Abstract
Background
KO-947, a potent, intravenously administered, extracellular signal-regulated kinase (ERK) inhibitor, has demonstrated activity in preclinical models. This phase I trial of KO-947 evaluated maximum tolerated dose (MTD), safety, and pharmacokinetics in patients with relapsed/refractory solid tumors.
Materials and methods
This multicenter, open-label, dose-escalation study evaluated KO-947 0.45-11.3 mg/kg in three schedules. Schedules 1 (0.45-5.4 mg/kg, 1- to 2-hour infusion) and 2 (4.8-9.6 mg/kg, 4-hour infusion) were administered once weekly on a 28-day cycle. Schedule 3 (3.6-11.3 mg/kg, 4-hour infusion) was administered on days 1, 4, and 8 (and on days 11 and 15 for two patients) on a 21-day cycle. The primary objective was determination of MTD and/or recommended phase II dose. Safety analysis included adverse events of special interest (AESIs), namely ocular toxicities and infusion-related reactions (e.g. hypotension, corrected QT interval prolongation). Results from the dose-escalation portion of the phase I study are presented due to trial termination before preplanned cohort expansion cohorts.
Results
All 61 enrolled patients (schedules 1/2, n = 34, schedule 3, n = 27) discontinued treatment, mostly owing to disease progression (88% and 67%). The MTD for schedule 1 was 3.6 mg/kg; the maximum administered doses for schedules 2 and 3 were 9.6 and 11.3 mg/kg, respectively. Treatment-related adverse events occurred in 88% of patients in schedules 1/2, and 92% in schedule 3; most common were blurred vision (schedules 1/2, 50.0%; schedule 3, 33.3%). AESIs occurred in 50% of patients in schedules 1/2, and 82% in schedule 3. In all schedules, the best overall response was stable disease.
Conclusions
Intravenous KO-947 had a generally tolerable safety profile with minimal gastrointestinal toxicity compared with oral administration of other ERK inhibitors.
Key words: solid tumors, ERK1/2 inhibition, KO-947, phase I, intravenous administration
Highlights
-
•
This phase 1 study tested KO-947 (ERK inhibitor) in solid tumors for MTD, safety, and pharmacokinetics.
-
•
Overall, KO-947 had a tolerable safety profile with minimal gastrointestinal toxicity.
-
•
In all schedules, the best clinical response was stable disease (schedules 1/2, 15%; schedule 3, 37%).
-
•
This study demonstrated the feasibility and value of alternate dose formulations to overcome treatment-related toxicity.
Introduction
The mitogen-activated protein kinase (MAPK) pathway, including Rat sarcoma protein (RAS), Rapidly accelerated fibrosarcoma (RAF), mitogen-activated extracellular signal-regulated kinase (MEK), and extracellular signal-regulated kinase (ERK) proteins, is a major driver of human malignancies, with RAS mutations alone occurring in up to one-third of all cancers.1 The BRAFV600E mutation occurs in ∼8% of human cancers, often in melanoma.2 Multiple inhibitors of BRAF and/or MEK have been approved for treatment of cancer including vemurafenib ± cobimetinib and dabrafenib ± trametinib for melanoma, encorafenib + cetuximab for BRAFV600E-mutated colorectal cancer,3, 4, 5, 6 and the small-molecule KRASG12C inhibitors sotorasib and adagrasib for non-small-cell lung cancer.7,8 However, MAPK inhibitor success has been hampered by primary and acquired resistance, often from failure to inhibit downstream ERK signaling.1,9,10
ERK1/2 kinases are the final node in the MAPK signaling pathway and are ideal treatment targets that may not be subject to feedback reactivation mechanisms undermining RAF or MEK blockade. As a downstream target of KRAS, NRAS, and BRAF, ERK inhibition may provide clinical benefit for patients with mutations in MAPK-associated proteins.9 ERK inhibitors have demonstrated clinical efficacy in solid tumors. In a phase I clinical trial, the oral ERK1/2 kinase inhibitor ulixertinib demonstrated clinical responses in patients with NRAS-, BRAFV600- and non-BRAFV600-mutant solid tumors; however, 32% of patients had dose reductions due to toxicity, and treatment-related adverse events (AEs) included frequent gastrointestinal events.11 The oral ERK1/2 inhibitors MK-8353, GDC-0994, LY3214996, and ASN007 have demonstrated preclinical and clinical antitumor effects in MAPK-altered tumors12, 13, 14, 15, 16, 17 although significant gastrointestinal toxicity was observed.12,14
KO-947, a potent and selective ERK1/2 kinase inhibitor with an enzymatic concentration that causes 50% inhibition of growth (IC50) value of 10.0 nM, has demonstrated inhibition of signaling for >4 h following washout in cell lines, suggesting prolonged ERK1/2 residency.18 KO-947 demonstrated extended pathway modulation after a single bolus dose, enabling flexible administration schedules (e.g. from daily to once weekly) and supporting intravenous (i.v.) administration. Antitumor activity of KO-947 occurred in multiple patient-derived xenograft (PDX) murine models of colon, lung, and pancreatic tumors bearing KRAS or BRAF mutations. In 11q13-amplified (11q-AMP) PDX models of esophageal squamous cell carcinoma (SCC), KO-947 had a disease control rate of 77% and an overall response rate of 51% versus 21% and 3% for 11q13WT, respectively.19 Complete responses and tumor regression were observed in 11q-AMP models of head and neck cancer.18 In mouse models of esophageal SCC/head and neck SCC, overall response rates for KO-947-treated mice with 11q-AMP exceeded those observed in 11q13WT populations (51%/56% versus 3%/9%), suggesting that 11q13 may be a biomarker for KO-947 activity.19 The 11q13 amplicon contains multiple potential oncogenes including three (cyclin D1, anoctamin-1, FAS-associated via death domain) associated with the MAPK pathway. Pleotropic effects of concerted overexpression of these genes may drive 11q-AMP SCC cells into MAPK pathway (i.e. ERK) addiction.19
Intravenous KO-947 offers a desirable route of administration, as oral ERK inhibitor formulations cause considerable gastrointestinal toxicity. Among ulixertinib-treated patients, diarrhea and nausea occurred in 48% and 42% of patients, respectively.11 Similar results were observed with MK-8353 (diarrhea, 44%; nausea, 28%), GDC-0994 (49%; 32%), and ASN007 (both 30%).12,14,17 Administering i.v. KO-947 on a weekly, intermittent dosing schedule may improve tolerability and decrease feedback from chronic ERK pathway suppression.
