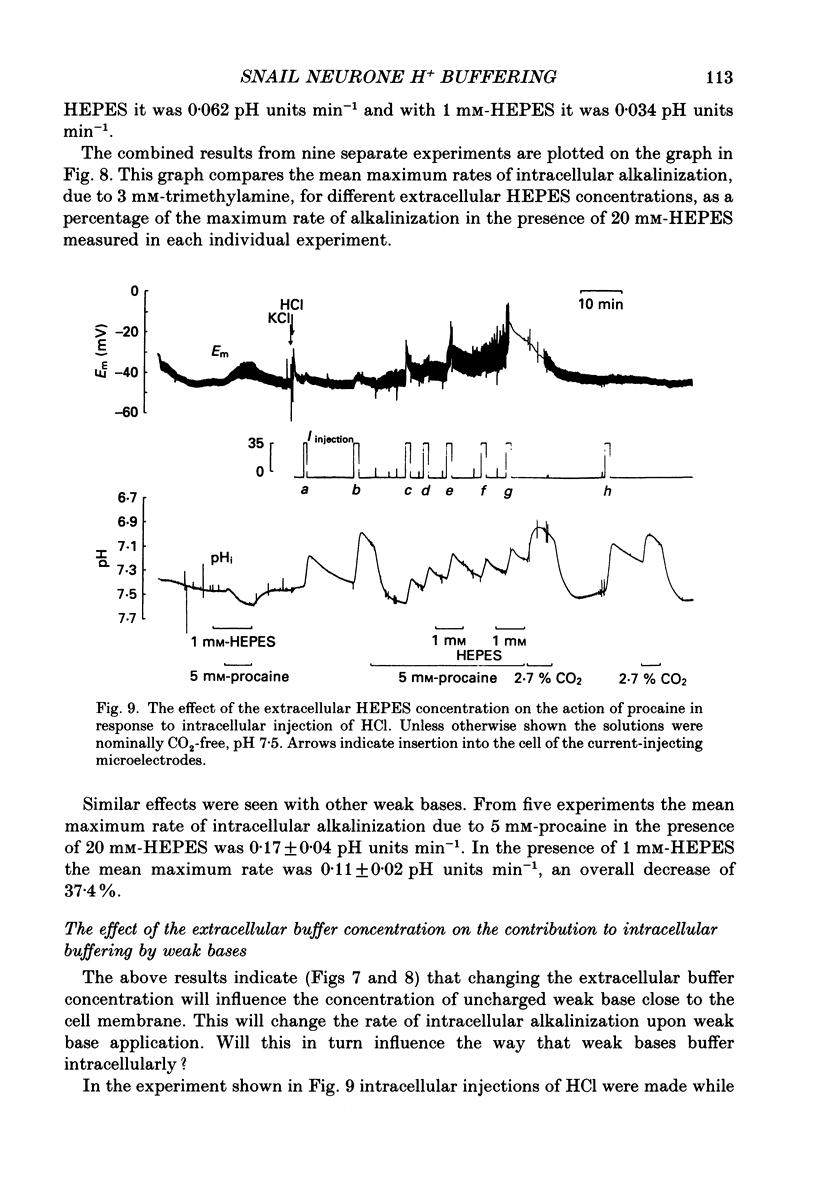
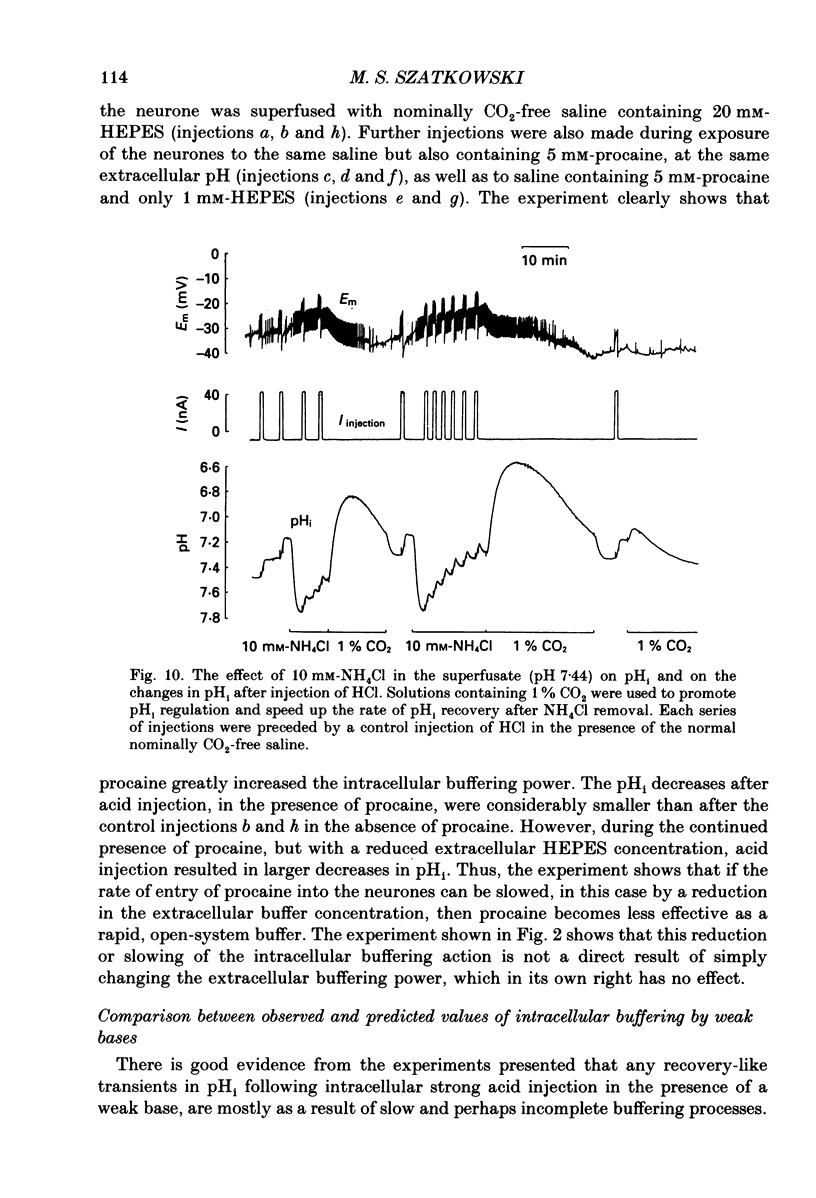
