Abstract
Background
Peripheral artery disease (PAD) is a major public health concern due to its high prevalence, severe impact on individuals' health and quality of life, and substantial economic burden. Pharmacological interventions are still limited with numbers needed-to-treat ranging from 6 (cilostazol) to 50 (aspirin, statins, and vorapaxar).
Objectives
This randomized, placebo-controlled, double-blinded crossover interventional trial aims to measure the effect of L-citrulline and tetrahydrobiopterin (H4Bip) on walking distance in patients with PAD, stratified by plasma levels of asymmetric dimethyl L-arginine (ADMA), the endogenous inhibitor of endothelial nitric oxide (NO) synthase.
Methods
We measured preinterventional ADMA levels in 51 patients with PAD in Australia and Germany with mean changes in absolute claudication distance (dACD) as the primary outcome upon orally supplementing the L-arginine precursor, L-citrulline (3 g) twice daily for 12 weeks, and, in one arm, additionally H4Bip (0.45 g) once per day for a further 2 weeks.
Results
Preinterventional ADMA levels were pathological (>0.4 μM) in 34 patients. Supplementation with L-citrulline significantly increased the mean plasma levels of both L-citrulline and L-arginine, from 41.8 ± 2.7 μmol/l to 246.3 ± 67.3 μmol/l (P = 0.004) and from 75.2 ± 4.2 μmol/l to 119.2 ± 6.9 μmol/l (P < 0.0001) respectively, when compared with placebo. dACD in % of control was significantly improved by L-citrulline vs placebo (20.11% ± 4.50% vs 5.73% ± 2.74%, respectively; P = 0.011). Further addition of H4Bip increased the mean percentage dACD to 28.15% ± 6.84% (P = 0.021), but only in patients with preinterventional pathological ADMA levels.
Conclusions
L-citrulline and, when ADMA levels are pathological, H4Bip are effective nutritional interventions in patients with PAD warranting further confirmatory trials.
Key words: ADMA, arginine, citrulline, nitric oxide synthase, peripheral artery disease, tetrahydrobiopterin
Central Illustration
Peripheral artery disease (PAD) is the narrowing or complete occlusion of the arteries supplying blood to the lower limbs due to atherosclerosis-associated plaque formation,1 the classification of which ranges from compensated symptomatic PAD to chronic limb-threatening ischemia. Pain in the leg muscles during ambulation, that is, intermittent claudication, is the earliest and most common symptom. Global prevalence of PAD and associated mortality have increased substantially,2 and only approximately 55% of this disease burden can be attributed to identified risk factors, with tobacco use, diabetes, and hypertension being the major contributors.3 Thus, the unmet medical need is high.
Currently, the primary treatment for intermittent claudication is physical therapy, that is, physical exercise with intermittent walking.4,5 Pharmacologically, the treatment for PAD aims at reducing plaque formation, that is, by statins, and preventing atherothrombosis by combining the antiplatelet acetylsalicylic acid with the anticoagulant rivaroxaban, which both improve prognosis.6 Revascularization is possible in selected cases with proximal obstructions. Nonopioid analgesics are used when critical ischemia occurs in cases unsuitable for surgical or endovascular treatment.7 Possible additional pathomechanisms include inflammation8 correlating with adhesion molecule CD11b, CD11c, and TREM-1 expression on monocytes and neutrophils.9 However, no therapy currently targets an identified causal mechanism or provides a curative treatment option.2
Atherosclerosis and its comorbidities, such as hypertension and diabetes, share several risk genes,10 suggesting common causal molecular mechanisms.11 One such mechanism appears to be related to nitric oxide (NO) formation,12,13 specifically observed also in PAD.11 NO is produced by NO synthases (NOS), which in the presence of the redox-sensitive cofactor, tetrahydrobiopterin (H4Bip), oxidizes the amino acid L-arginine to L-citrulline and NO. Different mechanisms can interfere with this: 1) the accumulation of asymmetric dimethyl L-arginine (ADMA),14 a competitive inhibitor of NOS at its substrate site;15,16 2) oxidation of H4Bip to dihydrobiopterin (H2Bip), which can inhibit NOS17 by uncoupling the activation of oxygen from the oxidation of L-arginine to NO;12 and 3) the chemical scavenging of NO by reactive oxygen species (ROS).18,19 It has thus been hypothesized that increasing or reinstalling NO production by overcoming ADMA-based inhibition and recoupling NOS by dietary supplementation with L-arginine may be a possible mechanism-based approach in atherosclerosis including PAD. However, clinical studies testing this hypothesis produced mixed results.20, 21, 22 Moreover, orally supplemented L-arginine has gastrointestinal side effects23 and low bioavailability with a high degree of interindividual variability.24 Therefore, L-citrulline has been suggested as an alternative food supplement, fully absorbed and converted to L-arginine.25, 26, 27 Indeed, L-citrulline doses as low as 3 g/d have been shown to improve NO formation.28
We hypothesize that the oral supplementation of L-citrulline significantly improves walking distance in PAD patients when compared to placebo, probably by reversing endothelial dysfunction, and that the effects can be further potentiated by H4Bip leading to recoupling of endothelial NOS. Therefore, we conducted the CIPER (CItrulline in PERipheral artery disease) trial in patients with PAD using the food supplement, L-citrulline, and change in absolute claudication distance (dACD) as the primary patient-relevant outcome parameter. Moreover, the combination of L-citrulline with H4Bip in a network pharmacology approach29 was also explored. However, because of its high costs and limited availability for human use in only one of our 2 sites. As potential mechanism-based stratification markers, plasma levels of ADMA and ROS-related biomarkers were measured.
