Abstract
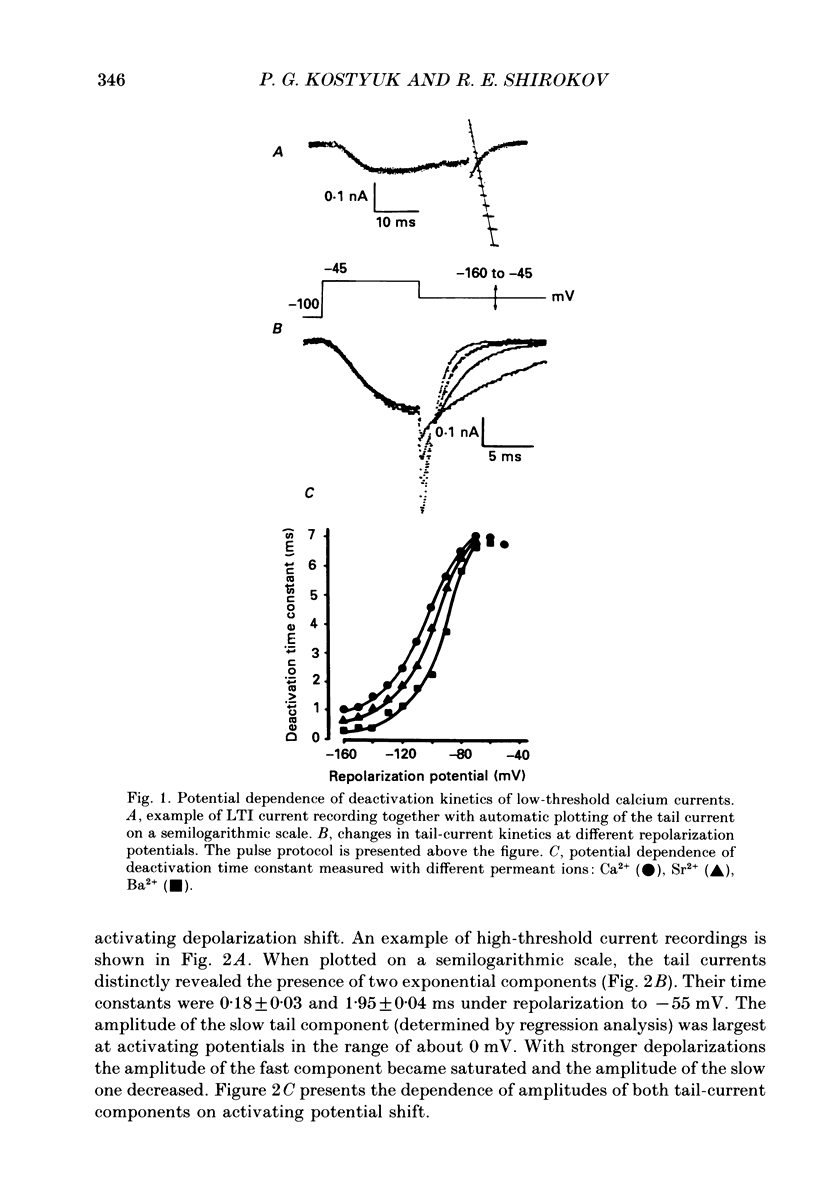
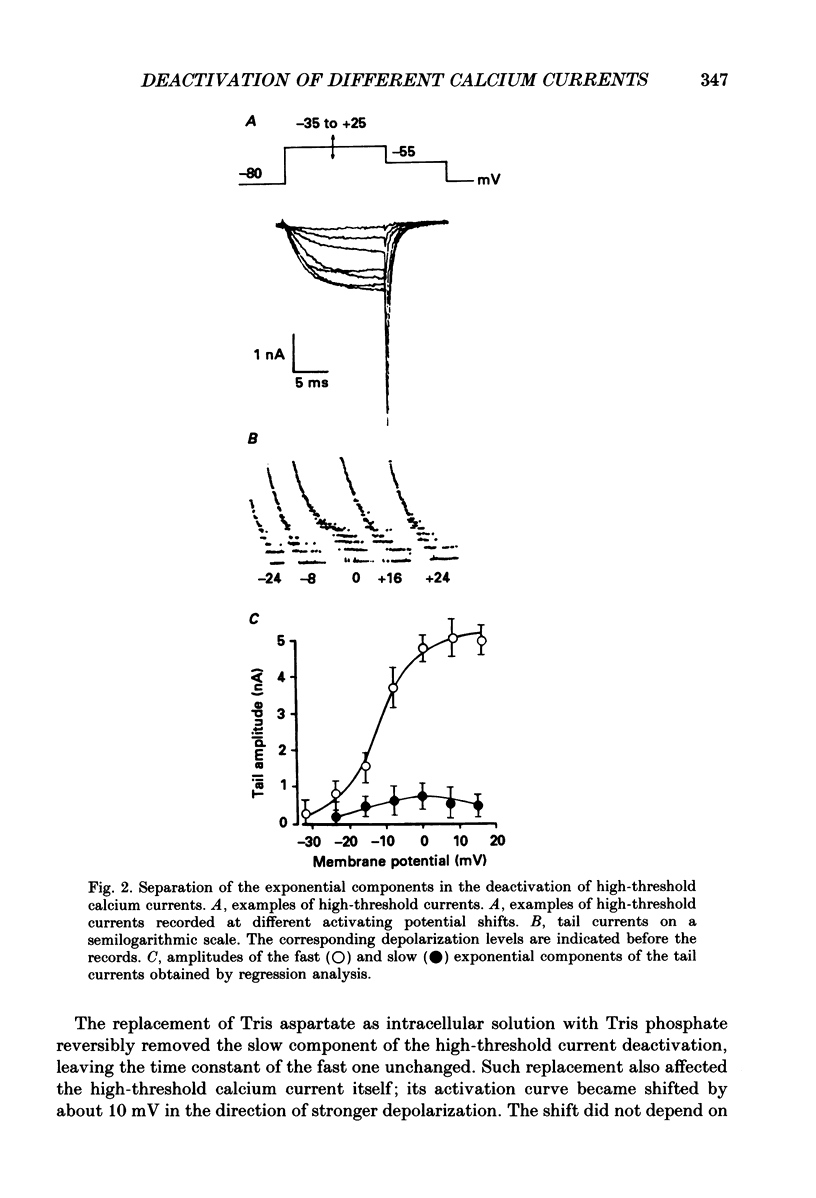
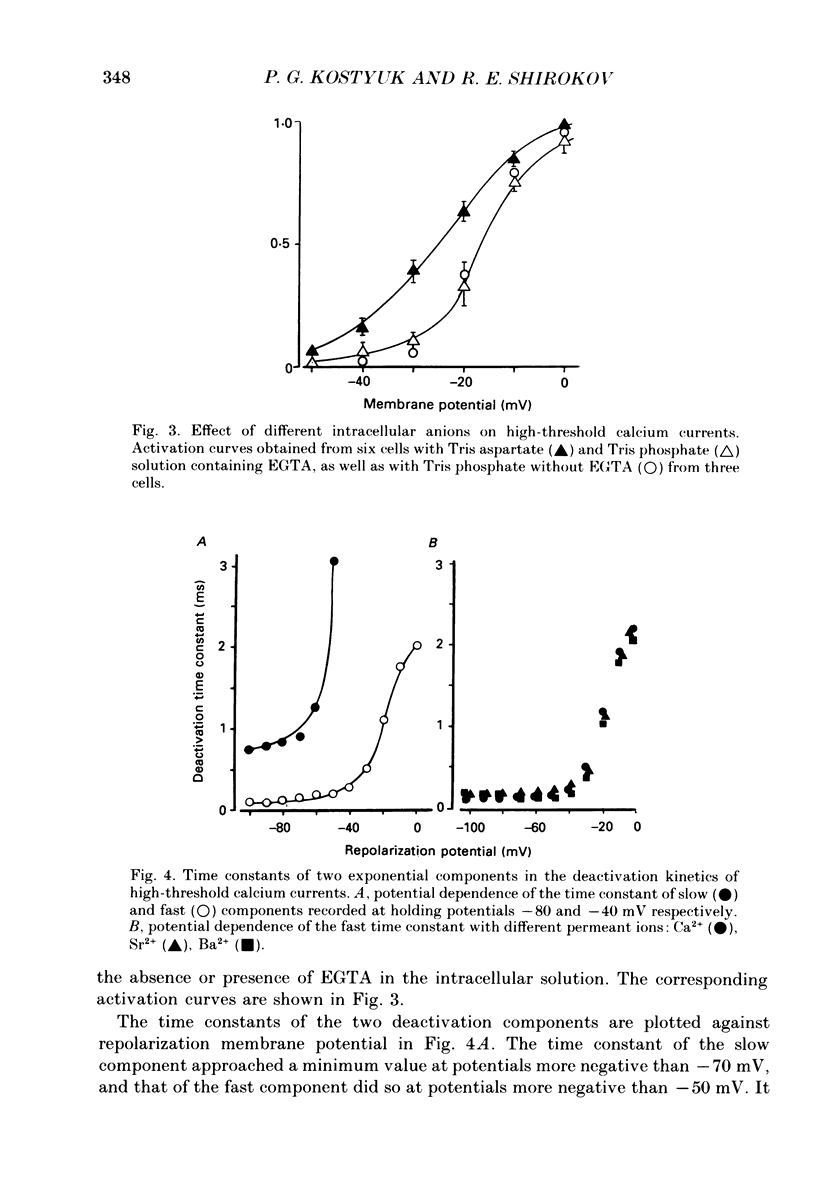
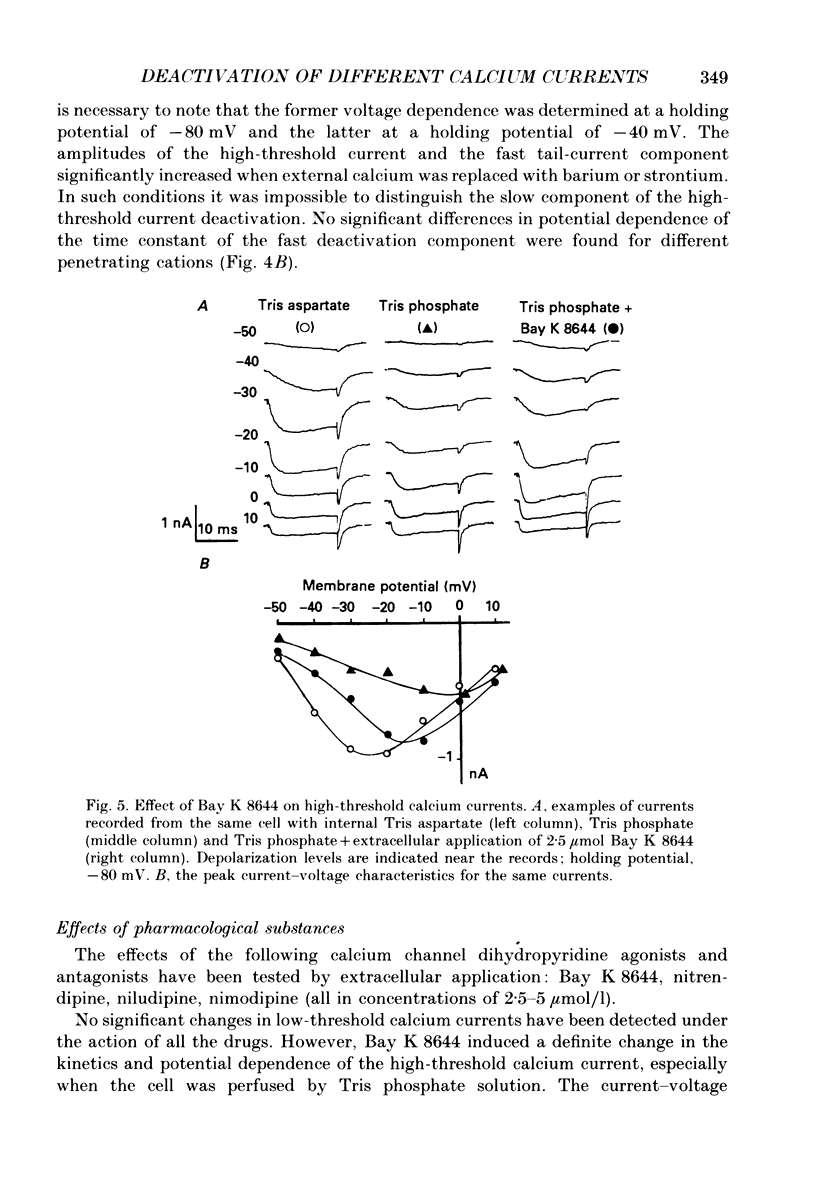
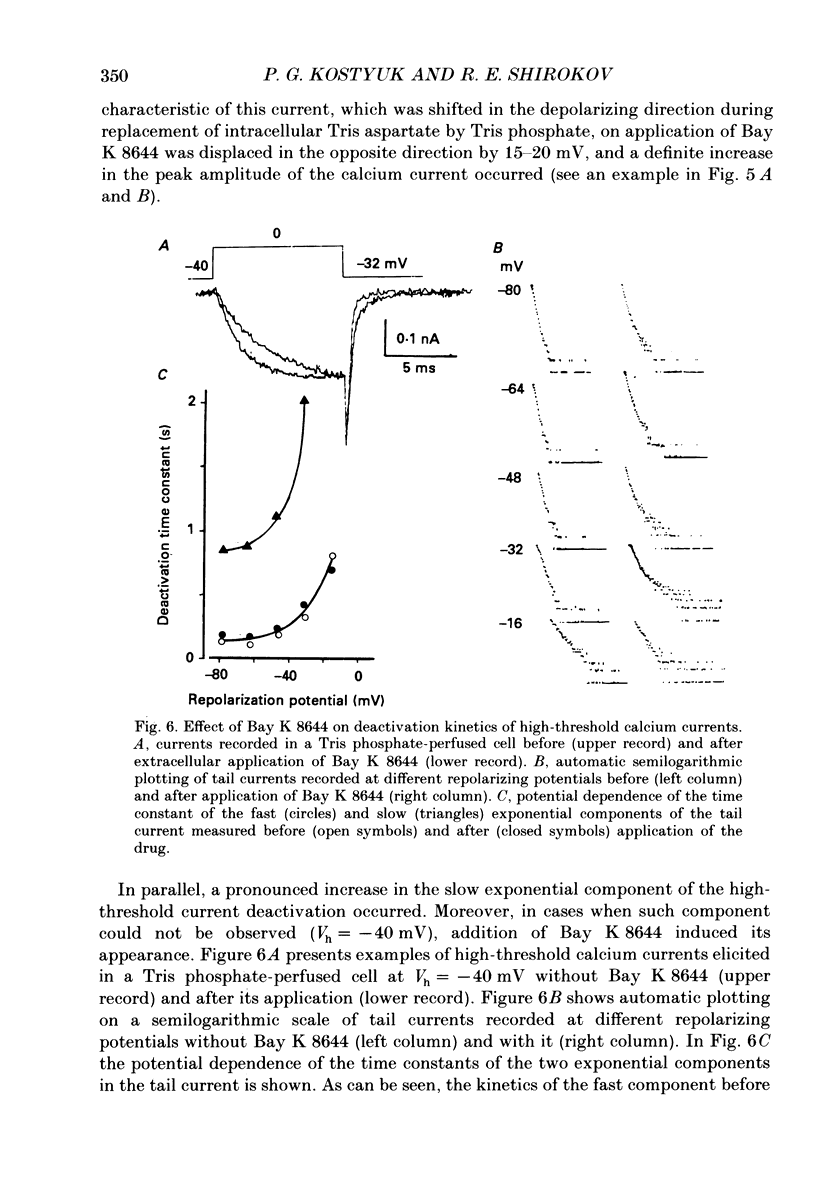
1. Deactivation kinetics of different components of calcium inward current were analysed by measuring 'tail currents' in intracellularly perfused sensory neurones isolated from dorsal root ganglia of newborn mice. 2. Deactivation of a low-threshold inactivating component had a mono-exponential time course with time constants that decreased as the potential was made more negative, and reached a limiting value of 1.0-1.2 ms at extreme hyperpolarizations. Replacement of Ca2+ by Ba2+ as a charge carrier decreased this value almost twofold. 