The objectives of this phase I trial (NCT03051035) were to determine the maximum tolerated dose (MTD), pharmacokinetics (PK), safety, and preliminary clinical activity of KO-947 monotherapy in adults with locally advanced unresectable or metastatic, relapsed and/or refractory nonhematological malignancies with MAPK pathway alterations or 11q-AMP.
Methods
Study design and objectives
The primary objective of this phase I, first-in-human, multicenter (USA, three sites; Spain, two sites), open-label, dose-escalation study was to determine the MTD and/or the recommended phase II dose of KO-947 in patients with locally advanced unresectable or metastatic, relapsed and/or refractory nonhematological malignancies. Secondary objectives included evaluating the safety and tolerability of KO-947; PK after a single i.v. dose and after multiple i.v. administrations; and preliminary clinical activity endpoints, including objective response rate, progression-free survival, and duration of response. Upon determination of the MTD/recommended phase II dose, tumor-specific expansion cohorts were planned. The trial was terminated before the planned expansion cohorts, therefore results detailed here are from the dose-escalation portion of the phase I study.
This trial was carried out in compliance with the Declaration of Helsinki, and the protocol and all amendments were approved by the relevant institutional review boards.
Dose escalation
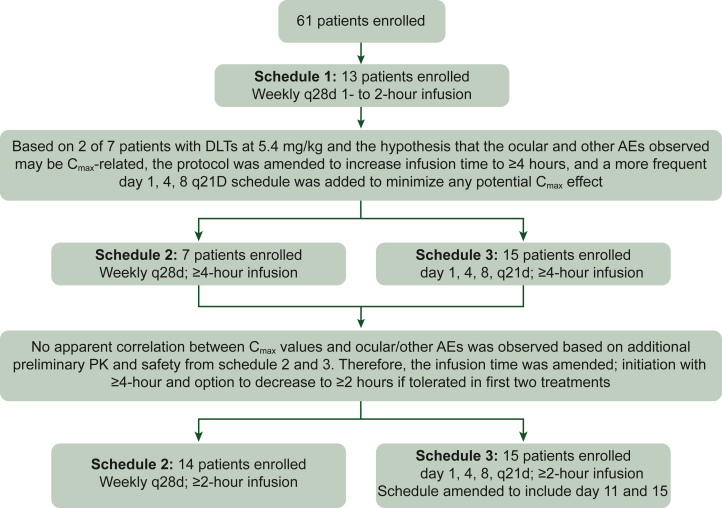
The dose-escalation portion of the trial (Figure 1) was initiated at a starting dose of i.v. KO-947 0.45 mg/kg, once weekly in 28-day cycles with a 1-hour infusion (schedule 1) using a modified Simon’s type 2 accelerated titration design.20 Patients were initially enrolled in single patient cohorts with 100% dose escalation (0.45-3.6 mg/kg cohorts). Dose cohorts at doses >3.6 mg/kg followed a standard 3 + 3 design. Upon determination of the MTD/recommended phase II dose based on the dose-limiting toxicities (DLTs) (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2025.104300) observed for a given schedule, all remaining patients in the dose-escalation portion for that schedule were eligible for dose escalation to the MTD/recommended phase II dose at the discretion of the investigator. Details on protocol amendments are in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2025.104300.
Figure 1.
Study design: dose-escalation phase. Schedule 1 (weekly, 28-day cycle) with a 1- or 2-hour infusion of 0.45 mg/kg (cohort 1), 0.9 mg/kg (cohort 2), 1.8 mg/kg (cohort 3), 3.6 mg/kg (cohort 4), or 5.4 mg/kg (cohort 5) was completed; 3.6 mg/kg (cohort 4) was determined to be the MTD because 2/6 patients experienced DLTs (grade 3 QTc prolongation and grade 3 retinopathy) with 5.4 mg/kg (cohort 5). Schedule 2 (weekly, 28-day cycle) initiated with a dose of 4.8 mg/kg (cohort 6) administered over 4 hours as part of amendment 3 and then escalated to 5.4 mg/kg (cohort 7) as part of amendment 4 with ≥4-hour infusion with further dose escalation to 7.2 mg/kg (cohort 8) and 9.6 mg/kg (cohort 9). Schedule 3 (days 1, 4, and 8, 21-day cycle) initiated with a dose of 3.6 mg/kg (cohort 1) administered over ≥4 hours with dose escalation to 4.8 mg/kg (cohort 2), 6.4 mg/kg (cohort 3), 7.2 mg/kg (cohort 4), and 11.3 mg/kg (cohort 5). In amendment 5, at 11.3 mg/kg (cohort 7), dosing on days 11 and 15 was added to schedule 3 (days 1, 4, 8, 11, and 15; 21-day cycle) to lessen the dosing holiday time between cycles. DLT, dose-limiting toxicity; AE, adverse event; MTD, maximum tolerated dose; PK, pharmacokinetics; q21d, every 21 days; q28d, every 28 days; QTc, corrected QT interval.
Patients
Eligible patients were adults with locally advanced unresectable or metastatic, relapsed and/or refractory nonhematological malignancies whose treatment with an approved agent considered standard of care either did not exist or had proved ineffective. Tumors had squamous histology or were nonsquamous with BRAF, KRAS, NRAS, or HRAS mutations. Patients had one lesion or more measurable by Response Evaluation Criteria in Solid Tumors (RECIST v1.1) at least 2 weeks following their last systemic or radiotherapy, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-1, and acceptable liver and renal function and hematological status. Exclusion criteria are given in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2025.104300. All patients provided written informed consent.
Treatment
Intravenous KO-947 was administered according to one of three schedules. In schedule 1, KO-947 was administered once weekly on a 28-day cycle, with a 1- to 2-hour infusion. In schedule 2, KO-947 was administered once weekly on a 28-day cycle, with a 4-hour infusion; administration time could be decreased (minimum 2-hour infusion) or increased per investigators based on tolerability following the first two treatments. In schedule 3, KO-947 was administered on days 1, 4, and 8 of a 21-day cycle, with a 4-hour infusion; additional administration on days 11 and 15 was added according to amendment 5 (Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2025.104300). As in schedule 2, infusion time could be adjusted per investigators.