Methods
The CIPER study (L-Citrulline in Peripheral Artery Disease; NCT02521220) was a multicenter, prospective, randomized, double-blinded, and placebo-controlled phase II trial. A crossover design was chosen because of its advantages to reduce the influence of covariates such as comedication (Supplemental Table 1), patients acting as their own control, and the higher statistical power and efficiency compared to parallel designs.30 Patients were recruited at 2 sites, the Department of Vascular Sciences, Monash Health, Melbourne, Australia (Melbourne site), and the Department of Cardiology and Angiology, Hannover Medical School, Hannover, Germany (Hannover site), both following the International Council for Harmonisation and the Declaration of Helsinki. The respective ethics committees and corresponding health authorities approved the protocol (Monash Health Human Research Ethics Committee, Melbourne, No. 08210B, and MHH Ethical Committee OE 9515, Hannover, No. 7015). All data and events were documented from sources, including, but not limited to, paper and electronic charts and laboratory test reports, and adjudicated by an independent clinical events committee, whose members were unaware of the trial-group assignments. Serious adverse events were reviewed by an independent data and safety monitoring board according to a predefined schedule. The full database is available from the corresponding author upon reasonable request.
Study patients and assessments
Males 40 years of age or older and postmenopausal women with a 6-month history of stable intermittent claudication due to PAD were eligible for enrollment. Intermittent claudication was defined by pain, ache, cramp, numbness, or severe fatigue involving muscles of one or both lower extremities, reproducibly provoked by walking and relieved by rest. Fontaine31 class III patients were eligible for screening, but excluded if unable to perform an exercise test, for example, anyone who had rest pain at the time of study. Further exclusion criteria were a PAD of nonatherosclerotic nature, Fontaine class IV, that is ulcer or gangrene, leg amputation above the ankle, peripheral vascular surgery, sympathectomy, peripheral angioplasty or stent insertion within the previous 3 months, myocardial infarction, unstable angina, percutaneous transluminal coronary angioplasty or coronary artery bypass graft surgery within the previous 3 months, uncontrolled hypertension (resting systolic blood pressure, above 190 mm Hg or a diastolic blood pressure, above 115 mm Hg), hypotension (systolic blood pressure <90 mm Hg), type I diabetes, proliferative retinopathy, history of disease state or surgery affecting gastrointestinal absorption, significant renal disease (serum creatinine >3.0 mg/dL), liver disease (transaminase >3 times the upper limit of normal, bilirubin >1.5 times the upper limit of normal), cerebrovascular infarct in the last 3 months, autoimmune disorders (eg, systemic lupus erythematosus, ulcerative colitis), and conditions other than PAD that limit walking distance.
Patients were randomized to one of the 2 crossover arms (AB or BA in Central Illustration) by a block-of-4 randomization,32 stratified by each center.33 Allocation was concealed by using sequentially numbered, opaque, sealed envelopes. An independent clinical trial pharmacist kept the coding list. The list was also provided to the packaging company to prepare boxes with the study compounds for each patient and treatment period labeled with the appropriate randomization number. L-citrulline and, as placebo, maltodextrin sachets were formulated and packaged, for Melbourne, by the Monash Medical Centre Hospital Pharmacy and, for Hannover, prepared at Fagron BV (Capelle ad Ijssel, the Netherlands) and packaged by Prestige Promotion (Kleinostheim, Germany). H4Bip tablets (stabilized with an equal amount in mg of ascorbic acid) were purchased from Schircks Laboratories (Bauma, Switzerland) in original blisters. Compliance was monitored by counting unused study medications during the study visits, allowing opportunity to discuss any issues or provide positive reinforcement about compliance.
Central Illustration.
The CIPER Study
Within the disease network (Diseasome,72 A), atherosclerosis, for example, PAD, is genetically linked within a cluster11,27 (B) of cardiovascular, metabolic, neurological, and respiratory phenotypes11,27,55,73,74 to Nitric Oxide-cyclic guanosine monophosphate signaling and interfering reactive oxygen. In the derived disease module12 (C) ADMA may inhibit NO synthase by competing With L-arginine. NOX5 is the sole superoxide (O2−)-generating protein-protein interaction partner of NO synthase (NOS)12 and causes oxidation of tetrahydrobiopterin (H4Bip) to H2Bip, leading to NOS uncoupling (Uc-NOS). Moreover, O2− scavenges NO and, through intermediate Peroxynitrite, can lead to protein 3′-tyrosine nitration (3-NT)s. A possible mechanism-based therapeutic intervention comprises L-citrulline, converted to L-arginine to outcompete ADMA, and H4Bip to replace H2Bip in Uc-NOS. In the trial design (D), AB and BA indicate the different crossover groups, receiving first L-Citrulline and then placebo, or vice versa. In addition, the study was extended only at the Melbourne site by a 2-week cointervention with L-citrulline plus H4Bip. At each visit, exercise treadmill and vascular function tests were performed, and blood samples were collected. H2Bip = dihydrobiopterin; NO = nitric oxide; PAD = peripheral artery disease; other abbreviations as in Figures 3 and 4.