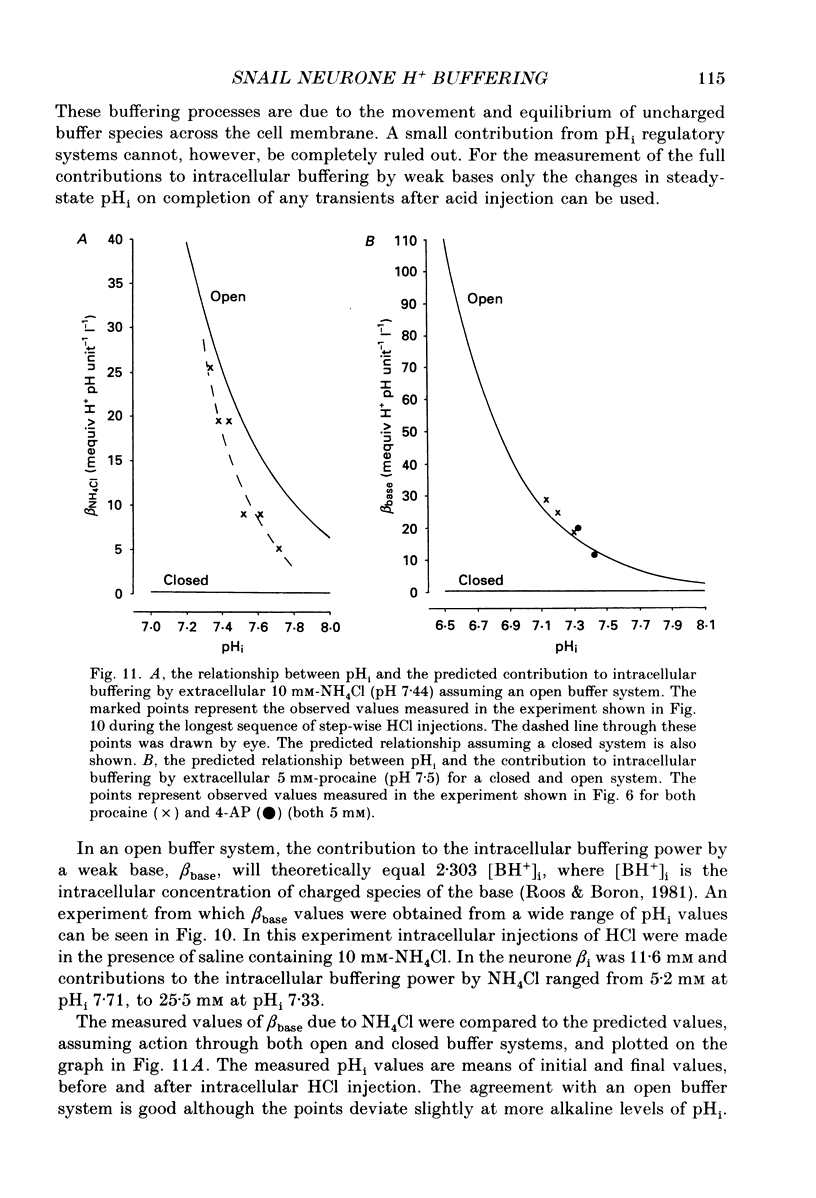
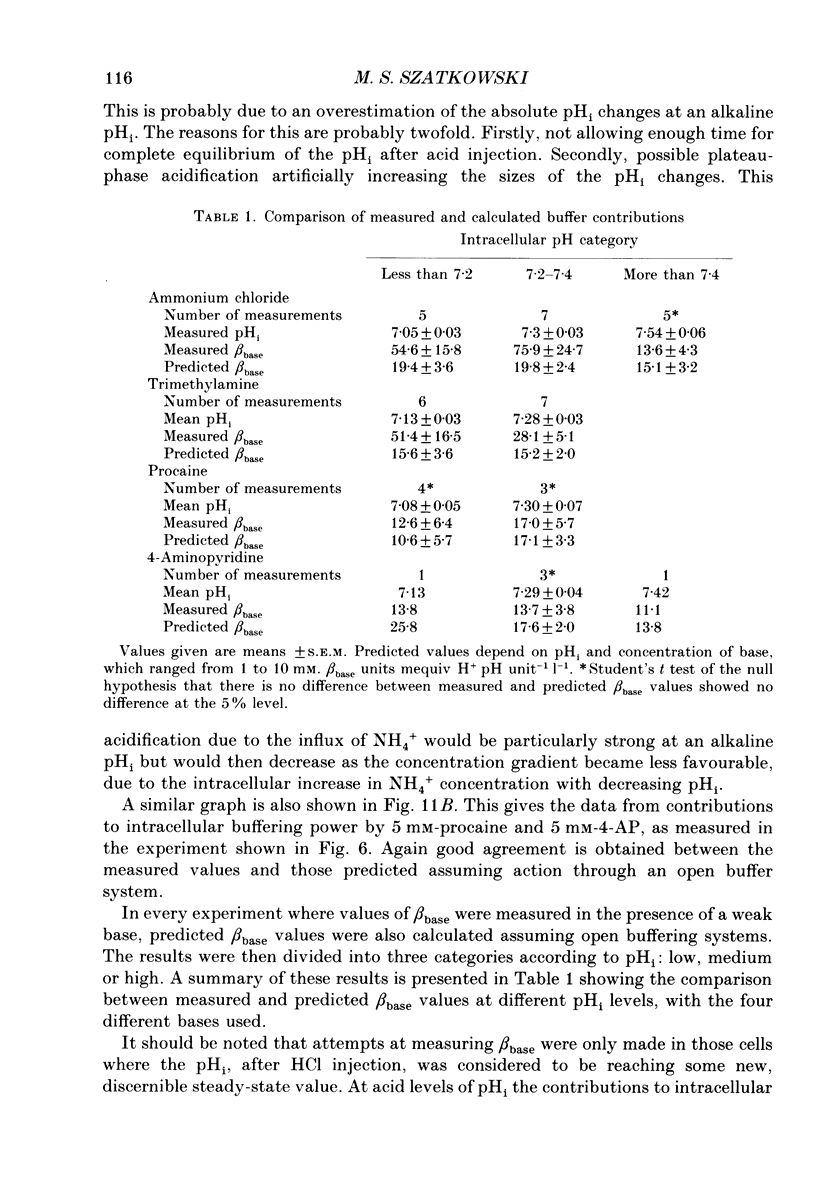




