Abstract
Due to bleomycin’s cytotoxic characteristics, which include cardiotoxicity, this investigation looked at the effectiveness of costus ethanolic extract in reducing cardiotoxicity in male rats receiving bleomycin therapy. Forty adult male rats (160–200 g) were evenly allocated into four groups: group (1) included normal rats serving as the control; group (2) included normal rats administered 200 mg/kg of costus ethanolic extract (CEE) orally for 6 weeks; group (3) consisted of rats receiving bleomycin (15 mg/kg twice weekly, ip) for 6 weeks; and group (4) involved rats treated orally with CEE (200 mg/kg/day) for 6 weeks following bleomycin intoxication. The results indicated that the CEE significantly reversed the cardiological deteriorations brought on by bleomycin; this was demonstrated by a considerable increase in cardiac SOD, GPx, GSH, and CAT, along with a substantial decrease in cardiac MDA, NO, and DNA fragmentation. Also, serum, LDH, CK-MB, CK- total, TNF-α, IL-4, IL-6 IL-10, IL-1β, triglycerides, cholesterol, and LDL were significantly reduced, while CD4 levels increased, and HDL declined significantly. The results of the histological and immunohistochemical analyses revealed a notable regeneration. In conclusion, CEE’s anti-cardiotoxic, anti-inflammatory, and antioxidant properties prove its ability to be a cardio-protective supplement. This may be mediated by its active constituents’ radical scavenging and antioxidant properties, particularly high phenolic content.
Keywords: Cardiotoxicity, Bleomycin, Saussurea costus, Anti-inflammatory, Rats
1. Introduction
Cancer was the second most prevalent cause of death in 2018.1 Along with radiotherapy and surgery, chemotherapy is a crucial cancer treatment. But its cardiotoxicity, which causes cardiomyopathy, is still a serious side effect.2 Heart failure results from cardiomyopathy, which causes the heart's pumping capacity to decline. It is the third contributing cause of heart failure after coronary insufficiency and hypertension.3, 4 Many types of cancer, such as Hodgkin's reticulosarcomas, malignant non-lymphomas, and certain reproductive cancers, can anticipate living longer and having a good quality of life appreciation to the widespread usage of various chemotherapy regimens, which improves their prognosis. The oncological pathology mentioned above is frequently treated with bleomycin.5 Bleomycin, an antibiotic that fights tumors, was discovered as an A2 fraction in a culture of Streptomyces verticillus. Its toxic effects on lung tissue, including the onset of pulmonary fibrosis, pleurisy with pain, and worsening respiratory failure, are among the first-known side effects. Infrequent toxic effects on blood vessels encompass cerebral arteritis, stroke, myocardial infarction, thrombotic microangiopathy, and Raynaud's syndrome.6 It may harm blood vessels, which may occasionally result in Raynaud's syndrome, cerebral arteritis, stroke, myocardial infarction, and thrombotic microangiopathy.5 The effects of medicine on the myocardium, particularly when administered intravenously, are also unknown. Cardiotoxicity is the most frequent symptom of treatment-related morbidity, even though modern advancements in cancer treatments, such as chemotherapy and radiation therapy, have boosted the survival rate of cancer patients.7 Cardiotoxicity refers to the complications that include various adverse effects that lead to cardiac dysfunction on the background of the drug and radiotherapy8. Bleomycin (BLM) is a medication produced by S. verticillus bacteria and is classified as a glycoprotein antibiotic. It is an antitumor drug used to treat malignancies such as lymphomas, testicular carcinoma, squamous cell carcinoma, and malignant pleural effusion.9 Among the frequent side effects of BLM medication include thrombotic microangiopathy, cerebral arteritis, myocardial infarction, pulmonary fibrosis, and Raynaud's syndrome.10 Reports indicate that the myocardium exhibits atrophic and inflammatory alterations following a single BLM injection.11.
There is currently no evidence connecting the acute chest pain brought on by bleomycin infusion to a pathophysiological cause. One likely reason is grave inflammation, which might manifest as acute pleuro-pericarditis or more widespread mucocutaneous toxicity related to bleomycin therapy. It is crucial to consider the vascular etiology of the pain because several pulmonary vascular illnesses, including pulmonary hypertension and pulmonary embolism, can result in pleuritic and substernal chest pain, even in the loss of infarction. Regrettably, there is still uncertainty about the anatomical mechanism behind the cardiotoxic effects of bleomycin. Due to their beneficial chemical components, herbal medicines have been utilized to treat numerous diseases for over 4,000 years. Essentially, the phytochemical components of plants that carry out precise pharmacological effects when consumed by humans are where their medicinal potential lies.12, 13.
The plant Saussurea costus, also known as Saussurea lappa Clarke,14 is a constituent of the Asteraceae family and is referred to as the Kuth root or Indian costus.15, 16 Costus was used as a medicinal plant to cure various conditions, including asthma, inflammatory diseases, ulcers, and stomach issues. It was utilized in modern medicine and was listed in the Prophet's medicine to treat numerous diseases.16, 17 A plant with a high concentration of antioxidants is Saussurea costus.18 Its pharmacological effects include anti-inflammatory, immune-modulating, hypoglycemic, anti-hepatotoxic, hypolipidemic, antiparasitic, antiviral, and anticancer properties.19, 20 Therefore, the current investigation aimed to evaluate the cardioprotective efficacy of CEE administration in bleomycin-induced cardiac damage in animal models.
2. Materials and methods
2.1. Chemicals
Bleomycin was sourced from Sigma Aldrich, located in St. Louis, MO, USA.
2.2. Plant materials and extraction
Scientific botanists identified and verified the costus roots before purchasing them from Imtinan Company in Nasr City, Cairo, Egypt. The plant was found to have a taxonomic serial number of 780691. According to the modified approach of21, the ethanolic extract of the dry powdered roots was performed using the previously published technique, and the 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity of CEE was assessed21. The extract's reducing power was evaluated using the method outlined.22The total phenolic content (TPC) of CEE was determind, using established biochemical method 59. The total extract yield was calculated according to 60
2.3. HPLC analysis of phenolic constituents
Using an Agilent 1260 series, the HPLC (high-performance liquid chromatography) test was performed for CEE screening. A Kromasil C18 column with a 4.6 mm ID and a 250 mm ID was used for the separation (5 m). Water and acetonitrile were the main components of the mobile phase, which had a flow rate of 1 ml/min and a trifluoroacetic acid concentration of 0.05 % (A and B). The mobile phase was programmed using a linear gradient at the following intervals: 0 min (82 % A), 0 min to 5 min (80 % A), 5–8 min to 6 min, 5–8 min to 12 min, 85 % A, and 15–16 min (82 % A). At a wavelength of 280 nm, the multiwavelength detector was observed. Then, all the sample solutions were exposed to an injection of adequate volume (10 ml). A constant 35 °C was kept in the column temperature.