The trial timeline (see Central Illustration) consisted of a run-in (screening 3 weeks prior), intervention 1 (L-citrulline or placebo; weeks 0-12), wash-out (weeks 12-16), intervention 2 (placebo or L-citrulline; weeks 16-28), intervention 3 (only in Melbourne, combining L-citrulline with H4Bip; weeks 28-30), and a follow-up phase. Patients received either 3 g of L-citrulline twice daily for 12 weeks, followed by a wash-out period of 4 weeks and then 12 weeks placebo intervention (AB in Central Illustration), or vice versa, that is, first placebo and then L-citrulline (BA). To explore whether the combination of L-citrulline with H4Bip is safe and has an additional beneficial effect, at the Melbourne site, a third open-label intervention was added for 2 weeks consisting of 3 g L-citrulline twice daily and 150 mg of the food supplement, H4Bip, 3 times daily. H4Bip was not tested on its own against placebo. Four weeks after the end of the study all participants were followed up by phone.
Study visits and consultations took place at weeks 0, 4, 12, 16, 20, 28, and (only in Melbourne) at week 30 after the initial screening phase (Supplemental Table 2) using the review form (Supplemental Method 1). Exercise treadmill tests (ETTs) were conducted using the Skinner-Gardner protocol.34 The purpose of the ETTs during the screening phase was to increase patient familiarization with the exercise and accommodate our inclusion criteria. Fasting (12 hours) blood samples were taken in 4 mL ethylenediaminetetraacetic acid tubes and centrifuged before the supernatant plasma was aliquoted into 300 μL Eppendorf tubes and stored at −80 °C until further analysis. This included routine safety laboratory measurements and the following biomarkers: plasma L-arginine, L-citrulline, ADMA, and, only for the Hannover cohort, 3-nitrotyrosine immunoreactivity (3-NT18), and nicotinamide adenine dinucleotide phosphate oxidase 5 protein (NOX512).
Trial endpoints
The primary efficacy parameter was absolute claudication distance (ACD) measured as maximum walking distance by treadmill and, upon intervention, to be independent of varying initial ACD values, the difference in ACD from baseline (dACD) expressed as % of control. The use of dACD eliminated any coincidental differences in baseline ACD between the 2 sequences (Table 1). To analyze the added benefit of H4Bip (Melbourne), the ACD at week 12 of the intervention phase was taken as the baseline. Secondary exploratory measures were flow-mediated dilatation,35 peripheral artery tonometry,36 and aortic (carotid-femoral) pulse wave velocity (Supplemental Method 2). As pharmacokinetic controls, at week 0 (basal) and week 12 (intervention), plasma L-citrulline was measured to validate bioavailability, and L-arginine to validate conversion (Supplemental Method 3).
Table 1.
Patient Baseline Characteristics
| Placebo First (AB) (n = 22) |
L-Citrulline First (BA) (n = 23) |
H4Bip (n = 25a) | |
|---|---|---|---|
| Sex | |||
| Male | 16 (72.7) | 20 (87) | 18 (72) |
| Female | 6 (27.2) | 3 (13) | 7 (28) |
| Age, y | 70.1 ± 1.9 | 67.2 ± 1.8; P < 0.001 | 67.6 ± 11.7 |
| Body weight, kg | 84.5 ± 12.1 | 91.5 ± 20.9 | 93 ± 17.8 |
| Waist-to-hip ratio | 0.99 ± 0.08 | 1.0 ± 0.08 | 0.97 ± 0.08 |
| Smoking | |||
| Current smoker | 4 (18.2) | 7 (30.4) | 7 (28) |
| Exsmoker | 14 (63.6) | 13 (56.5) | 14 (56) |
| Nonsmoker | 4 (18.2) | 3 (13) | 4 (16) |
| Blood pressure, mm Hg | |||
| Systolic | 142.6 ± 21.8 | 137.6 ± 17.8 | 143.8 ± 21.2 |
| Diastolic | 75.8 ± 11.1 | 77.2 ± 11.4 | 73.2 ± 10.7 |
| MAPb | 98.1 ± 11.3 | 97.3 ± 11.0 | 96.7 ± 11.4 |
| Clinical conditions | |||
| Hypertensionf | 12 (54.5) | 19 (82.6) | 17 (68) |
| Hypercholesterolemiac | 17 (77.3) | 13 (56.5) | 13 (52) |
| CVD other than PAD | 12 (54.5) | 9 (39.1) | 10 (40) |
| Ischemic heart disease | 4 (18.2) | 8 (34.8) | 9 (36) |
| Type 2 diabetes | 7 (31.8) | 8 (34.8) | 10 (40) |
| Blood levels | |||
| Fasting glucose, mmol/L | 7.0 ± 3.5 | 6.3 ± 2 | 6.7 ± 2.7 |
| Total cholesterol, mmol/L | 4.7 ± 1.3 | 4.2 ± 0.9 | 4.3 ± 1.1 |
| Triglycerides, mmol/L | 1.5 ± 0.7 | 1.8 ± 0.7 | 1.6 ± 0.8 |
| LDL, mmol/L | 2.9 ± 1.2 | 2.3 ± 0.8 | 2.5 ± 1.1 |
| Creatinine, μmol/L | 104.8 ± 42.6 | 89.3 ± 23.7 | 93.7 ± 38.8 |
| Urea, mmol/L | 7.8 ± 3.5 | 6.4 ± 2.5 | 7.1 ± 2.7 |
| Albumin, g/L | 42.0 ± 2.4 | 42.3 ± 3.2 | 41.5 ± 2.3 |
| eGFRd, mL/min/1.73 m2 | 67.6 ± 25.9 | 77.6 ± 21.5 | 75.7 ± 25.0 |
| ACDg, m | 207 ± 28.4 | 231 ± 25.5; P = 0.003 | 291 ± 24.3 |
| Vascular testing | |||
| Resting ABIe | 0.75 ± 0.16 | 0.79 ± 0.16 | 0.79 ± 0.16 |
| Postexercise ABIe | 0.58 ± 0.2 | 0.66 ± 0.26 | 0.72 ± 0.22 |
Values are mean ± SEM. P values are only provided if a significant difference between sequence was detected. Data were missing for the following characteristics: ABI (for 1 patient in both sequence AB and BA), creatinine, and eGFR (for 1 patient in sequence BA). Percentages may not total 100 because of rounding.