3. Deactivation of the high-threshold component of calcium current contained two exponential components with time constants of similar potential dependence. The fast time constant became practically constant (0.1-1.2 ms) during repolarizations beyond -50 mV, and the slow time constant became constant (0.80-0.85 ms) at repolarization beyond -70 mV. 4. Replacement of intracellular aspartate with phosphate, or a depolarized holding potential of -40 mV, abolished reversibly the slow component of the tail current; in parallel, the peak current-voltage relation of the current became shifted by 15-20 mV in the depolarizing direction. 5. Extracellular application of the calcium channel agonist Bay K 8644 (2.5-5 mumol/l) did not change deactivation kinetics of the low-threshold current; however, it increased considerably the slow exponential component of high-threshold current deactivation or induced such a component in conditions when the latter was absent before application (Vh = -40 mV; cell perfusion with Tris phosphate). 6. The data obtained are analysed on the basis of the presence of three types of calcium channels in the neuronal membrane which possess similar kinetic mechanisms of activation and deactivation but differ in the activation thresholds and the time constants of transitions between open and closed states. The three calcium channels differ also in interaction with different permeant ions and calcium channel agonists.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Matteson D. R. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985 Jan 4;227(4682):65–67. doi: 10.1126/science.2578071. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Tsuda Y., Wilson D. L. A description of activation and conduction in calcium channels based on tail and turn-on current measurements in the snail. J Physiol. 1983 Nov;344:549–583. doi: 10.1113/jphysiol.1983.sp014956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Chase P. B., Stimers J. R. Calcium current activation kinetics in neurones of the snail Lymnaea stagnalis. J Physiol. 1984 Mar;348:187–207. doi: 10.1113/jphysiol.1984.sp015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W. Evidence for two voltage-dependent calcium currents in the membrane of the ciliate Stylonychia. J Physiol. 1984 Oct;355:137–159. doi: 10.1113/jphysiol.1984.sp015411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-II. Calcium currents. Neuroscience. 1981;6(12):2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Long-opening mode of gating of neuronal calcium channels and its promotion by the dihydropyridine calcium agonist Bay K 8644. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2178–2182. doi: 10.1073/pnas.82.7.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Veselovskii N. S., Fedulova S. A. Dva tipa kal'tsievykh kanalov v somaticheskoi membrane neironov spinal'nykh gangliev krys. Dokl Akad Nauk SSSR. 1983;268(3):747–750. [PubMed] [Google Scholar]
- Veselovskii N. S., Kostiuk P. G., Fedulova S. A., Shirokov R. E. Deaktivatsiia kal'tsievykh tokov v some neironov spinal'nykh gangliev pri vykliuchenii depoliarizuiushchego smeshcheniia membrannogo potentsiala. Neirofiziologiia. 1985;17(5):682–691. [PubMed] [Google Scholar]


