Abstract
4D printing polymeric biomaterials can change their morphology or performance in response to stimuli from the external environment, compensating for the shortcomings of traditional 3D-printed static structures. This paper provides a systematic overview of 4D printing polymeric biomaterials for tissue regeneration and provides an in-depth discussion of the principles of these materials, including various smart properties, unique deformation mechanisms under stimulation conditions, and so on. A series of typical polymeric biomaterials and their composites are introduced from structural design and preparation methods, and their applications in tissue regeneration are discussed. Finally, the development prospect of 4D printing polymeric biomaterials is envisioned, aiming to provide innovative ideas and new perspectives for their more efficient and convenient application in tissue regeneration.
Keywords: 4D printing, Polymeric biomaterials, Tissue regeneration
Graphical abstract
Highlights
-
•
The core elements of4D printing polymeric biomaterials are summarized and elaborated.
-
•
The research advances of 4D printing polymeric biomaterials in structure, design, and application are analyzed.
-
•
The latest achievements and breakthroughs of 4D printing polymeric biomaterials in tissue regeneration are introduced.
1. Introduction
4D printing is an advanced manufacturing technology developed based on traditional 3D printing, which adds a time dimension to the 3D structure created by 3D printing, enabling the printed object to automatically undergo shape or morphological changes when subjected to external stimuli as shown in Fig. 1. 4D printing polymeric biomaterials, as a typical class of 4D printing materials, are widely used in tissue regeneration [[1], [2], [3]]. These materials are often characterized by shape memory, responsiveness, or self-assembly, showing great potential for applications in smart structures, soft robots, and biomedical devices [[4], [5], [6], [7], [8]]. For example, shape-memory polymers are capable of transforming from a temporary shape to a permanent one at a specific temperature [6,9], while environmentally responsive polymers can adjust their structure in response to changes in the environment, such as changes in humidity or pH [7,10]. The smart behavior of these materials provides unprecedented creative scope for developing new products that can adapt to environmental changes, repair themselves, or perform complex tasks.
Fig. 1.
Basic elements of 4D printing polymeric biomaterials. Some image elements are referenced.[5] Licensed under a Creative Commons CC BY license. [11] Copyright 2019, Springer Nature. [12] Copyright 2011, Royal Society of Chemistry
In recent years, research has been proliferation into 4D printing polymeric biomaterials for tissue regeneration [[13], [14], [15]]. On one hand, these materials have unique properties, such as processability, biocompatibility, degradability, and bioactivity, which are necessary for medical implants [16,17]. On the other hand, the ability of 4D printing polymeric biomaterials to dynamically adapt their shape and function in response to changes in time or external stimuli (e.g., temperature, humidity, light, etc.) is just enough to mimic the unique properties of natural tissues effectively. Biomaterials for natural tissue regeneration involve complex three-dimensional structures, microstructures, and extracellular matrix (ECM) compositions, as well as dynamic changes to adapt tissues with unique functions [18]. Most of these dynamic functional conformational changes are caused by intrinsic mechanisms in response to internal or external stimuli, which are unable to be mimicked by traditional 3D printing static structures [18]. Therefore, 4D printing polymeric biomaterials demonstrate great potential for repairing, supporting, replicating, or enhancing the function of human tissues and organs [[19], [20], [21], [22]].
This review aims to comprehensively summarize the principles and research progress of 4D printing polymeric biomaterials for adaptive tissue regeneration. Moreover, it delves into the challenges faced in current research, offering insights and inspiration for the innovative application of biomedical implants in the era of personalized medicine.
2. Basic elements of 4D printing polymeric biomaterials
2.1. General elements
2.1.1. Printability
The basic structures of 4D printing materials are constructed through 3D printing technology [[23], [24], [25]], so the printability of these polymers is crucial for the construction of their 3D structures. It is important to choose a printing method that matches the properties of the materials. For inkjet printing methods, the viscosity of the ink is very important. The molecular weight and concentration of polymers are usually important factors affecting the viscosity of the ink. In general, high molecular weights and concentrations lead to an increase in the entanglement of the polymer chains in the system, increasing the viscosity of the ink. When the viscosity is too high, the nozzle will be blocked and when the viscosity is too low, the ink droplets passing through the nozzle will flow on the printing platform, hindering the accumulation of 3D structures. Therefore, the viscosity should be controlled within an appropriate range [26]. For the inks used in SLA and DLP methods, in addition to the requirements just mentioned above, the ink also needs the ability of photocuring, and the type and concentration of the photocuring material also influence the printing process. For this printing method of FDM achieved by extruding filament, the ink is required to be thermoplastic. It can be processed into fine filament and has a certain mechanical strength after cooling. At the same time, the rheological properties of the ink, including shear stress, shear thinning, and thixotropic, are crucial to the printing results [14,[27], [28], [29], [30], [31], [32]]. Nanoparticles such as nanosilicates [26,33], nanocellulose [34], and nanoclay [35] have been used to impart shear thinning behavior to the inks and significantly suppress the shrinkage and expansion of printed structures during crosslinking, thereby improving printing fidelity [36].
As mentioned above, the printability of materials is related to the viscosity, rheological properties, and physical and chemical properties of the materials themselves [37]. From this perspective, the evaluation of material printability is an extremely complex process, so far, there is no comprehensive evaluation theory and parameters. It is difficult for us to evaluate whether a material has printability based on the results of a certain experiment. Some scholars have made attempts in this regard, attempting to obtain a universal empirical conclusion to quickly evaluate the printability of materials. Lee et al. studied rheological parameters including G′, G″, and tan δ. Through the research on the printing process of alginate/gelatin-based gel, it was found that G′ and G″ were more related to the printing pressure, while tan δ was more related to the shape fidelity and extrusion uniformity [38]. Through model prediction and statistical analysis, it was found that materials could be printed when they exhibit high yield viscosity and low plasticity before flowing. When high-elastic materials have greater elasticity in lower frequency ranges and high viscosity in higher frequency ranges, they are often easier to print [39].
2.1.2. Biocompatibility
Biocompatibility includes not only biological inertness, but also biostability and biological function, which depends on the properties of materials (such as crystallinity, charge, wettability, and hardness), and the interactions with the biological environment of the target tissue (protein adsorption, inflammatory processes, contacting with blood). When biomaterials are implanted into the body, they will have an impact and effect on specific biological tissue environments, and the biological tissue will also influence the biomaterials. This interaction persists until an equilibrium is reached or the implant disappears. These materials not only need to coexist safely with biological tissues without causing any damage reactions but also possess functional properties that positively affect the targeted tissues, such as promoting cell differentiation and combating inflammation, etc. [14,40]. It is also essential to identify the potential changes that biomaterials may undergo within the body, such as interactions with the internal environment or their degradation, and to ensure that the resulting products do not adversely affect biological tissues.
The strong dependence of biocompatibility on polymer materials is mainly reflected in the following aspects. First, the chemical composition and structural characteristics of the material are the foundation, which directly determines the interaction mode between the material and the organism. Second, the surface properties of materials, such as hydrophilicity, hydrophobicity, charge distribution, roughness, etc., also significantly affect cell adhesion, cell proliferation, cell differentiation, and other behaviors. Furthermore, the immune response, metabolic processes, and long-term physiological adaptability of organisms to materials are essential factors in evaluating biocompatibility. Usually, a single untreated material is difficult to meet the requirements of multiple indicators. Therefore, researchers often composite different types of materials. For example, natural polymers are biocompatible but have poor mechanical properties, so they are often compounded with synthetic polymers to obtain better mechanical properties. Alternatively, biologically active components can be combined with polymers to develop new materials with good biocompatibility [41].
2.1.3. Biodegradability
The biodegradability of biomaterials in vivo refers to the ability of materials to gradually break down and decompose under specific biological activities or environmental conditions when implanted or introduced into the body, ultimately to be absorbed or metabolized by the organism, or eliminated from the body [42]. It is necessary to understand the degradation mechanism of materials. Polymer materials may interact with microorganisms, enzymes, water, and free radicals, causing molecular chain breakage or cross-linking, resulting in changes in the structure and properties [43]. Polymer materials vary in molecular weight, polydispersity, crystallinity, thermal transitions, and degradation rates, significantly affecting the performance of polymer scaffolds. Given the abundance of cells and the variety of enzymes in body fluids compared to in vitro conditions, this distinct solution environment can result in varied degradation behaviors for the same polymer [44]. The biodegradation of polymers is a complex process, so the dissolution method is commonly used to study the degradation process of materials.
2.2. Unique elements
2.2.1. Stimulation
4D printing materials spontaneously change their shape structure over time in response to external stimuli as shown in Fig. 2 [2,[45], [46], [47], [48], [49], [50], [51]]. So stimulus conditions play a crucial role in the dynamic changes of 4D printing polymeric biomaterials. The emergence of this approach provides a potential method to create smart materials of self-regulation.
Fig. 2.
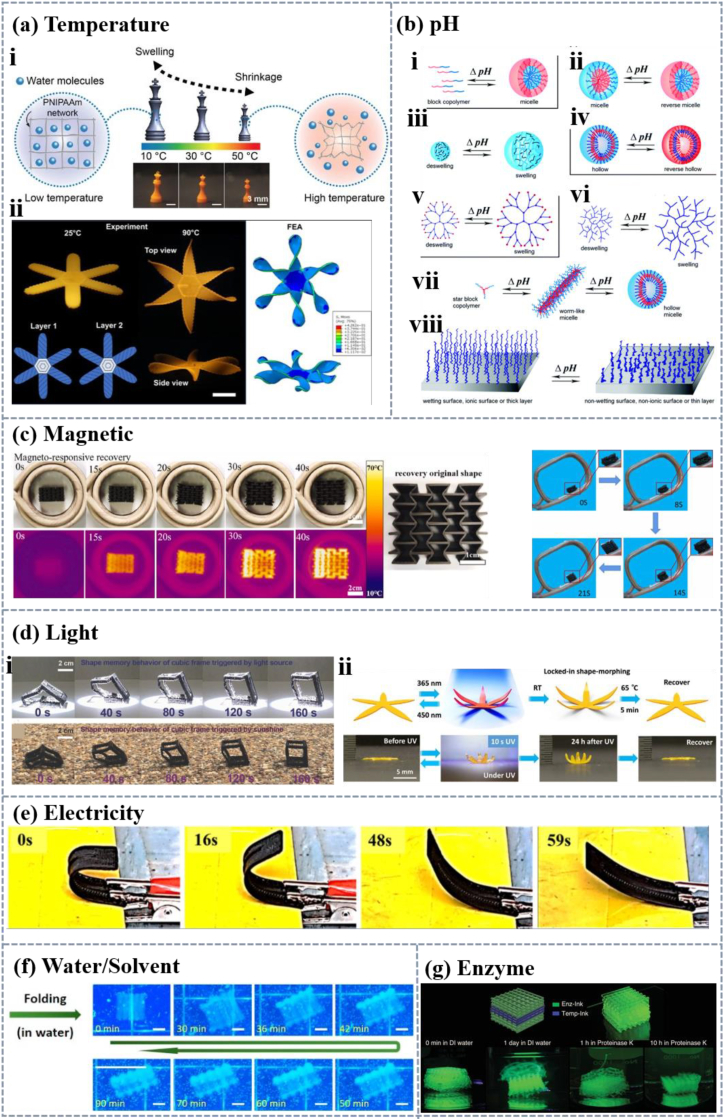
Stimulus-response of 4D printing polymeric biomaterials. (a) Temperature response: i) temperature-responsive swelling of 3D printed PNIPAAm hydrogel structures [52]. Licensed under a Creative Commons CC BY license. ii) petals twisted when bilayer printed structures prepared with different print paths are immersed in hot water at 90 °C [53]. Licensed under a Creative Commons CC BY license. (b) pH-responsive polymers of different architectures: i) unimer–micelle, ii) micelle–reverse micelle, iii) nanogels or microgels, iv) hollow– reverse hollow, v) dendrimer, vi) hyper-branched, vii) micelle morphology changes (from worm-like to hollow), and viii) brushes [54]. Copyright 2017, Royal Society of Chemistry. (c) Magnetic responsive shape memory processes of two kinds of polymeric materials [55,56]. Copyright 2023, Elsevier, and Copyright 2022, IOP Publishing, Ltd. (d) Light response: i) shape recovery process of the cubic frame under a light source of 87 mW cm−2 and insolation of 76 mW cm−2 [57]. Copyright 2017, John Wiley and Sons. ii) deformation process of the flower-like shape printed by polymer material under light conditions [58]. Copyright 2021, John Wiley and Sons. (e) Shape recovery process of U-structured electroactive materials at 35 V [59]. Copyright 2022, Elsevier. (f) Reversible shape change of tracheal hydrogels between pure water and brine [60]. Copyright 2020, Elsevier. (g) Three-dimensional structures were constructed using enzyme-responsive material as the top and bottom thirds and temperature-responsive material as the middle third. The structure was transferred to deionized water and the structure swelled. It was then transferred to proteinase K solution and the material deformed in response to the enzyme [61]. Copyright 2021, John Wiley and Sons.
2.2.1.1. Temperature
Thermal-stimulus response is the most common and extensively studied stimulus in 4D printing as shown in Fig. 2a, and the mechanisms of thermal stimulus response vary greatly among different polymers [62] including glass transition, melting transition, crystallization transition, low critical solution temperature (LCST) and high critical solution temperature (UCST). The ability of materials to accept stimuli and undergo changes in shape or properties is related to their molecular structure and physical and chemical properties. The core of the temperature stimulus-response of shape memory polymers (SMPs) is the phase transition behavior, including glass transition, melting transition, and crystallization transition, which are accompanied by molecular chain movement as the driving force of shape memory [7]. The principle of glass transition is based on the generation and release of stresses in the molecular cross-linking network induced by the transition between the glassy and rubbery states above and below the glass phase transition temperature [63]. Polyurethane (PU) is usually constructed as SMPs through the glass transition temperature. For example, Staszczak et al. prepared PU materials with a glass transition temperature of 45 °C. The material has good shape memory ability, with a shape fixation ratio of about 98 % in three heat engine cycles and little change in subsequent cycles [64]. The principle of the melt transition, which is mainly based on a change in the crystalline structure of the polymer, is another important phase transition process in SMPs. At low temperatures, the polymer molecular chains are well-aligned and form a crystalline structure. When the temperature rises above the transition temperature (melt temperature) the thermal movement of the polymer molecular chains increases, the crystalline structure is disrupted and the material is in the amorphous state. For both transformation modes, the molecular weight and structure of the soft and hard sections of the material will affect the transformation temperature [6], for example, the more rigid groups, the higher the degree of crosslinking, the greater the molecular weight will lead to an increase in the transformation temperature. The crystalline transition is the process by which a polymer is transformed from an amorphous to a crystalline state and is the third important phase transition process in polymers. The principle of crystalline transition is mainly based on the regular arrangement of polymer molecular chains. For this type of transformation, the rate of crystallization transition and crystallinity are important parameters, they determine the speed and degree of shape recovery of the material. Ma et al. fused the two effects to prepare ethylene-vinyl acetate copolymer/poly(propylene carbonate) (EVA/PPC) blends with a triple shape memory effect, which is realized by its unique phase structure and the different transition temperatures of the two polymers. EVA and PPC are incompatible and thus form a distinct phase separation during melt blending, including an island structure (EVA as the continuous phase and PPC as the dispersed phase) and an interconnected structure (PPC as the continuous phase and EVA as the dispersed phase), which provides the necessary phase structure basis for the shape memory effect. During the deformation recovery process, PPC undergoes a glass transition at lower temperatures, and the material returns to its temporary shape, while EVA undergoes a melting/crystallization transition, and the material returns to its initial shape. Reversible phase transitions of EVA and PPC provide reversible deformation and recovery mechanisms for shape memory effects [65]. The temperature response mechanism of hydrogels is mainly based on LCST or UCST [66]. When the temperature is below the LCST, the polymer molecular chains are miscible with water due to the formation of hydrogen bonding with water molecules and the gel swells. Once the temperature is heated up to the LCST, the increase in the activity of H2O molecules results in the breakage of the hydrogen bonds, then the water molecules are released and the gel volume shrinks [67].
2.2.1.2. pH value
pH-responsive polymers are usually formed by cross-linked polyelectrolytes with weakly acidic or weakly alkaline groups, and may also be composed of amphoteric polyelectrolytes with both groups simultaneously. These weak acid or weak base groups accept or release protons in response to changes in the pH of the environment. pH response mechanism is commonly used in gel materials (Fig. 2b). For example, the polymer polyacrylic acid (PAA) with a carboxyl group is pH-sensitive [68]. When the pH of the dispersing medium is larger than the ionization constant (pK), the weak acid groups on the molecular chain ionize to form anion groups with negative charges, and the molecular chain stretches due to electrostatic repulsive forces, thus generating a large osmotic pressure inside the hydrogel causing it to swell [69]. And protonation at lower pH values causes the molecular chain to shrink [70]. In addition to this, weakly acid groups that are pH responsive also include sulfonic acid, phosphoric acid, and boronic acid [54].
As for basic polymers, polymers with amine groups are typical of weakly basic pH-responsive polymers such as poly[(2-diisopropylamino)ethyl methacrylate], and nylon [71]. When the pH of the dispersion medium is less than the pK of the weakly basic polyelectrolyte, the weakly basic group on the molecular chain receives protons to form a positively charged cationic group causing swells due to the increase of the internal osmotic pressure of the hydrogel [72]. Therefore, the ionization degree of the molecular chains of pH-responsive hydrogels will change greatly due to the small change in the environmental pH, resulting in changes in their internal osmotic pressure, leading to swelling or shrinking of hydrogels. The hydrophilicity of hydrogels in water can be adjusted by ion transformation, resulting in an increase or decrease in the solubility of polymer segments or swelling and de-swelling of hydrogels [54].
2.2.1.3. Magnetic field
Compared to other stimulus response methods, magnetic field induced heating can be applied to the positions that are difficult to be heated directly, such as the interior of the human body [73]. Therefore, magnetic response SMP has potential application prospects in the field of intelligent medical devices, and the magnetic response studies are listed in Fig. 2c. For polymers, which are not inherently magnetically responsive, it is usually prepared by compounding magnetic nanoparticles with SMP matrix through blending or covalent bonding. There are two commonly used magnetic induction methods [74,75]. The first way is to directly add magnetic particles to the material and the material can generate heat after being stimulated in the alternating magnetic field to achieve deformation behavior [76,55]. Wang et al. combined iron carbide nanoparticles with hydrogels, where the magnetic particles can generate heat in the presence of an applied magnetic field (the magnetothermal effect), leading to an increase in the temperature of the polymer matrix causing it to undergo a huge volume contraction at high temperatures [77]. In another work, Fe3O4 magnetic particles were precipitated into a polymer network of Poly(N-isopropyl acrylamide) (PNIPAAm) hydrogels by in-situ precipitation method, and the PNIPAAm composite gel and elastomer were designed into a double-layer structure, due to the selective heating of the double-layer gel under the action of an alternating magnetic field, the corresponding layer shrinkage caused the structure to bend achieving self-folding [78].
The second way is to use magnetic particles as an active filler in polymers [55], where the material induces a magnetic moment to change in physicochemical properties, and ultimately a change in shape or properties. Common magnetic particles include iron [79,80], magnetic Fe3O4 nanoparticles [55,[80], [81], [82]], and NdFeB [83]. Inorganic nanoparticles tend to aggregate due to their incompatibility with polymers [84]. To improve the compatibility and dispersion of Fe3O4 with the matrix, the magnetic nanoparticles are coated, modified [85], or functionalized [86] by oleic acid. Although there are advantages to directly inducing changes in magnetic response materials through magnetic fields, there are still some points to note, such as adjusting the frequency of the external magnetic field to a safe range to prevent any potential harm to living tissues.
2.2.1.4. Light
Light-driven polymers introduce photoresponsive functional groups into the polymer network [87]. When the material is irradiated with light of a specific wavelength, molecules undergo optical isomerization reactions, thereby changing the form of the chain segments and achieving light-induced deformation (Fig. 2d) [57,58]. There are many photoresponsive materials which can be mainly divided into two categories. One is based on the photochemical conversion of functional groups to achieve light stimulation response, mainly including azobenzene groups, benzopyran groups, triphenylmethane groups, cinnamic acid groups, etc [46,50,88]. These active groups change their configuration or form charged groups after exposure to light, leading to changes in the conformation or hydrophobicity of the polymer molecular chain, resulting in a shape change of the SMPs or swelling/unswelling of the hydrogel [46]. For example, the azobenzene group is a photoactive group with two isomers, cis and trans. When exposed to specific wavelengths of ultraviolet light, the trans structure of the azobenzene group will transform into a cis structure and when exposed to blue light, the cis structure will transform into a trans structure [58,[89], [90], [91]]. Another kind is based on the principle of photothermal effect, which requires materials to have the ability to convert light energy into heat, raise the temperature of the material above its transition temperature, and thus achieve light stimulus responsiveness [92]. Usually, materials with photothermal effects such as carbon black [57], graphene [93,94], and carbon nanotubes [95] are combined or covalently connected with thermally responsive polymers to achieve photoresponsiveness [96]. For example, graphene has a high photothermal conversion efficiency in the near-infrared region. Near-infrared light or photons strongly interact with graphene or oxidized graphene through resonance vibration, further converting kinetic energy vibration into thermal energy.
2.2.1.5. Electricity
Similar to magnetic-responsive polymers, electric field responsive polymers can also be constructed by loading conductive elements and changing their shape or function in the presence of the electric field. The response of electrical stimulation in 4D printing has been widely studied (Fig. 2e), and the response can be achieved by constructing intrinsic conductive materials and conductive composite materials. The commonly used conductive materials include carbon black [97], carbon nanofiber [98], carbon nanotube [99], polyaniline, polypyrrole, and polythiophen [47,49]. These conductive fillers are usually mixed with printing resins through melting or solution for printing. The response modes of electrical stimulation-responsive materials also usually include two kinds. One is the transfer of heat caused by electrical signals. Dong et al. mixed polylactic acid (PLA) and carbon nanotubes (CNTs) by melting. CNTs have good electrical conductivity, and form a continuous conductive network when added to the PLA matrix, which endows the composite with electrical conductivity. The composite was locally warmed by the Joule heat generated by the CNTs when a voltage was applied, and the PLA molecular chains were oriented and rearranged to deform [59]. Another kind is that when the external electric field is applied, the ions migrate in the hydrogel resulting in changes in osmotic pressure which causes the hydrogel to swell or contract [100]. The extent of the hydrogel expansion/contraction deformation depends on the internal properties of the gel (such as the stiffness and charge density of the gel) and the external properties (such as the strength of the applied voltage) [46]. Sarmad et al. synthesized chitosan-based polymer films in which the cations (Na+) in the solution migrated toward the cathode and the anions (Cl−) and the mobile anions (Ac−) from the chitosan migrated toward the anode when the films were immersed in a sodium chloride solution and a direct current electric field was applied. Since the -NH3+ ions on the chitosan backbone are fixed and cannot migrate, the chitosan film is bent by the electrostatic attraction from the cathode. The ionic migration caused by the electric field results in an osmotic pressure difference, which further pushes the polymer film to bend toward the cathode [101].
2.2.1.6. Water/solvent
Solvent stimulation is usually used for hydrogel materials including various solvents, such as water or other liquids, to change the shape and characteristics of the 4D printing structure as shown in Fig. 2f, [51,102]. There are two kinds of response mechanisms of solvent-responsive polymers. One of them is that solvent molecules enter the material and cause plasticization, greatly reducing the transition temperature of the polymer matrix. The stretched molecular chains return to the most stable state, leading to the recovery of temporary shapes. A work investigated the water response behavior of PU. PU absorbs water when soaked in water, and the hydrogen bond between water and polymer chains weakens that between molecular chains, thereby reducing the glass transition temperature, so that the shape recovery is achieved by absorbing water at room temperature. By controlling the amount of water absorbed by different parts of the polymer, different glass transition temperatures can be generated, thus achieving sequential control of shape recovery [103]. Another response mechanism is using functional groups that respond to solvent stimuli. For example, hydrogen bonding or network structure serves as reversible phases for shape memory, such as the reversible network of cellulose nanowhiskers [104]. When composite materials containing cellulose nanowhiskers absorb water, the network structure of the material is disrupted due to the formation of competitive hydrogen bonds, resulting in a significant decrease in modulus and a change in shape. The network structure can be reformed then the shape can be fixed after drying.