2.4. Animals and the Design of Experiments
At the National Research Centre in Giza, Egypt's Animal Colony provided male albino rats (weighing between 160 and 200 g). After acclimatization to the experimental conditions, the animals were divided into four groups of ten. In the first group, designated as a control group, healthy animals received standard food and an intraperitoneal injection of 1 ml isotonic saline. The second group consisted of good-health animals that received 200 mg/kg/day of CEE (costus ethanolic extract) orally for six weeks.23 The third group included animals receiving 15 mg/kg of bleomycin (IP) twice a week.24 The fourth group served as positive control, which included rats administered CEE orally once daily for six weeks following bleomycin intoxication.
2.5. Samples of blood and tissue
The rats underwent overnight fasting and weighing after the treatment period (6 weeks). After the intramuscular administration of sodium pentobarbital (9.1 mg/kg in a sterile 0.9 % NaCl solution), blood samples were collected from the retro-orbital plexus utilizing heparinized and sterilized glass capillaries. The blood samples underwent centrifugation for 10 min at 1000 rpm under cooling conditions to facilitate the separation of sera. The sera were subsequently divided into aliquots and stored at −80 °C. After blood collection, the animals were euthanized, and each heart was examined. For biochemical analysis, one portion of the heart was cleaned in saline, dried, and wrapped in aluminum foil. Formalin-saline (10 %) buffer was used to soak a second section of the heart for histological and immunohistochemical processing and microscopic examination.
2.6. Biochemical determinations
All biochemical measurements were performed using a Shimadzu spectrophotometer (UV–vis 1201, Japan). The serum lipid profile was determined using kits obtained from DiaSys Diagnostic Systems GmbH in Germany. Reagent kits from BioVision, based in South Milpitas, California, USA, were utilized to conduct colorimetric assays for LDH, CK-total, and CK-MB.
2.7. Markers of oxidative stress in cardiac tissue
To assess the cardiac stress, the SOD, GPx, GSH, CAT, NO, and MDA activities in cardiac rat tissues were determined based on the instructions in the enclosed guides of the commercial kits (My BioSource, San Diego, USA).
2.8. Determination of pro-inflammatory cytokine and apoptotic biomarker
Commercial rat ELISA kits were procured from MyBioSource Company (San Diego, USA). The pro-inflammatory cytokine and apoptotic biomarkers comprising IL-4, IL-6, IL-10, TNF-α, IL-1β, and CD4 were evaluated by ELISA Kits using a commercially existing highly sensitive ELISA kit to the manufacturer’s directions following the methods.25, 26.
2.9. Cardiac DNA fragmentation %
The DNA fragmentation in cardiac tissues was assessed using ELISA Kits using the quantitative method.27.
2.10. Histopathology assay
After the experiment, heart samples were removed and fixed in neutral buffered formalin (10 %). Before being divided into tiny pieces (5 m), the samples were washed in xylene, embedded in paraffin, and then gradually dehydrated in ethyl alcohol. Following deparaffinization, samples were stained with Hematoxylin and Eosin under 100X light microscope examination.22
2.11. Immunohistochemistry study
The immunohistochemical procedure was hired to determine the immunoexpressing of Caspase 3 in cardiac tissues by utilizing the rabbit polyclonal antibody CASPASE 3 as described by28, 29. The immunostaining intensity of anti-Caspase 3 in cardiac rat tissues was assessed using the Image J program.28, 29.
2.12. Statistical analysis
A post hoc (Tukey) multiple comparisons test at p > 0.05 was employed after a one-way analysis of variance (ANOVA) to compare means. This was accomplished using the statistical analysis system (S.A.S.) computer software; Copyright (c) 1998 by S.A.S. Institute Inc., Cary, North Carolina, U.S.A.
3. Results
3.1. HPLC analysis of phenolic constituents
The statistics below demonstrate the radical scavenging activity (RSA), yield, total phenolic content, and reduction of the power of the CEE (Fig. 1, Fig. 2). Most of the 16 phenolic compounds were discovered using HPLC analysis in CEE. Among the chemicals found were high amounts of naringenin, taxifolin, ferulic acid, gallic acid, chlorogenic acid, and coffeic acid (Table 1 and Fig. 3).
Fig. 1.
The yield percentage of total phenolic content and radical scavenging activity percentage of three replicates of ethanolic extract from costus dry powdered roots.
Fig. 2.
Assessment of the reducing power of three replicates of the ethanolic extract from powdered dry roots of Costus.
Table 1.
The primary phenolic compounds of the CEE (ethanolic extract of costus) were identified using HPLC analysis.
| Constituents | Area | Concentration (µg/ml = µg/ 6.8 mg) |
Concentration (µg/g) |
|---|---|---|---|
| Naringenin | 654.73 | 40.05 | 1494.47 |
| Chlorogenic acid | 508.86 | 39.58 | 1477.00 |
| Gallic acid | 77.66 | 6.23 | 232.49 |
| Ferulic acid | 537.79 | 16.74 | 624.59 |
| Taxifolin | 82.11 | 9.36 | 349.28 |
| Coffeic acid | 87.66 | 3.17 | 118.27 |
| Pyro catechol | 28.89 | 2.80 | 104.38 |
| Coumaric acid | 150.73 | 2.43 | 90.79 |
| Vanillin | 143.40 | 2.24 | 83.58 |
| Syringic acid | 63.85 | 2.17 | 81.15 |
| Kaempferol | 20.59 | 1.68 | 62.50 |
| Methyl gallate | 4.14 | 0.06 | 2.33 |
| Cinnamic acid | 70.85 | 0.75 | 27.91 |
| Ellagic acid | 6.58 | 0.38 | 14.08 |
| Rutin | 0.00 | 0.00 | 0.00 |
| Catechin | 0.00 | 0.00 | 0.00 |
Fig. 3.