Assessments
Screening evaluations were completed within 4 weeks (28 days) of cycle 1 day 1 for patients in the 28-day schedule, or within 3 weeks (21 days) of cycle 1 day 1 for patients in the 21-day schedule. Schedules of tumor assessments are included in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2025.104300.
Determination of objective tumor response was carried out by the investigator per RECIST v1.1. Patients were included in the clinical activity-evaluable population if they received one or more KO-947 doses and had both baseline and one or more post-treatment tumor assessments.
Upon disease progression, all patients were followed up every ∼12 weeks for survival and use of subsequent therapy until either death or 12 months after completion of study accrual, whichever occurred first. All patients were followed for safety during treatment and up to ∼30 days (30 ± 7 days) after treatment discontinuation or until initiation of another anticancer therapy, whichever occurred first.
Safety was evaluated using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Adverse events of special interest (AESIs) included symptoms of retinal dysfunction (e.g. blurred vision, floaters, flashing lights) and any documented retinal disorders (e.g. retinopathy, retinal tear, retinal detachment, other: subretinal fluid, macula edema). If a patient experienced a constellation of signs and symptoms consistent with an infusion reaction, the AE was grouped under the term ‘infusion reaction’; if a patient experienced only one AE, it was reported as that AE alone (e.g. hypotension). The Fridericia correction formula was used to calculate the corrected QT (QTc) interval.
Pharmacokinetic analysis
The PK of KO-947 were characterized after a single i.v. administration and at steady state after multiple i.v. administrations. Additional data on timepoints for blood samples are in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2025.104300. KO-947 plasma concentration was determined from collected blood samples by validated high-performance liquid chromatography with a mass spectrometric detection method. Plasma concentration–time data were analyzed to derive PK parameters calculated using a model-independent approach (noncompartmental analysis). The following PK endpoints related to this objective include area under the concentration–time curve (AUC) over the dosing interval (AUCtau), AUC from time zero to infinity (AUCinf), maximum plasma concentration (Cmax), time to Cmax (tmax), half-life (t½), clearance (CL), and volume of distribution (Vd).
Results
Patients
Overall, 61 patients were enrolled and received treatment (schedules 1 and 2, n = 34; schedule 3, n = 27). Demographics were largely similar between patients in schedules 1 and 2 and those in schedule 3; exceptions included the number of men and women [schedules 1 and 2, 47% men (53% women) versus schedule 3, 70% men (30% women)], proportion of patients with an ECOG PS of 0 at baseline (33% versus 63%, respectively), and cancer type (Table 1). The most common cancer types in schedules 1 and 2 were colorectal (35%) and pancreatic (27%), followed by lung and other (both 12%). In schedule 3, the most common cancer types were colorectal (52%), followed by ovarian, esophagogastric, and other (11% each). In both groups, patients had a median of four prior lines of therapy, and KRAS was the most common RAS/BRAF mutation at screening (schedules 1 and 2, 68%; schedule 3, 52%); no patients had documented 11q-AMP. KO-947 exposure is shown in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2025.104300. Across all groups, 23% of patients received prior therapies targeting the MAPK pathway, including BRAF, MEK, RAF, and ERK inhibitors.
Table 1.
Demographics, baseline characteristics, and disease history
| Schedules 1 and 2 (n = 34) | Schedule 3 (n = 27) | Total (N = 61) | |
|---|---|---|---|
| Mean age (standard deviation), years | 57 (12) | 61 (10) | 59 (11) |
| Men, n (%) | 16 (47) | 19 (70) | 35 (57) |
| Race, n (%) | |||
| Asian | 5 (15) | 0 | 5 (8) |
| Black or African American | 2 (6) | 0 | 2 (3) |
| White | 27 (79) | 26 (96) | 53 (87) |
| Other | 0 | 26 (96) | 26 (43) |
| ECOG performance status, n (%) | |||
| 0 | 3 (33) | 17 (63) | 20 (33) |
| 1 | 20 (59) | 10 (37) | 30 (49) |
| Cancer type, n (%) | |||
| Colorectal | 12 (35) | 14 (52) | 26 (43) |
| Pancreatic | 9 (27) | 0 | 9 (15) |
| Lung | 4 (12) | 0 | 4 (7) |
| Ovarian | 0 | 3 (11) | 3 (5) |
| Esophagogastric | 0 | 3 (11) | 3 (5) |
| Cholangiocarcinoma | 1 (3) | 2 (7) | 3 (5) |
| Head and neck | 2 (6) | 0 | 2 (3) |
| Melanoma | 0 | 2 (7) | 2 (3) |
| Cervical | 2 (6) | 0 | 2 (3) |
| Other | 4 (12)a | 3 (11)b | 7 (11) |
| Median number of prior lines of therapy, n (range) | |||
| Systemic anticancer therapy | 4 (1-9) | 4 (1-8) | 4 (1-9) |
| Surgery | 4 (1-14) | 2 (1-10) | 3 (1-14) |
| Radiation | 2 (1-4) | 1 (1-3) | 1 (1-4) |
| Gene mutations at screening, n (%) | |||
| KRAS | 23 (68) | 14 (52) | 27 (44) |
| G12A | 1 (3) | 0 | 1 (2) |
| G12C | 1 (3) | 1 (4) | 2 (3) |
| G12D | 6 (18) | 3 (11) | 9 (15) |
| G12R | 2 (6) | 2 (7) | 4 (7) |
| G12S | 2 (6) | 0 | 2 (3) |
| G12V | 6 (18) | 2 (7) | 8 (13) |
| G13D | 0 | 1 (4) | 1 (2) |
| Other | 5 (15) | 5 (19) | 10 (16) |
| NRAS | 1 (3) | 3 (11) | 4 (7) |
| Q61L | 1 (3) | 0 | 1 (2) |
| Q61K | 0 | 2 (7) | 2 (3) |
| Q61R | 0 | 1 (4) | 1 (2) |
| HRASG13R | 1 (3) | 0 | 1 (2) |
| BRAFV600 | 2 (6) | 6 (22) | 8 (13) |
| BRAF – other | 3 (9) | 1 (4) | 4 (7) |
| Not available | 5 (15) | 3 (11) | 8 (13) |
| Proportion of patients with BRAFV600 mutation receiving prior BRAK/MEK inhibitor, n/N (%)c | 2/5 (40) | 2/7 (29) | 4/12 (33) |
ECOG, Eastern Cooperative Oncology Group.