2.2.1.7. Others
In addition to the stimulus factors discussed above, there are other stimulus factors in the 4D printing process, such as enzyme (Fig. 2g) [61], mechanical stress, microwave [105], etc., which can activate changes in supramolecular structure and surface properties [106]. With the development of stimulus diversity, the research direction of stimulus-responsive materials is moving towards multi-stimulus responses like magnetic field/temperature, temperature/solvent, etc [88]. More multi-responsive materials can be created with different structures and superior performance and are widely used in various industries [[107], [108], [109]].
2.2.2. Smart performance
2.2.2.1. Shape memory
The shape memory is one of the most common and widely used properties of 4D printing materials. The deformation behaviors considered in 4D printing include twisting, folding, curling, bending, linear or nonlinear expansion/contraction, etc. [110,111], and the shape changes can be made from 1D to 2D, 2D to 2D, 1D to 3D, 2D to 3D, and so on [110,[112], [113], [114], [115]]. The shape memory capability of SMPs is related to the molecular structure, which usually consists of a fixed phase that defines the initial shape of the material and a reversible phase that controls the temporary shape [7]. The reversible phase is usually the portion of the polymer that undergoes phase transitions including glass transition, crystallization transition, and melt transition, which is discussed in more detail in the section on polymer materials and temperature response. SMPs can be divided into two main categories including unidirectional shape memory and bidirectional shape memory [116]. Unidirectional shape memory materials include two kinds, the first is shape-altering materials, which change shape under stimulus conditions (Fig. 3a–i), and the second type is a material that can be programmed to one or more temporary shapes, and can gradually recover to a permanent shape through multiple temporary shapes under external stimuli. Hydrogels with swelling properties are the representative materials of this type. In the thermosensitive hydrogel, the PNIPAAm layer began to lose water and shrink when the temperature was raised, and after the shape was bent and cooled, the PNIPAM layer absorbed water and expanded again, and the structure returned to the initial plane state [117]. The deformation process is irreversible, and after recovering to a permanent shape, a reprogramming step is required to rebuild the temporary shape (Fig. 3a–ii) [9]. The bidirectional shape memory property is characterized by its reversibility, whereby the polymer is capable of changing the shape in response to a certain stimulus, and then recovering when the stimulus is removed (Fig. 3a–iii). Anthamatten prepared a PCL shape memory material by introducing cross-linking of molecular chains followed by stretching. The resulting network has built-in stress and anisotropy along the stretching direction, conferring a bidirectional shape memory effect in the absence of external loading. The molecular chains can switch between nearly isotropic coils and oriented crystals during thermal cycling. When cooled, the material elongates as crystallization further aligns the PCL chains along the tensile direction, and when heated, the material shrinks back to its relaxed state [101].
Fig. 3.
Smart performance of 4D printing polymeric biomaterials. (a) Different ways of shape memory. (b) Shape memory processes in thermoplastic and thermoset SMPs [118]. Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International license (https://creativecommons.org/licenses/by/4.0). (c–d) Self-healing process of polymeric materials [119,120]. Licensed under a Creative Commons CC BY license and Copyright 2024, John Wiley and Sons. (e) Various reversible chemical covalent and noncovalent bonding interactions behind dynamic self-healing materials [121]. Licensed under a Creative Commons CC BY license. (f) Transformation of polymeric materials from free molecules to micelles and macroaggregates in response to changes in pH and temperature [122]. Licensed under a Creative Commons CC BY license. (g) Self-assembly of GEL-CUR hybrid hydrogels [4]. Reprinted with permission from [4]. Copyright 2024, American Chemical Society.
From the properties of the material, SMPs can be divided into thermoset SMPs and thermoplastic SMPs. As shown in Fig. 3b, the molecules of thermosetting polymers consist of a three-dimensional network, and because of the crosslinked network structure, these molecules do not melt and have a well-defined fixed original shape. When the temperature is higher than the transition temperature, the molecular chains of the reversible phase move and deform under the action of external forces, and are frozen upon cooling down so a temporary shape is defined. When the temperature is raised above the transition temperature again, the reversible phase moves under the action of the intramolecular stresses, and the shape of the material is restored to its original shape. The process of shape memory process of thermoplastic SMPs is similar to that of thermoset molecules (Fig. 3b) [118]. However, the molecular structure of these two SMPs is different. Thermoplastic SMPs are usually composed of linear molecules, the molecular chains are broken and free to slide after heating, so the material changes into the melting state, thus their permanent shape could be defined many times [63].
2.2.2.2. Self-healing
Self-healing properties refer to the ability of smart materials to restore or approach their original properties by automatically repairing the damage through internal mechanisms or external stimuli after damage (Fig. 3c and d) [123]. The determining factor for the rationalized design of biomedical self-healing hydrogels is the cross-linking mechanism that includes reversible chemical covalent bonding cross-linking and physical noncovalent interactions [[121], [124], [125]]. Common reversible covalent bonding includes the Schiff reaction [126,127], Diels-Alder reaction [128,129], borate bond [130], and disulfide bond (Fig. 3e) [131,132] and these chemical bonds have dynamic and reversible characteristics. The dynamic covalent bonding in the molecular network of the material is constantly changing dynamically, and there are always uncrosslinked polymer chains with reactive groups in the network, which can be crosslinked at the damaged place many times in a repetitive reaction, and ultimately, the material will recover to the original structure and function. In addition to these chemical bonds, several non-covalent physical interactions can also provide biomedical hydrogels with self-healing properties, such as hydrophobic interactions [124,133], hydrogen bonding [8], ionic interactions [11], and host-guest interactions (Fig. 3e) [134].
These methods are often applied to material matrices to improve their mechanical properties [125]. For example, in the hydrogel material composed of polyvinyl alcohol (PVA)/borax and carboxymethyl chitosan, borax and PVA molecules combine to form a borate ester crosslinked network, which can be reversibly broken and re-formed when damaged by external forces, to achieve self-healing. At the same time, the network structure caused by crosslinking enhances the strength and flexibility of the hydrogel [135]. In addition, self-healing properties are used to give shape memory polymers the ability to be reprogrammed and reconstructed. Currently, most SMPs only have one permanent shape. Ge's team introduced covalent adaptability networks (CANs) into SMPs and successfully prepared MRC-SMPs with high deformability, high glass transition temperature, and high modulus. By processing at higher temperatures, the initial shape can be reconstructed multiple times [119].
The self-healing properties of the materials are closely related to the 3D-printed structures. For example, some complex structures may rupture during the printing process, leading to a decrease in the self-healing ability [136]. Moreover, there may be some microcracks in the 3D printed structure, which are difficult to detect and localize, thus affecting the self-healing effect. In addition, when combining 3D printing and the self-healing properties of materials, it is also important to fully consider the physical and chemical properties of the materials themselves to ensure that they can be adapted to the printing method [136].
2.2.2.3. Self-assembly
Self-assembly refers to the spontaneous organization of macromolecules into ordered structures through non-covalent bonding interactions [137,138]. The self-assembly process does not require external guidance or templates, and the molecules form a stable structure relying on their chemical properties and external conditions [139]. The key to the self-assembly properties lies in the molecular recognition ability including the spatial complementarity of the molecular shape of the molecular shape or non-covalent interaction, which determines the unique orientation of the molecules [5]. Under externally stimulated conditions, the non-covalent interactions of the molecules will change, leading to a change of properties in some molecular chains, such as different crystallization ability or different hydrophilicity between different molecular chain segments, which can be used as a directional assembly condition for self-assembly, thus changing the nature or hydrophilicity of the whole system [5]. Li et al. synthesized peptide-like double-block copolymers by introducing carboxyl groups on the side chains, and the materials exhibit temperature and pH responsiveness in acidic solutions. As the pH decreases or the temperature increases, the hydrophilicity of the material decreases and aggregation occurs to form micelles (Fig. 3f). The formation and disappearance of micelles are reversible, which gives them potential applications in the field of drug carriers [122]. The nanofibers and high helix hydrogels (GEL-CUR) prepared by Wang et al. were able to induce the formation of nanofiber networks within the gel network by the denaturing/renaturing and solvent exchange process (Fig. 3g), and by controlling the drying and swelling conditions, the density and orientation of the CUR nanofibers can be regulated, thus affecting the mechanical properties and cytocompatibility of the hydrogels. The gels supported the adhesion and growth of neural cells, which has great potential for use in neural tissue engineering [4].
Meanwhile, the self-assembly process can provide a basis for the design of shape memory materials. Through the interactions mentioned above, molecules self-assemble to form ordered structures that can be used as the blocks of shape memory materials, enabling shape change and recovery ability under specific conditions. Bao et al. combined a pH-responsive peptide (MA-FIID) with a hydrogel material. Under acidic conditions (such as pH 2.0), the MA-FIID peptide molecules self-assemble to form a fiber with a β-folded structure, and the three-dimensional network structure increases the internal resistance of the hydrogel, thereby limiting the entry of water molecules, resulting in the volume contraction of the hydrogel. By changing the pH of the solution, the process of self-assembly and depolymerization can be controlled, thus regulating the volume expansion and contraction of the hydrogel [140]. Overall, self-assembly is a process of multiple driving forces interacting and combining, and molecular guidance effects are crucial for the formation of highly ordered assembly structures [5].
3. Polymers for 4D printing biomaterials
With the development of the industry and the continuous growth of medical demand, exploring 4D printing polymeric materials with excellent performance has become the focus of current research. Polymers can be categorized into synthetic and natural polymers. In this section, we list some common 4D printing biopolymers and discuss their structures and applications. The properties of different polymers are listed in Table 1.
Table 1.
Properties of different 4D printing polymers.
| Polymers | Physicochemical properties | Stimulation | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| PNIPAAm | Temperature-sensitive | Temperature | The response temperature is close to the body temperature | Low mechanical strength | [141] |
| PVA | Water-soluble polymer with good adhesion and film-forming properties | Temperature, Water |
Good water solubility; easy to process; good biocompatibility | Low mechanical strength; poor stability | [[142], [143], [144],145] |
| PCL | Semi-crystalline polymer with low melting point and glass transition temperature | Temperature, pH | Good biocompatibility, biodegradability, and flexibility; slow degradation rate; suitable for long-term application | Low mechanical strength; general thermal stability; printing accuracy may be limited | [146,147,148] |
| PLA | High crystallinity | Temperature, pH | Good biocompatibility and degradability; good mechanical properties | Great brittleness and insufficient toughness; degradation products may result in a locally acidic environment | [149,150,151,152] |
| PU | Good mechanical properties, flexibility, and wear resistance | Temperature | Adjustable properties by changing the molecular structure and composition | Relatively poor biodegradability | [153,154,155] |
| Alginate | Natural polymer with good biocompatibility and gel formation ability | Ion, pH |
Rich source; low cost; good biocompatibility and gel properties. | Limited mechanical strength; the rate of degradation is difficult to control precisely | [156,157,158] |
| Chitosan | Natural polysaccharide with good biocompatibility and biodegradability | Enzyme, pH | Good biocompatibility and bioactivity; antibiosis | Poor mechanical properties; difficult to process | [159,160,161] |
3.1. Synthetic polymers
Synthetic polymers are prepared by chemical synthesis methods, such as poly(ε-caprolactone) (PCL), PLA, etc. They have excellent physical and chemical properties and can be customized and modified to meet different biomedical needs.
3.1.1. PNIPAAm
PNIPAAm is a typical thermo-responsive polymer with biocompatibility, and the hydrophilicity of PNIPAAm changes at a LCST of 32 °C, leading to hydration and dehydration phenomena [162,163], thus exhibiting temperature-regulated stretching and shrinkage of the material [164]. Since the PNIPAAm consists of hydrophilic amide groups and hydrophobic isopropyl groups, and when the temperature falls below the LCST, the hydrophilicity of PNIPAAm is enhanced because of the formation of hydrogen bonds between amide groups on the PNIPAAm chains and the water molecules, so the polymer chains are stretched (Fig. 4a and b) [165,166]. When the temperature is warmed up to the LCST, the hydrogen bonding between water molecules and PNIPAAm is weakened, so the water molecules are released, resulting in a globular conformation of the polymer chains (Fig. 4a and b). This kind of thermo-responsive polymer is widely used in biomedical applications due to its relatively low LCST (close to physiological temperature) [166]. Zhao et al. [167] prepared temperature-triggered PNIPAAm hydrogels, which were able to efficiently adhere to biological tissues and actively contract at body temperature to promote wound healing in the absence of any drug as shown in Fig. 4c. Siadati [168] prepared a temperature-sensitive hydrogel drug scaffold by PNIPAAm and niosomal. Encapsulation of rosuvastatin in niosomal allows targeted delivery and controlled release of the drug, thereby promoting the repair of myocardial tissue. By adjusting the composition and structure of the hydrogel and niosomal, the release rate and duration of the drug can be controlled to achieve the therapeutic purpose. Since the drug is encapsulated inside the hydrogel, the degradation rate of the hydrogel affects the rate of drug release. When the hydrogel begins to degrade, the drug is gradually released as the hydrogel network structure is destroyed. Therefore, by adjusting the cross-linking density of the hydrogel, the degradation rate of the hydrogel can be controlled, thus indirectly controlling the release rate of the drug. In addition to this method, LCST modulation is another way to control the drug release rate [165]. Lowering the LCST of PNIPAAm can keep the hydrogel in a sol-gel state at body temperature, thus slowing down the drug release rate. This is suitable for situations where a slow release of drugs is required, such as long-term administration. Elevating the LCST of PNIPAAm allows the hydrogel to remain in a gel state at body temperature, thereby accelerating the rate of drug release. In general, both the molecular weight and the component polymerized affect its LCST [165,169]. It has been reported that high molecular weights enhanced polymer-polymer interactions, which reduced LCST [169]. The LCST of PNIPAM can be modulated by copolymerization with other monomers, which is achieved by adjusting the hydrophilicity. For example, the introduction of hydrophobic monomers may increase the LCST, whereas the introduction of hydrophilic monomers may decrease the LCST. Staubitz et al. [170] found that the LCST of PNIPAAM decreased to about 20 °C by modified with an azobenzene-containing blend illustrated in Fig. 4d.
Fig. 4.
(a) Bonding changes of the amide group of HA-PNIPAAm hydrogen above and below LCSTs[171]. Copyright 2018, John Wiley and Sons. (b) Molecular chain changes in PNIPAAm in response to temperature [164]. Licensed under a Creative Commons CC BY license. (c) Schematic diagram of the synthesis, mechanical activity, and biological applications of SIS-PNIPAm hydrogels [167]. Copyright 2022, Royal Society of Chemistry. (d) DSC plot showing a decrease in LCST of PNIPAAm copolymer after copolymerization with azo [170]. Licensed under a Creative Commons CC BY license. (e) Preparation strategy of DNA-PNIPAAm hydrogel [172]. Copyright 2023, Springer Nature.
PNIPAAm hydrogel has sensitive temperature responsiveness, but its mechanical properties and tunability are limited [173]. To compensate for these shortcomings, many methods were applied. Jiang et al. [172] formed an interpenetrating network structure by combining PNIPAAm with polyacryloyl-modified Y-type DNA, and a crosslinking network was formed between DNA and PNIPAAM via a crosslinking agent (Fig. 4e), which increased the structural stability of the material. The modulus of elasticity (G′) of DNA-PNIPAAm hydrogels is about 25 times higher than that of PNIPAAm hydrogels, and the DNA-PNIPAAm hydrogels have higher elasticity and deformation resistance. In addition, the non-biodegradability of PNIPAAm hydrogels is another limitation for use in biomedical applications [174,175] It could be improved by introducing different biodegradable monomers or natural polymers [176]. Pal et al. [177] utilized the advantages of synthetic and natural biopolymers to synthesize covalently crosslinked hydrogels with stimulating properties based on dextrin (DXT) and PNIPAAm. The hydrogels showed good degradation in lysozyme/phosphate buffered saline (PBS) medium, losing 80 % of their mass in only 21 days.
3.1.2. PVA
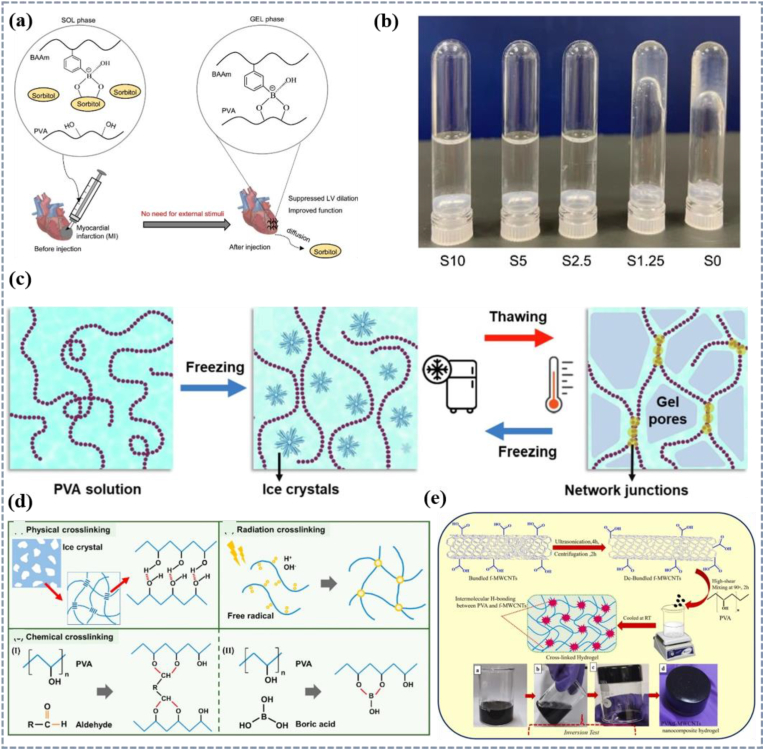
PVA is a semi-crystalline, water-soluble linear synthetic polymer polymerized from vinyl acetate, with a molecular weight ranging from 20000 to 400000 g/mol depending on the degree of hydrolysis [[142], [143], [144], [178]]. PVA molecules contain a large number of hydroxyl groups, which can form intramolecular and intermolecular hydrogen bonds. Due to these hydrogen bonds, PVA has lower water vapor and gas permeability [[142], [143], [144]]. PVA has good mechanical properties, hydrophilicity, good biocompatibility and biodegradability, and low protein adsorption tendency, so it is often used as a hydrogel in the biomedical field [143,178]. PVA can change shape in response to temperature and water and is therefore often used in drug delivery systems. Its shape memory behavior is mainly dependent on the crystalline behavior of the molecular chains, with the crystalline and cross-linked portions of the molecular chains acting as stationary phases and the amorphous portions of the molecular chains acting as active phases that are capable of moving when the temperature reaches Tg. When PVA is in contact with water molecules, swelling behavior occurs due to hydrogen bonding [179]. Yamaoka [180] studied an injectable composite solution for the treatment of myocardial infarction. This material is composed of poly(3-acrylamidophenylboronic acidco-acrylamide) (BAAm), PVA, and sorbitol (S) (Fig. 5a). The high concentration of sorbitol in the system blocks the cross-linking between BAAm and PVA, keeping it in a solution state (Fig. 5b). After injection into the heart, the sorbitol in the solution can diffuse out spontaneously, resulting in a decrease in the content of the sorbitol and decrease in the viscoelasticity of the system. Under physiological conditions, the groups between BAAm and PVA are cross-linked to achieve gelation.
Fig. 5.
(a) Schematic representation of the composition, delivery, and in situ gelling mechanisms and (b) sol-gel state of PVA injectable materials [180]. Copyright 2024, Elsevier. (c) Preparation of PVA hydrogels by the F-T method [178]. Copyright 2022, Elsevier. (d) Different crosslinking methods of PVA [181]. Licensed under a Creative Commons CC BY license. (e) Schematic diagram of PVA/f-MWCNTs nanocomposite hydrogels prepared by natural cooling at room temperature [182]. Copyright 2024, Elsevier.
The shape memory behavior of PVA can be modulated by cross-linking and crosslinking methods including physical crosslinking (Fig. 5c), chemical crosslinking, and radiation crosslinking and the molecular chain changes are as shown in Fig. 5d, [144]. Vieira et al. [183] prepared PVA hydrogel film by physical crosslinking and chemical crosslinking. It was found that the swelling degree of PVA film prepared by chemical method was lower and it decreased with the increase of crosslinking time. The thermosensitive PNPAAm micro-gel and superparamagnetic iron oxide nanoparticles were confined in the PVA nanofiber membrane. Both crosslinking methods can ensure the thermal and magnetic response properties of the film. Sharma [182] prepared PVA/f-MWCNTs high-strength nanocomposite hydrogels at room temperature by physical crosslinking and natural cooling. PVA/f-MWCNTs nanoparticles can be evenly dispersed in the PVA matrix and the intermolecular hydrogen bonds are formed between the hydroxyl group in the PVA molecule and the carboxyl group in PVA/f-MWCNT to build a hydrogel network (Fig. 5e). The hardness of the composite hydrogel was significantly improved compared with PVA hydrogel and due to the interface interaction and hydrogen bonding, the introduction of f-MWCNTs into PVA matrix can also effectively improve the thermal, mechanical and dielectric properties of the hydrogel.
3.1.3. Polyesters
PLA, and PCL are the typical polyesters for 4D printing biomaterials with excellent biocompatibility and biodegradability, which lays the foundation for its application in biomedical fields [[146], [147], [184], [185], [186], [187]]. They can be completely degraded to carbon dioxide and water in a complex body fluid environment and the degradation rate can be modulated by molecular weight, crystallinity, and degradation conditions [188], ranging from a few months to a few years [146].
Linear PCL does not have a shape memory function, but cross-linked PCL can be used as a shape memory material. The cross-linked structure therein acts as the stationary phase of polycaprolactone, which can produce crystallization with temperature and the molten crystalline phase becomes a reversible phase. However, many of the reported PCL-based shape memory blends have encountered the challenge of inadequate shape recovery, and the stability of shape memory properties remains largely unexplored.
As a semi-crystalline polymer, PLA has a certain shape memory ability, with its crystalline and amorphous regions serving as the stationary phase and reversible phase, respectively [[149], [150], [189]]. Due to the different molecular configurations, including poly D-lactic acid (PDLA), poly L-lactic acid (PLLA), and a randomly arranged structural isomer called poly DL-lactic acid (PDLLA) (Fig. 6a), there may also be some differences in their physical and chemical properties, for example, the regularity of PDLLA molecular chains is poor, so they do not have crystallization ability, while PLLA and PDLA are both semi-crystalline polymers. The melting point of PLA can be controlled by adjusting the ratio of PLLA and PDLA in the material. Through this method, SMPs with practical application potential can also be constructed with higher fineness [190]. Due to the relatively high modulus of glassy and elastic states, PLA has a high shape fixation ratio. However, the inherent brittleness greatly limits the performance of the shape memory characteristics. It is prone to brittle fracture under tensile stress at room temperature, and the deformation and recovery can only occur when the temperature rises to or above Tg (60 °C) which is prone to cause cell damage when used in the human body. In addition, due to the high rigidity of PLA molecular chains, the crystallization rate and crystallinity are low, and the rigidity of the molecular chain and the weak ability of amorphous zone movement lead to a low shape recovery rate.
Fig. 6.
(a) Two configurations of PLA [190]. Licensed under a Creative Commons CC BY license. (b) Preparation process of PCL-PLA nanofiber-based smart nanoporous scaffolds [191]. Copyright 2024, Elsevier. (c) i: Preparation of mCS and mCS/PLA/PCL blends, ii: 4D printing of mCS/PLA/PCL [192]. Licensed under a Creative Commons CC BY license.