The results of HPLC screening of phenolic ingredients of CEE (costus ethanolic extract).
3.2. Biochemical determinations and oxidative stress markers of cardiac tissue
Contrary to predictions, bleomycin intoxication caused a significantly higher level of triglycerides, LDL, and cholesterol in the blood and a significantly inferior level of HDL related to the standard treatment. Administering CEE to bleomycin-intoxicated rats resulted in a discernible enhancement in the lipid profile as indicated by the significant reductions in LDL, triglycerides, and cholesterol, as well as the observable augments in the levels of HDL when compared with the bleomycin-treated rats (Table 2).
Table 2.
Effects of Bleomycin and/or CEE oral administration on serum lipid profile of adult male Wistar.
| Control | CEE1 | Bleomycin | Bleomycin ∼ CEE | |
|---|---|---|---|---|
| Cholesterol (mg/dl) | 130.3±4.6c | 128.3±6.6c | 320±13.5a | 204±3.9b |
| Triglycerides (mg/dl) | 155±8.6c | 152±7.7c | 364±20.1 a | 215±8.3b |
| HDL-C (mg/dl) | 46.5±5.4c | 45.6±4.7c | 28.5±2.9 a | 36.7±4.0b |
| LDL-C (mg/dl) | 84.5±5.5c | 83.7±4.9c | 196.5 ± 12.5 a | 110.5±6.4b |
1CEE; costus ethanolic extract. The values are stated as the mean ± SE.
a, b, c Rows have significantly diverse superscripts (a, b, and c).
The significant deterioration of cardiac oxidative stress induced by bleomycin toxicity was shown by a marked increase in cardiac MDA and NO levels, with a considerable reduction in the activities of CAT, SOD, and GPx, as well as GSH concentrations (Table 3). Rats that were given CEE displayed a significant decrease in cardiac MDA and NO levels compared to the bleomycin-intoxicated group and a noticeably increased level of GSH, CAT, SOD, and GPx activities.
Table 3.
Effect of Bleomycin and/or CEE oral administration on cardiac oxidative stress.
| Control | CEE1 | Bleomycin | Bleomycin ∼ CEE | |
|---|---|---|---|---|
| MDA (pg/mL) | 213.5±16.5c | 210.1 ± 18.1c | 487.4±40.3 a | 263.7±25.3b |
| NO (µmol/L) | 25.1±1.4c | 24.4±2.2c | 62.5±3.1 a | 39.3±2.8b |
| GSH (ng/mL) | 66.2±4.3c | 70.3±2.8c | 24.11±2.3 a | 52.08±3.4b |
| SOD (U/L) | 35.2±0.1c | 38.4±1.3c | 14.4±1.0 a | 28.3±1.2b |
| GPx (U/L) | 762±47c | 789±37c | 369±27.3 a | 599±51.3b |
| CAT (U/L) | 11.9±0.8c | 12.7±0.4c | 5.4±0.2 a | 9.8±3.1b |
1CEE; costus ethanolic extract. The values are stated as the mean ± SE.
a, b, c Rows have significantly diverse superscripts (a, b, and c).
3.3. Determination of pro-inflammatory cytokine, apoptotic biomarker, cardiac enzymes, and cardiac DNA fragmentation %
The data collected showed a significant increase in IL-10, IL-6, TNF-α, IL-4, IL-1β, LDH, CK-total, and CK-MB and DNA damage in the bleomycin group as related to the controlled one. Intriguingly, the administration of CEE to bleomycin-treated rats improved all inflammatory cytokines, apoptotic markers, DNA damage, and cardiac enzyme to within normal values while significantly lowering levels of TNF-α, IL1β, IL-4-, IL-6, IL-10, DNA damage LDH, CK-MB, and CK-total and increasing CD4 compared to bleomycin (Fig. 4a-j).
Fig. 4.
(a – j). Levels of TNF-α, IL-1β, IL-4, IL-6, IL-10, CD4, CK-MB, CK-total, and LDH, together with cardiac DNA fragmentation in control, bleomycin-intoxicated, and CEE-treated male rats. * Exhibits a significant difference from the control group, while # demonstrates a significant difference from the bleomycin group (p ≤ 0.05).
3.4. Histopathology
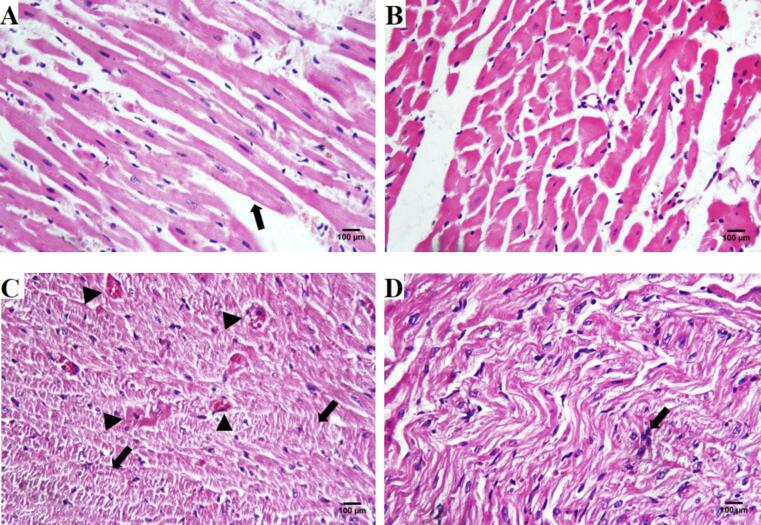
Examining the histological architecture of H&E-stained tissues of the control group revealed normal myocardial fiber. The histological architecture of H&E-stained tissues of the control group revealed normal myocardial fiber. The histological architecture of H&E-stained tissues of the control group revealed normal myocardial fiber arrangement with typical striation and branching of muscle fibers. The cardiomyocytes exhibited central oval nuclei and acidophilic sarcoplasm. The intercellular spaces infiltrated with few blood capillaries (Fig. 5a). CEE–treated group showed approximately typical cardiac muscle structure (Fig. 5b). Histological examination of the bleomycin-intoxicated group revealed marked distortion, fragmentation, signs of vacuolar degeneration in the form of hyperacid philia and cytoplasmic vacuolation, dull striations following the necrosis of myofibrillar tissue and degenerated myofibrils lost their nuclei as compared to the control group (Fig. 5c). Bleomycin-intoxicated and CEE–treated group showed marked improvement and preservation of intercellular spaces and morphology of cardiomyocytes compared to the bleomycin-treated group. Normal cardiac muscle cells with minimal cytoplasmic vacuolization were observed (Fig. 5-d).