ABI = ankle-brachial index; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; H4Bip = tetrahydrobiopterin; LDL = low-density lipoprotein; MAP = mean arterial pressure; PAD = peripheral artery disease.
Refers to patients in the Melbourne cohort that received H4Bip as a third intervention.
Calculated by doubling the diastolic blood pressure and adding the sum to the systolic blood pressure, then dividing by 3.
Defined as LDL at or above 4.1 mmol/L and total cholesterol at or above 6.2 mmol/L.
Calculated using the CKD-EPI equation with reference ranges of 59 to 104 and 45 to 84 mL/min/1.73 m2 for males and females, respectively.
Normal ABI defined as between 0.9 and 1.25; <0.9, claudication; <0.5 = rest pain or critical limb ischemia. If no pulse was detectable at rest, then postexercise ABI was not considered.
Defined as a blood pressure at or above 140/90 mm Hg.
Regarding gender-dependent differences, ACD at baseline was 195 ± 20.5 m and 227 ± 55.6 m in males and females, respectively (P = 0.603).
Statistical analysis
Oka and coworkers20 reported a baseline mean walking distance of 300 ± 150 m in a similar population. No information was available at the start of the study for both within-subject correlation or variance of the difference for a 2-period crossover study of L-citrulline vs placebo. We computed the sample size to compare 2 paired means. Taking into account typical drop-out rates, a sample size of 50 was calculated to have an 80% power to detect a clinically meaningful 20% difference in ACD with a 2-sided alpha of 5%, a within-subject correlation of >0.60 and an SD of the population of 75 m.
Baseline characteristics were described as mean and SD, median and IQR, or as count and percentage. For patient-reported outcomes, we analyzed the fraction of maximal walking distance and speed scores by dividing the total scores by their respective maximum possible scores of 6,060 and 34.5, the percentage changes of these scores during each trial phase, and the degree of walking impairment, specifically pain or aching in calves and thighs, using a scale from 0 (much) to 3 (none) for significant differences using paired sample t-tests. The presence of any training effect was tested for by using simple linear regression, taking time as the independent variable and the aggregate ACD of all patients during the placebo phases of each sequence as the dependent variable. We used a 2-way repeated measures ANOVA to test for a carry-over effect between the 2 sequences (AB/BA) to evaluate whether, after the washout period, there were any residual effects. We used paired sample t-tests to analyze differences in ACD, L-citrulline, and L-arginine levels between placebo and L-citrulline as well as placebo and L-citrulline + H4Bip. The relationship of ACD with ADMA (or 3-NT and NOX5) was analyzed by linear regression; the relationship between plasma L-citrulline and L-arginine, by nonlinear regression. To measure and monitor any adverse events during the study, we used descriptive statistics to evaluate safety parameters.
Results
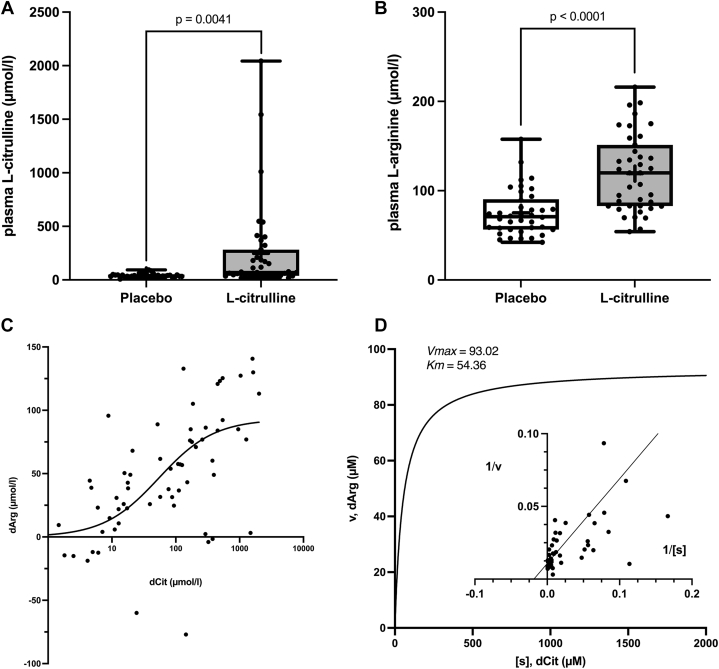
A total of 51 patients with stable intermittent claudication due to PAD were recruited (Figure 1), thus reaching the necessary total sample size: 24 patients at the Hannover site for interventions 1 and 2; 27 at the Melbourne site for interventions 1 to 3 according to their visit schedule (Supplemental Table 2). All patients provided written informed consent before enrollment. Baseline characteristics were similar in patients allocated to either sequence (Table 1). Patients were recruited and followed-up between 2009 and 2012 in Melbourne and from 2017 up until the closure of the trial in 2019 in Hannover. Upon oral L-citrulline supplementation and conversion to L-arginine, both plasma L-citrulline (mean 246.3 ± 67.3 μM, median 70.5 μM; placebo: mean 41.8 ± 2.7 μM, median 38.8 μM) and L-arginine (mean 119.2 ± 6.9 μM, median 120.0 μM; placebo: mean 75.2 ± 4.2 μM, median 70.9 μM) were increased (Figures 2A and 2B). Moreover, the increases in plasma L-citrulline (dCit) and L-arginine (dArg) followed sigmoidal (Figure 2C) and apparently saturable (Figure 2D) kinetics (r = 0.40, 95% CI: 0.19-0.58; P ≤ 0.001) with a 0.54 μmol/L increase in dArg for every 10 μmol/L increase of dCit.