Given the flexibility of PCL and the rigidity of PLA, the two are often combined to improve their shape memory capabilities and mechanical properties [185,193,194]. The different phase transition temperatures of PLA and PCL provide a shape memory capability for their composite system [148]. PLA nanofibers between PCL polymer matrices were constructed to introduce intelligent performance and prepared a variable new nanoporous intelligent scaffold (Fig. 6b) [191]. However, some researchers discovered that the composite system still had difficulty in supporting multiple repeated deformations which was due to the weak interfacial force between PCL and PLA and the low internal stress of deformation [195]. So another work introduced chitosan into the PCL/PLA system to improve the compatibility [192]. The modified chitosan was obtained by grafting low molecular weight PLA and PCL onto the side chains of chitosan (Fig. 6c–i). PCL and PLA on the side chains were well compatible with the matrix of the composite system, and the modified chitosan acted as a connecting bridge between amorphous regions of PLA and PCL, which improved the interfacial force of the blends. The compounds did not break during the shape memory process, while the compatibility also improved and a co-continuous structure was formed, which significantly enhanced the shape memory properties of the material (Fig. 6c–ii) [196]. In addition, high-toughness polymers such as PU [197], or inorganic particles such as calcium phosphate, hydroxyapatite (HA), alumina, zirconia, etc [[198], [199], [200], [201]] are usually used to construct PLA-based shape memory systems to improve the mechanical strength and shape recovery ability.
3.1.4. PU
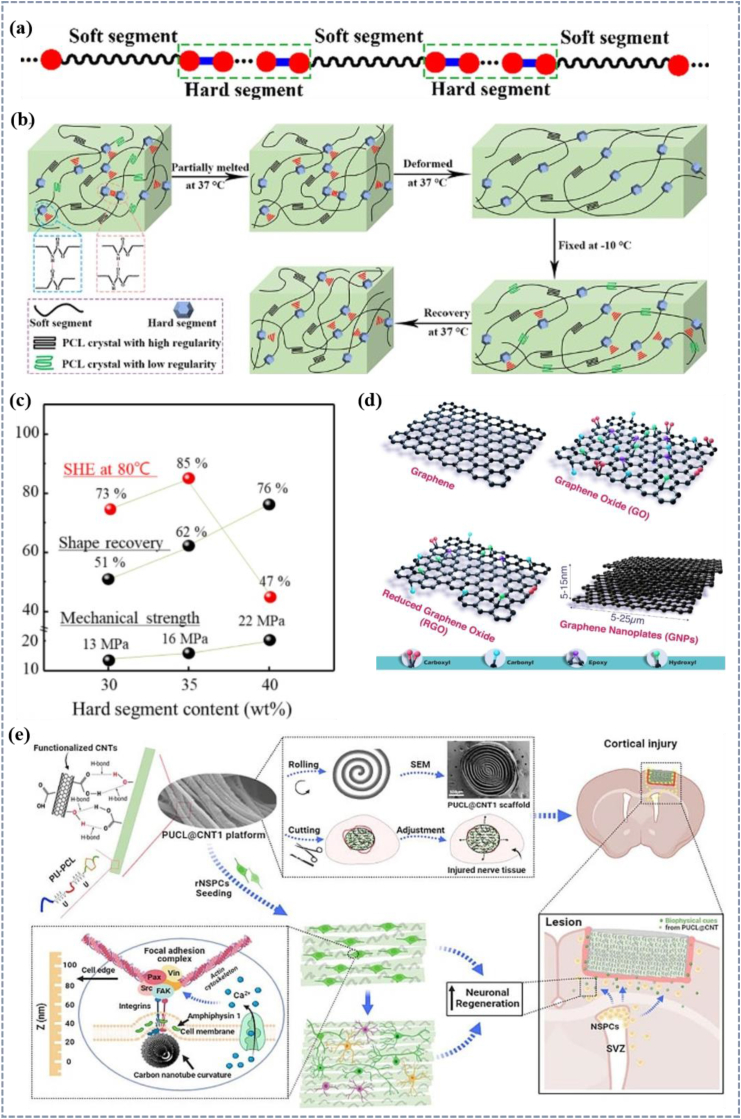
PU is a thermo-responsive synthetic polymer consisting of urethane-linked organic units that have been widely used in biomedical applications because of their excellent physical properties and biostability [195]. PU is composed of two parts, soft and hard segments (Fig. 7a), the soft segments are generally polyether, polyester or polyolefin, etc., and the hard segments consist of isocyanate and chain extender. The structure of PUs with separated soft and hard segments gives them shape memory capability (Fig. 7b), with the hard segments forming a network structure through cross-linking, which serves as a permanent shape for the process of shape memory, and the soft segments acting as molecular switches [202,203]. The selection of the hard and soft segments is critical to the structure and properties of PU materials, and factors such as the ratio and type of the two affect the degree of phase separation and hydrogen bonding [204], leading to differences in shape memory and mechanical properties between different PUs [203]. For example, Zhang et al. found that the addition of rosin chain extenders to PU systems increased the degree of phase separation between the soft and hard segments, and the thermodynamic incompatibility between them became more pronounced [205]. The addition of tetrahydrofuran solvent to PU also modifies the phase separation behavior of the system. The solvent leads to an increase in the fluidity, which makes it easier to form purely soft and hard regions, and with the increase in ordered hydrogen bonding and cross-linking of the hard segments, the size of the phase region increases, the degree of phase separation is increased, and the shape memory capability is enhanced [203]. The final PU properties can be customized based on the properties, structure, and molar mass of the polyols and the diisocyanate involved in the synthesis [206,153].
Fig. 7.
(a) Soft and hard segment structures and (b) shape memory mechanism of PU [207]. Copyright 2022, Elsevier.(c) Relationship between self-healing efficiency, shape recovery, mechanical strength, and hard segment content [154]. Copyright 2019, Springer Nature. (d) Schematic diagram of Graphene, Graphene Oxide, Reduced Graphene Oxide, and Graphene nanoplatelets used as a nanofiller for nanocomposite fabrication [208]. Copyright 2023, John Wiley and Sons. (e) Diagram of preparation, deformation, and the mechanism of promoting neural tissue regeneration of PUCL@CNT [209]. Copyright 2023, Elsevier.
The molecular structure of the soft and hard segments has an impact on the properties of PUs, e.g., the position of the amine groups in the side chains has a significant effect on the properties of PUs. Amino groups in the soft segments enhance the interaction between the hard and soft segments, improve mechanical properties, and reduce surface hydrophobicity, while amino groups in the hard segments disrupt the crystallization of the hard segments, enhance microphase separation, reduce mechanical properties, but increase surface hydrophilicity [210]. Within a certain range, the shape recovery properties of PUs increase as the concentration of hard segments in the PU matrix increases, due to the physical cross-linking between the hard segments that improves the shape recovery and mechanical properties of the PU (Fig. 7c) [154,207]. However, when the content of the hard segments is too high or too low, the shape memory capability decreases [153]. An increase in the length of the soft segments increases the crystallization behavior of the soft segments, which increases the glassy and rubbery modulus. A higher glassy state modulus results in greater shape fixation after deformation, while a higher rubbery state modulus enhances shape recovery after deformation. By adjusting the molecular weight of the soft segments, the recovery temperature of the shape memory behavior can be adjusted to be close to the body temperature, which meets the basic requirements for medical implantation. Karasu et al. prepared PUs using poly(ethylene sebacate) (PES) with different molecular weights and found that the transition temperature of the PUs increased with increasing molecular weight of polyester [211].
Although PU has many advantages and has great potential for application in the field of shape memory materials. However, PU still has some drawbacks, such as poor electrical and thermal conductivity. So in recent years the research on PU mainly focuses on PU composites, expecting to improve the performance by compositing with other materials that are easy to conduct electricity and heat [208,212] including carbon nanotubes, graphene, and so on (Fig. 7d) [208,213]. Li et al. prepared a PU-PCL (PUCL) and CNTs composite scaffolds (PUCL@CNT). CNT-modified materials have good shape memory properties as well as electrical conductivity, and the composites induced a large number of endogenous neural stem cells (NSCs) including neurons near the injury area in a rat corticotomy model, illustrating the potential for the regeneration of neural tissues as shown in Fig. 7e, [209]. Dispersion of conductive materials from the PU matrix is still an important issue, and many approaches including melt blending, situ polymerization, and ultrasonically dispersed solution mixing have been attempted to promote dispersion [214].
3.2. Natural polymers
Natural polymers such as proteins (e.g. collagen, fibronectin), polysaccharides (e.g. chitosan, sodium alginate, hyaluronic acid), etc. are derived from nature and can be obtained from a wide variety of sources such as plants, animals, and microorganisms. Natural polymers have better compatibility with human tissues, reducing the risk of rejection reactions, and can be degraded within the living organisms, and ultimately be absorbed or metabolized by the body without the need for surgical removal. In addition, natural polymers have a structure similar to that of the human ECM, thus providing a suitable microenvironment for cell adhesion, growth, and differentiation. Although most natural polymers do not have the capability of 4D printing by themselves, they can still be used as a class of high-performance matrix materials that can be modified or post-treated to obtain stimulus-responsive capabilities for application in the field of tissue regeneration.
3.2.1. Alginate
Alginate is an anionic natural polysaccharide extracted from brown algae, composed of mannuronic acid (MA) and glucuronic acid (GA) [155]. It has low cytotoxicity and good biocompatibility and can be rapidly biodegraded and cross-linked. Meanwhile, due to its ability to rapidly gel under mild physiological conditions without producing harmful by-products, it is widely used as a bio-printing material [156]. When exposed to divalent cations such as calcium, barium, strontium, etc., the material can chelate with carboxyl groups in the molecular chain, inducing ion crosslinking to form a stable structure shown in Fig. 8a [157]. Different cations exhibit varying affinities for alginate, and the concentration of ions can affect the stability of cross-linking. Research has shown that at low concentrations, Ca2+ binds temporarily to alginate chains, and higher concentrations of Ca2+ promote relatively permanent binding [215]. The properties of alginate can be improved by crosslinking [158], which is achieved by the carboxyl and hydroxyl groups in the molecular chains. By combining functional groups with carboxyl and hydroxyl groups, alginate can be modified to adjust the physical and chemical properties [216]. Cao et al. [157] prepared a 4D printing methacrylate alginate (SA-MA) hydrogel capable of stimulating response. The degree of crosslinking increases when SA-MA hydrogel is immersed in Ca2+ and chitosan solution successively. The double-layer SA-MA hydrogels have different degrees of crosslinking density, resulting in different degrees of shrinkage and self-folding behavior (Fig. 8b). Xu [217] diffused high molecular weight chitosan into polyvinyl alcohol/sodium alginate/chitosan (PSCS) hydrogel, and successfully prepared PSCS double web hydrogel through physical crosslinking. The gel has good PH sensitivity, when the hydrogel is immersed in different PH solutions, the swelling ratio of buffer solution with pH = 2 is lower than that with pH = 12 (Fig. 8c). The reason showing the behavior is that the pKa of SA is 3.2 and in the solution with PH = 2, most COO− groups are converted into -COOH groups, resulting in the increased intermolecular hydrogen bonds, more compact hydrogel structure and low swelling ratio. Based on the different swelling degrees of hydrogels under different PH, a double-layer hydrogel structure was constructed using two hydrogels with large swelling differences, and the double-layer hydrogel can bend spontaneously due to swelling deformation.
Fig. 8.
(a) Deformation mechanism of photocrosslinked methacrylated alginate [157]. (b) Deformation schemes and images of SA-MA bilayer films under the action of Ca2+ solution. The scale bars are 5 mm and 10 mm [157]. Reprinted with permission from [157]. Copyright 2021, American Chemical Society. (c) Deformation of hydrogels in different pH media [217]. Copyright 2024, Elsevier. (d) Intermolecular hydrogen bonds between chitosan and PVA polymeric chains [218]. Licensed under a Creative Commons CC BY license. (e) Schematic diagram of the self-healing mechanism of emulsion gel [219]. Copyright 2024, Elsevier. (f) Adhesion, self-healing properties and (g) self-healing mechanism of CPT hydrogels [220]. Copyright 2024, Elsevier.
3.2.2. Chitosan
Chitosan is obtained by deacetylation of chitin, which is widely present in nature. This natural polymer has unique biological characteristics including antibacterial properties, mucous adhesion, and excellent properties such as compatibility and microbial degradation. Chitosan can be absorbed by the human body system and processed into porous structures that are highly suitable for cell transplantation and tissue regeneration applications. Therefore, it has been widely used in biomedical fields such as wound healing, drug release, and tissue engineering [221]. Pure chitosan-based hydrogel has poor mechanical stability and slow gel speed, so it is often compounded with other materials to improve its performance. For example, when PVA is added to the chitosan matrix, the hydrogen bonds between the hydroxyl group in PVA and the carboxyl group in chitosan help to improve the stability, tensile strength, and toughness of the material (Fig. 8d) [218].In recent years, a series of chitosan derivatives have been developed, including carboxymethyl chitosan (CMC), chitosan methacrylate, ethylene glycol chitosan, maleic anhydride modified chitosan, hydroxybutyl chitosan, etc., which are widely used in drug delivery systems and tissue repair [159]. Chitosan-based materials are commonly used to build self-healing hydrogel systems [[160], [222], [223]], so physical or chemical cross-linking of chitosan is needed to build a dynamic polymer three-dimensional network. The abundant hydroxyl and amine groups in the molecular chains of chitosan provide the structural basis for the realization of the cross-linking structures [161]. A dynamic three-dimensional network structure is constructed through non-covalent interactions, including hydrogen bonds [224], hydrophobic interaction [225], electrostatic interaction [226], etc., and dynamic covalent bonds such as disulfide bonds [227] and imine bonds [228], endowing hydrogels the characteristics with self-healing properties. Zhou et al. [219] cross-linked CMC stabilized lotion with dialdehyde cellulose nanocrystals (DACNC) to prepare self-healing emulsion gel (Fig. 8e). In the absence of any external stimulation, the two independent emulsion gels can be reassembled, which is due to the reversible imine bond between the amino group of CMC and the aldehyde group of DACNC, giving the emulsion gels good self-healing ability [229]. Xiong et al. [220] prepared a series of pH-responsive CPT multifunctional hydrogel dressings by embedding natural plant extract Tannins (TA) as a non-antibiotic crosslinking agent in CMC and TA with different concentrations of TA. The synthesized CPT hydrogel is pH-responsive, which can accelerate dissolution under the pH value of infected wounds, release TA faster, and effectively enhance the antioxidant and antibacterial effects of the hydrogel. CPT hydrogel shows excellent self-healing, adaptive, and adhesive properties (Fig. 8f) due to the hydrogen bonding interaction (Fig. 8g).
4. Applications of 4D polymeric biomaterials in tissue regeneration
The uniqueness of 4D printing biomaterials lies in their ability to accurately simulate complex dynamic processes within living organisms, demonstrating a high degree of intelligent responsiveness and tissue structure matching and providing unprecedented flexibility and controllability for tissue regeneration [230]. This technology solves the difficult problems that traditional methods cannot overcome and brings new hope for the development of tissue regeneration.
4.1. Bone
Autologous or allogeneic bone transplantation is the traditional way to solve bone defects. However, autologous bone transplantation inevitably leads to damage to the donor site, while allogeneic transplantation carries the risk of immune rejection. 4D printing polymeric materials provide an effective means for bone tissue regeneration therapy with their unique characteristics, especially demonstrating extraordinary potential in handling complex bone repair problems [14]. The materials can customize and design bone tissue scaffolds that highly match the defect site of the patient based on their individualized pathological condition and rehabilitation trajectory [[231], [232], [233]]. The scaffold dynamically adjusts its shape according to the rehabilitation process to support and guide the growth of new bone tissue in the optimal way [234,235], thereby improving the bone tissue repair process and promoting bone regeneration [235].
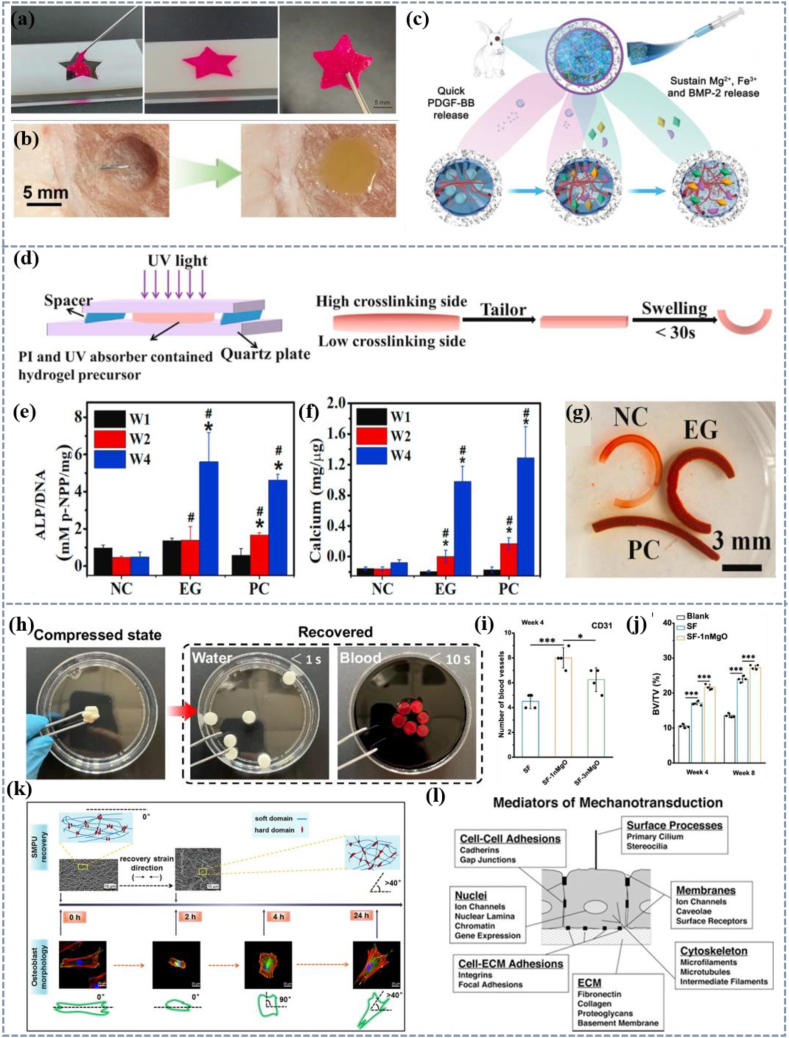
At present, the commonly used materials for bone tissue regeneration include stimulus-responsive hydrogel [[236], [237], [238], [239], [240]] and shape memory scaffold. Injectable stimulus-responsive hydrogel is an important way to treat bone defects by injecting hydrogel-containing cells into the bone defect site. The hydrogel responds to temperature stimulation to achieve a gel state and dynamically adjusts its shape to fully match the bone defect (Fig. 9a) [237]. Lv et al. added MgFe layered double hydroxides (LDH) nanosheets functionalized by bone morphogenetic protein 2 (BMP-2) to the chitosan/silk fibroin (CS) hydrogel loaded with platelet-derived growth factor bb (PDGF-BB) to construct an intelligent injectable thermal response hydrogel (CSP-LB). The hydrogel solution accurately fits pig tissue defects and quickly solidifies under the condition of a 37 °C water bath (Fig. 9b). Hydrogel realizes the explosive release of PDGF-BB (platelet-derived growth factor BB), the continuous release of BMP-2, bioactive Mg2+and Fe3+ ions (Fig. 9c), with good angiogenesis and osteogenesis performance. Compared with CS hydrogel, its bone volume density increases by 4.5 times and 3.6 times, respectively [241].
Fig. 9.
Applications of 4D printing of polymer biomaterials for bone tissue. (a) The injectable hydrogel is irradiated by blue light to form a stable scaffold [237]. Copyright 2024, Elsevier. (b) Image of injection performance of CS-L hydrogel on pig tissue mode [241]. (c) Biological properties of CSP-LB hydrogel [241]. Copyright 2022, John Wiley and Sons. (d) The diagram of the preparation device and deformation process of crosslinked gradient hydrogel [242]. (e) ALP activity normalized to DNA content [242]. (f) Calcium content normalized to DNA content in the cell-laden hydrogels at varying time points [242]. NC (negative control): cell-laden hydrogels obtained in the presence of a UV absorber and cultured in cell growth medium, EG (experimental group): cell-laden hydrogels obtained in the presence of a UV absorber and cultured in osteogenic medium, PC (positive control): cell-laden hydrogels obtained in the absence of a UV absorber and cultured in osteogenic medium (g) Alizarin red stained scaffolds after 4-week in vitro culture. [242].Licensed under a Creative Commons CC BY license. (h) Photographs of the shape recovery process of silk fibroin/magnesium composite scaffold scaffolds in water and blood [243]. (i) Statistical analysis of stent vessels at 4 weeks after operation [243]. (j) Bone volume changes (BV/BT) in a blank group, fibroin group, and silk fibroin/magnesium group observed at 4 and 8 weeks [243]. Licensed under a Creative Commons CC BY license. (k) The shape restoration process of SMPU affects the morphology of the Osteoblast [244]. Copyright 2019, Elsevier. (l) The mediators of cellular mechanical transduction include ECM, cell-ECM and cell-cell adhesion, membrane components, special surface processes, cytoskeletal filaments, and nuclear structures [245]. Copyright 2006, John Wiley and Sons.
The preparation of hydrogel materials with a cross-linking gradient is a design strategy to realize the deformation function and apply it to bone repair [246]. The mixed solution of polymer, photoinitiator, and UV absorber is spun between two quartz plates. The hydrogel is crosslinked under the irradiation of UV light and the degree of crosslinking of the hydrogel decreases with the increase of the distance from the light source, to obtain the hydrogel with cross-linking gradient and realize the deformation function (Fig. 9d). By adjusting the light irradiation time, intensity, type, and concentration of photoinitiator and polymer system, the gradient of hydrogel can be precisely controlled. Human mesenchymal stem cells (hMSCs) showed strong osteogenic differentiation in gradient hydrogel scaffolds when cultured in an osteoblastic culture medium, indicating the potential for bone tissue regeneration (Fig. 9e–g) [242]. Meanwhile, SMP-based bone repair scaffolds can also effectively cooperate with osteoblasts, promoting cell adhesion and proliferation, and constructing an ideal micro ecosystem for natural regeneration of bone tissue [247]. The stent can be compressed and implanted into the body, fully restoring its original shape upon contact with water or blood (Fig. 9h). Then, the close contact with surrounding tissues promotes the proliferation and adhesion of bone cells, as well as the osteogenic differentiation of bone marrow mesenchymal stem cells in vitro, thereby promoting the growth of vascular and new bone (Fig. 9i and j) [243].
Compared to 3D-printed static scaffolds, the dynamic characteristics of SMP scaffolds also have an impact on tissue regeneration [248]. Luo's team investigated the key role of recovery strain and phase separation nanostructures of shape memory polyurethane (SMPU) in regulating cell morphology and found that the deformation caused by recovery strain in osteoblasts leads the orientation perpendicular to the strain direction. The nanostructures on the surface of the responding SMPUs direct the orientation of the osteoblasts so that they tend to align along the nanostructures (Fig. 9k) [244]. Moreover, the deformation process of the material is accompanied by mechanical forces, which have an influence on cell proliferation, growth, and tissue formation (Fig. 9l) [249,245]. Therefore, the mechanism of 4D printing materials in promoting bone tissue is complex, with factors such as the microenvironment and dynamic properties of the materials acting synergistically to promote bone tissue regeneration through multiple channels.
4.2. Cartilage
Cartilage, as a crucial bridge connecting different bones, plays an indispensable role in maintaining joint stability and reducing friction [250]. It usually cannot return to the original state through self-repair when damaged but will be replaced by scar tissue of which the function and structure are not as good as the original cartilage, greatly affecting the load-bearing capacity of the joint [14].