Small bowel (n = 1), optic pathway glioma—pilocytic astrocytoma (n = 1), appendiceal (n = 1), and skin cancer/squamous cell carcinoma (n = 1).
Penile (n = 1), vaginal (n = 1), and sarcoma (gastrointestinal stromal tumor) (n = 1).
Among patients with a BRAF mutation.
Most patients discontinued owing to disease progression (schedules 1 and 2/schedule 3: 88%/67%); other reasons included AEs (3%/15%), symptomatic deterioration (3%/7%), patient request (6%/7%), and physician decision (0%/4%). Thirteen patients received a second computed tomography scan.
MTD determination
In schedule 1, there were no DLTs in the six patients enrolled in cohorts 1-4 (0.45-3.6 mg/kg). In cohort 5 (5.4 mg/kg), one patient had a DLT of prolonged QTc interval on electrocardiogram (ECG), and the cohort was expanded to six patients; an additional patient had a DLT of macular edema and subretinal fluid. To exclude a possible Cmax effect, the infusion time for cohort 5 was increased to 2 hours. MTD for schedule 1 was determined to be 3.6 mg/kg administered over 1-2 hours.
Schedules 2 and 3 were initiated to determine if the ocular DLT and other AEs observed in schedule 1 were due to Cmax-associated toxicities temporally related to the short duration of i.v. administration. PK modeling of available KO-947 concentration data from patients (n = 13) treated at KO-947 dose levels 0.45-5.4 mg/kg was carried out to guide the infusion duration for doses >3.6 mg/kg for schedules 2 and 3. Simulated profiles predicted that with a 4-hour and a 6-hour infusion, escalation up to 9.6 mg/kg (inclusive) and up to 12.8 mg/kg (inclusive), respectively, would result in Cmax at or below the highest Cmax of 3.6 mg/kg, 1-hour infusion (3630 ng/ml), with no ocular AEs observed. As a result, infusion durations for amendments 2 and 3 were adjusted to ≥4 hours for doses up to 9.6 mg/kg and ≥6 hours for doses over 9.6 mg/kg up to a maximum of 12.8 mg/kg.
Despite longer infusion duration, ocular AEs were reported in schedules 2 and 3. Follow-up PK analysis of patients enrolled in schedules 2 and 3 (total n = 26) was carried out. The total AUC for 3.6 mg/kg (cohort 1, schedule 3) and 5.4 mg/kg (cohort 7, schedule 2) were comparable to those from patients in schedule 1 at the same dose levels. As expected with the longer infusion time in schedules 2 and 3, the KO-947 Cmax was lower than that observed in schedule 1 and achieved near the end of the infusion (∼4 hours). No apparent correlation of Cmax values with ocular toxicity occurrence was observed based on the PK and safety data in schedules 2 (4.8-5.4 mg/kg) and 3 (3.6-5.4 mg/kg). These results suggested that reducing Cmax does not clearly mitigate ocular toxicity. In amendment 5, the infusion duration was amended to ≥4-hour infusion with flexibility per the investigator’s discretion to decrease to 2 hours if the infusion was tolerated in the first two treatments.
Among patients enrolled in schedule 2, no DLTs occurred in cohorts 5-8 (4.8-7.2 mg/kg). One patient experienced a DLT of QTc prolongation in cohort 9 (9.6 mg/kg), resulting in expansion of the cohort to six patients; a second patient in this expansion also experienced a DLT of QTc prolongation. Therefore, the MTD for schedule 2 was 7.2 mg/kg administered over 2 to 4 hours.
In schedule 3, no DLTs were observed in the six patients enrolled in cohorts 1 or 2 (3.6-4.8 mg/kg). One DLT of hypotension was observed in cohort 3 (6.4 mg/kg), which was expanded to six patients; no further DLTs occurred. No DLTs occurred in cohort 4 (8.5 mg/kg, n = 3). In cohort 5 (11.3 mg/kg), one patient had a DLT of an infusion-related reaction, expanding the cohort to six patients; no further DLTs occurred. In cohort 6 (11.3 mg/kg, with additional dosing on days 11 and 15), one patient had a DLT of infusion-related reaction. The maximum administered dose for schedule 3 was 11.3 mg/kg (cohort 6). A formal MTD based on DLTs was not determined for schedule 3, due to fewer DLTs versus the higher 11.3 mg/kg dose, as well as PK exposure predicted to have antitumor activity; however, a recommended dose of 8.5 mg/kg using the schedule of i.v. administration on days 1, 4, 8, 11, and 15 every 21 days with ≥4-hour infusion was selected for further investigation of the safety, tolerability, PK/pharmacodynamics, and antitumor activity of KO-947.
Safety
All patients experienced at least one treatment-emergent AE (TEAE; Supplementary Tables S3 and S4, available at https://doi.org/10.1016/j.esmoop.2025.104300). Grade ≥3 TEAEs occurred in 17 patients (50%) in schedules 1 and 2 and 18 (67%) in schedule 3 (Supplementary Tables S5 and S6, available at https://doi.org/10.1016/j.esmoop.2025.104300). The most common grade ≥3 TEAEs in schedules 1 and 2 included increased gamma-glutamyl transferase (n = 5, 15%); increased alanine aminotransferase, increased aspartate aminotransferase, and QTc prolongation on ECG (each n = 3, 9%); and increased blood alkaline phosphatase, lymphopenia, hypokalemia, and rash (each n = 2, 6%). In schedule 3, the most common grade ≥3 TEAEs were respiratory tract infection, asthenia, increased transaminases, anemia, infusion-related reaction, and dyspnea (each n = 2, 7%).
Treatment-related TEAEs occurred in 88% of patients in schedules 1 and 2 and 93% of those in schedule 3 (Table 2). Grade ≥3 treatment-related TEAEs occurred in 27% of patients in schedules 1 and 2 and 15% of those in schedule 3. In schedules 1 and 2, the most common treatment-related TEAEs were blurred vision (50%), subretinal fluid (32%), nausea (18%), vomiting, QTc prolongation, dermatitis acneiform (15% each), and fatigue and headache (12% each). In schedule 3, the most common treatment-related TEAEs were blurred vision (33%), subretinal fluid, diarrhea, dermatitis acneiform, and infusion-related reaction (22% each), retinal detachment, macular edema, and photopsia (15% each), and vitreous floaters, dyspepsia, and QTc prolongation (11% each).