Selected References
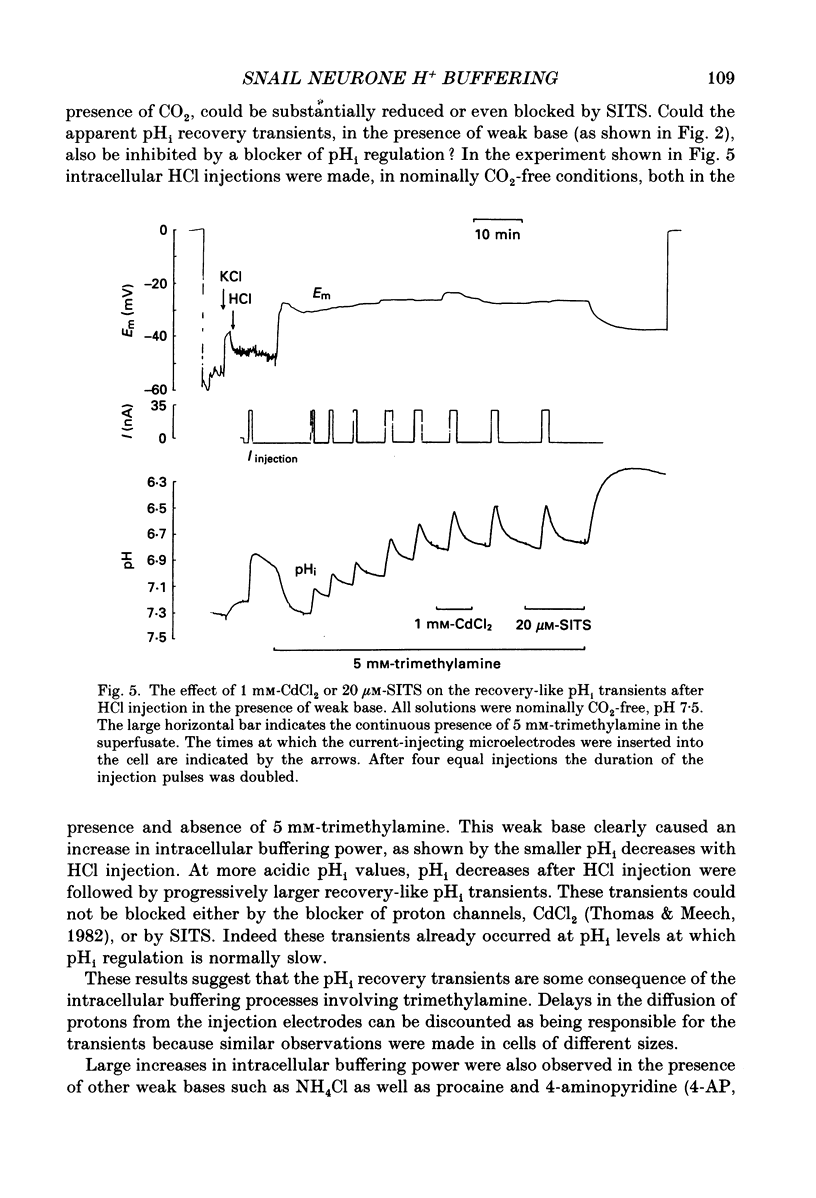