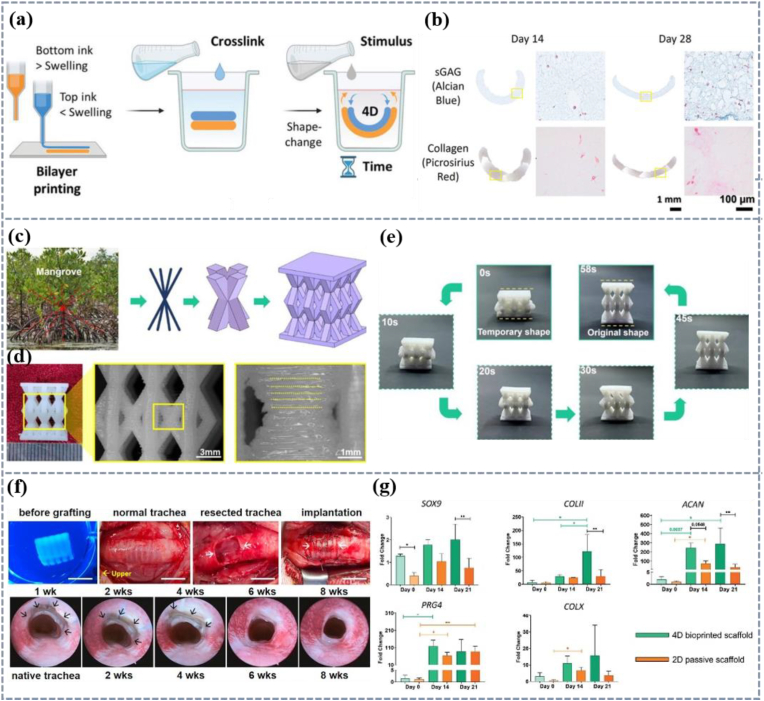
Natural polymers are widely used as cartilage tissue repair materials due to their excellent biocompatibility and biodegradability [251,252]. Hyaluronic acid with a high swelling capacity and alginate with a low swelling capacity were used to prepare double-layer intelligent structure hydrogel. Due to the different swelling degrees, the material has the characteristic of self-bending (Fig. 10a). After culturing the scaffold loaded with cells in a cartilage culture medium for 28 days, the formation of cartilage-like matrix can be observed (Fig. 10b) [253]. However, natural polymers usually have a certain disadvantage in strength, and synthetic polymers can compensate for this disadvantage and provide better mechanical support for materials. A shape memory composite material for cartilage defects was prepared by adding nano HA to the PU matrix. Due to the hydrogen bonding between HA and PU, good mechanical properties and biocompatibility are obtained. Based on the structural characteristics of mangrove forests, a biomimetic 4D printing cartilage scaffold has been designed (Fig. 10c and d), which can recover from a temporary shape to a permanent shape within 60 s at the temperature close to human physiological environment (Fig. 10e). The strength of the composite material increased by 200 %, while the shape recovery ratio and fixation ratio remained above 90 % with application prospects in minimally invasive and cartilage defects [254]. Trachea, as a key cartilage tube connecting the throat and lungs, has a unique structure consisting of 16–20 C-shaped transparent cartilage rings tightly connected by tracheal muscles, ensuring smooth breathing [230]. Kim and his colleagues prepared Sil-MA composite double-layer hydrogel. The TBSCs of the respiratory mucosa and chondrocytes of the tracheal cartilage were loaded on different layers and placed in a culture solution, and the water-induced material curled into a shape similar to the tracheal. The structure loaded with cells was implanted into the injured trachea of rabbits (Fig. 10f). After 8 weeks, it was found that the 4D printing trachea naturally fused with the host trachea to form epithelium and cartilage indicating the potential of this material in the field of tracheal tissue regeneration therapy (Fig. 10f) [60]. Given the complexity of the trachea structure, 4D printing of the trachea can not only accurately reproduce its unique C-ring structure, but also promote the proliferation and differentiation of cells through its dynamic stimulation. Chiesa et al. compared the ability of ear cartilage progenitor cells to differentiate into mature chondrocytes on 4D printing and 2D static scaffolds (Fig. 10g) and found that the cells on the 4D scaffold formed mature cartilage after 21 days of culture which means that the 4D bio-printing self-folding scaffold can promote cartilage formation, indicating that the self-folding scaffold may mimic the role of smooth muscle cell progenitor cells to regulate the shape of tracheal and promote the formation of cartilage [255].
Fig. 10.
Applications of 4D printing of polymer biomaterials for cartilage tissue.(a) The construction process of double-layer hydrogel [253]. (b) Representative histological images of alcian blue staining for sGAG in dark blue and picrosirius red staining for collagen in dark pink at two different magnifications at 14 and 28 days of culture. The squared boxes represent a zoomed-in region of interest [253]. Licensed under a Creative Commons CC BY license. (c) Bionic 4D printed cartilage scaffold with mangrove morphology [254]. (d) Structural details of the 4D printed bionic cartilage scaffold [254]. (e) Shape recovery process of the bionic 4D printed cartilage scaffold at 45 °C [254]. Reprinted with permission from [254]. Copyright 2023, American Chemical Society.(f) 4D bio-printed trachea implanted into a damaged rabbit trachea at a scale of 1 cm and images of the native trachea and the 4D bio-printed tissue-engineered trachea at 1, 2, 4, 6, and 8 weeks after the surgery [60]. Copyright 2020, Elsevier. (g) RT-PCR for CPCs seeded and cultured on the 4D bio-printed scaffolds (green) and 2D passive control (orange) for the chondrogenic genes SOX9, COLII, ACAN, and PRG4, and the hypertrophic gene COLX [255]. Copyright 2024, John Wiley and Sons.
4.3. Heart and vascular tissue
In recent years, 4D bioprinting technology has stood out with its unique advantages with good resolution in the construction of the cardiovascular system [256], achieving accurate control of vascular diameter [257] and structure [[258], [259], [260]]. For example, the 3D printed scaffolds can be scaled from a convenient implantation shape to a desired shape under light conditions or solution immersion conditions, which has application prospects in the treatment of vascular diseases and minimally invasive treatment [261]. Poly (D, L-lactide-co-trimethylene carbonate) (PDLA-co-TMC) and gelatin methacrylate (GelMA) were used to prepare porous bilayer shaped deformable scaffolds. The scaffold automatically transformed into a tubular shape when heated to human body temperature (Fig. 11a). GelMA hydrogel layer is used to load vascular endothelial growth factor, and realize the controllable release of VEGF from the stent by controlling the photocrosslinking time. In vitro release experiments have found that vascular endothelial growth factor can be rapidly released within the first 3 days, and stably and continuously released within 21 days (Fig. 11b and c). It is expected to be applied in tissue regeneration such as blood vessels [24].
Fig. 11.
Applications of 4D printing of polymer biomaterials for heart and vascular tissue. (a) Schematic of preparation of bilayer PDLA-co-TMC/GelMA scaffolds by 3D printing [24]. (b) VEGF release rate of PDLA-co-TMC/GelMA-VEGF bilayer scaffolders with different UV crosslinking times in vitro [24]. (c) Fluorescence images (at 4 × and 10 × magnifications) of rMSCs seeded on shape morphing PDLLA-co-TMC/GelMA bilayer scaffolds with or without VEGF after 3-day culture (scale bar: 250 μm) [24]. Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International license (https://creativecommons.org/licenses/by/4.0). (d) Changes in the curvature of heart tissue during diastole and systole [262]. (e) CAD design of the three-dimensional retractable structure of the heart [262]. Licensed under a Creative Commons CC BY license. (f) 4D printing workflow diagram of personalized LAA occluder, transformation, implantation and in situ shape recovery[263]. (g) Formation of new tissue on tube after 21-day implantation shown by H&E staining images. Scale bar: 100 μm [263]. Copyright 2024, Elsevier.
A 4D hydrogel heart patch consisting of GelMA and polyethylene glycol diacrylate (PEGDA) was developed using beam scanning stereolithography printing technology. The gradient internal stress induced by light and the material relaxation induced by solvent drive the autonomous deformation of the printed object, achieving a conformation almost identical to the curvature of the heart surface (Fig. 11d). The material has a highly stretchable microstructure that can switch fiber arrangement from wavy mode to mesh mode to simulate the relaxation and contraction of the cardiac cycle (Fig. 11d and e). By cultivating cardiomyocytes, mesenchymal stromal cells, and endothelial cells, anisotropic epicardial fibers and vascular network structures were printed. After implantation in a mouse myocardial infarction model, anisotropic mechanical adaptation promoted the maturation and vascularization of myocardial cells, demonstrating great potential for application [264].
A Study of 4D-printed obstructors for LAA occlusion advances 4D printing for cardiac applications. A cross-linked PGDA polyacrylic acid (PAA) copolymer was synthesized, with a phase transition temperature range near physiological temperature and considerable elasticity above and below the phase transition temperature. This material can simulate natural tissues and maintain robust mechanical properties even after cyclic deformation. In the in vitro validation of left atrial appendage occlusion via catheterization, the heart of a 23-year-old patient was scanned and the heart model was constructed by 3D printing for in vitro validation based on relevant data. By programming and compressing the PGDA-PAA Left Atrial Appendage Occluder (LAAO) to implant it into a heart model in a minimally invasive manner (Fig. 11f), the shape of the LAAO is restored to its original shape after release near the LAA hole, and the LAAO can effectively seal the LAA. PGDA-PAA tubes are biodegradable, so the graft can be replaced by the patient's own tissue as time goes on (Fig. 11g), thereby improving the integrity and function of blood vessels [263].
4.4. Skin
Wound dressings play an indispensable role as they can absorb exudate, maintain a moist environment, and promote the process of wound healing. These dressings can also deliver drugs or growth factors during the healing process, precisely regulating cell growth and differentiation [14,30]. However, such dressings usually only have a static structure and lack the flexibility to respond to environmental changes. 4D bio-printing creates skin tissues that can change shape over time, which not only improves the accuracy of wound coverage but also provides a powerful tool for studying the dynamic interactions between skin cells and ECM. By precise manipulation of fibroblasts and myofibroblasts [265,266], the in vitro 4D skin model can be constructed which can simulate the dynamic characteristics of skin cells and reveal their complex behavior at the microscopic level [265,267]. During the healing process, temperature [268,269], pH [270,271], glucose levels [[272], [273], [274]], etc. can all affect the process of wound healing. It is of great application value if the wound dressing can respond to these factors and promote wound healing.
Wang et al. [275] prepared a copper peroxide-loaded gelatin/oxidized dextran hydrogel (CGO) that can respond to temperature and pH values (Fig. 12a). As shown in Fig. 12b, when the wound is infected (PH > 6), the hydrogel releases copper peroxide under near-infrared laser irradiation, producing copper ions and hydrogen peroxide for sterilization. When the bacteria are removed from the wound, the pH of the wound becomes acidic, and the hydrogel releases copper peroxide through pH response. Copper ions and oxygen produced by copper peroxide accelerate the wound-healing process and promote the growth of skin tissue. Thermo-reactive injectable hydrogel (MCK) was prepared from keratin (K) and MC in the presence of sodium sulfate. The gel undergoes a sol-gel transition between 30 °C and 33 °C under the interaction between protein and polymer (Fig. 12c). In the evaluation experiment of wound healing in vivo, the wound treated with water gel showed that the degree of wound healing was significantly improved by∼75 % on the 12th day, and by the 18th day, the wound was healed by∼95 % (Fig. 12d and e) [276].
Fig. 12.
Applications of 4D printing of polymer biomaterials for skin tissue. (a) The preparation process and (b) the antimicrobial mechanism of CGO hydrogels [275]. Copyright 2024, Elsevier. (c) Diagram of hydrogel formation process [276]. (d) Images of wounds treated with MC_30K hydrogel and Tegaderm (control group) at days 0, 6, 12, and 18 [276]. (e) Healing rates of wound treated with MC_30K and untreated at different time points [276]. Copyright 2024, Elsevier. (f) Schematic diagram of shape fixation of PU/PVB polymer chains under the action of electric field during electrostatic spinning [277]. (g) Shrinkage behavior of different shapes of PU/PVB electrospun fiber membranes. Scale bar indicates 1 cm [277]. Copyright 2024, John Wiley and Sons. (h) Preparation process and application of wound dressing[278]. (i) Colour change images showing the progression of the infection and the mechanism of color change [278]. Licensed under a Creative Commons CC BY license.
Chronic skin wounds are often characterized by reduced tissue contractility, and cell survival may also be inhibited in the wound environment. Mechanical force has been proven to be an effective method in promoting contraction and healing of the wound [266,279]. Zhang's team [280] developed NIPAM-co-AAC micro gel by using N- isopropylacrylamide (NIPAM) and acrylic acid (AAC) monomers through improved sedimentation polymerization. Instantaneous self-contraction occurs when exposed to the near-physiological temperature, which drags on the wound and promotes healing. In another work, a thermally triggered shrinkage and shape recoverable nanofiber ribbon with a “rigid-elastic” binary synergistic component was constructed through electrospinning. The contracted film has good stretchability and toughness. During the electrospinning process, the PU in the material is significantly stretched, and the stretched molecular chains are fixed in the rigid PVB network, forming a temporary shape (Fig. 12f). Under the stimulation of temperature, molecular chain migration leads to rapid shrinkage behavior of the material and FMs showed rapid healing and good healing effect in rat skin wound models (Fig. 12g) [277].
In addition to the smart materials that directly promote the growth of skin tissue mentioned above, there is also a kind of material detecting the process of wound healing that indirectly plays a significant role in skin tissue regeneration. This dressing is loaded with antimicrobial drugs (STAC) and methylene blue (MB) in the base material (Fig. 12h). The color of MB in the dressing changes when the amount of bacterial loading exceeds the bacterial inhibition threshold of the dressing, through which method, the infection situation of the wound can be visually observed, and corresponding measures can be taken in time for treatment (Fig. 12h and i) [278].
4.5. Nerves
Neural tissue regeneration is a complex biological phenomenon, and the ability to regenerate is greatly limited when the damage to nerves is severe [47,281]. Moreover, after peripheral nerve injury, the problems of slow nerve regeneration and axonal regeneration mismatch are also difficult to solve. Multi-channel neural guide catheters (NGCs) have been widely studied and applied in the field of neural tissue regeneration to simulate the natural structure of nerves [259]. The hydrogel [[282], [283], [284], [285], [286]] with good folding ability and the oriented electrospun fibers [287,288] have become the ideal matrix materials for nerve tissue regeneration.
A two-component hydrogel system composed of alginate and MC (A/MC) was prepared. The content of the two components was adjusted to ensure the different swelling ratios of the material. The printed gel sheet was implanted into the rat sciatic nerve defect model, and the shape of the gel changed under the stimulation of water to form a catheter (Fig. 13a–c). The potential of this material in repairing and promoting the regeneration of peripheral nerves was also confirmed (Fig. 13d) [286]. A study combined poly(L-lactide-co-trimethylene carbonate) (PLATMC) and Ti3C2Tx MXene nanosheets to prepare a conduit for neural tissue repair and regeneration (Fig. 13e–f). This material can be activated at body temperature to spontaneously curl into a filling structure, covering damaged nerves and promoting nerve tissue regeneration (Fig. 13g and h). The material is printed into a structure with oriented micropores (Fig. 13i), which accelerate the directional migration of cells along the pore direction during the repair process to repair the nerve tissue faster. Meanwhile, the addition of electroactive material MXene can improve the conductivity of the conduit, forming a complete electrical pathway during nerve repair (Fig. 13j), effectively promoting cell proliferation and microvascular formation, thereby promoting the repair of peripheral nerve defects [289]. Two-dimensional nerve conduits constructed by electrospinning can be rapidly restored into three-dimensional tubes in water at 40 °C, transforming them into three-channel nerve conduits with three embedded tubes. As shown in Fig. 13, Fig. 12 weeks after implantation of the catheter in a 10 mm nerve defect in the rat sciatic nerve, erythrocytes were detected in the newly formed blood vessels between the regenerated nerve tissues, suggesting the potential application of this material in promoting the regeneration of nerve tissues [288]. In addition, the orientation effect caused by electrostatic spinning can induce the directional extension of neurons and promote the repair of damaged nerves [285].
Fig. 13.
Applications of 4D printing of polymer biomaterials for nerve tissue. (a) 4D printing process of nerve catheter [286]. (b) Schematic diagram of scaffold implantation in the SD rat sciatic nerve transection model [286]. (c) Digital image of the scaffold implantation site on postoperative day 45 (scale bar: 1 cm) [286]. (d) Pictures of transverse slices of H&E stained rat sciatic nerve. (i-iii, vii-ix) scale bar: 200 μm; (iv-vi) scale bar: 50 μm [286]. Copyright 2023, John Wiley and Sons. (e) PLATMC synthesis process [289]. (f) 3D printing of MXPLT scaffolds. (g) MXPLT scaffolds spontaneously curled at body temperature to (h) repair sciatic nerve injury in rats [289]. (i) SEM imaging of MXPLT cross-layer structure [289]. (j) Photos of MXPLT lighting up an LED [289]. Copyright 2024, John Wiley and Sons. (k) H&E staining of a cross-section of regenerated nerve tissue after 12 weeks (black arrows indicate deeply stained closed circles). Scale: 400 μm, 50 μm [288]. (l) Quantitative analysis of neonatal microvessels density and (m) diameter [288]. Copyright 2024, John Wiley and Sons.
5. Challenges and prospects
5.1. Exploring the effective method to construct bio-ink
In the in-depth exploration of the innovation of 4D printing technology, the meticulous preparation of intelligent bio-ink undoubtedly occupies a core position. This type of ink designed specifically for the human environment requires a series of high standards, such as excellent biocompatibility, degradability, and mechanical properties adjusted based on implant site specificity, aiming to integrate and promote the recovery process of the organism. An efficient and forward-looking strategy is to integrate stimulus-response mechanisms into existing 3D printing inks, such as embedding magnetic responsive particles to enable the ink to accurately respond to changes in external magnetic fields or introducing photosensitive chemical groups to endow them with the ability to control light deformation, thereby expanding their application scenarios in the medical field. Another method is to modify and upgrade the basic materials deeply through physical and chemical modification such as incorporating flexible PCL segments into a rigid molecular skeleton to balance and enhance the mechanical strength and stimulus-response sensitivity of the material. In addition, synthesizing polymer monomers with specific functional groups through molecular design, and then forming polymer materials with specific properties through polymerization reactions is also an effective method. Meanwhile, nanotechnology can also be used to composite polymers with inorganic nanoparticles, biomolecules, etc., forming multifunctional composite materials with excellent properties. From the perspective of practical applications and biomimetic design, researchers are committed to constructing stimulus-responsive structures that are highly compatible with living organisms. This strategy is deeply rooted in the principles of bionics, designing bio-inks that can intelligently respond to environmental changes, and promote tissue repair and regeneration by simulating the complex structures and functional mechanisms of organisms in nature. The process not only requires a profound understanding of biomaterials science but also the integration and innovation of interdisciplinary knowledge to jointly promote the development of 4D printing bio-ink technology towards a more intelligent and personalized direction [14].
5.2. Constructing responsive materials for multiple stimuli
4D printing polymer biomaterials are developing towards multiple stimulus responsiveness which indicates that materials will no longer be limited to the response of a single stimulus source (such as temperature, humidity), but can flexibly respond to various external changes including light, electric fields, magnetic fields, and even the synergistic effects of these stimulus sources. By regulating the molecular structure and response mechanism of materials, it is expected to realize the structural remodeling and functional transformation of materials in complex environments. 4D printing polymer materials must be perfectly integrated and adapted to the complex ecosystem of the human body when applied in the biomedical field which requires the materials to have both excellent biocompatibility and the ability to accurately perceive and respond to diverse stimuli in the human body. Therefore, the development of intelligent materials that can autonomously respond to various internal regulatory mechanisms of the human body, such as neural regulation and fluid regulation, will be the focus of future research [48] In the future, we may witness a revolutionary transformation: materials can adjust themselves according to human needs without external stimulation, achieving true "intelligence" integration which is necessary to use multi-level and multi-scale methods to ensure precise control of material behavior [290]. Meanwhile, exploring design strategies with multiple states, configurations, and transformations will promote the development of materials towards more flexible, adaptable, and programmable directions, establishing a solid foundation for building an intelligent medical system.
5.3. Constructing theoretical models and design methods
It is crucial to construct an accurate and comprehensive theoretical model to explore the complex and varied stimulus-response behavior of 4D printing polymer materials. The model system requires deep integration of multiple disciplines, such as materials science, mechanics, chemistry, and computer science, to achieve precise prediction of the dynamic evolution of material conformation and functional implementation path in specific environments through highly refined mathematical simulations and complex calculations. The construction of theoretical models can break through the boundaries of traditional design and achieve precise design and control of material structures from micro to macro levels. At the same time, the introduction of intelligent algorithms ensures that each output achieves dual optimization of performance and application by continuously optimizing printing parameters and process paths. It is worth noting that the application prospects of 4D printed polymer materials in the field of medical implants are particularly broad, but this also means that the evaluation of their performance and effectiveness needs to be more rigorous and comprehensive. Therefore, improving the accuracy of clinical imaging technology, accelerating the upgrade of modeling software, enhancing the controllability and flexibility of deformation programming, and developing specialized software that can accurately simulate the deformation process in vivo have become key issues that urgently need to be addressed. Furthermore, we need to be aware that the advancement of theoretical models and design methods is a dynamic and cyclical process. On the one hand, the improvement of theoretical models requires continuous absorption of experimental data and feedback from practical applications; On the other hand, innovation in design methods will provide new ideas and possibilities for experimental design and practical applications. The two aspects complement each other and jointly promote the development of 4D printing polymer materials science, bringing revolutionary changes to multiple fields such as medicine, engineering, aerospace, etc.
5.4. Establishing a comprehensive evaluation system
To thoroughly and deeply analyze the comprehensive performance of 4D printing polymer materials and their effectiveness in practical applications, it is urgently need to construct a multidimensional, multi-level, and comprehensive evaluation system. This system will not be limited to traditional mechanical performance testing but will be extended to multiple dimensions such as shape memory properties of materials, biocompatibility evaluation, and quantitative analysis of stimulus-response accuracy, thus forming a three-dimensional and dynamic evaluation network. In this system, each evaluation indicator plays a crucial role, collectively forming the standard for assessing the quality of materials. By comprehensively utilizing these indicators, it is possible to comprehensively and accurately reveal the intrinsic characteristics and external performance of materials, providing solid data support and theoretical basis for the improvement and optimization of materials.
It is particularly noteworthy that we need to emphasize the close connection between manufacturing processes and material structure design. Different manufacturing processes and structural designs often have an impact on the final performance of materials. Therefore, in the evaluation system, it is necessary to introduce an analysis module of the correlation between manufacturing processes and structural design, explore the inherent relationship between the two by constructing a detailed model, and validate and optimize the model with experimental data. This process not only helps to understand the mechanism by which manufacturing processes affect material properties but also provides scientific guidance for optimizing manufacturing processes and improving structural design.
In summary, building a comprehensive performance evaluation system is the key to deepening the understanding of 4D printing polymer materials, promoting technological development and application expansion. By establishing and improving this system, we can have a more comprehensive and in-depth understanding of the performance characteristics and application advantages of materials, providing strong support and guarantee for scientific research and technological innovation in related fields [291].
5.5. Promoting cell compatibility to cell regulation
How to achieve harmonious coexistence between cells and materials has become a key issue in the construction and application of smart materials. The compatibility issue between cells and materials is particularly prominent especially when loading tissue cells into material structures. Organic small molecules and photoinitiators in traditional 4D printing materials may be a potential threat to cells. Therefore, researchers are committed to developing new additives with low toxicity and high biocompatibility and optimizing the preparation process to reduce harmful residues. At the same time, strategies such as surface modification are used to isolate harmful substances from the material, ensuring the safety and health of cells. In addition, for light-stimulated materials, it is necessary to conduct in-depth research on the toxicity of their photoinitiators and the impact of photothermal conversion effects on tissues, to ensure that the performance of the material is stimulated without damaging the vitality of cells. During the printing process, protecting cells from mechanical damage such as shear forces is equally important. The introduction of additives such as nanoclay not only enhances the mechanical properties of the material but also effectively reduces the damage to cells [292]. In addition, the combined application of copolymers and bioactive peptides has opened up new methods for improving cell adhesion and vitality. By adjusting the ratio of these components, the material formula can be further optimized to promote the growth of cells in 4D bio-printing structures [100].