Figure 1.
The CONSORT Diagram
All 51 patients enrolled in the study consented to participate. Reasons are given for patients who discontinued the trial regimen at each stage of the study. $Patients enrolled in Melbourne received L-citrulline and H4Bip for 2 weeks after period 2. Aforementioned patients who discontinued the trial regimen include those who did not complete period 3. ∗Discontinued due to limited storage life of the study medications. Affected patients were followed per-protocol until week 32, if applicable. CONSORT = Consolidated Standards of Reporting Trials; H4Bip = tetrahydrobiopterin.
Figure 2.
Increase in Plasma Levels of L-Citrulline and L-Arginine
Plasma L-citrulline (A) and L-arginine (B) levels at week 4 and week 12 (AB in Figure 1) and week 20 and 28 (BA in Figure 1) vs placebo. apparent concentration-dependence (C) and saturation kinetics (D) of the increase in plasma L-arginine (dArg) depending on the increase in plasma L-citrulline (dCit) in linear format and as lineweaver-burk plot (Insert).
L-citrulline improves ACD
L-citrulline supplementation with or without H4Bip was safe (Supplemental Table 3) and reached the primary efficacy endpoint, dACD (mean 20.1% ± 4.5% of control or 38.8 ± 7.9 m, median 15.7% of control), vs placebo (mean 5.7% ± 2.7% of control or 5.4 ± 9.3 m, median 0.6% of control, P = 0.011; Figure 3A). No significant difference between sexes was seen in the response of L-citrulline on ACD (mean 21.3% ± 6.7% and 37.4 ± 17 to 7% of control for male and female patients, respectively; P = 0.420). Regarding an exercise training effect, we found a significant relationship (P < 0.001) during the placebo phase between time and ACD (r = 0.96, 95% CI: 0.63-0.99), with an increase of 4.37 ± 0.58 m in ACD for every further week the participant was taking part in the study. However, with respect to the crossover design, no order effect was detected (P = 0.360), suggesting that this training effect did not influence the response to L-citrulline. Secondary surrogate vascular function outcomes, namely ankle-brachial index (Supplemental Figure 1), flow-mediated dilatation, peripheral artery tonometry, pulse wave velocity-central (data not shown), and the PAD Walking Impairment Questionnaire (Supplemental Table 4) were not significantly different between L-citrulline/H4Bip and placebo. Similarly, L-citrulline had no effect on blood pressure (mean change in MAP of −1.1 ± 1.6 and −2.3 ± 4.1 mm Hg during L-citrulline and L-citrulline + H4Bip, respectively, vs −3.7 ± 1.6 mm Hg during placebo; P = 0.300 and 0.899, respectively).
Figure 3.
Primary Outcome of L-Citrulline and Tetrahydrobiopterin on ACD
Boxplots demonstrating the changes in ACD induced by placebo, L-citrulline, or L-citrulline plus H4Bip expressed in % of baseline (week 0 or 16, respectively) in either (A) all patients (n = 45); or (B) only those with pathological high ADMA plasma levels (“ADMA positive”), that is, above 0.4 μM (n = 38).37 ADMA = asymmetric dimethyl L-arginine; other abbreviation as in Figure 1.
Adding H4Bip to L-citrulline further improves ACD
Adding H4Bip to L-citrulline for 2 weeks (Melbourne site), further increased both the mean and median ACD, but also its variability, so that a numerical increase in ACD did not reach significance when including all patients at this site (mean 31.0% ± 7.6% of control, median 31.0% of control, P = 0.060) (Figure 3A) with a small standardized effect size expressed as Cohen's D of 0.10 when comparing both treatment arms. However, when using pathological ADMA levels as an additional, post hoc stratification criterion, adding H4Bip to L-citrulline further increased ACD (mean 28.2% ± 6.8% of control, median 33.3% of control, P = 0.021) in patients with pathological ADMA levels (Figure 3B).37
Patients with pathological plasma ADMA have lower ACD
In a post hoc analysis to gain mechanistic understanding and to develop hypotheses for future studies, baseline, that is, preinterventional, plasma ADMA levels tended to negatively correlate with baseline ACD without reaching statistical significance (r = 0.28, 95% CI: −0.55 to 0.03; P = 0.079). However, when patients with pathological ADMA levels, that is, above 0.4 μM, were grouped, their basal ACD was nearly 50% reduced vs patients with physiological or lower ADMA levels (Figure 4A) (402.3 ± 51.9 m vs 172.8 ± 17.5 m, P < 0.0001). Similarly additional biomarkers related to reactive oxygen formation, plasma NOX5, and plasma 3-NT, demonstrated pathological levels38 in all patients and, when analyzed in combination, negatively correlated with ACD (Figures 4B to 4D). Upon intervention, no changes in the frequency distribution of ADMA and, only analyzed in the Hannover cohort, NOX5 or 3-NT were observed (Supplemental Figure 2).