Table 2.
Treatment-related TEAEs occurring in >10% of patients in schedules 1 and 2, and in schedule 3
| Schedules 1 and 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 (n = 1) | Cohort 2 (n = 1) | Cohort 3 (n = 1) | Cohort 4 (n = 3) | Cohort 5 (n = 7) | Cohort 6 (n = 4) | Cohort 7 (n = 3) | Cohort 8 (n = 5) | Cohort 9 (n = 9) | Total (N = 34) | |
| Dose, mg/kg | 0.45 | 0.9 | 1.8 | 3.6 | 5.4 | 4.8 | 5.4 | 7.2 | 9.6 | |
| Total number of treatment-related TEAEs, n | 2 | 0 | 1 | 7 | 60 | 25 | 18 | 14 | 39 | 166 |
| Patients with ≥1 treatment-related TEAE, n (%) | 1 (100) | 0 | 1 (100) | 2 (67) | 7 (100) | 3 (75) | 3 (100) | 5 (100) | 8 (89) | 30 (88) |
| Blurred vision | 0 | 0 | 0 | 0 | 4 (57) | 1 (25) | 2 (67) | 2 (40) | 8 (89) | 17 (50) |
| Subretinal fluid | 0 | 0 | 0 | 0 | 3 (43) | 1 (25) | 2 (67) | 2 (40) | 3 (33) | 11 (32) |
| Nausea | 0 | 0 | 0 | 1 (33) | 1 (14) | 1 (25) | 2 (67) | 0 | 1 (11) | 6 (18) |
| Vomiting | 0 | 0 | 0 | 1 (33) | 0 | 0 | 1 (33) | 1 (20) | 2 (22) | 5 (15) |
| QTc prolongation | 0 | 0 | 0 | 0 | 1 (14) | 0 | 0 | 0 | 4 (44) | 5 (15) |
| Dermatitis acneiform | 0 | 0 | 0 | 0 | 0 | 1 (25) | 1 (33) | 2 (40) | 1 (11) | 5 (15) |
| Fatigue | 1 (100) | 0 | 0 | 0 | 1 (14) | 0 | 1 (33) | 0 | 1 (11) | 4 (12) |
| Headache | 0 | 0 | 0 | 1 (33) | 1 (14) | 1 (25) | 1 (33) | 0 | 0 | 4 (12) |
|
Schedule 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 (n = 3) | Cohort 2 (n = 3) | Cohort 3 (n = 9) | Cohort 4 (n = 3) | Cohort 5 (n = 6) | Cohort 6 (n = 3) | Total (N = 27) | ||||
| Dose, mg/kg | 3.6 | 4.8 | 6.4 | 7.2 | 11.3 | 11.3 | ||||
| Total number of treatment-related TEAEs, n | 11 | 9 | 19 | 38 | 50 | 9 | 136 | |||
| Patients with ≥1 treatment-related TEAE, n (%) | 2 (67) | 3 (100) | 8 (89) | 3 (100) | 6 (100) | 3 (100) | 25 (93) | |||
| Blurred vision | 0 | 0 | 3 (33) | 1 (33) | 4 (67) | 1 (33) | 9 (33) | |||
| Subretinal fluid | 1 (33) | 1 (33) | 2 (22) | 0 | 1 (17) | 1 (33) | 6 (22) | |||
| Diarrhea | 0 | 1 (33) | 1 (11) | 2 (67) | 2 (33) | 0 | 6 (22) | |||
| Dermatitis acneiform | 0 | 0 | 0 | 1 (33) | 4 (67) | 1 (33) | 6 (22) | |||
| Infusion-related reaction | 0 | 0 | 2 (22) | 1 (33) | 2 (33) | 1 (33) | 6 (22) | |||
| Retinal detachment | 0 | 1 (33) | 1 (11) | 1 (33) | 1 (17) | 0 | 4 (15) | |||
| Macular edema | 0 | 0 | 1 (11) | 1 (33) | 2 (33) | 0 | 4 (15) | |||
| Photopsia | 0 | 0 | 1 (11) | 1 (33) | 1 (17) | 1 (33) | 4 (15) | |||
| Vitreous floaters | 2 (67) | 0 | 0 | 0 | 1 (17) | 0 | 3 (11) | |||
| Dyspepsia | 0 | 0 | 1 (11) | 1 (33) | 1 (17) | 0 | 3 (11) | |||
| QTc prolongation | 0 | 0 | 0 | 0 | 1 (17) | 2 (67) | 3 (11) | |||
QTc, corrected QT interval; TEAE, treatment-emergent adverse event.
To evaluate the effect of KO-947 on eye disorders, AESI were recorded for all schedules (Table 3). Most AESI reported were either grade 1 or 2, except for the initial event of subretinal fluid recorded, which was grade 3. In schedules 1 and 2, 50% of patients had at least one AESI, as did 82% of those in schedule 3. The most common AESI were blurred vision (schedules 1 and 2, 35%; schedule 3, 33%) and subretinal fluid (24% and 22%, respectively). There were no reports of retinal vein occlusion. In cycle 1, the median time to onset of ocular events was up to 24 hours (range, 0-6 days) of dosing and median time to resolution was 3 days (range, 0-114 days). No modifications to dosing or other medical interventions were typically required; however, three patients had their dose reduced and one patient required dose interruption owing to ocular toxicity.
Table 3.