5.6. Promoting commercial development
From the perspective of practical clinical applications, the commercialization process of 4D printing biomaterials still faces great challenges. The first is the scalability of the material. As 4D printing materials have “dynamic” characteristics, requiring complex design and precise control, maintaining the quality and stability of the material in mass production is extremely challenging. Second, from preliminary research to practical application, to ensure that printed biomaterials meet strict quality and safety standards, a large number of tests and validations are required, which undoubtedly needs extremely high costs in the early stage. Despite the unique properties of printed biomaterials, their production efficiency still needs to be improved, and the benefits generated in the short term are difficult to compete with traditional biomaterials manufacturing methods. Finally, in terms of legal regulation, as an emerging technology, the safety and efficacy evaluation standards are not perfect enough, and the existing laws and regulations may not be able to fully cover its complex characteristics and clinical application scenarios. Moreover, 4D printing biomaterials show great advantages in the minimally invasive field, and as implantable materials, their legal regulation process is more stringent. However, with the development of the technology and the popularization of its application, the legal regulation is expected to usher in a positive change to adapt to the rapid development of the technology and strengthen the supervision of the whole process of research and development, production and application of 4D printing biomaterials. In conclusion, balancing these factors in research and technology development is key to being success and sustainability, which requires interdisciplinary collaboration, in-depth market research, and a deep understanding of regulations.
6. Conclusion
This paper provides a comprehensive and systematic overview of 4D printing polymeric biomaterials in tissue regeneration. With their dynamically tunable shape and properties, such materials open up innovative paths for the customized design of tissue scaffolds and the intelligent construction of drug delivery systems. In the process of transformation from materials to biological tissues, polymers must strictly follow a series of core principles, such as biocompatibility, controlled degradation, and efficient stimulus responsiveness, to ensure their safe and effective application in living organisms. Based on the satisfaction of these basic principles, 3D printing technology combined with polymer materials can precisely construct complex structures suitable for the regeneration of various tissues, such as bone, cartilage, blood vessels, and skin, demonstrating its great potential in the field of tissue engineering. In this paper, we further discuss the research progress of typical polymers used for 4D printing, deeply analyze the stimulus-response mechanism of these polymers and their composites at the structural level, and elaborate on their respective advantages and relevant application examples. Although polymer biomaterials for 4D printing still face many challenges in practical application, it is undeniable that, as the core components of 4D printing technology, these materials have shown broad application prospects and unlimited possibilities in promoting the development of tissue engineering, regenerative medicine, and personalized medicine and other cutting-edge fields. In the future, with the continuous innovation of technology and optimization of materials, 4D printing polymeric biomaterials are expected to achieve more far-reaching influence and change in the field of biomedicine.
CRediT authorship contribution statement
Zhe Wang: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Conceptualization. Duo Ma: Methodology, Formal analysis. Juan Liu: Methodology, Formal analysis. Shi Xu: Methodology, Formal analysis. Fang Qiu: Methodology, Formal analysis. Liqiu Hu: Methodology, Formal analysis. Yueming Liu: Writing – review & editing, Supervision. Changneng Ke: Writing – review & editing, Supervision, Methodology, Formal analysis. Changshun Ruan: Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key R&D Program (Grant No. 2022YFB3804403), the National Natural Science Foundation of China (Grant Nos. 92468106 and 82402533), the Natural Science Foundation of Guangdong Province in China (Grant No. 2024B1515040018), the Shenzhen Fundamental Research Foundation (Grant Nos. JCYJ20220818101613028, and JSGGKQTD20210831174330015), the Shenzhen Medical Research Fund (Grant No. B2402016), the Shenzhen Science and Technology Program (JCYJ20240813153012017).
Footnotes
Peer review under the responsibility of KeAi Communications Co., Ltd.
This manuscript is a review article and no animal-related experiments were performed.
Contributor Information
Yueming Liu, Email: 12022889@qq.com.
Changneng Ke, Email: kekey88@163.com.
Changshun Ruan, Email: cs.ruan@siat.ac.cn.
References
- 1.Karima B., Habibi M., Laperriere L. 4D printing of fiber-Reinforced Auxetic structures: the building blocks: a review. Smart Mater. Struct. 2024;33(6) doi: 10.1088/1361-665X/ad469d. [DOI] [Google Scholar]
- 2.Radzuan N.A.M., Foudzi F.M., Sulong A.B., Al-Furjan M.S.H., Royan N.R.R. Quick insight into the dynamic dimensions of 4D printing in polymeric composite mechanics. Rev. Adv. Mater. Sci. 2024;63(1) doi: 10.1515/rams-2024-0011. [DOI] [Google Scholar]
- 3.Gopinath S., Adarsh N.N., Radhakrishnan Nair P., Mathew S. Recent Trends in thermo-responsive elastomeric shape memory polymer nanocomposites. Polym. Compos. 2023;44(8):4433–4458. doi: 10.1002/pc.27464. [DOI] [Google Scholar]
- 4.Liang M., Liu Q., Chen Q., Wu Y., Wu C., Wang Y. Self-assembling gelatin-Curdlan Fibril hydrogels for oriented neural cell growth. ACS Appl. Mater. Interfaces. 2024;16(13):15741–15751. doi: 10.1021/acsami.3c17379. [DOI] [PubMed] [Google Scholar]
- 5.Jiang C., Xu G., Gao J. Stimuli-responsive macromolecular self-assembly. Sustainability. 2022;14(18) doi: 10.3390/su141811738. [DOI] [Google Scholar]
- 6.Zhao W., Li N., Liu L., Leng J., Liu Y. Mechanical behaviors and applications of shape memory polymer and its composites. Appl. Phys. Rev. 2023;10(1) doi: 10.1063/5.0126892. [DOI] [Google Scholar]
- 7.Dayyoub T., Maksimkin A.V., Filippova O.V., Tcherdyntsev V.V., Telyshev D.V. Shape memory polymers as smart materials: a review. Polymers. 2022;14(17):3511. doi: 10.3390/polym14173511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Li F., Luo Y., Shi Y., Ma X., Zhang M., Boukhvalov D.W., Luo Z. A self-healing elastomer based on an intrinsic non-covalent cross-linking mechanism. J. Mater. Chem. A. 2019;7(25):15207–15214. doi: 10.1039/c9ta03775f. [DOI] [Google Scholar]
- 9.Zhou J., Sheiko S.S. Reversible shape‐Shifting in polymeric materials. J. Polym. Sci. B Polym. Phys. 2016;54(14):1365–1380. doi: 10.1002/polb.24014. [DOI] [Google Scholar]
- 10.Zende R., Ghase V., Jamdar V. A review on shape memory polymers. Polymer-Plastics Technology and Materials. 2022;62(4):467–485. doi: 10.1080/25740881.2022.2121216. [DOI] [Google Scholar]
- 11.Chen W., Bu Y., Li D., Liu C., Chen G., Wan X., Li N. High-strength, Tough, and self-healing hydrogel based on carboxymethyl cellulose. Cellulose. 2019;27(2):853–865. doi: 10.1007/s10570-019-02797-z. [DOI] [Google Scholar]
- 12.G. Stoychev, N. Puretskiy, L. Ionov, Self-Folding All-Polymer Thermoresponsive Microcapsules, Soft Matter. 7 (7), (2011), 3277, 10.1039/c1sm05109a. [DOI]
- 13.Amukarimi S., Rezvani Z., Eghtesadi N., Mozafari M. Smart biomaterials: from 3D printing to 4D bioprinting. Methods. 2022;205:191–199. doi: 10.1016/j.ymeth.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Lai J., Liu Y., Lu G., Yung P., Wang X., Tuan R.S., Li Z.A. 4D bioprinting of programmed dynamic tissues. Bioact. Mater. 2024;37:348–377. doi: 10.1016/j.bioactmat.2024.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alshahrani H.A. Review of 4D printing materials and Reinforced composites: behaviors, applications and challenges. J. Sci.: Advanced Materials and Devices. 2021;6(2):167–185. doi: 10.1016/j.jsamd.2021.03.006. [DOI] [Google Scholar]
- 16.Keshavarz M., Jahanshahi M., Hasany M., Kadumudi F.B., Mehrali M., Shahbazi M.-A., Alizadeh P., Orive G., Dolatshahi-Pirouz A. Smart alginate inks for tissue engineering applications. Materials Today Bio. 2023;23 doi: 10.1016/j.mtbio.2023.100829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mtibe A., Muniyasamy S., Mokhena T.C., Ofosu O., Ojijo V., John M. Recent insight into the biomedical applications of Polybutylene succinate and Polybutylene succinate-based materials. Express Polym. Lett. 2023;17(1):2–28. doi: 10.3144/expresspolymlett.2023.2. [DOI] [Google Scholar]
- 18.Zhang X., Yang Y., Yang Z., Ma R., Aimaijiang M., Xu J., Zhang Y., Zhou Y. Four-dimensional printing and shape memory materials in bone tissue engineering. Int. J. Mol. Sci. 2023;24(1):814. doi: 10.3390/ijms24010814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan W., Zhou J., Liu K., Li X., Xu W., Song H., Shan G., Bao Y., Zhao Q., Pan P. Sequence-rearranged Cocrystalline polymer network with shape Reconfigurability and tunable switching temperature. ACS Macro Lett. 2020;9(4):588–594. doi: 10.1021/acsmacrolett.0c00075. [DOI] [PubMed] [Google Scholar]
- 20.Gugulothu S.B., Chatterjee K. Visible light-based 4D-Bioprinted tissue scaffold. ACS Macro Lett. 2023;12(4):494–502. doi: 10.1021/acsmacrolett.3c00036. [DOI] [PubMed] [Google Scholar]
- 21.Kelly S.S., Suarez C.A., Mirsky N.A., Slavin B.V., Brochu B., Vivekanand Nayak V., El Shatanofy M., Witek L., Thaller S.R., Coelho P.G. Application of 3D printing in Cleft Lip and Palate repair. J. Craniofac. Surg. 2024 doi: 10.1097/scs.0000000000010294. [DOI] [PubMed] [Google Scholar]
- 22.Li S., Huan Y., Zhu B., Chen H., Tang M., Yan Y., Wang C., Ouyang Z., Li X., Xue J., Wang W. Research progress on the biological modifications of implant materials in 3D printed Intervertebral fusion Cages. J. Mater. Sci. Mater. Med. 2022;33(1):2. doi: 10.1007/s10856-021-06609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu H., Gao C., Liu K., Fu H., Liu Z., Kouwer P.H.J., Han Z., Ruan C. Gradient Matters via filament diameter-Adjustable 3D printing. Nat. Commun. 2024;15(1) doi: 10.1038/s41467-024-47360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai J., Li J., Wang M. 3D printed porous tissue engineering scaffolds with the self-folding ability and controlled release of growth factor. MRS Communications. 2020;10(4):579–586. doi: 10.1557/mrc.2020.65. [DOI] [Google Scholar]
- 25.Lantean S., Barrera G., Pirri C.F., Tiberto P., Sangermano M., Roppolo I., Rizza G. 3D printing of Magnetoresponsive polymeric materials with tunable mechanical and magnetic properties by Digital light processing. Advanced Materials Technologies. 2019;4(11) doi: 10.1002/admt.201900505. [DOI] [Google Scholar]
- 26.Wilson S.A., Cross L.M., Peak C.W., Gaharwar A.K. Shear-thinning and thermo-reversible Nanoengineered inks for 3D bioprinting. ACS Appl. Mater. Interfaces. 2017;9(50):43449–43458. doi: 10.1021/acsami.7b13602. [DOI] [PubMed] [Google Scholar]
- 27.Lim K.S., Galarraga J.H., Cui X., Lindberg G.C.J., Burdick J.A., Woodfield T.B.F. Fundamentals and applications of Photo-cross-linking in bioprinting. Chem. Rev. 2020;120(19):10662–10694. doi: 10.1021/acs.chemrev.9b00812. [DOI] [PubMed] [Google Scholar]
- 28.Anandakrishnan N., Ye H., Guo Z., Chen Z., Mentkowski K.I., Lang J.K., Rajabian N., Andreadis S.T., Ma Z., Spernyak J.A., Lovell J.F., Wang D., Xia J., Zhou C., Zhao R. Fast stereolithography printing of large-scale biocompatible hydrogel models. Adv. Healthcare Mater. 2021;10(10) doi: 10.1002/adhm.202002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Q., Niu X., Shao L., Zhou L., Lin Z., Sun A., Fu J., Chen Z., Hu J., Liu Y., He Y. 3D printing of complex Gelma-based scaffolds with nanoclay. Biofabrication. 2019;11(3) doi: 10.1088/1758-5090/ab0cf6. [DOI] [PubMed] [Google Scholar]
- 30.Bartolo P., Malshe A., Ferraris E., Koc B. 3D bioprinting: materials, processes, and applications. Cirp Annals-Manufacturing Technology. 2022;71(2):577–597. doi: 10.1016/j.cirp.2022.06.001. [DOI] [Google Scholar]
- 31.Schwab A., Levato R., D'Este M., Piluso S., Eglin D., Malda J. Printability and shape fidelity of Bioinks in 3D bioprinting. Chem. Rev. 2020;120(19):11028–11055. doi: 10.1021/acs.chemrev.0c00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das A., Gilmer E.L., Biria S., Bortner M.J. Importance of polymer Rheology on material extrusion additive manufacturing: Correlating process Physics to print properties. ACS Appl. Polym. Mater. 2021;3(3):1218–1249. doi: 10.1021/acsapm.0c01228. [DOI] [Google Scholar]
- 33.Lokhande G., Carrow J.K., Thakur T., Xavier J.R., Parani M., Bayless K.J., Gaharwar A.K. Nanoengineered injectable hydrogels for wound healing application. Acta Biomater. 2018;70:35–47. doi: 10.1016/j.actbio.2018.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Träger A., Naeimipour S., Jury M., Selegård R., Aili D. Nanocellulose Reinforced Hyaluronan-based Bioinks. Biomacromolecules. 2023;24(7):3086–3093. doi: 10.1021/acs.biomac.3c00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao K., Varghese P J G., Chen P., Hu J. Developing a Transcatheter injectable nanoclay- alginate gel for minimally invasive Procedures. J. Mech. Behav. Biomed. Mater. 2024;152 doi: 10.1016/j.jmbbm.2024.106448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie M., Su J., Zhou S., Li J., Zhang K. Application of hydrogels as three-dimensional bioprinting ink for tissue engineering. Gels. 2023;9(2):88. doi: 10.3390/gels9020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naghieh S., Chen X. Printability–a key issue in extrusion-based bioprinting. Journal of Pharmaceutical Analysis. 2021;11(5):564–579. doi: 10.1016/j.jpha.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao T., Gillispie G.J., Copus J.S., Pr A.K., Seol Y.-J., Atala A., Yoo J.J., Lee S.J. Optimization of gelatin–alginate composite Bioink printability using rheological parameters: a systematic approach. Biofabrication. 2018;10(3) doi: 10.1088/1758-5090/aacdc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadernezhad A., Groll J. Machine Learning reveals a general understanding of printability in Formulations based on Rheology additives. Adv. Sci. 2022;9(29) doi: 10.1002/advs.202202638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai J., Wang C., Wang M. 3D printing in biomedical engineering: processes, materials, and applications. Appl. Phys. Rev. 2021;8(2) doi: 10.1063/5.0024177. [DOI] [Google Scholar]
- 41.Hasanzadeh R., Mihankhah P., Azdast T., Rasouli A., Shamkhali M., Park C.B. Biocompatible tissue-engineered scaffold polymers for 3D printing and its application for 4D printing. Chem. Eng. J. 2023;476 doi: 10.1016/j.cej.2023.146616. [DOI] [Google Scholar]
- 42.Hussain M., Khan S.M., Shafiq M., Abbas N., Sajjad U., Hamid K. Advances in biodegradable materials: degradation mechanisms, mechanical properties, and biocompatibility for Orthopedic applications. Heliyon. 2024;10(12) doi: 10.1016/j.heliyon.2024.e32713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubey A., Vahabi H., Kumaravel V. Antimicrobial and biodegradable 3D printed scaffolds for Orthopedic infections. ACS Biomater. Sci. Eng. 2023;9(7):4020–4044. doi: 10.1021/acsbiomaterials.3c00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng X., Dong K., Wu Z., Wang J., Wang Z.L. A review on emerging biodegradable polymers for environmentally Benign transient Electronic skins. J. Mater. Sci. 2021;56(30):16765–16789. doi: 10.1007/s10853-021-06323-0. [DOI] [Google Scholar]
- 45.Ramezani M., Ripin Z.M. 4D printing in biomedical engineering: Advancements, challenges, and future directions. J. Funct. Biomater. 2023;14(7):347. doi: 10.3390/jfb14070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nain A., Chakraborty S., Jain N., Choudhury S., Chattopadhyay S., Chatterjee K., Debnath S. 4D hydrogels: fabrication strategies, stimulation mechanisms, and biomedical applications. Biomater. Sci. 2024;12(13) doi: 10.1039/d3bm02044d. [DOI] [PubMed] [Google Scholar]
- 47.Faber L., Yau A., Chen Y. Translational biomaterials of Four-dimensional bioprinting for tissue regeneration. Biofabrication. 2024;16(1) doi: 10.1088/1758-5090/acfdd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Z., Tang W., Yang J., Fan C. Application of 4D printing and bioprinting in cardiovascular tissue engineering. Biomater. Sci. 2023;11(19):6403–6420. doi: 10.1039/d3bm00312d. [DOI] [PubMed] [Google Scholar]
- 49.Noroozi R., Arif Z.U., Taghvaei H., Khalid M.Y., Sahbafar H., Hadi A., Sadeghianmaryan A., Chen X. 3D and 4D bioprinting Technologies: a Game changer for the biomedical sector? Ann. Biomed. Eng. 2023;51(8):1683–1712. doi: 10.1007/s10439-023-03243-9. [DOI] [PubMed] [Google Scholar]
- 50.Mahmood A., Akram T., Shenggui C., Chen H. Revolutionizing manufacturing: a review of 4D printing materials, stimuli, and cutting-edge applications. Composites Part B-Engineering. 2023;266 doi: 10.1016/j.compositesb.2023.110952. [DOI] [Google Scholar]
- 51.Wan X., Xiao Z., Tian Y., Chen M., Liu F., Wang D., Liu Y., Bartolo P.J.D.S., Yan C., Shi Y., Zhao R.R., Qi H.J., Zhou K. Recent advances in 4D printing of advanced materials and structures for functional applications. Adv. Mater. 2024;36(34) doi: 10.1002/adma.202312263. [DOI] [PubMed] [Google Scholar]
- 52.Han D., Lu Z., Chester S.A., Lee H. Micro 3D printing of a temperature-responsive hydrogel using Projection micro-stereolithography. Sci. Rep. 2018;8(1):1963. doi: 10.1038/s41598-018-20385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z., Wang Z., Zheng Y., He Q., Wang Y., Cai S. Three-dimensional printing of functionally graded liquid crystal elastomer. Sci. Adv. 2020;6(39):eabc0034. doi: 10.1126/sciadv.abc0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kocak G., Tuncer C., Bütün V. pH-Responsive Polymers. Polym. Chem. 2017;8(1):144–176. doi: 10.1039/c6py01872f. [DOI] [Google Scholar]
- 55.Liu H., Wang F., Wu W., Dong X., Sang L. 4D printing of mechanically robust PLA/TPU/Fe3O4 Magneto-responsive shape memory polymers for smart structures. Compos. B Eng. 2023;248 doi: 10.1016/j.compositesb.2022.110382. [DOI] [Google Scholar]
- 56.Zhou W., Dong X., He Y., Zheng W., Leng J. In-vitro and in-vivo studies of 4D printed shape memory scaffolds with bioactive fillers and coating for enhanced bone tissue regeneration. Smart Mater. Struct. 2022;31(10) doi: 10.1088/1361-665X/ac884a. [DOI] [Google Scholar]
- 57.Yang H., Leow W.R., Wang T., Wang J., Yu J., He K., Qi D., Wan C., Chen X. 3D printed photoresponsive devices based on shape memory composites. Adv. Mater. 2017;29(33) doi: 10.1002/adma.201701627. [DOI] [PubMed] [Google Scholar]
- 58.Lu X., Ambulo C.P., Wang S., Rivera‐Tarazona L.K., Kim H., Searles K., Ware T.H. 4D‐Printing of Photoswitchable Actuators. Angew. Chem. Int. Ed. 2021;60(10):5536–5543. doi: 10.1002/anie.202012618. [DOI] [PubMed] [Google Scholar]
- 59.Dong X.Y., Zhang F.H., Wang L.L., Liu Y.J., Leng J.S. 4D printing of electroactive shape-changing composite structures and their programmable behaviors. Compos. Appl. Sci. Manuf. 2022;157 doi: 10.1016/j.compositesa.2022.106925. [DOI] [Google Scholar]
- 60.Kim S.H., Seo Y.B., Yeon Y.K., Lee Y.J., Park H.S., Sultan M.T., Lee J.M., Lee J.S., Lee O.J., Hong H., Lee H., Ajiteru O., Suh Y.J., Song S.-H., Lee K.-H., Park C.H. 4D-Bioprinted silk hydrogels for tissue engineering. Biomaterials. 2020;260 doi: 10.1016/j.biomaterials.2020.120281. [DOI] [PubMed] [Google Scholar]
- 61.Narupai B., Smith P.T., Nelson A. 4D printing of multi‐stimuli responsive protein‐based hydrogels for autonomous shape transformations. Adv. Funct. Mater. 2021;31(23) doi: 10.1002/adfm.202011012. [DOI] [Google Scholar]
- 62.Doberenz F., Zeng K., Willems C., Zhang K., Groth T. Thermoresponsive polymers and their biomedical application in tissue engineering – a review. J. Mater. Chem. B. 2020;8(4):607–628. doi: 10.1039/c9tb02052g. [DOI] [PubMed] [Google Scholar]
- 63.Kouka M.A., Abbassi F., Habibi M., Chabert F., Zghal A., Garnier C. 4D printing of shape memory polymers, blends, and composites and their advanced applications: a comprehensive Literature review. Adv. Eng. Mater. 2022;25(4) doi: 10.1002/adem.202200650. [DOI] [Google Scholar]