Fig. 5.
(a-d). Histopathological examination of cardiac tissues of the groups. (a) Photomicrograph of the cardiac tissue of the control group showing the normal histological pattern of myocardial fibers (arrow) (b) Photomicrograph of CEE-treated group showing the cross-section of preserved cardiomyocyte morphology with central oval vesicular nuclei, exhibiting approximately normal histological architecture. (c) Photomicrograph of the bleomycin-intoxicated group showing degenerated cardiomyocytes (arrow). Also, vacuolization within myocardial fibers (arrowhead) indicated vacuolar degeneration in cardiomyocytes. The vacuoles could represent cardiac muscle fiber auto-phagocytosis. (d) Photomicrograph of bleomycin-intoxicated and CEE–treated group showing reduced perivascular fibrosis, with approximately normal pattern of myocardial fibers, with some residual fibers with enlarged nuclei (arrow).
3.5. Immunohistochemistry
The Caspase-3 expression was modest in control of animals. At the same time, it was moderate in the CEE group (Fig. 6a, b). Rats exposed to bleomycin displayed highly expressed caspase-3 (Fig. 6c). A modest Caspase-3 positivity was seen in the bleomycin-intoxicated and CEE-treated group, which was less immunoreactive than the bleomycin group (Fig. 6d; Fig. 7)
Fig. 6.
(a-d). Photomicrographs of cardiac tissues of various groups immune-stained with Caspase-3 (x400). (a): rats in the control groups have normal weak cytoplasmic Caspase-3 IHC positivity. (b): Rats treated with CEE display mild cytoplasmic Caspase-3 activity. (c): Rats from the bleomycin-intoxicated group display a severe Caspase-3 activity (arrow). (d): Bleomycin-intoxicated and CEE–treated group viewing weak Caspase-3 activity.
Fig. 7.
For a positive Caspase 3 immune reaction, intensity amounts are measured as mean ± SE * is considerably diversified in comparison to the control treatment, and # is significantly diversified with the bleomycin-treated rats (p ≤ 0.05).
4. Discussion
Many anticancer medications risk causing severe cardiotoxicity as a side effect that builds up over time and is dose-dependent. This risk exists for patients receiving treatment and healthcare professionals handling antiblastic medications. In reality, numerous investigations have demonstrated that certain cardiotoxic medicines, such as bleomycin, doxorubicin, epirubicin, cyclophosphamide, and 5-fluorouracil, were frequently found in the urine of exposed personnel.30 Cardiotoxicity symptoms include arrhythmias, cardiomyopathy, and minor blood pressure changes.31Anticlastic medicines cause cardiotoxicity by producing free oxygen radicals, which cause cellular impairment, and stimulating immunogenic reactions due to antigen-presenting cells in the heart.30.
LDH, CK-total, and CK-MB activities were significantly increased in adult male rats after receiving bleomycin 15 mg/kg twice a week, IP; these results are consistent with earlier studies that indicate bleomycin-induced oxidative stress might cause lipid peroxidation and release of these enzymes into the serum32. Bleomycin also caused cytotoxicity, apoptosis, and inflammation, as seen by the rise in LDH, CK-total, and CK-MB activities.2.
The bleomycin treatment significantly increased cardiac NO and MDA levels relative to the untreated one and decreased cardiac GSH, GPx, SOD, and CAT. These outcomes are consistent with33 and.4, 34 In our illustration, bleomycin led to an oxidative stress condition in the heart, as indicated by a decline in SOD activity and GSH level and an increase in NO activity. A key pathophysiological mechanism for heart injury is oxidative stress.35 Internal antioxidant glutathione protects the heart from internal or external cardiac toxins.36 The enzyme SOD catalyzes the formation of H2O2 from superoxide-free radical anions (O2).37.
Bleomycin could bind Fe (II) ions and create a complex that, when exposed to O2, oxidizes to Fe (III), producing ROS such as superoxide, hydroxyl, and Fe (III) radicals. This bleomycin complex can bind nucleophilically with the DNA helix, breaking DNA strands, peroxiding membrane lipids, and ultimately damaging membranes.38, 39 In particular, the activity of the antioxidant system in the heart is weaker than in other tissues. Therefore, cardiomyocytes are vulnerable to attack by free radicals. Bleomycin has also been found to increase NO levels via an increase in inducible nitric oxide synthase (iNOS) expression40. Increasing oxidative stress and overproduction of nitric oxide are implicated in the pathogenesis of cardiovascular diseases.41.
In the current study, oral treatment of ethanolic extract of costus for six weeks significantly raised the action of antioxidant defensives such (CAT, SOD, GSH, and GPx) in cardiac tissue but lowered MDA and NO accumulation, a lipid peroxidation indicator. These outcomes agree with those of.20, 42, 43 In this line,44 The Costaceae plant species Costus pictus, a relative of S. costus, contained two chief polysaccharides (SLT-3 and SLT-4) that effectively decreased ROS production, controlled GPx activities, and hindered M.D.A. creation. Additionally, S. costus can contribute electrons to reactive radicals, turning them into more constant and unreactive classes, as demonstrated by.45 According to research by,46 the traditional natural antioxidant tocopherol is comparable in strength to the preventive activities of S. costus extract in protecting against toxicant-induced oxidative stress and the depletion of marker enzymes. The high levels of flavonoids, phenolic acids, steroids, and chlorogenic acid in this action may be linked to decreased membrane fluidity and deterioration.47 Additionally, it has been demonstrated that prominent bioactive from S. costus, such as dehydrocostus lactone and costunolide, play significant roles as antioxidants by conjugating with particular proteins and mercapto (S.H.)-groups to influence several important biological functions in cells.48 The plant's capacity to protect tissue macromolecules from the detrimental impacts of ROS-intermediated lipid peroxidation may account for the lower MDA concentrations in the Saussurea costus-treated group.49.