AESI in schedules 1 and 2, and in schedule 3
| Schedules 1 and 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 (n = 1) | Cohort 2 (n = 1) | Cohort 3 (n = 1) | Cohort 4 (n = 3) | Cohort 5 (n = 7) | Cohort 6 (n = 4) | Cohort 7 (n = 3) | Cohort 8(n = 5) | Cohort 9 (n = 9) | Total (N = 34) | |
| Dose, mg/kg | 0.45 | 0.9 | 1.8 | 3.6 | 5.4 | 4.8 | 5.4 | 7.2 | 9.6 | |
| Total number of AESI, n | 0 | 0 | 0 | 0 | 0 | 3 | 5 | 5 | 16 | 29 |
| Patients with ≥1 AESI, n (%) | 0 | 0 | 0 | 0 | 0 | 1 (25) | 3 (10) | 5 (10) | 8 (89) | 17 (50) |
| Eye disorders, n (%) | 0 | 0 | 0 | 0 | 0 | 1 (25) | 3 (10) | 5 (10) | 8 (89) | 17 (50) |
| Blurred vision | 0 | 0 | 0 | 0 | 0 | 0 | 2 (67) | 2 (40) | 8 (89) | 12 (35) |
| Subretinal fluid | 0 | 0 | 0 | 0 | 0 | 1 (25) | 2 (67) | 2 (40) | 3 (33) | 8 (24) |
| Retinal edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (20) | 1 (11) | 2 (6) |
| Vitreous floaters | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33) | 0 | 1 (11) | 2 (6) |
| Macular edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (11) | 1 (3) |
|
Schedule 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 (n = 3) | Cohort 2 (n = 3) | Cohort 3 (n = 9) | Cohort 4 (n = 3) | Cohort 5 (n = 6) | Cohort 6 (n = 3) | Total (N = 27) | ||||
| Dose, mg/kg | 3.6 | 4.8 | 6.4 | 7.2 | 11.3 | 11.3 | ||||
| Total number of AEs, n | 4 | 2 | 11 | 14 | 16 | 3 | 50 | |||
| Patients with ≥1 AE, n (%) | 2 (67) | 2 (67) | 7 (78) | 3 (100) | 6 (100) | 2 (67) | 22 (82) | |||
| Eye disorders, n (%) | 2 (67) | 2 (67) | 7 (78) | 3 (100) | 6 (100) | 2 (67) | 22 (82) | |||
| Blurred vision | 0 | 0 | 3 (33) | 1 (33) | 4 (67) | 1 (33) | 9 (33) | |||
| Subretinal fluid | 1 (33) | 1 (33) | 2 (22) | 0 | 1 (17) | 1 (33) | 6 (22) | |||
| Macular edema | 0 | 0 | 1 (11) | 1 (33) | 2 (33) | 0 | 4 (15) | |||
| Photopsia | 0 | 0 | 1 (11) | 1 (33) | 1 (17) | 1 (33) | 4 (15) | |||
| Retinal detachment | 0 | 1 (33) | 1 (11) | 1 (33) | 1 (17) | 0 | 4 (15) | |||
| Vitreous floaters | 2 (67) | 0 | 0 | 0 | 1 (17) | 0 | 3 (11) | |||
| Detachment of retinal pigment epithelium | 0 | 0 | 0 | 1 (33) | 1 (17) | 1 (33) | 1 (4) | |||
AESI, adverse events of special interest.
There were several events of acute QTc prolongation and hypotension following KO-947 administration. Seven grade 3-4 events occurred in five patients: four with grade 3 QTc prolongation and one patient with grade 3 hypotension that resolved by the next day. These AEs occurred at doses ranging from 5.4 mg/kg to 11.3 mg/kg, and most events occurred during infusion, around tmax. The mean free Cmax in the overall study was about one-tenth of the IC50 value for the inhibitory effect of KO-947 on the human ether-a-go-go-related gene (hERG) potassium channel (4.4 μM). Preliminary exposure–response analysis indicated that KO-947 exposure had a positive relationship with QTc prolongation and a small, negative relationship with both diastolic and systolic blood pressure.
Dose interruptions and reductions due to toxicity occurred in 14 and 7 patients, respectively. One patient (3%) in schedules 1 and 2 and four patients (15%) in schedule 3 discontinued owing to AEs. No deaths due to treatment-related TEAEs occurred.
Pharmacokinetics
KO-947 PK were evaluated and available from patients in the dose-escalation cohorts (Figure 2). Based on noncompartmental analysis, KO-947 exposures after a single dose increased in a slightly greater than dose-proportional manner (slope = 1.2). KO-947 Cmax was observed at or near the end of the infusion, with KO-947 plasma concentration then declining in a biphasic manner. At 3.6 mg/kg (i.e. MTD for schedule 1), the observed Cmax ranged from 1410 ng/ml to 3630 ng/ml, with a median infusion time of 1 hour and mean t½ of ∼6.65 hours. None to minimal accumulation was observed with all dosing schedules. Total AUCs for 3.6 mg/kg (schedule 3) and 5.4 mg/kg (schedule 2) were comparable to those obtained from participants in the schedule 1 group at the same dose levels. In schedules 2 and 3, KO-947 Cmax was lower than that observed in schedule 1 at the same dose levels and was achieved near the end of the infusion (∼4 hours). KO-947 demonstrated a moderate plasma CL, moderate Vd, and a t½ of ∼6.5-24.0 hours. At the 11.3 mg/kg dose (cohort 5 of schedule 3 with a 6-hour infusion), the PK profile appeared distinct from early dose levels as patients were exposed to KO-947 at the plasma concentration of ∼3600 ng/ml for an extended period of time (∼4 hours).
Figure 2.
Mean (± standard deviation) plasma KO-947 concentration–time plots by dose. (A) Schedule 1: cycle 1 day 1 weekly administration every 28 days. (B) Schedule 2: cycle 1 day 1 weekly administration every 28 days. (C) Schedule 3: cycle 1 days 1, 4, 8, 11, and 15 administration every 21 days.
Clinical activity
No complete or partial responses were observed; best overall response for both schedules 1 and 2 and schedule 3 was stable disease [n = 5 (15%) and n = 10 (37%), respectively; Supplementary Table S7, available at https://doi.org/10.1016/j.esmoop.2025.104300]. The median duration of stable disease for the 15 patients was 58 days. Six of these patients had stable disease for ≥12 weeks, with one patient having stable disease for >6 months. Five of the six patients with stable disease for >100 days had a tumor type and/or genetics [germline neurofibromatosis type 1 (NF1)-associated glioma-pilocytic astrocytoma, cervical HRAS G13R squamous carcinoma, vaginal squamous carcinoma, ovarian KRAS G12R carcinoma, colon BRAF-mutated carcinoma] consistent with those responsive to other MAPK pathway inhibitors.
Discussion
In this first-in-human, phase I clinical trial, the i.v. ERK inhibitor KO-947 demonstrated excellent gastrointestinal tolerability but a high frequency of ocular toxicities in patients with advanced solid tumors. Emerging TEAEs led to dynamic and responsive amendments to the protocol to explore alternative doses and schedules to help mitigate toxicity; these revisions included extending the infusion duration and changing the dosing schedule, increasing ophthalmological monitoring, and adding retinal AEs and disorders to AEs of significant interest.