- 64.Staszczak M., Nabavian Kalat M., Golasiński K.M., Urbański L., Takeda K., Matsui R., Pieczyska E.A. Characterization of polyurethane shape memory polymer and Determination of shape Fixity and shape recovery in subsequent Thermomechanical cycles. Polymers. 2022;14(21):4775. doi: 10.3390/polym14214775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi G., Huang C., Cao X., Liu M., Zhang J., Zheng K., Ma Y. Triple shape memory effect of ethylene-vinyl acetate copolymer/poly(propylene carbonate) blends with broad composite ratios and phase Morphologies. Polymer. 2021;231 doi: 10.1016/j.polymer.2021.124144. [DOI] [Google Scholar]
- 66.Wang X., Shan M., Zhang S., Chen X., Liu W., Chen J., Liu X. Stimuli‐responsive antibacterial materials: molecular structures, design principles, and biomedical applications. Adv. Sci. 2022;9(13) doi: 10.1002/advs.202104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X., Li M., Tang L., Shi D., Lam E., Bae J. 3D shape morphing of stimuli-responsive composite hydrogels. Mater. Chem. Front. 2023;7(23):5989–6034. doi: 10.1039/d3qm00856h. [DOI] [Google Scholar]
- 68.Pourmadadi M., Farokh A., Rahmani E., Eshaghi M.M., Aslani A., Rahdar A., Ferreira L.F.R. Polyacrylic acid mediated targeted drug delivery nano-systems: a review. J. Drug Deliv. Sci. Technol. 2023;80 doi: 10.1016/j.jddst.2023.104169. [DOI] [Google Scholar]
- 69.Huang S.-H., Juang R.-S. Biochemical and biomedical applications of multifunctional magnetic nanoparticles: a review. J. Nanoparticle Res. 2011;13(10):4411–4430. doi: 10.1007/s11051-011-0551-4. [DOI] [Google Scholar]
- 70.Dubey R., Shende P. Potential of Brush and Mushroom conformations in biomedical applications. Chem. Pap. 2024;78(12):6873–6889. doi: 10.1007/s11696-024-03602-3. [DOI] [Google Scholar]
- 71.Schoeller J., Itel F., Wuertz-Kozak K., Fortunato G., Rossi R.M. pH-responsive electrospun nanofibers and their applications. Polym. Rev. 2021;62(2):351–399. doi: 10.1080/15583724.2021.1939372. [DOI] [Google Scholar]
- 72.Tian B., Liu J. Smart stimuli-responsive chitosan hydrogel for drug delivery: a review. Int. J. Biol. Macromol. 2023;235 doi: 10.1016/j.ijbiomac.2023.123902. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt A.M. Electromagnetic activation of shape memory polymer networks containing magnetic nanoparticles. Macromol. Rapid Commun. 2006;27(14):1168–1172. doi: 10.1002/marc.200600225. [DOI] [Google Scholar]
- 74.Pardo A., Gómez-Florit M., Barbosa S., Taboada P., Domingues R.M.A., Gomes M.E. Magnetic nanocomposite hydrogels for tissue engineering: design Concepts and Remote Actuation strategies to control cell fate. ACS Nano. 2021;15(1):175–209. doi: 10.1021/acsnano.0c08253. [DOI] [PubMed] [Google Scholar]
- 75.Huang T.-Y., Huang G.-L., Zhang C.-Y., Zhuang B.-W., Liu B.-X., Su L.-Y., Ye J.-Y., Xu M., Kuang M., Xie X.-Y. Supramolecular photothermal Nanomedicine mediated distant Tumor inhibition via Pd-1 and Tim-3 blockage. Front. Chem. 2020;8:1. doi: 10.3389/fchem.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wan X., He Y., Liu Y., Leng J. 4D printing of multiple shape memory polymer and nanocomposites with biocompatible, programmable and selectively Actuated properties. Addit. Manuf. 2022;53 doi: 10.1016/j.addma.2022.102689. [DOI] [Google Scholar]
- 77.Tang J., Yin Q., Qiao Y., Wang T. Shape morphing of hydrogels in alternating magnetic field. ACS Appl. Mater. Interfaces. 2019;11(23):21194–21200. doi: 10.1021/acsami.9b05742. [DOI] [PubMed] [Google Scholar]
- 78.Tang J., Tong Z., Xia Y., Liu M., Lv Z., Gao Y., Lu T., Xie S., Pei Y., Fang D., Wang T.J. Super Tough magnetic hydrogels for Remotely triggered shape morphing. J. Mater. Chem. B. 2018;6(18):2713–2722. doi: 10.1039/c8tb00568k. [DOI] [PubMed] [Google Scholar]
- 79.Zhu P., Yang W., Wang R., Gao S., Li B., Li Q. 4D printing of complex structures with a Fast response time to magnetic stimulus. ACS Appl. Mater. Interfaces. 2018;10(42):36435–36442. doi: 10.1021/acsami.8b12853. [DOI] [PubMed] [Google Scholar]
- 80.Yang Y., Zhang S., Ji Y., Wei Y., Wang J., He X. Magneto-responsive liquid crystalline elastomer nanocomposites. Mater. Today. 2024;74:167–186. doi: 10.1016/j.mattod.2024.02.001. [DOI] [Google Scholar]
- 81.Dai H., Li K., Yang D. Controllable regulation of Diesel Oil-in-water Pickering emulsion stability by Multiresponsive Recyclable magnetic polymer Brush microvessels. ACS Appl. Mater. Interfaces. 2024;16(14):17715–17727. doi: 10.1021/acsami.4c03069. [DOI] [PubMed] [Google Scholar]
- 82.Doostmohammadi H., Baniassadi M., Bodaghi M., Baghani M. 4D printing of Magneto‐thermo‐responsive PLA/Pmma/Fe3O4 nanocomposites with superior shape memory and Remote Actuation. Macromol. Mater. Eng. 2024;309(9) doi: 10.1002/mame.202400090. [DOI] [Google Scholar]
- 83.Kim Y., Yuk H., Zhao R., Chester S.A., Zhao X. Printing Ferromagnetic Domains for Untethered Fast-transforming soft materials. Nature. 2018;558(7709):274–279. doi: 10.1038/s41586-018-0185-0. [DOI] [PubMed] [Google Scholar]
- 84.Zhang S., Zhang Y., Wu Y., Yang Y., Chen Q., Liang H., Wei Y., Ji Y. A magnetic Solder for assembling Bulk covalent adaptable network blocks. Chem. Sci. 2020;11(29):7694–7700. doi: 10.1039/d0sc01678k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herrera-Posada S., Mora-Navarro C., Ortiz-Bermudez P., Torres-Lugo M., McElhinny K.M., Evans P.G., Calcagno B.O., Acevedo A. Magneto-responsive liquid crystalline elastomer nanocomposites as potential Candidates for dynamic cell culture Substrates. Mater. Sci. Eng. C. 2016;65:369–378. doi: 10.1016/j.msec.2016.04.063. [DOI] [PubMed] [Google Scholar]
- 86.Ditter D., Blümler P., Klöckner B., Hilgert J., Zentel R. Microfluidic synthesis of liquid crystalline elastomer particle Transport systems which can Be Remote‐controlled magnetically. Adv. Funct. Mater. 2019;29(29) doi: 10.1002/adfm.201902454. [DOI] [Google Scholar]
- 87.Lendlein A., Shastri V.P. Stimuli‐sensitive polymers. Adv. Mater. 2010;22(31):3344–3347. doi: 10.1002/adma.201002520. [DOI] [PubMed] [Google Scholar]
- 88.Pourmasoumi P., Moghaddam A., Mahand S.N., Heidari F., Moghaddam Z.S., Arjmand M., Kuehnert I., Kruppke B., Wiesmann H.-P., Khonakdar H.A. A review on the recent progress, Opportunities, and challenges of 4D printing and bioprinting in regenerative medicine. Journal of Biomaterials Science. 2023;(1):108–146. doi: 10.1080/09205063.2022.2110480. [DOI] [PubMed] [Google Scholar]
- 89.Ma Z., Wu J., Tan Y., Tan C. Azobenzene-based Conjugated polymers: synthesis, properties, and biological applications. Macromol. Rapid Commun. 2024;45(12) doi: 10.1002/marc.202400048. [DOI] [PubMed] [Google Scholar]
- 90.Aundhia C., Parmar G., Talele C., Talele D., Seth A.K. Light sensitive Liposomes: a Novel strategy for targeted drug delivery. Pharm. Nanotechnol. 2024;13(1):41–54. doi: 10.2174/0122117385271651231228073850. [DOI] [PubMed] [Google Scholar]
- 91.Bretel G., Tran D.-T., Morandi G., Lapinte V., Marais S., Hespel L. Synthesis of an original Oxazoline based monomer containing a photosensitive azobenzene Moiety and investigation of the behavior in solid state, in Aqueous and vapor media of a Photo and thermo-responsive Polyoxazoline copolymer. Polymer. 2023;271 doi: 10.1016/j.polymer.2023.125812. [DOI] [Google Scholar]
- 92.Kabirian F., Mela P., Heying R. 4D printing applications in the development of smart cardiovascular implants. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.873453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Z.J., Li C.Y., Zhao X.Y., Wu Z.L., Zheng Q. Thermo- and Photo-responsive composite hydrogels with programmed deformations. J. Mater. Chem. B. 2019;7(10):1674–1678. doi: 10.1039/c8tb02896f. [DOI] [PubMed] [Google Scholar]
- 94.Chen L., Weng M., Zhou P., Huang F., Liu C., Fan S., Zhang W. Graphene‐based Actuator with integrated‐Sensing function. Adv. Funct. Mater. 2018;29(5) doi: 10.1002/adfm.201806057. [DOI] [Google Scholar]
- 95.Hua D., Zhang X., Ji Z., Yan C., Yu B., Li Y., Wang X., Zhou F. 3D printing of shape changing composites for constructing flexible paper-based photothermal bilayer Actuators. J. Mater. Chem. C. 2018;6(8):2123–2131. doi: 10.1039/c7tc05710e. [DOI] [Google Scholar]
- 96.Yang X., Chen W., Liu H., Yang B., Xie Y., Wang Y., Lei Y., Xue L. Light‐driven shape memory of 3D‐printed Peek for programmable Actuations. Advanced Materials Technologies. 2022;7(11) doi: 10.1002/admt.202200266. [DOI] [Google Scholar]
- 97.Shao Z., Liu Y., Cai P., Wang Q., Xiao Z., Zhang L., Tong B., Wang B., Zhao Y., Zhang W., Xia Y. Poly(M-Phenylene Isophthalamide)/carbon black nanoparticle composite film as high-temperature electric heater. J. Electron. Mater. 2024;53(8):4601–4612. doi: 10.1007/s11664-024-11147-0. [DOI] [Google Scholar]
- 98.Kumar S., Ojha N., Ramesh M.R., Balan A.S.S., Doddamani M. Shape memory behavior of 4D printed CF/Pekk high temperature composite under subsequent Thermomechanical cycles. Mater. Lett. 2024;366 doi: 10.1016/j.matlet.2024.136567. [DOI] [Google Scholar]
- 99.Ruan K., Shi X., Zhang Y., Guo Y., Zhong X., Gu J. Electric‐field‐induced Alignment of functionalized carbon nanotubes inside thermally conductive liquid crystalline Polyimide composite films. Angew. Chem. Int. Ed. 2023;62(38) doi: 10.1002/anie.202309010. [DOI] [PubMed] [Google Scholar]
- 100.Li Y.-C., Zhang Y.S., Akpek A., Shin S.R., Khademhosseini A. 4D bioprinting: the Next-generation technology for Biofabrication enabled by stimuli-responsive materials. Biofabrication. 2016;9(1) doi: 10.1088/1758-5090/9/1/012001. [DOI] [PubMed] [Google Scholar]
- 101.Sarmad S., Yenici G., Gürkan K., Keçeli G., Gürdağ G. Electric field responsive chitosan–poly(N,N-Dimethyl acrylamide) semi-Ipn gel films and their dielectric, thermal and swelling Characterization. Smart Mater. Struct. 2013;22(5) doi: 10.1088/0964-1726/22/5/055010. [DOI] [Google Scholar]
- 102.Mulakkal M.C., Trask R.S., Ting V.P., Seddon A.M. Responsive cellulose-hydrogel composite ink for 4D printing. Mater. Des. 2018;160:108–118. doi: 10.1016/j.matdes.2018.09.009. [DOI] [Google Scholar]
- 103.Huang W.M., Yang B., An L., Li C., Chan Y.S. Water-driven programmable polyurethane shape memory polymer: Demonstration and mechanism. Appl. Phys. Lett. 2005;86(11) doi: 10.1063/1.1880448. [DOI] [Google Scholar]
- 104.Wang J., Zhou Z., Li X., Chang C. Cellulose nanocrystals-based optical Organohydrogel fiber with customizable Iridescent colors for strain and humidity response. Int. J. Biol. Macromol. 2024;275 doi: 10.1016/j.ijbiomac.2024.133501. [DOI] [PubMed] [Google Scholar]
- 105.Lv Y., Wang B., Cheng Y., Lv W., Zeng S., Xiao H. Printing characteristics and microwave infrared-induced 4D printing of Chestnut Powder composite Paste. J. Food Eng. 2024;382 doi: 10.1016/j.jfoodeng.2024.112197. [DOI] [Google Scholar]
- 106.Pingale P., Dawre S., Dhapte-Pawar V., Dhas N., Rajput A. Advances in 4D printing: from stimulation to simulation. Drug Delivery and Translational Research. 2022;13(1):164–188. doi: 10.1007/s13346-022-01200-y. [DOI] [PubMed] [Google Scholar]
- 107.Li Z., Fan Z., Xu Y., Lo W., Wang X., Niu H., Li X., Xie X., Khan M., Guan J. pH-sensitive and thermosensitive hydrogels as stem-cell carriers for cardiac therapy. ACS Appl. Mater. Interfaces. 2016;8(17):10752–10760. doi: 10.1021/acsami.6b01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kanaan A.F., Piedade A.P., de Sousa H.C., Dias A.M.A. Semi-interpenetrating chitosan/ionic liquid polymer networks as Electro-responsive biomaterials for potential wound dressings and Iontophoretic applications. Mater. Sci. Eng. C. 2021;121 doi: 10.1016/j.msec.2020.111798. [DOI] [PubMed] [Google Scholar]
- 109.Shokrani H., Shokrani A., Seidi F., Mashayekhi M., Kar S., Nedeljkovic D., Kuang T., Saeb M.R., Mozafari M. Polysaccharide-based biomaterials in a Journey from 3D to 4D printing. Bioengineering & Translational Medicine. 2023;8(4) doi: 10.1002/btm2.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Momeni F., Mehdi HassaniN S.M., Liu X., Ni J. A review of 4D printing. Mater. Des. 2017;122:42–79. doi: 10.1016/j.matdes.2017.02.068. [DOI] [Google Scholar]
- 111.Liu Y., Genzer J., Dickey M.D. “2d or not 2d”: shape-programming polymer sheets. Prog. Polym. Sci. 2016;52:79–106. doi: 10.1016/j.progpolymsci.2015.09.001. [DOI] [Google Scholar]
- 112.Villar G., Graham A.D., Bayley H. A tissue-like printed material. Science. 2013;340(6128):48–52. doi: 10.1126/science.1229495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu J., Yuan C., Ding Z., Isakov M., Mao Y., Wang T., Dunn M.L., Qi H.J. Multi-shape active composites by 3D printing of Digital shape memory polymers. Sci. Rep. 2016;6(1) doi: 10.1038/srep24224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Q., Zhang K., Hu G. Smart three-dimensional Lightweight structure triggered from a Thin composite sheet via 3D printing Technique. Sci. Rep. 2016;6(1) doi: 10.1038/srep22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Naficy S., Gately R., Gorkin R., Xin H., Spinks G.M. 4D printing of reversible shape morphing hydrogel structures. Macromol. Mater. Eng. 2017;302(1) doi: 10.1002/mame.201600212. [DOI] [Google Scholar]
- 116.Bonetti L., Scalet G. 4D fabrication of shape-changing systems for tissue engineering: state of the Art and perspectives. Progress in Additive Manufacturing. 2024 doi: 10.1007/s40964-024-00743-5. [DOI] [Google Scholar]
- 117.Appavoo D., Azim N., Elshatoury M., Antony D.-X., Rajaraman S., Zhai L. Four-dimensional printing of multi-material Origami and Kirigami-Inspired hydrogel self-folding structures. Materials. 2024;17(20):5028. doi: 10.3390/ma17205028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yan S., Zhang F., Luo L., Wang L., Liu Y., Leng J. Shape memory polymer composites: 4D printing. Smart Structures, and Applications, Research. 2023;6 doi: 10.34133/research.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li H., Zhang B., Ye H., Jian B., He X., Cheng J., Sun Z., Wang R., Chen Z., Lin J., Xiao R., Liu Q., Ge Q. Reconfigurable 4D printing via mechanically robust covalent adaptable network shape memory polymer. Sci. Adv. 2024;10(20) doi: 10.1126/sciadv.adl4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lai J., Xie M., Zhao Q., Zhang C., Wang Z., Xia H. Underwater non‐contact and Ultra‐Fast adaptive self‐healing elastomers based on programmable Dissociation of dynamic bonds. Adv. Funct. Mater. 2024 doi: 10.1002/adfm.202415732. [DOI] [Google Scholar]
- 121.Nezhad-Mokhtari P., Hasany M., Kohestanian M., Dolatshahi-Pirouz A., Milani M., Mehrali M. Recent advancements in Bioadhesive self-healing hydrogels for effective Chronic wound Care. Adv. Colloid Interface Sci. 2024;334 doi: 10.1016/j.cis.2024.103306. [DOI] [PubMed] [Google Scholar]
- 122.Liu D., Yang K., Xu L., Shen X., Feng L., Jiang Y., Ali A., Lu J., Guo L. Self-assembly study of block Copolypeptoids in response to pH and temperature stimulation. Polymers. 2024;16(8):1082. doi: 10.3390/polym16081082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takashima Y., Yonekura K., Koyanagi K., Iwaso K., Nakahata M., Yamaguchi H., Harada A. Multifunctional stimuli-responsive supramolecular materials with stretching, coloring, and self-healing properties functionalized via host–guest interactions. Macromolecules. 2017;50(11):4144–4150. doi: 10.1021/acs.macromol.7b00875. [DOI] [Google Scholar]
- 124.Quan L., Xin Y., Wu X., Ao Q. Mechanism of self-healing hydrogels and application in tissue engineering. Polymers. 2022;14(11):2184. doi: 10.3390/polym14112184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.An Z.-W., Xue R., Ye K., Zhao H., Liu Y., Li P., Chen Z.-M., Huang C.-X., Hu G.-H. Recent advances in self-healing polyurethane based on dynamic covalent bonds combined with other self-healing methods. Nanoscale. 2023;15(14):6505–6520. doi: 10.1039/d2nr07110j. [DOI] [PubMed] [Google Scholar]
- 126.Chen T., Chen Y., Rehman H.U., Chen Z., Yang Z., Wang M., Li H., Liu H. Ultratough, self-healing, and tissue-adhesive hydrogel for wound dressing. ACS Appl. Mater. Interfaces. 2018;10(39):33523–33531. doi: 10.1021/acsami.8b10064. [DOI] [PubMed] [Google Scholar]
- 127.Xu J., Liu Y., Hsu S.-h. Hydrogels based on Schiff base Linkages for biomedical applications. Molecules. 2019;24(16):3005. doi: 10.3390/molecules24163005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Terryn S., Brancart J., Roels E., Verhelle R., Safaei A., Cuvellier A., Vanderborght B., Van Assche G. Structure–property relationships of self-healing polymer networks based on reversible Diels–Alder chemistry. Macromolecules. 2022;55(13):5497–5513. doi: 10.1021/acs.macromol.2c00434. [DOI] [Google Scholar]
- 129.Vauthier M., Serra C.A. Controlled reversible aggregation of Thermoresponsive polymeric nanoparticles by interfacial Diels-Alder reaction. Colloids Surf. A Physicochem. Eng. Asp. 2022;648 doi: 10.1016/j.colsurfa.2022.129321. [DOI] [Google Scholar]
- 130.Smithmyer M.E., Deng C.C., Cassel S.E., LeValley P.J., Sumerlin B.S., Kloxin A.M. Self-healing boronic acid-based hydrogels for 3D Co-cultures. ACS Macro Lett. 2018;7(9):1105–1110. doi: 10.1021/acsmacrolett.8b00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lei Z.Q., Xiang H.P., Yuan Y.J., Rong M.Z., Zhang M.Q. Room-temperature self-Healable and Remoldable cross-linked polymer based on the dynamic exchange of disulfide bonds. Chem. Mater. 2014;26(6):2038–2046. doi: 10.1021/cm4040616. [DOI] [Google Scholar]
- 132.Ma J., Wen S., Zhou Z. Bioinspired thermoplastic elastomer with flexible, self-healing capabilities. Langmuir. 2022;38(29):8862–8870. doi: 10.1021/acs.langmuir.2c00955. [DOI] [PubMed] [Google Scholar]
- 133.Devi V. K A., Shyam R., Palaniappan A., Jaiswal A.K., Oh T.-H., Nathanael A.J. Self-healing hydrogels: preparation, mechanism and advancement in biomedical applications. Polymers. 2021;13(21):3782. doi: 10.3390/polym13213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yu R., Yang Y., He J., Li M., Guo B. Novel supramolecular self-healing silk fibroin-based hydrogel via host–guest interaction as wound dressing to enhance wound healing. Chem. Eng. J. 2021;417 doi: 10.1016/j.cej.2020.128278. [DOI] [Google Scholar]
- 135.Palungan J., Luthfiyah W., Mustopa A.Z., Nurfatwa M., Rahman L., Yulianty R., Wathoni N., Yoo J.-W., Hasan N. The Formulation and Characterization of wound dressing releasing S-Nitrosoglutathione from polyvinyl alcohol/borax Reinforced carboxymethyl chitosan self-healing hydrogel. Pharmaceutics. 2024;16(3):344. doi: 10.3390/pharmaceutics16030344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Almutairi M.D., Aria A.I., Thakur V.K., Khan M.A. Self-healing mechanisms for 3D-printed polymeric structures: from Lab to reality. Polymers. 2020;12(7):1534. doi: 10.3390/polym12071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu D., Yang S., Peng S., Chen Y., Zhang L., Tan J. Simultaneous synthesis and self‐assembly of Bottlebrush block copolymers at room temperature via photoinitiated Raft dispersion polymerization. Macromol. Rapid Commun. 2022;43(8) doi: 10.1002/marc.202100921. [DOI] [PubMed] [Google Scholar]
- 138.Dergham M., Lin S., Geng J. Supramolecular self‐assembly in living cells. Angew. Chem. Int. Ed. 2022;61(18) doi: 10.1002/anie.202114267. [DOI] [PubMed] [Google Scholar]
- 139.Dong S., Luo Y., Yan X., Zheng B., Ding X., Yu Y., Ma Z., Zhao Q., Huang F. A dual‐responsive supramolecular polymer gel formed by Crown ether based molecular recognition. Angew. Chem. Int. Ed. 2011;50(8):1905–1909. doi: 10.1002/anie.201006999. [DOI] [PubMed] [Google Scholar]
- 140.Xiang Y., Liu C., Ma S., Wang X., Zhu L., Bao C. Stimuli‐responsive peptide self‐assembly to construct hydrogels with Actuation and shape memory behaviors. Adv. Funct. Mater. 2023;33(34) doi: 10.1002/adfm.202300416. [DOI] [Google Scholar]
- 141.Frazar E.M., Shah R.A., Dziubla T.D., Hilt J.Z. Multifunctional temperature‐responsive polymers as advanced biomaterials and beyond. J. Appl. Polym. Sci. 2019;137(25) doi: 10.1002/app.48770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mallakpour S., Tabesh F., Hussain C.M. A new Trend of using poly(vinyl alcohol) in 3D and 4D printing Technologies: process and applications. Adv. Colloid Interface Sci. 2022;301 doi: 10.1016/j.cis.2022.102605. [DOI] [PubMed] [Google Scholar]
- 143.Rivera-Hernández G., Antunes-Ricardo M., Martínez-Morales P., Sánchez M.L. Polyvinyl alcohol based-drug delivery systems for Cancer treatment. Int. J. Pharm. 2021;600 doi: 10.1016/j.ijpharm.2021.120478. [DOI] [PubMed] [Google Scholar]