High blood cholesterol levels, which are also considered one of the principal menace issues for myocardial infarction, speed up the development of atherosclerosis.50 The Bleomycin injection resulted in hyperlipidemia and markedly elevated blood lipid levels. In experimental hypertriglyceridemia, triglyceride absorption from the systemic circulation is reduced due to decreased lipoprotein lipase action in the myocardium in bleomycin-injected rats. These findings concur with the investigation.34 According to several authors51 The protective role of the S. costus roots may be explained by their high content of active ingredients with strong anti-inflammatory and antioxidant properties. This may also explain the observed improvement in lipid profiles, particularly the costus ethanolic extract.
In the existing investigation, intraperitoneal injection of bleomycin only resulted in a substantial increase in inflammatory protein levels (IL-6, IL-4, IL-10, and IL-1β) and a significant drop in CD4. These results support the findings.25, 44, 52 The interaction of bleomycin with iron and DNA produces ROS and initiates the inflammatory process.36 Cardiovascular cells liberate inflammatory arbitrators and cytokines such as IL-4, IL-6, IL-1, TNF-α, and IL-10 in response to the ROS brought on by bleomycin. TNF-α synthesis impacted the mitochondria's ability to produce ROS and the inflammatory cells' creation of TNF-α and IL1β.53.
Accumulating evidence has indicated that abnormalities in the production or function of cytokines, such as the pro-inflammatory cytokines TNF-α and IL-1beta, play a fundamental role in many inflammatory lesions.54 Bleomycin has been reported to elevate ROS and trigger the production of TNF-α and IL-1β by creating the DNA/Fe2+/BLM complex.55 The increased release of these cytokines causes an increase in TGF-β1 expression.56.
The results show the extract's biological safety by lowering levels of cytokines and chemokines linked to inflammation, such as TNF-α, IL-6, IL-10, IL1β, and IL-4, in adult male albino rats after costus administration. In contrast to the standard treatment, these verdicts are consistent with those.42 Because it decreases the pro-inflammatory attributes such as TNF-α, IL-6, inducible NO synthase (NOS), and COX-2 (Cyclooxygenase) in numerous in vivo and in vitro trials, the latter plant has been identified as one of the plants with strong anti-inflammatory effects.57 Helper CD4 T cells modify the situation and the action of other immune cells by secreting cytokines.37.
The study of the cardiac myocytes histo-pathologically revealed a notable improvement. In contrast to rats treated with bleomycin, it kept the morphology of the heart muscle and decreased fibrosis and vacuolar degeneration. CEE's anti-inflammatory and antioxidant properties were said to be responsible for these results. The present findings were consistent with Ashry's investigation into the effectiveness of CEE in reducing cardio-hematotoxicity associated with Oxaloplatin therapy, in which he reported that CEE. had a protective role against Oxaloplatin in cardiac muscle tissue.51 Caspase3 expression is increased in experimental animals that experience heart failure and apoptosis, and people who have end-stage heart failure have activated caspase3 in their myocardium.48 Caspase3 has been suggested as a marker for cardiotoxicity.58.
5. Conclusions
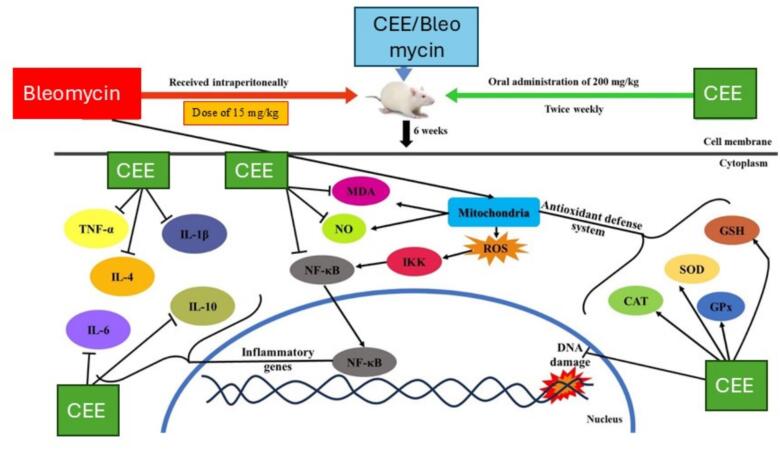
According to the current study, post-treatment with S. costus extract may mitigate bleomycin-induced heart injury by reducing oxidative stress and inflammation that damage DNA and membranes (Fig. 8) These positive effects could result from these supplements' direct free radical scavenging properties. Therefore, the current study could recommend S. costus extract as supplemental medication to minimize the cardiotoxicity associated with the anticancer drugs.
Fig. 8.
Schematic diagram depicting the Cardioprotective, antioxidant, and anti-inflammatory efficacy of Coustus ethanolic extract against Bleomycin-Induced Cardiotoxicity in rats.
Ethics approval.
The Faculty of Science at Al-Azhar University in Assiut, Egypt's Animal Care and Use Committee provided all animals with humane treatment following its rules. The Faculty of Science, Al-Azhar University, Assiut, Egypt, which ethically approved the proposal with a number AZHAR 16/2023.
Consent to participate
All the authors agree to participate in this paper.
Consent for publication
All the authors agree with the publication of this paper.
CRediT authorship contribution statement
Barakat M. Alrashdi: Writing – review & editing, Resources, Data curation. Hussam Askar: Writing – review & editing, Supervision, Investigation, Formal analysis, Data curation, Conceptualization. Mousa O. Germoush: Formal analysis, Data curation. Maged Fouda: Writing – original draft, Formal analysis. Diaa Massoud: Writing – review & editing, Formal analysis, Data curation. Sarah Alzwain: Formal analysis, Data curation. Naser Abdelsater: Writing – review & editing, Formal analysis, Data curation. Laila M.S. Salim: Formal analysis, Data curation. Mohamed H.A. Gadelmawla: Writing – original draft, Formal analysis, Data curation. Mahmoud Ashry: Writing – review & editing, Supervision, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors of the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through project number 223202.
Contributor Information
Barakat M. Alrashdi, Email: bmalrashdi@ju.edu.sa.
Hussam Askar, Email: Hussamaskar@azhar.edu.eg.