Figure 4.
Correlation of Initial Asymmetric Dimethyl L-Arginine and Composite Exploratory Biomarker Plasma Levels With Preinterventional ACD
Correlation of ACD before intervention (week 0 or 12, Resp) with (A) ADMA (circles and crosses indicate participants from the Hannover and Melbourne site, respectively), or, only for participants from the Hannover site (B) 3-NT, (C) NOX5, or (D) a composite marker of 3-NT and NOX5 (cROS, in % of max). The bar graph summarizes ACD in patients at or below (402 ± 51 m) and above (173 ± 18 m) physiological ADMA levels (P = 0.0008). The red dashed lines indicate pathological threshold levels for ADMA (0.4 μM),37 3-NT (0.12 μM),38 and NOX5 (105 ng/mL, unpublished observation in cardiovascular patients), respectively. (A) A pathological ADMA level (>0.4 μM) predicted a lower ACD (right), however, without a linear correlation (left). (D) The composite ROS marker of NOX5 and 3-NT, cROS (in % of each maximal value), but not the individual markers (B and C), negatively correlated with ACD (r = 0.48, 95% CI: −0.76 to −0.04; P = 0.034) with a 30.3 ± 13.2 m decrease in ACD for every 10 percentage points increase. 3-NT = 3-nitrotyrosine; NOX5 = nicotinamide adenine dinucleotide phosphate oxidase 5; ROS = reactive oxygen species; other abbreviation as in Figure 3.
Discussion
In this phase II interventional trial, we find that the food supplement L-citrulline increases plasma L-arginine levels and improves ACD in patients with PAD. In patients with pathologically high ADMA levels, this effect was further augmented by adding H4Bip. L-citrulline, neither alone nor in combination with H4Bip, induced any adverse events.
ETTs produce a training effect, possibly also due to improved coordination, finding optimal stride length, and overcoming anxiety. Performance (without intervention) usually reaches a plateau after 2 tests within a week. Our study's run-in phase, therefore, accounted for some of the early familiarization responses to the walking test before the commencement of the treatment arm, thereby reducing this component of a placebo effect. The crossover design minimized confounders such as additional exercise training, possibly explaining why the overall effect size of L-citrulline on ACD was smaller than for other study formats.39 Moreover, walking distance is a complex variable that could reflect many factors. For example, Ashley et al40 showed that L-citrulline increased oxygen uptake during walking exercise in men but not women. Yet no significant difference between sexes was seen when we compared the ACD at baseline and after 12 weeks of L-citrulline.
Previously, L-arginine has also been given as a food supplement in PAD, yielding however inconsistent results, with one study even suggesting a reduced walking distance.22 Here, the daily supplementation of only 3 g L-arginine, that is, half the amount of the more bioavailable L-citrulline as in the present study, may have been insufficient. Conversely, an 18-month intervention with 6.4 g L-arginine daily in diabetic patients improved endothelial function.41 Such apparent variability in the effects of L-arginine supplementation is likely due to considerable first-pass metabolism42 and underscores our choice for L-citrulline in the present study.
L-citrulline is approximately twice as effective concerning augmenting NO formation compared to L-arginine because it is not acted on by arginases and skips first-pass extraction before being converted to L-arginine by renal argininosuccinate lyase. Moreover, L-citrulline suppresses arginase activity, thereby also indirectly enhancing L-arginine levels.43 Thus, L-citrulline appears to be a more effective precursor and sustained source of L-arginine than oral supplementation of L-arginine itself.44 Our daily supplementation of 6 g L-citrulline reached blood levels of 205.6 μmol/L. The sigmoidal dependence of the subsequent increase in plasma L-arginine on the increase in plasma L-citrulline is consistent with any of the 2 above enzymatic mechanisms.
Mechanistically, the effect of L-citrulline in this context may be explained by L-arginine outcompeting the endogenous NOS inhibitor, ADMA.14 The alternative explanation of L-arginine causing ADMA extrusion, as observed in vasospastic angina45 and heart failure,46 and thereby indirectly deinhibiting of NOS is unlikely because one would have expected an increase in plasma ADMA induced by L-citrulline, which we did not observe. A third possibility for L-arginine would be recoupling of uncoupled, that is, superoxide-forming, NOS.47, 48, 49, 50 However, ADMA itself is unlikely to selectively uncouple NOS, but rather downregulates its electron flow entirely to reduce both NO and superoxide formation.51
While the effect of L-citrulline is therefore most likely related to ADMA competing at the L-arginine binding site, the additional effect of H4Bip in the present study may suggest that endothelial NOS (NOS3) was at least partially H4Bip-deficient and uncoupled.52,53 Positive cardiovascular effects of H4Bip have been observed before.54 Why the effect of H4Bip on ACD was limited to those patients with pathological ADMA levels (defined as ≥0.4 μM)37 is unclear, but may involve renal effects.55 Nevertheless, our findings make ADMA a likely mechanism-based inclusion criterion in future trials aimed at modulating the NOS3-sGC disease module.