- 144.Jin S.G. Production and application of biomaterials based on polyvinyl alcohol (PVA) as wound dressing. Chem. Asian J. 2022;17(21) doi: 10.1002/asia.202200595. [DOI] [PubMed] [Google Scholar]
- 145.Zhong Y., Lin Q., Yu H., Shao L., Cui X., Pang Q., Zhu Y., Hou R. Construction methods and biomedical applications of PVA-based hydrogels. Front. Chem. 2024;12 doi: 10.3389/fchem.2024.1376799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dwivedi R., Kumar S., Pandey R., Mahajan A., Nandana D., Katti D.S., Mehrotra D. Polycaprolactone as biomaterial for bone scaffolds: review of Literature. Journal of Oral Biology and Craniofacial Research. 2020;10(1):381–388. doi: 10.1016/j.jobcr.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Olubamiji A.D., Izadifar Z., Si J.L., Cooper D.M.L., Eames B.F., Chen D.X.B. Modulating mechanical Behaviour of 3D-printed cartilage-Mimetic PCL scaffolds: influence of molecular weight and pore Geometry. Biofabrication. 2016;8(2) doi: 10.1088/1758-5090/8/2/025020. [DOI] [PubMed] [Google Scholar]
- 148.Kovaleva P.A., Pariy I.O., Chernozem R.V., Zadorozhnyy M. Yu, Permyakova E.S., Kolesnikov E.A., Surmeneva M.A., Surmenev R.A., Senatov F.S. Shape memory effect in hybrid Polylactide-based polymer scaffolds functionalized with reduced graphene oxide for tissue engineering. Eur. Polym. J. 2022;181 doi: 10.1016/j.eurpolymj.2022.111694. [DOI] [Google Scholar]
- 149.Zhang S., Liu T., Zhao B., Verdi C., Liu W., Hao C., Zhang J. Shape memory poly(lactic acid) binary blends with Unusual Fluorescence. Polymer. 2020;209 doi: 10.1016/j.polymer.2020.122980. [DOI] [Google Scholar]
- 150.DeStefano V., Khan S., Tabada A. Applications of PLA in modern medicine. Engineered Regeneration. 2020;1:76–87. doi: 10.1016/j.engreg.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mehrpouya M., Vahabi H., Janbaz S., Darafsheh A., Mazur T.R., Ramakrishna S. 4D printing of shape memory polylactic acid (PLA) Polymer. 2021;230 doi: 10.1016/j.polymer.2021.124080. [DOI] [Google Scholar]
- 152.Bavaliya K.J., Vala N.S., Raj M., Raj L. A review on biodegradable composites based on poly (lactic acid) with various bio fibers. Chem. Pap. 2024;78(5):2695–2728. doi: 10.1007/s11696-023-03298-x. [DOI] [Google Scholar]
- 153.Mondal S. Temperature responsive shape memory polyurethanes. Polymer-Plastics Technology and Materials. 2021:1–28. doi: 10.1080/25740881.2021.1906903. [DOI] [Google Scholar]
- 154.Ha Y.-M., Seo H.C., Kim Y.-O., Khil M.-S., Cho J.W., Lee J.-S., Jung Y.C. Effects of hard segment of polyurethane with disulfide bonds on shape memory and self-healing ability. Macromol. Res. 2019;28(3):234–240. doi: 10.1007/s13233-020-8027-y. [DOI] [Google Scholar]
- 155.Agarwal K., Srinivasan V., Lather V., Pandita D., Vasanthan K.S. Insights of 3D bioprinting and focusing the Paradigm Shift towards 4D printing for biomedical applications. J. Mater. Res. 2023;38(1):112–141. doi: 10.1557/s43578-022-00524-2. [DOI] [Google Scholar]
- 156.El-Husseiny H.M., Mady E.A., Doghish A.S., Zewail M.B., Abdelfatah A.M., Noshy M., Mohammed O.A., El-Dakroury W.A. Smart/stimuli-responsive chitosan/gelatin and other polymeric macromolecules natural hydrogels Vs. Synthetic hydrogels systems for Brain tissue engineering: a state-of-the-Art review. Int. J. Biol. Macromol. 2024;260 doi: 10.1016/j.ijbiomac.2024.129323. [DOI] [PubMed] [Google Scholar]
- 157.Cao P., Tao L., Gong J., Wang T., Wang Q., Ju J., Zhang Y. 4D printing of a sodium alginate hydrogel with step-Wise shape deformation based on Variation of crosslinking density. ACS Appl. Polym. Mater. 2021;3(12):6167–6175. doi: 10.1021/acsapm.1c01034. [DOI] [Google Scholar]
- 158.Xie F., Gao C., Avérous L. Alginate-based materials: enhancing properties through Multiphase Formulation design and processing innovation. Mater. Sci. Eng. R Rep. 2024;159 doi: 10.1016/j.mser.2024.100799. [DOI] [Google Scholar]
- 159.Agarwal T., Chiesa I., Costantini M., Lopamarda A., Tirelli M.C., Borra O.P., Varshapally S.V.S., Kumar Y.A.V., Reddy G.K., De Maria C., Zhang L.G., Maiti T.K. Chitosan and its derivatives in 3D/4D (bio) printing for tissue engineering and drug delivery applications. Int. J. Biol. Macromol. 2023;246 doi: 10.1016/j.ijbiomac.2023.125669. [DOI] [PubMed] [Google Scholar]
- 160.J M.E., Solanki R., Dhanka M., Thareja P., Bhatia D. Self-healing, injectable chitosan-based hydrogels: structure, properties and biological applications. Materials Advances. 2024;5(13):5365–5393. doi: 10.1039/d4ma00131a. [DOI] [Google Scholar]
- 161.Pan S., Zhu C., Wu Y., Tao L. Chitosan-based self-healing hydrogel: from fabrication to biomedical application. Polymers. 2023;15(18):3768. doi: 10.3390/polym15183768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ge L., Li S.-P., Lisak G. Advanced sensing Technologies of Phenolic compounds for Pharmaceutical and biomedical analysis. J. Pharmaceut. Biomed. Anal. 2020;179 doi: 10.1016/j.jpba.2019.112913. [DOI] [PubMed] [Google Scholar]
- 163.Nagase K., Matsuda J., Takeuchi A., Ikemoto Y. Hydration and dehydration behaviors of poly(N-Isopropylacrylamide)-Grafted Silica Beads. Surf. Interfaces. 2023;40 doi: 10.1016/j.surfin.2023.103058. [DOI] [Google Scholar]
- 164.Nagase K. Bioanalytical Technologies using temperature-responsive polymers. Anal. Sci. 2024;40(5):827–841. doi: 10.1007/s44211-024-00545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shaibie N.A., Ramli N.A., Mohammad Faizal N.D.F., Srichana T., Mohd Amin M.C.I. Poly(N‐Isopropylacrylamide)‐Based polymers: recent overview for the development of temperature‐responsive drug delivery and biomedical applications. Macromol. Chem. Phys. 2023;224(20) doi: 10.1002/macp.202300157. [DOI] [Google Scholar]
- 166.Yu Y., Cheng Y., Tong J., Zhang L., Wei Y., Tian M. Recent advances in thermo-sensitive hydrogels for drug delivery. J. Mater. Chem. B. 2021;9(13):2979–2992. doi: 10.1039/d0tb02877k. [DOI] [PubMed] [Google Scholar]
- 167.Chen X.-Y., Wang Y., Ma S.-Q., Huang Y.-Q., Jing W., Wei P.-F., Yu X.-Q., Zhao B. Mechanically active small Intestinal Submucosa hydrogel for accelerating Chronic wound healing. J. Mater. Chem. B. 2022;10(33):6279–6286. doi: 10.1039/d2tb01355j. [DOI] [PubMed] [Google Scholar]
- 168.Malektaj H., Imani R., Siadati M.H. Study of injectable Pnipaam hydrogels containing niosomal Angiogenetic drug delivery system for potential cardiac tissue regeneration. Biomedical Materials. 2021;16(4) doi: 10.1088/1748-605X/abdef8. [DOI] [PubMed] [Google Scholar]
- 169.Razmimanesh F., Sodeifian G. Evaluation of a temperature-responsive Magnetotocosome as a magnetic targeting drug delivery system for Sorafenib Tosylate Anticancer drug. Heliyon. 2023;9(11) doi: 10.1016/j.heliyon.2023.e21794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Colaco R., Appiah C., Staubitz A. Controlling the Lcst-phase transition in azobenzene-functionalized poly (N-Isopropylacrlyamide) hydrogels by light. Gels. 2023;9(2):75. doi: 10.3390/gels9020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ekerdt B.L., Fuentes C.M., Lei Y., Adil M.M., Ramasubramanian A., Segalman R.A., Schaffer D.V. Thermoreversible Hyaluronic Acid‐Pnipaam Hydrogel Systems for 3D Stem Cell Culture. Advanced Healthcare Materials. 2018;7(12) doi: 10.1002/adhm.201800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li Y., Huang C., Duan A., Li M., Zhang X., Lu L., Yu L., Yu L., Liu P., Chen K., Jiang Y. A temperature-sensitive DNA-Pnipaam hydrogel prepared by base Pairing. Colloid Polym. Sci. 2023;301(4):383–388. doi: 10.1007/s00396-023-05071-8. [DOI] [Google Scholar]
- 173.Xue X., Hu Y., Wang S., Chen X., Jiang Y., Su J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022;12:327–339. doi: 10.1016/j.bioactmat.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Klouda L., Mikos A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008;68(1):34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lü S., Liu M., Ni B. Degradable, injectable poly(N-Isopropylacrylamide)-Based hydrogels with low gelation concentrations for protein delivery application. Chem. Eng. J. 2011;173(1):241–250. doi: 10.1016/j.cej.2011.07.052. [DOI] [Google Scholar]
- 176.Lanzalaco S., Armelin E. Poly(N-Isopropylacrylamide) and copolymers: a review on recent Progresses in biomedical applications. Gels. 2017;3(4):36. doi: 10.3390/gels3040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Das D., Ghosh P., Ghosh A., Haldar C., Dhara S., Panda A.B., Pal S. Stimulus-responsive, biodegradable, biocompatible, covalently cross-linked hydrogel based on dextrin and poly(N-isopropylacrylamide) for in vitro/in vivo controlled drug release. ACS Appl. Mater. Interfaces. 2015;7(26):14338–14351. doi: 10.1021/acsami.5b02975. [DOI] [PubMed] [Google Scholar]
- 178.Adelnia H., Ensandoost R., Shebbrin Moonshi S., Gavgani J.N., Vasafi E.I., Ta H.T. Freeze/thawed polyvinyl alcohol hydrogels: present, Past and future. Eur. Polym. J. 2022;164 doi: 10.1016/j.eurpolymj.2021.110974. [DOI] [Google Scholar]
- 179.Mahmoud D.B., Schulz‐Siegmund M. Utilizing 4D printing to design smart Gastroretentive, Esophageal, and Intravesical drug delivery systems. Adv. Healthcare Mater. 2023;12(10) doi: 10.1002/adhm.202202631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Le H.T., Mahara A., Fukazawa K., Nagasaki T., Yamaoka T. Widely distributable and Retainable in-situ gelling material for treating myocardial infarction. Acta Biomater. 2024;176:221–233. doi: 10.1016/j.actbio.2024.01.013. [DOI] [PubMed] [Google Scholar]
- 181.Li H., Wu C., Yu X., Zhang W. Recent advances of PVA-based hydrogels in cartilage repair application. J. Mater. Res. Technol. 2023;24:2279–2298. doi: 10.1016/j.jmrt.2023.03.130. [DOI] [Google Scholar]
- 182.Sharma S., Bhende M., Verma H.R., Kumar S. Physically cross-linked PVA/F-Mwcnts nanocomposite hydrogel with enhanced thermal, mechanical, and dielectric properties. Mater. Today Commun. 2024;40 doi: 10.1016/j.mtcomm.2024.109400. [DOI] [Google Scholar]
- 183.Gonçalves A., Cabrita R., Matos J., Rodrigues I., Vieira T., Borges J.P., Soares P.I.P. Dual-stimuli-responsive poly(vinyl alcohol) nanofibers for localized Cancer treatment: magnetic Hyperthermia and drug release studies. J. Drug Deliv. Sci. Technol. 2024;94 doi: 10.1016/j.jddst.2024.105492. [DOI] [Google Scholar]
- 184.Tamay D.G., Dursun Usal T., Alagoz A.S., Yucel D., Hasirci N., Hasirci V. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 2019;7:164. doi: 10.3389/fbioe.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.He W., Zhou D., Gu H., Qu R., Cui C., Zhou Y., Wang Y., Zhang X., Wang Q., Wang T., Zhang Y. A biocompatible 4D printing shape memory polymer as emerging strategy for fabrication of Deployable medical devices. Macromol. Rapid Commun. 2022;44(2) doi: 10.1002/marc.202200553. [DOI] [PubMed] [Google Scholar]
- 186.Sharma A., Rai A. Fused Deposition Modelling (Fdm) based 3D & 4D printing: a state of Art review. Mater. Today Proc. 2022;62:367–372. doi: 10.1016/j.matpr.2022.03.679. [DOI] [Google Scholar]
- 187.Slavković V., Hanželič B., Plesec V., Milenković S., Harih G. Thermo-mechanical behavior and strain rate sensitivity of 3D-printed polylactic acid (PLA) below glass transition temperature (Tg) Polymers. 2024;16(11):1526. doi: 10.3390/polym16111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mondal D., Griffith M., Venkatraman S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: current scenario and challenges. International Journal of Polymeric Materials and Polymeric Biomaterials. 2016;65(5):255–265. doi: 10.1080/00914037.2015.1103241. [DOI] [Google Scholar]
- 189.Carlson M., Li Y. Development and kinetic evaluation of a low-Cost temperature-sensitive shape memory polymer for 4-dimensional printing. Int. J. Adv. Des. Manuf. Technol. 2020;106(9–10):4263–4279. doi: 10.1007/s00170-020-04927-5. [DOI] [Google Scholar]
- 190.Su S., Kopitzky R., Tolga S., Kabasci S. Polylactide (PLA) and its blends with poly(butylene succinate) (Pbs): a Brief review. Polymers. 2019;11(7):1193. doi: 10.3390/polym11071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kumar R., Singh R., Kumar V., Ranjan N., Gupta J., Bhura N. On 3D printed Thermoresponsive PCL-PLA nanofibers based architected smart nanoporous scaffolds for tissue reconstruction. J. Manuf. Process. 2024;119:666–681. doi: 10.1016/j.jmapro.2024.04.008. [DOI] [Google Scholar]
- 192.Song M., Li S., Zhu G., Guo J. Compatibilised and Toughened of PLA/PCL blends via modified-chitosan linking amorphous regions: 4D printing and shape memory processes. Polym. Test. 2023;125 doi: 10.1016/j.polymertesting.2023.108105. [DOI] [Google Scholar]
- 193.Pandey H., Mohol S.S., Kandi R. 4D printing of tracheal scaffold using shape-memory polymer composite. Mater. Lett. 2022;329 doi: 10.1016/j.matlet.2022.133238. [DOI] [Google Scholar]
- 194.Li A., Chen X.-G., Zhang L.-Y., Zhang Y.-F. Temperature and Infill density effects on thermal, mechanical and shape memory properties of polylactic acid/poly(ε-caprolactone) blends for 4D printing. Materials. 2022;15(24):8838. doi: 10.3390/ma15248838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang Y., Li X. 4D-Printed Bi-material composite Laminate for manufacturing reversible shape-change structures. Compos. B Eng. 2021;219 doi: 10.1016/j.compositesb.2021.108918. [DOI] [Google Scholar]
- 196.Ma X., Wu N., Liu P., Cui H. Fabrication of highly efficient Phenylphosphorylated chitosan bio-based Flame Retardants for Flammable PLA biomaterial. Carbohydr. Polym. 2022;287 doi: 10.1016/j.carbpol.2022.119317. [DOI] [PubMed] [Google Scholar]
- 197.Hamidi M.N., Abdullah J., Shuib R.K., Aziz I., Namazi H. 4D printing of polylactic acid (PLA)/Thermoplastic polyurethane (TPU) shape memory polymer - a review. Engineering Research Express. 2024;6(1) doi: 10.1088/2631-8695/ad337e. [DOI] [Google Scholar]
- 198.Choudhary N., Sharma V., Kumar P. Reinforcement of polylactic acid with Bioceramics (alumina and Ysz composites) and their Thermomechanical and physical properties for biomedical application. J. Vinyl Addit. Technol. 2021;27(3):612–625. doi: 10.1002/vnl.21837. [DOI] [Google Scholar]
- 199.Kumar M., Ghosh S., Kumar V., Sharma V., Roy P. Tribo-mechanical and biological Characterization of Pegda/Bioceramics composites fabricated using stereolithography. J. Manuf. Process. 2022;77:301–312. doi: 10.1016/j.jmapro.2022.03.024. [DOI] [Google Scholar]
- 200.Kumar M., Sharma V. Shape memory effect of Four-dimensional printed polylactic acid-based scaffold with nature-Inspired structure. 3D Print. Addit. Manuf. 2024;11(1):10–23. doi: 10.1089/3dp.2022.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zimina A., Nikitin A., Lvov V., Bulygina I., Kovaleva P., Vodopyanov S., Zadorozhnyy M., Peshkina E., Karshieva S., Choudhary R., Abakumov M., Senatov F. Impact of Cofe2o4 magnetic nanoparticles on the physical and mechanical properties and shape memory effect of Polylactide. Journal of Composites Science. 2024;8(2):48. doi: 10.3390/jcs8020048. [DOI] [Google Scholar]
- 202.Park S., Moon J., Cho M., Lee Y.S., Chung H., Yang S. Multiscale study of shape-memory behavior of Semicrystalline polyurethane nanocomposites Doped with Silica nanoparticles based on Coarse-Grained molecular dynamics simulation. ACS Appl. Polym. Mater. 2024;6(6):3192–3206. doi: 10.1021/acsapm.3c02968. [DOI] [Google Scholar]
- 203.Du J., Zhang W., Yang W., Sun B., Xian C., Li Y., Xia T., Yang C. Study of shape memory mechanism and performance of shape memory polyurethane prepared based on tetrahydrofuran. J. Mol. Struct. 2024;1302 doi: 10.1016/j.molstruc.2024.137484. [DOI] [Google Scholar]
- 204.Liu J., Chen Z., Hu C., Yang W., Wang J., Xu W., Wang Y., Ruan C., Luo Y. Fluorescence Visualization directly Monitors microphase separation behavior of shape memory polyurethanes. Appl. Mater. Today. 2021;23 doi: 10.1016/j.apmt.2021.100986. [DOI] [Google Scholar]
- 205.Zhang L., Jiang Y., Xiong Z., Liu X., Na H., Zhang R., Zhu J. Highly recoverable rosin-based shape memory polyurethanes. J. Mater. Chem. A. 2013;1(10):3263. doi: 10.1039/c3ta01655b. [DOI] [Google Scholar]
- 206.Lee B.S., Chun B.C., Chung Y.-C., Sul K.I., Cho J.W. Structure and Thermomechanical properties of polyurethane block copolymers with shape memory effect. Macromolecules. 2001;34(18):6431–6437. doi: 10.1021/ma001842l. [DOI] [Google Scholar]
- 207.Wang Y., Zhu M., Hao C., Dai R., Huang M., Liu H., He S., Liu W. Development of semi-crystalline polyurethane with self-healing and body temperature-responsive shape memory properties. Eur. Polym. J. 2022;167 doi: 10.1016/j.eurpolymj.2022.111060. [DOI] [Google Scholar]
- 208.Pathak R., Punetha V.D., Bhatt S., Punetha M. Advances in graphene‐Reinforced polyurethane nanocomposites: an advanced shape memory material. ChemistrySelect. 2023;8(45) doi: 10.1002/slct.202303261. [DOI] [Google Scholar]
- 209.Li Y.-M., Patel K.D., Han Y.-K., Hong S.-M., Meng Y.-X., Lee H.-H., Park J.H., Knowles J.C., Hyun J.K., Lee J.-H., Kim H.-W. Electroconductive and Mechano-Competent PUCL@CNT Nanohybrid scaffolds guiding neuronal Specification of neural stem/progenitor cells. Chem. Eng. J. 2023;466 doi: 10.1016/j.cej.2023.143125. [DOI] [Google Scholar]
- 210.Tantisuwanno C., Jain T., Tseng Y.-M., Joy A. Pendant amines in the hard or soft segments of PCL-Polyurethanes have Contrasting effects on the mechanical and surface properties. Macromolecules. 2024;57(9):4448–4459. doi: 10.1021/acs.macromol.3c02292. [DOI] [Google Scholar]
- 211.Wu L., Bao L., Wang Z., Yu Z., Wang B., Chen Q., Ling Y., Qin Y., Tang K., Cai Y., Huang R. Emulation of Synaptic scaling based on Mos2 Neuristor for self‐Adaptative Neuromorphic computing. Advanced Electronic Materials. 2021;7(4) doi: 10.1002/aelm.202001104. [DOI] [Google Scholar]
- 212.Punetha V.D., Rana S., Yoo H.J., Chaurasia A., McLeskey J.T., Ramasamy M.S., Sahoo N.G., Cho J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: a Comparison study between CNT and graphene. Prog. Polym. Sci. 2017;67:1–47. doi: 10.1016/j.progpolymsci.2016.12.010. [DOI] [Google Scholar]
- 213.Jiang R., Zheng X., Zhu S., Li W., Zhang H., Liu Z., Zhou X. Recent advances in functional polyurethane chemistry: from structural design to applications. ChemistrySelect. 2023;8(11) doi: 10.1002/slct.202204132. [DOI] [Google Scholar]
- 214.Idumah C.I., Odera R.S., Ezeani E.O., Low J.H., Tanjung F.A., Damiri F., Luing W.S. Construction, Characterization, properties and multifunctional applications of stimuli-responsive shape memory polymeric Nanoarchitectures: a review. Polymer-Plastics Technology and Materials. 2023;62(10):1247–1272. doi: 10.1080/25740881.2023.2204936. [DOI] [Google Scholar]
- 215.George M., Abraham T.E. Polyionic Hydrocolloids for the Intestinal delivery of protein drugs: alginate and chitosan — a review. J. Contr. Release. 2006;114(1):1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 216.Neves M.I., Moroni L., Barrias C.C. Modulating alginate hydrogels for improved biological performance as cellular 3D microenvironments. Front. Bioeng. Biotechnol. 2020;8:665. doi: 10.3389/fbioe.2020.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xu J., Song W., Ren L., Wu N., Zeng R., Wang S., Wang Z., Zhang Q. Reinforced hydrogel building via Formation of alginate-chitosan double network with pH & Salt-responsiveness and electric conductivity for soft Actuators. Int. J. Biol. Macromol. 2024;263 doi: 10.1016/j.ijbiomac.2024.130282. [DOI] [PubMed] [Google Scholar]
- 218.Nathan K.G., Genasan K., Kamarul T. Polyvinyl alcohol-chitosan scaffold for tissue engineering and regenerative medicine application: a review. Mar. Drugs. 2023;21(5):304. doi: 10.3390/md21050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhou X., Zhang B., Huang W. Carboxymethyl chitosan and dialdehyde cellulose nanocrystal based injectable self-healing emulsion gel. Carbohydr. Polym. 2024;338 doi: 10.1016/j.carbpol.2024.122211. [DOI] [PubMed] [Google Scholar]
- 220.Xiong M., Chen Y., Hu H.-J., Cheng H., Li W.-X., Tang S., Hu X., Lan L.-M., Zhang H., Jiang G.-B. Multifunctional pH-responsive hydrogel dressings based on carboxymethyl chitosan: synthesis, Characterization Fostering the wound healing. Carbohydr. Polym. 2024;341 doi: 10.1016/j.carbpol.2024.122348. [DOI] [PubMed] [Google Scholar]