Appendix A. . List of acronyms
| Acronyms | Full Form |
|---|---|
| (iNOS) | inducible nitric oxide synthase |
| BLM | Bleomycin |
| Caspase 3 | Cysteine-aspartic acid protease 3 |
| CAT | Catalase |
| CD4 | Cluster of Differentiation 4 |
| CEE | costus ethanolic extract |
| CK- total | Creatine kinase-total |
| CK-MB | Creatine kinase-MB |
| COX-2 | Cyclooxygenase |
| DNA | Deoxyribonucleic acid |
| Fe (II) ions | iron (II) or ferrous ion |
| Fe (III), | iron (III) or ferric |
| GPx | Glutathione Peroxidase |
| GSH | Reduced glutathione |
| H2O2 | Hydrogen peroxide |
| HDL | density high-density lipoprotein |
| HPLC | High-performance liquid chromatography |
| IHC | Immunohistochemistry |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1beta |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-9 |
| LDH | lactic acid dehydrogenase |
| LDL | Low-density lipoprotein |
| MDA | Malondialdehyde |
| NO | Nitric oxide |
| O2 | Dioxygen |
| ROS | Reactive oxygen species |
| RSA | Radical Scavenging Activity |
| S. costus | Saussurea costus |
| S. verticillus | Streptomyces verticillus |
| SOD | Superoxide dismutase |
| TGF-β1 | Transforming growth factor-beta 1 |
| TNF-α | Tumor necrosis factor-alpha |
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal AJCacjfc. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2018;68(6):394-424. [DOI] [PubMed]
- 2.McGowan J.V., Chung R., Maulik A., et al. Anthracycline Chemotherapy and Cardiotoxicity. 2017;31(1):63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansour H.H., Omran M.M., Hasan H.F., El Kiki S.M. Modulation of bleomycin-induced oxidative stress and pulmonary fibrosis by N-acetylcysteine in rats via AMPK/SIRT1/NF-kappabeta. Clin Exp Pharmacol Physiol. Dec 2020;47(12):1943–1952. doi: 10.1111/1440-1681.13378. [DOI] [PubMed] [Google Scholar]
- 4.Ali H.M., Ashry M. Potential ameliorative effect of Melissa Officinalis Ethanolic Extract on Bleomycin Induced Cardiotoxicity in rats. J Home Econ. 2023;33(1):113–127. [Google Scholar]
- 5.Cabrera S., Maciel M., Hernandez-Barrientos D., et al. Delayed resolution of bleomycin-induced pulmonary fibrosis in absence of MMP13 (collagenase 3) Am J Physiol Lung Cell Mol Physiol. 2019;316(5):L961–L976. doi: 10.1152/ajplung.00455.2017. [DOI] [PubMed] [Google Scholar]
- 6.Jaheed E., Mohamed A.H., Hassan N.M.F., Mahran K.M.A., Nasr S.M., Abou-Zeina H.A.A. Evaluation of the curative effect of Balanites aegyptiaca fruits ethanolic extract on Haemonchosis experimentally induced in Egyptian Baladi goats: phytoanalytical, parasitological and hematological studies. J Parasit Dis. Dec 2019;43(4):638–650. doi: 10.1007/s12639-019-01143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akazawa H. Cardiotoxicity of Cancer Chemotherapy - Mechanisms and Therapeutic Approach. Gan To Kagaku Ryoho. Dec 2017;44(13):2058–2063. [PubMed] [Google Scholar]
- 8.Gozhenko A., Bestanchuk O., Kaschenko O., Narbutova T. Cumulative cardiotoxic effect of bleomycin in experiment. Journal of Education, Health and Sport. 2021;11(6):301–308. [Google Scholar]
- 9.Pacola P.R., Rostey R.R.L., Rizzo F.F.A. Chemotherapeutical treatment of basal cell carcinoma with bleomycin via microinfusion of the drug into the skin (MMP(R)) An Bras Dermatol. Sep-Oct. 2023;98(5):587–594. doi: 10.1016/j.abd.2022.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gozhenko A, Bestanchuk E, Kashchenko O, Narbutova T, Badiuk N. Changes in the myocard during chemotherapy with intravenous bleomycin in testic cancer: a clinical case. 2021;.
- 11.Bestanchuk E., Gozhenko A., Kashchenko O., Narbutova T., Berezovskyi O., Zakharova V. Cardiotoxic effect of bleomycin with a single administration in the experiment. Journal of Education, Health and Sport. 2021;11(10):242–251. [Google Scholar]
- 12.Eliza J., Daisy P., Ignacimuthu S.-J.-C.-B.-I. Antioxidant activity of costunolide and eremanthin isolated from Costus speciosus (Koen ex. Retz) Sm. 2010;188(3):467–472. doi: 10.1016/j.cbi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Ashry MJPJoBSP. Cardioprotective and Antioxidant Efficiency of Balanites aegyptiaca Extract Against Doxorubicin® Complication. 2022;25(3):270-281. [DOI] [PubMed]
- 14.Parmar M., Negi L.S., Ramola S.J.S. Seeds Germination and Seedlings Analysis of Saussurea Costus Royle Ex Benth. In High and Low Altitudinal Villages of District Uttarkashi (uttarakhand). 2012;2(6):25–30. [Google Scholar]
- 15.Gwari G., Bhandari U., Andola H.C., Lohani H. Chauhan NJPr. Volatile Constituents of Saussurea Costus Roots Cultivated in Uttarakhand Himalayas, India. 2013;5(3):179. doi: 10.4103/0974-8490.112424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amara U., Khan A., Laraib S., et al. Conservation Status and Therapeutic Potential of Saussurea Lappa: an Overview. 2017;8(3):602–614. [Google Scholar]
- 17.Al-Kuraishy H, Hussein RJRJoO. Caspase-3 levels (CASP-3) in doxorubicin induced-cardiotoxicity: role of metformin pretreatment. 2017;1(1).