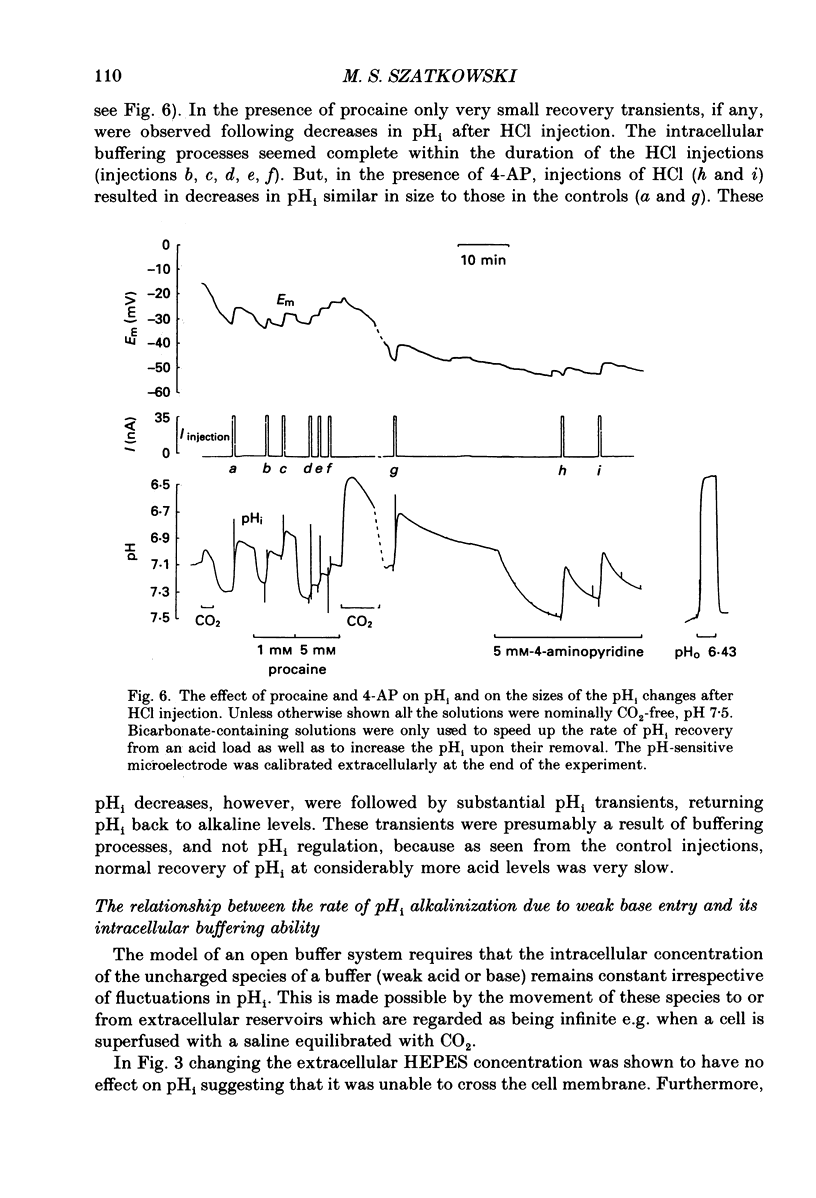
These references are in PubMed. This may not be the complete list of references from this article.