The 8.5 mg/kg dose administered i.v. with at least a 4-hour infusion on days 1, 4, 8, 11, and 15 on a 21-day cycle was selected as the RP2D schedule to investigate antitumor activity of KO-947 in two tumor-specific cohorts. This selection was based on overall safety, tolerability, and PK, as no DLTs were observed in three patients treated at this dose; one patient tolerated the dose for seven cycles; DLTs were observed at the higher doses of 9.6 mg/kg and 11.3 mg/kg. Preclinical studies suggested the 8.5 mg/kg dose should provide drug exposure for antitumor activity and dosing on days 1, 4, 8, 11, and 15 provides a more intense dose schedule, likely providing more effective inhibition of the ERK pathway while the length of infusion minimizes Cmax and risk of QTc prolongation. The selected RP2D schedule was not further characterized as the study was terminated early.
KO-947-related diarrhea and nausea were less common than gastrointestinal toxicity reported for oral ERK inhibitors, for which diarrhea was reported in >40% of patients receiving ulixertinib, MK-8353, or GDC-0994.11,12,14 This is likely due to the i.v. administration and absence of local effect on the gastrointestinal tract. Ocular toxicity was frequent in this study, consistent with ocular toxicity as a class effect of ERK inhibitors; ocular TEAEs have been reported by numerous oral ERK inhibitors, including ulixertinib/BVD-523 (retinopathy, 14%), MK-8353 (oropharyngeal pain and blurred vision, each 8%), GDC-0994 (blurred vision, 13%), and LY3214996 (blurred vision, 15%).11, 12, 13, 14, 15,21 Increased incidence of ocular toxicity with KO-947 may have been due to i.v. administration versus the oral route used by other ERK inhibitors.11,21 The temporal association between administration and onset of DLTs initially suggested that this may have been due to a high Cmax resulting from the short infusion time (1-2 hours); however, preliminary PK and safety data from patients in schedules 2 and 3 did not identify a correlation between Cmax and ocular toxicities. It is also possible that more active monitoring for ocular AEs in this study contributed to increased detection. Importantly, ERK inhibitor-related ocular events are nearly always reversible and self-limited.
Preclinical studies of KO-947 demonstrated compelling antitumor activity in both adenocarcinomas with activating mutations in the MAPK pathway and in SCCs wild type for BRAF and RAS, with the majority of regressions observed in esophageal SCC and head and neck SCC with 11q-AMP.18,19 The study plan was to evaluate clinical activity in the MTD and tumor expansion cohorts, in which patients were to be selected based on tumor type and genomics (e.g. head and neck SCC and esophageal SCC with 11q-AMP) shown to respond in the preclinical studies. Evaluation of the level of ERK inhibition using validated pERK and pRSK assays in pre- and post-treatment biopsies was planned; however, the study was terminated before initiating this phase. The best overall response observed was stable disease, seen in 15 patients. The lack of clinical activity may have been due to patient selection, incomplete pathway inhibition, feedback re-activation of ERK signaling, and/or activation of parallel pathways. While the observed on-target retinal toxicity and rash suggest that KO-947 inhibited ERK at least transiently, the low drug accumulation and relatively short half-life for the dosing schedule may have impacted the level of ERK inhibition. Additionally, the dose-escalation phase detailed in this paper did not include patients who may have the greatest potential for response to KO-947, as no patient had a documented 11q-AMP; more than half of patients had a KRAS mutation as the qualifying genomic alteration, which may signal in part through the parallel phosphatidylinositol 3'-kinase (PI3K) pathway. Lastly, direct inhibition of ERK may result in relief of ERK-dependent feedback inhibition, leading to re-activation of the MAPK pathway and activation of compensatory pathways such as the PI3K pathway.22
This first-in-human trial of KO-947 supports that i.v. administration of ERK inhibitors can minimize gastrointestinal toxicities and overcome other challenges, such as difficulty swallowing pills, when these issues may compromise dose optimization and compliance. Although KO-947 is not being developed further, the on-target ocular toxicity observed demonstrates the feasibility of effectively targeting oncogenic pathways with i.v. therapeutics.
Acknowledgements
Medical writing and editorial assistance were provided by Meghan Sullivan, PhD, of MedVal Scientific Information Services, LLC, Princeton, NJ, USA and were funded by Kura Oncology, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3.
Funding
This work was supported by Kura Oncology, Inc (no grant number).
Disclosure
AMS: advisory board: Mersana Therapeutics, Merus, PMV Pharma, Relay Therapeutics, Schrodinger. Consulting: Blueprint Bio, Flagship Pioneering, Redona Therapeutics. Steering committee: Merus, Pfizer, Relay Therapeutics. Research to institution: AstraZeneca, ArQule, BeiGene/Springworks, Black Diamond Therapeutics, Elevation Oncology, Kura Oncology, Eli Lilly, Merus, Northern Biologics, Pfizer, PMV Pharma, Relay Therapeutics, Repare Therapeutics, Revolution Medicines, Surface Oncology. AMS is supported by the National Cancer Institute (NCI P30 CA008748 CCITLA) and the American Society of Clinical Oncology (ASCO) Conquer Cancer Foundation Career Development Award. VB: consultant or advisory role: CytomX Therapeutics, Guidepoint, IDEAYA Biosciences; Loxo Oncology, Puma Biotechnology, Oncoart. Honoraria (speaking): Eli Lilly. Institutional financial support for clinical trials from: AbbVie, ACEO, Adaptimmune, Amcure, Amgen, ArQule, Astellas Pharma, BeiGene, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb CytomX, Daiichi Sankyo, Debiopharm, Dynavax Technologies, GlaxoSmithKline, Genentech/Roche, H3, Incyte Corporation, Inovio, Janssen Pharmaceuticals, Kura Oncology, Lilly, Loxo Oncology, Nektar Therapeutics, MacroGenics, Menarini, Merck Sharp & Dohme, Mersana Therapeutics, Merus, Millennium Pharmaceuticals, Nanobiotix, Nektar, Novartis, ORCA Therapeutics, Pfizer, PharmaMar, Principia Biopharma, PsiOxus, Puma Biotechnology, Regeneron Pharmaceuticals, Rigontec, Sanofi, Seattle Genetics, Spectrum, Synthon, Taiho Pharmaceutical Co., Takeda, Tesaro, Transgene, Zenith. AJO: institutional financial support for clinical trials from: Anaveon, Anegene, GSK, HiFiBio, Immatics, J&J, Kura, Lantern, Pfizer, Regeneron, Replimune, Shionogi, Takeda, Viewpoint. Advisory board: Bristol Myers Squibb, IO Biotech, Merck, Oncosec, Pfizer, Replimune. Data and Safety Monitoring Board role (no financial renumeration): Takeda, Pfizer. MV: research: AstraZeneca, BeiGene, C4 Therapeutics, Novartis, Roche, Taiho Oncology, Thermo Fisher Scientific. Consultant or advisory role: BMS. Speaker’s bureau: Novocure. Travel/accommodation expenses: Merck, Roche, Serono. Other: BMS, Debiopharm Group, HutchMed, Incyte, Mundipharma, Novartis, PharmaMar, Roche, Servier, Taiho Oncology. MK: consultant: Kura Oncology, Inc. JMA: employment: Kura Oncology, Inc. BT: employment: Kura Oncology, Inc. EG: research: Novartis, Roche, Thermo Fisher, AstraZeneca, Taiho, BeiGene, Janssen. Consultant or advisory role: Roche, Ellipses Pharma, Boehringer Ingelheim, Janssen Global Services, Seattle Genetics, Thermo Fisher, MabDiscovery, Anaveon, Hengrui, Sanofi, Incyte, Medscape, Pfizer, Amgen. Speaker’s bureau role: Merck Sharp & Dohme, Roche, Thermo Fisher, Novartis, SeaGen. Employment: NEXT Oncology. Stocks: 1TRIALSP. All other authors have declared no conflicts of interest.