- 18.Saleem T.M., Lokanath N., Prasanthi A., et al. Aqueous Extract of Saussurea Lappa Root Ameliorate Oxidative Myocardial Injury Induced by Isoproterenol in Rats. 2013;4(2):94. doi: 10.4103/2231-4040.111525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahara K, Tabassum S, Sabir S, et al. A review of therapeutic potential of Saussurea lappa-An endangered plant from Himalaya. Asian Pac J Trop Med. Sep 2014;7S1:S60-9. doi:10.1016/S1995-7645(14)60204-2. [DOI] [PubMed]
- 20.Binobead M.A., Aziz I.M., Ibrahim S.M., Aljowaie R.M. Chemical composition and bioactivities of the methanol root extracts of Saussurea costus. Open Chem. 2024;22(1):20240002. [Google Scholar]
- 21.Nogala-Kalucka M., Korczak J., Dratwia M., Lampart-Szczapa E., Siger A., Buchowski M. Changes in antioxidant activity and free radical scavenging potential of rosemary extract and tocopherols in isolated rapeseed oil triacylglycerols during accelerated tests. Food Chem. 2005;93(2):227–235. [Google Scholar]
- 22.Sethiya N.K., Trivedi A., Mishra S. The total antioxidant content and radical scavenging investigation on 17 phytochemical from dietary plant sources used globally as functional food. Biomed Prev Nutr. 2014;4(3):439–444. [Google Scholar]
- 23.Al-Duais M.A.H., Al-Awthan Y.S.M. Hepatoprotective effect of costus roots extract against carbon tetrachloride (CCl4)-induced liver injury in Guinea Pigs. J Life Sci. 2017;11:176–184. [Google Scholar]
- 24.El-Fakharany EM, El-Gendi H, El-Maradny YA, et al. Inhibitory effect of lactoferrin-coated zinc nanoparticles on SARS-CoV-2 replication and entry along with improvement of lung fibrosis induced in adult male albino rats. Int J Biol Macromol. Aug 1 2023;245:125552. doi:10.1016/j.ijbiomac.2023.125552. [DOI] [PMC free article] [PubMed]
- 25.Cetiner S., Demirhan O., Inal T.C., Tastemir D., Sertdemir Y. Analysis of peripheral blood T-cell subsets, natural killer cells and serum levels of cytokines in children with Down syndrome. Int J Immunogenet. Aug 2010;37(4):233–237. doi: 10.1111/j.1744-313X.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- 26.Hata H., Sakaguchi N., Yoshitomi H., et al. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. Aug 2004;114(4):582–588. doi: 10.1172/JCI21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMillan E.M., Quadrilatero J. Differential apoptosis-related protein expression, mitochondrial properties, proteolytic enzyme activity, and DNA fragmentation between skeletal muscles. Am J Physiol Regul Integr Comp Physiol. Mar 2011;300(3):R531–R543. doi: 10.1152/ajpregu.00488.2010. [DOI] [PubMed] [Google Scholar]
- 28.Gadelmawla M.H., Alazzouni A.S., Farag A.H., Gabri M.S., Hassan B.N. Enhanced effects of ferulic acid against the harmful side effects of chemotherapy in colon cancer: docking and in vivo study. The Journal of Basic and Applied Zoology. 2022;83(1):28. [Google Scholar]
- 29.Suvarna K.S., Layton C., Bancroft J.D. Bancroft's theory and practice of histological techniques. Elsevier Health Sciences. 2018 [Google Scholar]
- 30.Luckheeram RV, Zhou R, Verma AD, Xia BJC, immunology d. CD4+ T cells: differentiation and functions. 2012;2012. [DOI] [PMC free article] [PubMed]
- 31.Schimmel KJ, Richel DJ, Van den Brink RB, Guchelaar H-JJCtr. Cardiotoxicity of cytotoxic drugs. 2004;30(2):181-191. [DOI] [PubMed]
- 32.Khazri O, Mezni A, Limam F, Aouani EJD-R. Bleomycin-Induced Damage in Rat Lung: Protective Effect of Grape Seed and Skin Extract. 2022;20(4):15593258221131648. [DOI] [PMC free article] [PubMed]
- 33.Bestanchuk E, Gozhenko A, Kashchenko O, Narbutova T, Berezovskyi O, Zakharova V. Cardiotoxic effect of bleomycin with a single administration in the experiment. 2021;.
- 34.Khazri O, Mezni A, Limam F, Ezzeddine A. Bleomycin-Induced Damage in Rat Lung: Protective Effect of Grape Seed and Skin Extract. Dose Response. Oct-Dec 2022;20(4):15593258221131648. doi:10.1177/15593258221131648. [DOI] [PMC free article] [PubMed]
- 35.Zaafan M.A., Zaki H.F., El-Brairy A.I., Kenawy S.A. Pyrrolidinedithiocarbamate attenuates bleomycin-induced pulmonary fibrosis in rats: Modulation of oxidative stress, fibrosis, and inflammatory parameters. Exp Lung Res. Oct-Dec. 2016;42(8–10):408–416. doi: 10.1080/01902148.2016.1244578. [DOI] [PubMed] [Google Scholar]
- 36.Turgut N.H., Kara H., Elagoz S., Deveci K., Gungor H., Arslanbas E. The Protective Effect of Naringin against Bleomycin-Induced Pulmonary Fibrosis in Wistar Rats. Pulm Med. 2016;2016:7601393. doi: 10.1155/2016/7601393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luckheeram R.V., Zhou R., Verma A.D., Xia B. CD4(+)T cells: differentiation and functions. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Wahhab KG, Ashry M, Hassan LK, et al. Hepatic and immune modulatory effectiveness of lactoferrin loaded Selenium nanoparticles on bleomycin induced hepatic injury. Sci Rep. Sep 10 2024;14(1):21066. doi:10.1038/s41598-024-70894-6. [DOI] [PMC free article] [PubMed]
- 39.El-Fakharany EM, El-Maradny YA, Ashry M, Abdel-Wahhab KG, Shabana ME, El-Gendi H. Green synthesis, characterization, anti-SARS-CoV-2 entry, and replication of lactoferrin-coated zinc nanoparticles with halting lung fibrosis induced in adult male albino rats. Sci Rep. Sep 23 2023;13(1):15921. doi:10.1038/s41598-023-42702-0. [DOI] [PMC free article] [PubMed]
- 40.Gurujeyalakshmi G., Wang Y., Giri S.N. Suppression of bleomycin-induced nitric oxide production in mice by taurine and niacin. Nitric Oxide. Aug 2000;4(4):399–411. doi: 10.1006/niox.2000.0297. [DOI] [PubMed] [Google Scholar]