- Boron W. F. Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol. 1977 Sep;233(3):C61–C73. doi: 10.1152/ajpcell.1977.233.3.C61. [DOI] [PubMed] [Google Scholar]
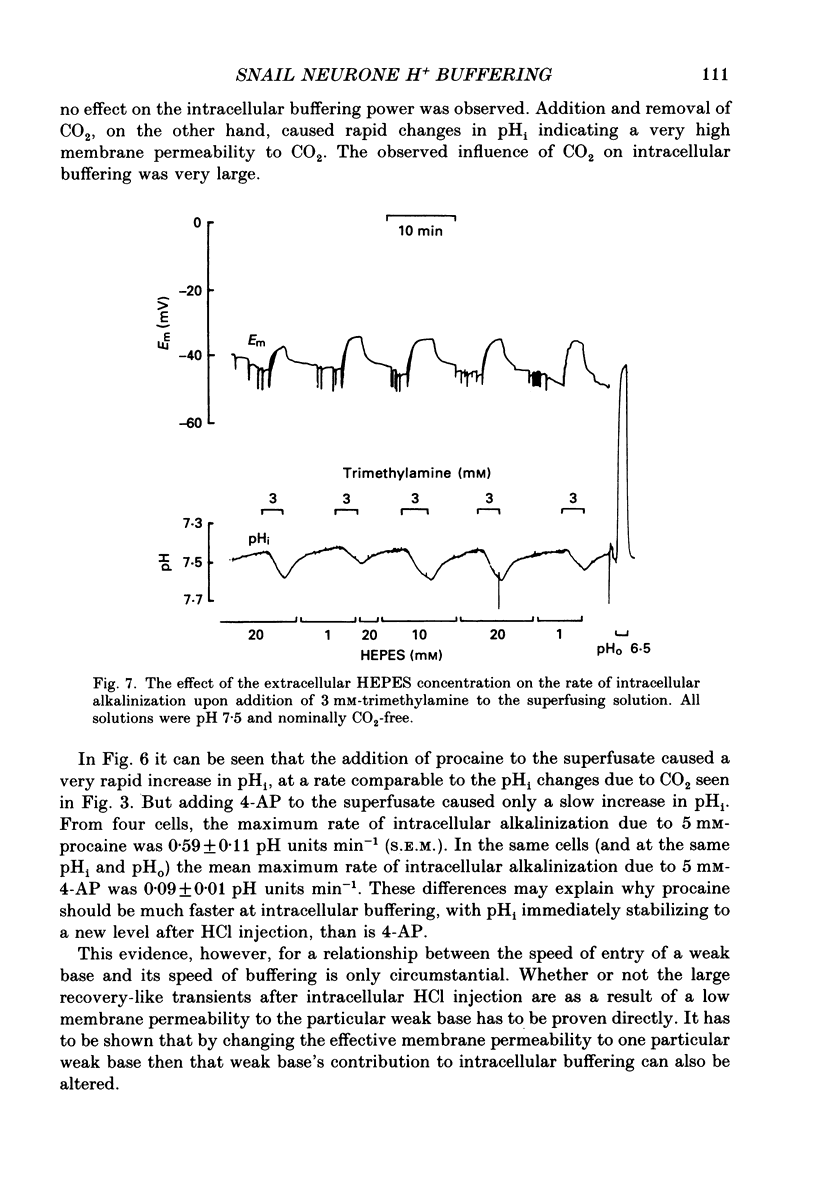
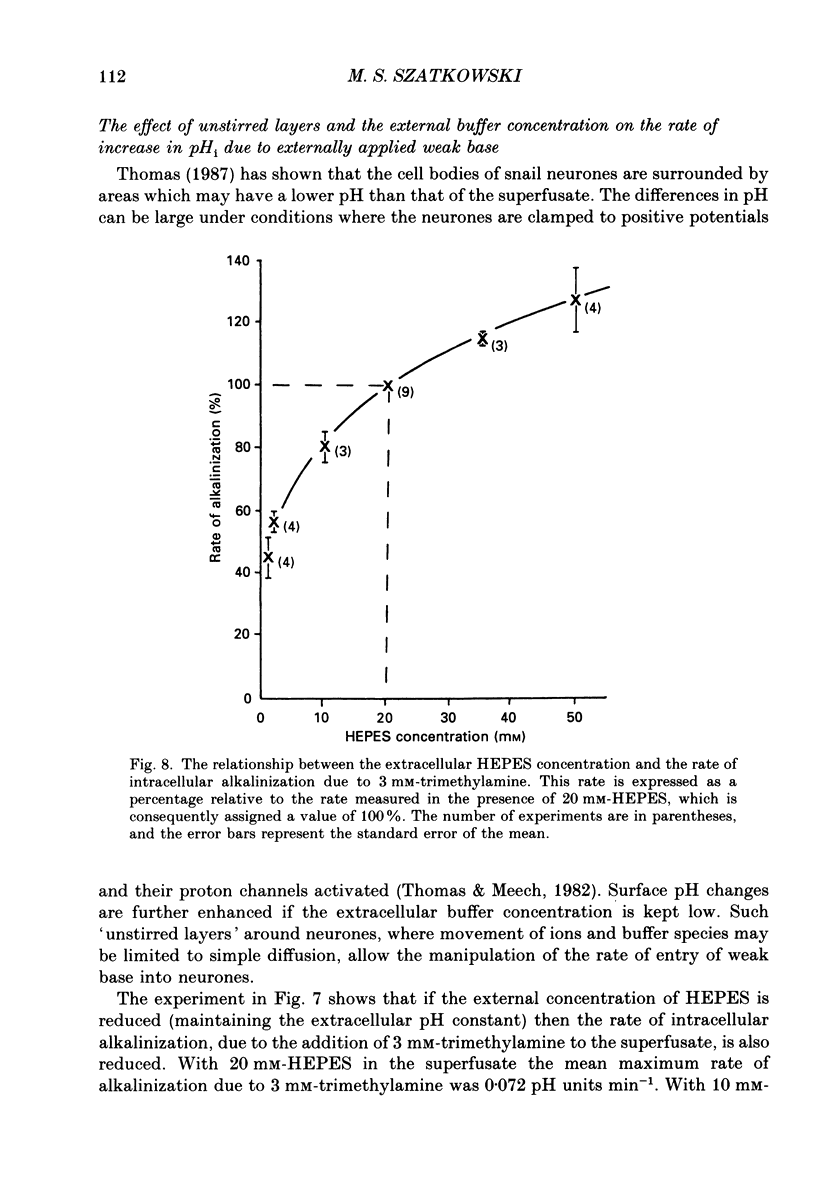
- Hudgins P. M., Putney J. W., Jr Distribution of local anesthetics and the intracellular pH in vascular smooth muscle. J Pharmacol Exp Ther. 1972 Jun;181(3):538–546. [PubMed] [Google Scholar]
- Johnson R. G., Carlson N. J., Scarpa A. deltapH and catecholamine distribution in isolated chromaffin granules. J Biol Chem. 1978 Mar 10;253(5):1512–1521. [PubMed] [Google Scholar]
- Mason M. J., Mainwood G. W., Thoden J. S. The influence of extracellular buffer concentration and propionate on lactate efflux from frog muscle. Pflugers Arch. 1986 May;406(5):472–479. doi: 10.1007/BF00583369. [DOI] [PubMed] [Google Scholar]
- Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977 Jun;267(3):791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol. 1981 Sep;90(3):656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B., Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981 Sep;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. The buffer value of weak acids and bases: origin of the concept, and first mathematical derivation and application to physico-chemical systems. The work of M. Koppel and K. Spiro (1914). Respir Physiol. 1980 Apr;40(1):1–32. doi: 10.1016/0034-5687(80)90002-x. [DOI] [PubMed] [Google Scholar]
- Szatkowski M. S., Thomas R. C. The intrinsic intracellular H+ buffering power of snail neurones. J Physiol. 1989 Feb;409:89–101. doi: 10.1113/jphysiol.1989.sp017486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Changes in the surface pH of voltage-clamped snail neurones apparently caused by H+ fluxes through a channel. J Physiol. 1988 Apr;398:313–327. doi: 10.1113/jphysiol.1988.sp017044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984 Sep;354:3P–22P. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Extracellular acidification at the surface of depolarized voltage-clamped snail neurones detected with eccentric combination pH microelectrodes. Can J Physiol Pharmacol. 1987 May;65(5):1001–1005. doi: 10.1139/y87-158. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Meech R. W. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982 Oct 28;299(5886):826–828. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Influence of surface pH on intracellular pH regulation in cardiac and skeletal muscle. Am J Physiol. 1986 May;250(5 Pt 1):C748–C760. doi: 10.1152/ajpcell.1986.250.5.C748. [DOI] [PubMed] [Google Scholar]