Data sharing
The data generated in this study are available in the article and supplementary data files. Additional data are available upon request from the corresponding author.
Supplementary data
References
- 1.Roskoski R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol Res. 2019;144:19–50. doi: 10.1016/j.phrs.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Bond C.E., Whitehall V.L.J. How the BRAF V600E mutation defines a distinct subgroup of colorectal cancer: molecular and clinical implications. Gastroenterol Res Pract. 2018;2018 doi: 10.1155/2018/9250757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman P.B., Hauschild A., Robert C., et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larkin J., Ascierto P.A., Dreno B., et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 5.Hauschild A., Grob J.J., Demidov L.V., et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 6.Kopetz S., Grothey A., Yaeger R., et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 7.Hong D.S., Fakih M.G., Strickler J.H., et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janne P.A., Riely G.J., Gadgeel S.M., et al. Adagrasib in non-small-cell lung cancer harboring a KRAS(G12C) mutation. N Engl J Med. 2022;387(2):120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 9.Samatar A.A., Poulikakos P.I. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13(12):928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 10.O’Leary C.G., Andelkovic V., Ladwa R., et al. Targeting BRAF mutations in non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(6):1119–1124. doi: 10.21037/tlcr.2019.10.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan R.J., Infante J.R., Janku F., et al. First-in-class ERK1/2 inhibitor ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: results of a phase I dose-escalation and expansion study. Cancer Discov. 2018;8(2):184–195. doi: 10.1158/2159-8290.CD-17-1119. [DOI] [PubMed] [Google Scholar]
- 12.Moschos S.J., Sullivan R.J., Hwu W.J., et al. Development of MK-8353, an orally administered ERK1/2 inhibitor, in patients with advanced solid tumors. JCI Insight. 2018;3(4) doi: 10.1172/jci.insight.92352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blake J.F., Burkard M., Chan J., et al. Discovery of (S)-1-(1-(4-chloro-3-fluorophenyl)-2-hydroxyethyl)-4-(2-((1-methyl-1H-pyrazol-5-yl)amino)pyrimidin-4-yl)pyridin-2(1H)-one (GDC-0994), an extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor in early clinical development. J Med Chem. 2016;59(12):5650–5660. doi: 10.1021/acs.jmedchem.6b00389. [DOI] [PubMed] [Google Scholar]
- 14.Varga A., Soria J.C., Hollebecque A., et al. A first-in-human phase I study to evaluate the ERK1/2 inhibitor GDC-0994 in patients with advanced solid tumors. Clin Cancer Res. 2020;26(6):1229–1236. doi: 10.1158/1078-0432.CCR-19-2574. [DOI] [PubMed] [Google Scholar]
- 15.Pant S., Bendell J.C., Sullivan R.J., et al. A phase I dose escalation (DE) study of ERK inhibitor, LY3214996, in advanced (adv) cancer (CA) patients (pts) J Clin Oncol. 2019;37(suppl 15):3001. [Google Scholar]
- 16.Portelinha A., Thompson S., Smith R.A., et al. ASN007 is a selective ERK1/2 inhibitor with preferential activity against RAS- and RAF-mutant tumors. Cell Rep Med. 2021;2(7) doi: 10.1016/j.xcrm.2021.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolcher A.W., Sullivan R.J., Rasco D.W., et al. Phase 1 clinical safety and efficacy of ASN007, a novel oral ERK1/2 inhibitor, in patients with RAS, RAF or MEK mutant advanced solid tumors. Mol Cancer Ther. 2019;18(suppl 12):TARG-19–PR09. [Google Scholar]
- 18.Burrows F., Kessler L., Chen J., et al. Abstract 5168: KO-947, a potent ERK inhibitor with robust preclinical single agent activity in MAPK pathway dysregulated tumors. Cancer Res. 2017;77(suppl 13):5168. [Google Scholar]
- 19.Burrows F., Kessler L., Wu T., et al. Abstract 3885: 11q13 amplification selects for sensitivity to the ERK inhibitor KO-947 in squamous cell carcinomas. Cancer Res. 2018;78(suppl 13):3885. [Google Scholar]
- 20.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 21.Francis J.H., Canestraro J., Haggag-Lindgren D., et al. Clinical and morphologic characteristics of extracellular signal-regulated kinase inhibitor-associated retinopathy. Ophthalmol Retina. 2021;5(12):1187–1195. doi: 10.1016/j.oret.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan M.B., Der C.J., Wang-Gillam A., Cox A.D. Targeting RAS-mutant cancers: is ERK the key? Trends Cancer. 2015;1(3):183–198. doi: 10.1016/j.trecan.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.