- 221.Mallakpour S., Sirous F., Hussain C.M. Current achievements in 3D bioprinting technology of chitosan and its hybrids. New J. Chem. 2021;45(24):10565–10576. doi: 10.1039/d1nj01497h. [DOI] [Google Scholar]
- 222.Omidian H., Wilson R.L., Gill E.J. Advancements and challenges in self-healing hydrogels for wound Care. Gels. 2024;10(4):241. doi: 10.3390/gels10040241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li A., Ma B., Hua S., Ping R., Ding L., Tian B., Zhang X. Chitosan-based injectable hydrogel with multifunction for wound healing: a critical review. Carbohydr. Polym. 2024;333 doi: 10.1016/j.carbpol.2024.121952. [DOI] [PubMed] [Google Scholar]
- 224.Baghaie S., Khorasani M.T., Zarrabi A., Moshtaghian J. Wound healing properties of PVA/Starch/Chitosan hydrogel membranes with nano Zinc oxide as antibacterial wound dressing material. Journal of Biomaterials Science. 2017;28(18):2220–2241. doi: 10.1080/09205063.2017.1390383. [DOI] [PubMed] [Google Scholar]
- 225.X. Hao, H. Liu, Y. Xie, C. Fang, H. Yang, Thermal-Responsive Self-Healing Hydrogel Based on Hydrophobically Modified Chitosan and Vesicle, Colloid Polym. Sci. 291 (7), 1749-1758, 10.1007/s00396-013-2910-4). [DOI]
- 226.Zhang J., Chen L., Shen B., Wang Y., Peng P., Tang F., Feng J. Highly transparent, self-healing, injectable and self-adhesive chitosan/Polyzwitterion-based double network hydrogel for potential 3D printing Wearable strain sensor. Mater. Sci. Eng. C. 2020;117 doi: 10.1016/j.msec.2020.111298. [DOI] [PubMed] [Google Scholar]
- 227.Fu B., Wang X., Chen Z., Jiang N., Guo Z., Zhang Y., Zhang S., Liu X., Liu L. Improved myocardial performance in infarcted rat heart by injection of disulfide-cross-linked chitosan hydrogels loaded with basic fibroblast growth factor. J. Mater. Chem. B. 2022;10(4):656–665. doi: 10.1039/d1tb01961a. [DOI] [PubMed] [Google Scholar]
- 228.Nisar S., Pandit A.H., Wang L.-F., Rattan S. Strategy to design a smart Photocleavable and pH sensitive chitosan based hydrogel through a Novel crosslinker: a potential Vehicle for controlled drug delivery. RSC Adv. 2020;10(25):14694–14704. doi: 10.1039/c9ra10333c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.El-Husseiny H.M., Mady E.A., Hamabe L., Abugomaa A., Shimada K., Yoshida T., Tanaka T., Yokoi A., Elbadawy M., Tanaka R. Smart/stimuli-responsive hydrogels: cutting-edge platforms for tissue engineering and other biomedical applications. Materials Today Bio. 2022;13 doi: 10.1016/j.mtbio.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang Y., Cui H., Esworthy T., Mei D., Wang Y., Zhang L.G. Emerging 4D printing strategies for Next‐generation tissue regeneration and medical devices. Adv. Mater. 2022;34(20) doi: 10.1002/adma.202109198. [DOI] [PubMed] [Google Scholar]
- 231.Yang J., Chen Z., Gao C., Liu J., Liu K., Wang X., Pan X., Wang G., Sang H., Pan H., Liu W., Ruan C. A mechanical-Assisted post-bioprinting strategy for challenging bone defects repair. Nat. Commun. 2024;15(1):3565. doi: 10.1038/s41467-024-48023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Arif Z.U., Khalid M.Y., Zolfagharian A., Bodaghi M. 4D bioprinting of smart polymers for biomedical applications: recent progress, challenges, and future perspectives. Reactive Funct. Polym. 2022;179 doi: 10.1016/j.reactfunctpolym.2022.105374. [DOI] [Google Scholar]
- 233.Wang C., Yue H., Liu J., Zhao Q., He Z., Li K., Lu B., Huang W., Wei Y., Tang Y., Wang M. Advanced Reconfigurable scaffolds fabricated by 4D printing for treating critical-size bone defects of Irregular shapes. Biofabrication. 2020;12(4) doi: 10.1088/1758-5090/abab5b. [DOI] [PubMed] [Google Scholar]
- 234.Chen Z., Wang X., Liu J., Liu K., Li S., Wu M., Wu Z., Wang Z., Shi Y., Ruan C. A Stone‐Cottage‐Inspired printing strategy to build Microsphere Patterned scaffolds for accelerated bone regeneration. Adv. Funct. Mater. 2024 doi: 10.1002/adfm.202417836. [DOI] [Google Scholar]
- 235.Li Z., Li S., Gao C., Liu J., Qu H., Yang J., Lu W.W., Ruan C., Niu X. Continuous manufacturing of Bioinspired bone‐Periosteum integrated scaffold to promote bone regeneration. Adv. Funct. Mater. 2024;34(41) doi: 10.1002/adfm.202403235. [DOI] [Google Scholar]
- 236.Vaidya G., Pramanik S., Kadi A., Rayshan A.R., Abualsoud B.M., Ansari M.J., Masood R., Michaelson J. Injecting hope: chitosan hydrogels as bone regeneration Innovators. Journal of Biomaterials Science. 2024;35(5):756–797. doi: 10.1080/09205063.2024.2304952. [DOI] [PubMed] [Google Scholar]
- 237.Song T., Zhao F., Yan L., Liu P., Yang J., Ruan C., Li D., Xiao Y., Zhang X. Structure driven bio-responsive ability of injectable nanocomposite hydrogels for efficient bone regeneration. Biomaterials. 2024;309 doi: 10.1016/j.biomaterials.2024.122601. [DOI] [PubMed] [Google Scholar]
- 238.Bandyopadhyay A., Ghibhela B., Mandal B.B. Current advances in engineering Meniscal tissues: insights into 3D printing, injectable hydrogels and physical stimulation based strategies. Biofabrication. 2024;16(2) doi: 10.1088/1758-5090/ad22f0. [DOI] [PubMed] [Google Scholar]
- 239.Ding H., Li B., Liu Z., Liu G., Pu S., Feng Y., Jia D., Zhou Y. Decoupled pH‐ and thermo‐responsive injectable chitosan/Pnipam hydrogel via Thiol‐Ene Click chemistry for potential applications in tissue engineering. Adv. Healthcare Mater. 2020;9(14) doi: 10.1002/adhm.202000454. [DOI] [PubMed] [Google Scholar]
- 240.Chen Q., Yan M., Hu A., Liang B., Lu H., Zhou L., Ma Y., Jia C., Su D., Kong B., Hong W., Jiang L., Dong J. Injectable Nanorobot-hydrogel Superstructure for Hemostasis and Anticancer therapy of spinal Metastasis. Nano-Micro Lett. 2024;16(1):259. doi: 10.1007/s40820-024-01469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lv Z., Hu T., Bian Y., Wang G., Wu Z., Li H., Liu X., Yang S., Tan C., Liang R., Weng X. A Mgfe‐Ldh Nanosheet‐Incorporated smart thermo‐responsive hydrogel with controllable growth factor releasing capability for bone regeneration. Adv. Mater. 2022;35(5) doi: 10.1002/adma.202206545. [DOI] [PubMed] [Google Scholar]
- 242.Ding A., Lee S.J., Ayyagari S., Tang R., Huynh C.T., Alsberg E. 4D Biofabrication via Instantly generated graded hydrogel scaffolds. Bioact. Mater. 2022;7:324–332. doi: 10.1016/j.bioactmat.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mao Z., Bi X., Yu C., Chen L., Shen J., Huang Y., Wu Z., Qi H., Guan J., Shu X., Yu B., Zheng Y. Mechanically robust and personalized silk fibroin-magnesium composite scaffolds with water-responsive shape-memory for Irregular bone regeneration. Nat. Commun. 2024;15(1):4160. doi: 10.1038/s41467-024-48417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xing J., Pan X., Zhang H., Wang J., Ma Y., Wang Y., Luo Y. Shape recovery strain and nanostructures on recovered polyurethane films and their regulation to osteoblasts morphology. J. Mech. Behav. Biomed. Mater. 2019;92:128–136. doi: 10.1016/j.jmbbm.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 245.Ingber D.E. Cellular Mechanotransduction: Putting all the Pieces Together again. FASEB J. 2006;20(7):811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 246.Yuan Z., Yuan X., Zhao Y., Cai Q., Wang Y., Luo R., Yu S., Wang Y., Han J., Ge L., Huang J., Xiong C. Injectable Gelma Cryogel Microspheres for Modularized cell delivery and potential vascularized bone regeneration. Small. 2021;17(11) doi: 10.1002/smll.202006596. [DOI] [PubMed] [Google Scholar]
- 247.Al-allaq A.A., Kashan J.S., Abdul-Kareem F.M. In vivo investigations of polymers in bone tissue engineering: a review study. International Journal of Polymeric Materials and Polymeric Biomaterials. 2024;73(18):1664–1684. doi: 10.1080/00914037.2024.2305227. [DOI] [Google Scholar]
- 248.Davis K.A., Burke K.A., Mather P.T., Henderson J.H. Dynamic cell behavior on shape memory polymer Substrates. Biomaterials. 2011;32(9):2285–2293. doi: 10.1016/j.biomaterials.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 249.Tajik A., Zhang Y., Wei F., Sun J., Jia Q., Zhou W., Singh R., Khanna N., Belmont A.S., Wang N. Transcription Upregulation via force-induced direct stretching of Chromatin. Nat. Mater. 2016;15(12):1287–1296. doi: 10.1038/nmat4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhang Y., Li D., Liu Y., Peng L., Lu D., Wang P., Ke D., Yang H., Zhu X., Ruan C. 3D-Bioprinted anisotropic Bicellular living hydrogels Boost Osteochondral regeneration via reconstruction of cartilage–bone interface. Innovation. 2024;5(1) doi: 10.1016/j.xinn.2023.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jeyaraman M., Jeyaraman N., Nallakumarasamy A., Ramasubramanian S., Yadav S. Critical challenges and Frontiers in cartilage tissue engineering. Cureus Journal of Medical Science. 2024;16(1) doi: 10.7759/cureus.53095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhao Z., Xia X., Liu J., Hou M., Liu Y., Zhou Z., Xu Y., He F., Yang H., Zhang Y., Ruan C., Zhu X. Cartilage-inspired self-assembly Glycopeptide hydrogels for cartilage regeneration via Ros Scavenging. Bioact. Mater. 2024;32:319–332. doi: 10.1016/j.bioactmat.2023.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Díaz‐Payno P.J., Kalogeropoulou M., Muntz I., Kingma E., Kops N., D'Este M., Koenderink G.H., Fratila‐Apachitei L.E., van Osch G.J.V.M., Zadpoor A.A. Swelling‐dependent shape‐based transformation of a human mesenchymal stromal cells‐laden 4D bioprinted construct for cartilage tissue engineering. Adv. Healthcare Mater. 2022;12(2) doi: 10.1002/adhm.202201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Deng Y., Zhang F., Liu Y., Zhang S., Yuan H., Leng J. 4D printed shape memory polyurethane-based composite for bionic cartilage scaffolds. ACS Appl. Polym. Mater. 2023;5(2):1283–1292. doi: 10.1021/acsapm.2c01833. [DOI] [Google Scholar]
- 255.Chiesa I., Esposito A., Vozzi G., Gottardi R., De Maria C. 4D bioprinted self-folding scaffolds enhance cartilage formation in the engineering of trachea. 2023. 318. [DOI]
- 256.Borse K., Shende P. 3D-to-4D structures: an exploration in biomedical applications. AAPS PharmSciTech. 2023;24(6):163. doi: 10.1208/s12249-023-02626-4. [DOI] [PubMed] [Google Scholar]
- 257.Kirillova A., Maxson R., Stoychev G., Gomillion C.T., Ionov L. 4D Biofabrication using shape‐morphing hydrogels. Adv. Mater. 2017;29(46) doi: 10.1002/adma.201703443. [DOI] [PubMed] [Google Scholar]
- 258.Nain A., Joshi A., Debnath S., Choudhury S., Thomas J., Satija J., Huang C.-C., Chatterjee K. A 4D printed Nanoengineered super bioactive hydrogel scaffold with programmable deformation for potential Bifurcated vascular channel construction. J. Mater. Chem. B. 2024;12(31):7604–7617. doi: 10.1039/d4tb00498a. [DOI] [PubMed] [Google Scholar]
- 259.Xie R., Cao Y., Sun R., Wang R., Morgan A., Kim J., Callens S.J.P., Xie K., Zou J., Lin J., Zhou K., Lu X., Stevens M.M. Magnetically driven Formation of 3D Freestanding soft Bioscaffolds. Sci. Adv. 2024;10(5) doi: 10.1126/sciadv.adl1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kitana W., Apsite I., Hazur J., Boccaccini A.R., Ionov L. 4D Biofabrication of T‐shaped vascular Bifurcation. Advanced Materials Technologies. 2022;8(1) doi: 10.1002/admt.202200429. [DOI] [Google Scholar]
- 261.Deng Y., Zhang F., Jiang M., Liu Y., Yuan H., Leng J. Programmable 4D printing of photoactive shape memory composite structures. Acs Applied Materials & Interfaces. 2022;14(37):42568–42577. doi: 10.1021/acsami.2c13982. [DOI] [PubMed] [Google Scholar]
- 262.H. Cui, C. Liu, T. Esworthy, Y. Huang, Z.-x. Yu, X. Zhou, H. San, S.-j. Lee, S. Y. Hann, M. Boehm, M. Mohiuddin, J. P. Fisher, L. G. Zhang, 4D Physiologically Adaptable Cardiac Patch: A 4-Month in Vivo Study for the Treatment of Myocardial Infarction, Science Advances. 6 (26), (2020), eabb5067, 10.1126/sciadv.abb5067. [DOI] [PMC free article] [PubMed]
- 263.Mahjoubnia A., Cai D., Wu Y., King S.D., Torkian P., Chen A.C., Talaie R., Chen S.-Y., Lin J. Digital light 4D printing of Bioresorbable shape memory elastomers for personalized biomedical implantation. Acta Biomater. 2024;177:165–177. doi: 10.1016/j.actbio.2024.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cui H., Liu C., Esworthy T., Huang Y., Yu Z.-x., Zhou X., San H., Lee S.-j., Hann S.Y., Boehm M., Mohiuddin M., Fisher J.P., Zhang L.G. 4D physiologically adaptable cardiac patch: a 4-Month in vivo study for the treatment of myocardial infarction. Sci. Adv. 2020;6(26):eabb5067. doi: 10.1126/sciadv.abb5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Douillet C., Nicodeme M., Hermant L., Bergeron V., Guillemot F., Fricain J.-C., Oliveira H., Garcia M. From local to global matrix organization by fibroblasts: a 4D laser-Assisted bioprinting approach. Biofabrication. 2022;14(2) doi: 10.1088/1758-5090/ac40ed. [DOI] [PubMed] [Google Scholar]
- 266.Dong R., Guo B. Smart wound dressings for wound healing. Nano Today. 2021;41 doi: 10.1016/j.nantod.2021.101290. [DOI] [Google Scholar]
- 267.Zhao L., Niu L., Liang H., Tan H., Liu C., Zhu F. pH and glucose dual-responsive injectable hydrogels with Insulin and fibroblasts as bioactive dressings for Diabetic wound healing. ACS Appl. Mater. Interfaces. 2017;9(43):37563–37574. doi: 10.1021/acsami.7b09395. [DOI] [PubMed] [Google Scholar]
- 268.Chen X., Song H., Song K., Zhang Y., Wang J., Hong J., Xie Q., Zhao J., Liu M., Wang X. Temperature‐sensitive hydrogel releasing Pectolinarin Facilitate Scarless wound healing. J. Cell Mol. Med. 2024;28(4) doi: 10.1111/jcmm.18130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Diao J., Meng Y., Wang L., Zhao G., Sun B., Du S., Niu Y., He G., Yu M., Jiang X. Temperature/pH Co-responsive Cs/Nipam-Based hydrogel with controlled release of Ga3+ for improved infected wound healing. Chem. Eng. Sci. 2024;294 doi: 10.1016/j.ces.2024.120104. [DOI] [Google Scholar]
- 270.Qiu Y., Yang T., Zhang H., Dai H., Gao H., Feng W., Xu D., Duan J. The application of pH-responsive hyaluronic acid-based essential Oils hydrogels with enhanced Anti-Biofilm and wound healing. Int. J. Biol. Macromol. 2024;275 doi: 10.1016/j.ijbiomac.2024.133559. [DOI] [PubMed] [Google Scholar]
- 271.Kruse C.R., Singh M., Targosinski S., Sinha I., Sørensen J.A., Eriksson E., Nuutila K. The effect of pH on cell Viability, cell migration, cell proliferation, wound closure, and wound Reepithelialization: in vitro and in vivo study. Wound Repair Regen. 2017;25(2):260–269. doi: 10.1111/wrr.12526. [DOI] [PubMed] [Google Scholar]
- 272.Huang Z., Wang M., Chai L., Chen H., Chen D., Li Y., Liu H., Wu Y., Yang X., He L., Xue L., Lei Y., Guo L. Glucose-responsive, self-healing, Wet adhesive and multi-Biofunctional hydrogels for Diabetic wound healing. Materials Today Bio. 2024;27 doi: 10.1016/j.mtbio.2024.101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Liu M., You J., Zhang Y., Zhang L., Quni S., Wang H., Zhou Y. Glucose-responsive self-healing bilayer drug Microneedles promote Diabetic wound healing via a Trojan-Horse strategy. ACS Appl. Mater. Interfaces. 2024;16(19):24351–24371. doi: 10.1021/acsami.4c03050. [DOI] [PubMed] [Google Scholar]
- 274.Xu Z., Liu G., Huang J., Wu J. Novel glucose-responsive antioxidant hybrid hydrogel for enhanced Diabetic wound repair. ACS Appl. Mater. Interfaces. 2022;14(6):7680–7689. doi: 10.1021/acsami.1c23461. [DOI] [PubMed] [Google Scholar]
- 275.Shen X., Zhao D., Shi J., Li C., Bai Y., Qiu L., Xuan Y., Wang J. Copper peroxide loaded gelatin/oxide dextran hydrogel with temperature and pH responsiveness for antibacterial and wound healing activity. Int. J. Biol. Macromol. 2024;274 doi: 10.1016/j.ijbiomac.2024.133258. [DOI] [PubMed] [Google Scholar]
- 276.Dixit K., Bora H., Chakrabarti R., Saha B., Dogra N., Biswas S., Sengupta T.K., Kaushal M., Rana S., Mukherjee G., Dhara S. Thermoresponsive keratin-Methylcellulose self-healing injectable hydrogel accelerating Full-Thickness wound healing by promoting rapid Epithelialization. Int. J. Biol. Macromol. 2024;263 doi: 10.1016/j.ijbiomac.2024.130073. [DOI] [PubMed] [Google Scholar]
- 277.Dong Y., Zhang X., Chen Y., Yu J., Li X., Ding B. “Stiff‐Elastic” binary synergistic fibrous Tape with thermal‐triggered shrinkable and shape recoverable performances for wound closure. Adv. Funct. Mater. 2024;34(7) doi: 10.1002/adfm.202402252. [DOI] [Google Scholar]
- 278.He M., Ou F., Wu Y., Sun X., Chen X., Li H., Sun D., Zhang L. Smart multi-layer PVA Foam/Cmc mesh dressing with integrated multi-functions for wound Management and infection Monitoring. Mater. Des. 2020;194 doi: 10.1016/j.matdes.2020.108913. [DOI] [Google Scholar]
- 279.Chang L., Du H., Xu F., Xu C., Liu H. Hydrogel-enabled mechanically active wound dressings. Trends Biotechnol. 2024;42(1):31–42. doi: 10.1016/j.tibtech.2023.06.004. [DOI] [PubMed] [Google Scholar]
- 280.Xie Q., Yan C., Liu G., Bian L., Zhang K. In situ triggered self‐contraction bioactive microgel assembly accelerates Diabetic skin wound healing by activating Mechanotransduction and Biochemical pathway. Adv. Mater. 2024;36(8) doi: 10.1002/adma.202406434. [DOI] [PubMed] [Google Scholar]
- 281.Esworthy T.J., Miao S., Lee S.-J., Zhou X., Cui H., Zuo Y.Y., Zhang L.G. Advanced 4D-bioprinting Technologies for Brain tissue modeling and study. International Journal of Smart and Nano Materials. 2019;10(3):177–204. doi: 10.1080/19475411.2019.1631899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhu N., Zhuang Y., Sun W., Wang J., Wang F., Han X., Han Z., Ni M., Cui W., Qiu Y. Multistructured hydrogel promotes nerve regeneration. Materials Today Advances. 2024;21 doi: 10.1016/j.mtadv.2024.100465. [DOI] [Google Scholar]
- 283.Xiang R., Chen J., Dang J., Zhu H., Ran Z., Dong Y., Wu X., Yang Y., Deng H., Xiong C., Huang Z., Xu P., Xu W., Xu H. Electrospun silk fibroin/polylactic acid conduit filled with Proangiogenic Carboxylated silk fibroin/chitosan hydrogel Facilitates peripheral nerve regeneration. International Journal of Polymeric Materials and Polymeric Biomaterials. 2024:1–14. doi: 10.1080/00914037.2024.2338138. [DOI] [Google Scholar]
- 284.Hong J., Wu D., Wang H., Gong Z., Zhu X., Chen F., Wang Z., Zhang M., Wang X., Fang X., Yang S., Zhu J. Magnetic Fibrin nanofiber hydrogel delivering iron oxide magnetic nanoparticles promotes peripheral nerve regeneration. Regenerative Biomaterials. 2024;11 doi: 10.1093/rb/rbae075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Xu D., Fu S., Zhang H., Lu W., Xie J., Li J., Wang H., Zhao Y., Chai R. Ultrasound‐responsive aligned Piezoelectric nanofibers derived hydrogel conduits for peripheral nerve regeneration. Adv. Mater. 2024;36(28) doi: 10.1002/adma.202307896. [DOI] [PubMed] [Google Scholar]
- 286.Joshi A., Choudhury S., Baghel V.S., Ghosh S., Gupta S., Lahiri D., Ananthasuresh G.K., Chatterjee K. 4D printed programmable shape‐morphing hydrogels as Intraoperative self‐folding nerve conduits for Sutureless Neurorrhaphy. Adv. Healthcare Mater. 2023;12(24) doi: 10.1002/adhm.202300701. [DOI] [PubMed] [Google Scholar]
- 287.Apsite I., Constante G., Dulle M., Vogt L., Caspari A., Boccaccini A.R., Synytska A., Salehi S., Ionov L. 4D Biofabrication of fibrous Artificial nerve graft for neuron regeneration. Biofabrication. 2020;12(3) doi: 10.1088/1758-5090/ab94cf. [DOI] [PubMed] [Google Scholar]
- 288.Song J., Dong J., Yuan Z., Huang M., Yu X., Zhao Y., Shen Y., Wu J., El‐Newehy M., Abdulhameed M.M., Sun B., Chen J., Mo X. Shape‐persistent conductive nerve guidance conduits for peripheral nerve regeneration. Adv. Healthcare Mater. 2024;13(26) doi: 10.1002/adhm.202401160. [DOI] [PubMed] [Google Scholar]
- 289.Wang Z., Zheng Y., Qiao L., Ma Y., Zeng H., Liang J., Ye Q., Shen K., Liu B., Sun L., Fan Z. 4D‐Printed Mxene‐based Artificial nerve guidance conduit for enhanced regeneration of peripheral nerve Injuries. Adv. Healthcare Mater. 2024;13(23) doi: 10.1002/adhm.202401093. [DOI] [PubMed] [Google Scholar]
- 290.Demoly F., Dunn M.L., Wood K.L., Qi H.J., André J.-C. The Status, Barriers, challenges, and future in design for 4D printing. Mater. Des. 2021;212 doi: 10.1016/j.matdes.2021.110193. [DOI] [Google Scholar]
- 291.Chen X., Han S., Wu W., Wu Z., Yuan Y., Wu J., Liu C. Harnessing 4D printing Bioscaffolds for advanced Orthopedics. Small. 2022;18(36) doi: 10.1002/smll.202106824. [DOI] [PubMed] [Google Scholar]
- 292.Cidonio G., Glinka M., Kim Y.-H., Kanczler J.M., Lanham S.A., Ahlfeld T., Lode A., Dawson J.I., Gelinsky M., Oreffo R.O.C. Nanoclay-based 3D printed scaffolds promote vascular Ingrowth Ex vivo and generate bone Mineral tissue in vitro and in vivo. Biofabrication. 2020;12(3) doi: 10.1088/1758-5090/ab8753. [DOI] [PubMed] [Google Scholar]