- 41.Mannaa F.A., Abdel-Wahhab K.G., Abdel-Wahhab M.A. Prevention of cardiotoxicity of aflatoxin B1 via dietary supplementation of papaya fruit extracts in rats. Cytotechnology. Mar 2014;66(2):327–334. doi: 10.1007/s10616-013-9579-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Rahman GIA, Behairy A, Elseddawy NM, et al. Saussurea lappa Ethanolic Extract Attenuates Triamcinolone Acetonide-Induced Pulmonary and Splenic Tissue Damage in Rats via Modulation of Oxidative Stress, Inflammation, and Apoptosis. Antioxidants (Basel). May 8 2020;9(5)doi:10.3390/antiox9050396. [DOI] [PMC free article] [PubMed]
- 43.Abdul M.R., Rahim S.M., Saleh A.H. Cardioprotective Activity of Costus Root Ethanol Extract in Experimentally-Induced Hypothyroidism in Female Albino Rats. HAYATI Journal of Biosciences. 2023;30(6):1054–1060. [Google Scholar]
- 44.Sathuvan M., Vignesh A., Thangam R., Palani P., Rengasamy R., Murugesan K. In vitro antioxidant and anticancer potential of bark of costus pictus D. Don. Asian Pacific Journal of Tropical Biomedicine. 2012;2(2):S741–S749. [Google Scholar]
- 45.Benedetto C, D'Auria M, Mecca M, et al. Chemical and biological evaluation of essential oil from Saussurea costus (Falc.) Lipsch. from Garhwal Himalaya collected at different harvesting periods. Nat Prod Res. Aug 2019;33(16):2355-2358. doi:10.1080/14786419.2018.1440219. [DOI] [PubMed]
- 46.Saleem T.S., Lokanath N., Prasanthi A., Madhavi M., Mallika G., Vishnu M.N. Aqueous extract of Saussurea lappa root ameliorate oxidative myocardial injury induced by isoproterenol in rats. J Adv Pharm Technol Res. Apr 2013;4(2):94–100. doi: 10.4103/2231-4040.111525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abd Eldaim M.A., Tousson E., El Sayed I.E.T., Awd W.M. Ameliorative effects of Saussurea lappa root aqueous extract against Ethephon-induced reproductive toxicity in male rats. Environ Toxicol. Feb 2019;34(2):150–159. doi: 10.1002/tox.22669. [DOI] [PubMed] [Google Scholar]
- 48.Condorelli G., Roncarati R., Ross J., Jr, et al. Heart-targeted overexpression of caspase3 in mice increases infarct size and depresses cardiac function. Proc Natl Acad Sci U S A. 2001;98(17):9977–9982. doi: 10.1073/pnas.161120198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anyasor G.N., Onajobi F., Osilesi O., Adebawo O., Oboutor E.M. Anti-inflammatory and antioxidant activities of Costus afer Ker Gawl. hexane leaf fraction in arthritic rat models. J Ethnopharmacol. 2014;155(1):543–551. doi: 10.1016/j.jep.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 50.Syahputra R.A., Harahap U., Dalimunthe A., Pandapotan M., Satria D. Protective effect of Vernonia amygdalina Delile against doxorubicin-induced cardiotoxicity. Heliyon. Jul 2021;7(7):e07434. doi: 10.1016/j.heliyon.2021.e07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashry M. Protective Effect of Costus (Saussurea Costus) Ethanolic Extract on Oxaloplatin®-Induced Histological Changes and Hemato-Cardiotoxicity in Adult Male Albino Rats. Egyptian Academic Journal of Biological Sciences, D Histology & Histochemistry. 2019;11(2):69–85. [Google Scholar]
- 52.Sadek K.M., Mahmoud S.F.E., Zeweil M.F., Abouzed T.K. Proanthocyanidin alleviates doxorubicin-induced cardiac injury by inhibiting NF-kB pathway and modulating oxidative stress, cell cycle, and fibrogenesis. J Biochem Mol Toxicol. Apr 2021;35(4):e22716. doi: 10.1002/jbt.22716. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Q., Ju Y., Ma Y., Wang T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: A randomized controlled trial. Medicine (Baltimore). Nov 2018;97(45):e13087. doi: 10.1097/MD.0000000000013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin C.T., Chen C.J., Lin T.Y., Tung J.C., Wang S.Y. Anti-inflammation activity of fruit essential oil from Cinnamomum insularimontanum Hayata. Bioresour Technol. Dec 2008;99(18):8783–8787. doi: 10.1016/j.biortech.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 55.Chitra P., Saiprasad G., Manikandan R., Sudhandiran G. Berberine attenuates bleomycin induced pulmonary toxicity and fibrosis via suppressing NF-kappaB dependant TGF-beta activation: a biphasic experimental study. Toxicol Lett. 2013;219(2):178–193. doi: 10.1016/j.toxlet.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Kandhare A.D., Bodhankar S.L., Mohan V., Thakurdesai P.A. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: Decisive role of Bax, Nrf2, NF-kappaB, Muc5ac, TNF-alpha and IL-1beta. Chem Biol Interact. 2015;237:151–165. doi: 10.1016/j.cbi.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 57.Selim S, Al Jaouni S. Anti-inflammatory, antioxidant and antiangiogenic activities of diosgenin isolated from traditional medicinal plant, Costus speciosus (Koen ex.Retz.) Sm. Nat Prod Res. Aug 2016;30(16):1830-3. doi:10.1080/14786419.2015.1065493. [DOI] [PubMed]
- 58.Al-Kuraishy H., Hussein R. Caspase-3 levels (CASP-3) in doxorubicin induced-cardiotoxicity: role of metformin pretreatment. Research. J Oncol. 2017;1(1) [Google Scholar]
- 59.Nogala-Kalucka M., Korczak J., Dratwia M., Lampart Szczapa E., Siger A., Buchowski M. Changes in antioxidant activity and free radical scavenging potential of rosemary extract and tocopherols in isolated rapeseed oil triacylglycer ols during accelerated tests. Food Chem. 2005;93(2):227–235. [Google Scholar]
- 60.Ashry M., El-Sahra D.G., Gaber D.A., Mustafa M.A., Abdel-Wahhab K.G. Nephroprotective effect of costus (Saussurea costus) ethanolic extract on Oxaliplatin®-induced nephrotoxicity in adult male wistar rats. Pak. J. Biol. Sci. 2021;24:830–839. doi: 10.3923/pjbs.2021.830.839. [DOI] [PubMed] [Google Scholar]