To explore this ROS-related component of the L-citrulline/H4Bip intervention, we conducted a post hoc analysis of several ROS-related biomarkers and their correlation with the observed ACD, that is, the ROS source and part of the NOS3-sGC disease module, NOX5, with a high degree of evidence in vascular disease,56, 57, 58, 59 and the metabolic ROS marker, 3-NT.12 Indeed, when analyzed jointly, ACD was negatively correlated. Upon the intervention, none of these markers, ADMA, NOX5, and 3-NT, changed. This is, however, also not to be expected since only the consequences of enhanced NOX5 expression and ROS formation (eg, uncoupled-NOS) are treated, but neither NOX5 expression and activity nor ROS formation. This may become an option once NOX5 inhibitory compounds become available.
Study limitations
A possible limitation of L-citrulline supplementation may be its short biological half-life of, for example, 0.9 hour for 5 g L-citrulline.60 However, L-citrulline and L-arginine may not require constantly elevated plasma levels as their site of action is within the vascular endothelium where they accumulate.61 Apart from NOS3, secondary or tertiary effects on inducible NOS (NOS2) activity and inflammation by H4Bip have been shown in many animal models. However, a consistent string of trial failures in rheumatoid arthritis and asthma makes a NOS2 an unlikely human therapeutic target.62 NOS2 may well be suited as a marker of inflammation. Thus the overall effect of combining L-citrulline and H4Bip is likely due to a causal mechanism-based effect in ADMA-positive patients, which could either be ADMA itself or an upstream effect such as ROS formation leading to both increased ADMA levels63 and oxidized H4Bip64 and thereby uncoupled NOS.64,65 The addition of H4Bip was not tested against a placebo. However, at that time point, the effect of L-citrulline on dACD (%) was reaching saturation (+4.3%/week from week 0 to week 4, but only +0.8%/week from week 4 to week 12), suggesting that the additional increase in ACD between week 12 and week 14 (+2.35%/week) was indeed due to H4Bip and not a further accumulation of the L-citrulline effect.
H4Bip was chosen in the present study because it is the physiological ligand of NOS, yet it comes with both kinetic and cost concerns. Under conditions of elevated ROS formation, H4Bip may be at least partially oxidized to H2Bip, which competes with H4Bip on its NOS binding site making H2Bip a functional H4Bip antagonist.53,66,67 Indeed, 2 human studies supplementing H4Bip showed no beneficial cardiovascular effect, possibly due to increased H2Bip.68,69 A key observation in this context may be that only when H4Bip was substituted in a mechanism-based manner, that is, only to ADMA-positive patients, was it of benefit. Dihydrofolate reductase can nicotinamide adenine dinucleotide phosphate-dependently regenerate H4Bip from H2Bip via the salvage pathway.70 Therefore, net H4Bip cellular bioavailability may reflect the steady state between de novo synthesis, loss, and regeneration.71 Still, ACD variability slightly increased in H4Bip-treated patients, possibly indicating some H2Bip formation and vascular side effects. From a health economic point of view, H4Bip supplementation is a costly intervention of more than 50 US$ per day. Hence, more cost-effective approaches, for example, folate or H4Bip precursors such as 5-methyltetrahydrofolate or sepiapterin, may be better suited than H4Bip. Furthermore, the H4Bip precursor 5-methyltetrahydrofolate can improve endothelial function and decrease superoxide production by improving vascular availability of H4Bip and preventing endothelial NOS uncoupling.72 Folate or H4Bip precursors may also be less susceptible to autoxidation.
Conclusions
L-citrulline and—in ADMA-positive patients—the combination with H4Bip appear to be effective interventions in patients with PAD as measured by mean dACD as primary outcome parameter of disease severity and functional impairment. These data warrant confirmatory trials in PAD and encourage similar nutritional interventions in related cardiovascular disease states with endothelial dysfunction. A switch to folate or 5-methyltetrahydrofolate instead of H4Bip should be considered not the least because of health economic reasons. Finally, this nutritional combination may be further potentiated with pharmacological approaches such as stimulators or activators of the NO receptor, soluble guanylate cyclase, sGC, or its ROS-induced heme-free form, apo-sGC.73
Perspectives.
COMPETENCY IN PATIENT CARE: In this pilot study, orally supplementing patients with supraphysiological amounts of the nonessential amino acid L-citrulline significantly increased plasma L-arginine levels and improved absolute claudication distance. In patients with pathological ADMA levels, further addition of H4Bip, a cofactor of NOS, increased this effect.
TRANSLATIONAL OUTLOOK: Future confirmatory trials need to consider using elevated ADMA levels as an inclusion criterion to supplement L-citrulline. H4Bip may be an additional add-on therapy or be replaced by more economical substitutes such as folate. On a larger dimension, this study may represent 1 of the first successful clinical applications redefining a common disease not by a symptom but molecular cause, allowing precision diagnosis using mechanism-based biomarkers (ADMA) and network pharmacology (L-citrulline + H4Bip).
Funding Support and Author Disclosures
This work was supported by the National Health and Medical Research Council, Australia (595964), the European Research Council Advanced Investigator programme (RadMed), the European Union's Horizon 2020 research and innovation programme under grant agreement No. 777111 (REPO-TRIAL), the REPO4EU project funded by the European Union under grant agreement No. 101057619. Views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of the European Union or European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them. This reflects only the author's view, and the European Commission is not responsible for any use that may be made of the information it contains. The funders had no role in the design, data collection, statistical analysis, writing of this manuscript, or decision to publish. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, tables, and figures, please see the online version of this paper.
Supplementary data
References
- 1.Kullo I.J., Rooke T.W. Clinical Practice. Peripheral artery disease. N Engl J Med. 2016;374(9):861–871. doi: 10.1056/NEJMcp1507631. [DOI] [PubMed] [Google Scholar]
- 2.Criqui M.H., Matsushita K., Aboyans V., et al. Lower extremity peripheral artery disease: contemporary epidemiology, management gaps, and future directions: a scientific statement from the American heart association. Circulation. 2021;144(9):e171–e191. doi: 10.1161/CIR.0000000000001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eid M.A., Mehta K., Barnes J.A., et al. The global burden of peripheral artery disease. J Vasc Surg. 2023;77(4):1119–1126.e1. doi: 10.1016/j.jvs.2022.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Pasqualini L., Bagaglia F., Ministrini S., et al. Effects of structured home-based exercise training on circulating endothelial progenitor cells and endothelial function in patients with intermittent claudication. Vasc Med. 2021;26(6):633–640. doi: 10.1177/1358863X211020822. [DOI] [PubMed] [Google Scholar]
- 5.Hageman D., Fokkenrood H.J., Gommans L.N., van den Houten M.M., Teijink J.A. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;4(4) doi: 10.1002/14651858.CD005263.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClure G.R., Kaplovitch E., Narula S., Bhagirath V.C., Anand S.S. Rivaroxaban and aspirin in peripheral vascular disease: a review of implementation strategies and management of common clinical scenarios. Curr Cardiol Rep. 2019;21(10):115. doi: 10.1007/s11886-019-1198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboyans V., Ricco J.B., Bartelink M.L.E.L., et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European stroke organization (ESO)the task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of cardiology (ESC) and of the European Society for vascular surgery (ESVS) Eur Heart J. 2018;39(9):763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 8.Steven S., Daiber A., Dopheide J.F., Münzel T., Espinola-Klein C. Peripheral artery disease, redox signaling, oxidative stress - basic and clinical aspects. Redox Biol. 2017;12:787–797. doi: 10.1016/j.redox.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dopheide J.F., Scheer M., Doppler C., et al. Change of walking distance in intermittent claudication: impact on inflammation, oxidative stress and mononuclear cells: a pilot study. Clin Res Cardiol. 2015;104(9):751–763. doi: 10.1007/s00392-015-0840-5. [DOI] [PubMed] [Google Scholar]
- 10.Langhauser F., Casas A.I., Dao V.T.V., et al. A diseasome cluster-based drug repurposing of soluble guanylate cyclase activators from smooth muscle relaxation to direct neuroprotection. NPJ Syst Biol Appl. 2018;4(1) doi: 10.1038/s41540-017-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt H.H.H.W., Walter U. NO at work. Cell. 1994;78(6):919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 12.Elbatreek M.H., Sadegh S., Anastasi E., et al. NOX5-induced uncoupling of endothelial NO synthase is a causal mechanism and theragnostic target of an age-related hypertension endotype. Lo C.W., editor. PLoS Biol. 2020;18(11) doi: 10.1371/journal.pbio.3000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler T., Schunkert H. Genomic strategies toward identification of novel therapeutic targets. Handb Exp Pharmacol. 2022;270:429–462. doi: 10.1007/164_2020_360. [DOI] [PubMed] [Google Scholar]
- 14.Williams G., Shi-Wen X., Abraham D., Selvakumar S., Baker D.M., Tsui J.C.S. Nitric oxide manipulation: a therapeutic target for peripheral arterial disease? Cardiol Res Pract. 2012;2012 doi: 10.1155/2012/656247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leiper J., Murray-Rust J., McDonald N., Vallance P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Natl Acad Sci USA. 2002;99(21):13527–13532. doi: 10.1073/pnas.212269799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallance P., Leone A., Calver A., Collier J., Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992;20(Suppl 1):S60–S62. doi: 10.1097/00005344-199204002-00018. [DOI] [PubMed] [Google Scholar]
- 17.Kotsonis P., Fröhlich L.G., Shutenko Z.V., Horejsi R., Pfleiderer W., Schmidt H.H.H.W. Allosteric regulation of neuronal nitric oxide synthase by tetrahydrobiopterin and suppression of auto-damaging superoxide. Biochem J. 2000;346(Pt 3):767–776. doi: 10.1042/0264-6021:3460767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pirro M., Schillaci G., Mannarino M.R., et al. Effects of rosuvastatin on 3-nitrotyrosine and aortic stiffness in hypercholesterolemia. Nutr Metab Cardiovasc Dis. 2007;17(6):436–441. doi: 10.1016/j.numecd.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Gryglewski R.J., Palmer R.M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 20.Oka R.K., Szuba A., Giacomini J.C., Cooke J.P. A pilot study of L-arginine supplementation on functional capacity in peripheral arterial disease. Vasc Med. 2005;10(4):265–274. doi: 10.1191/1358863x05vm637oa. [DOI] [PubMed] [Google Scholar]
- 21.Jabłecka A., Checiński P., Krauss H., Micker M., Ast J. The influence of two different doses of L-arginine oral supplementation on nitric oxide (NO) concentration and total antioxidant status (TAS) in atherosclerotic patients. Med Sci Monit. 2004;10(1):CR29–CR32. [PubMed] [Google Scholar]
- 22.Wilson A.M., Harada R., Nair N., Balasubramanian N., Cooke J.P. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116(2):188–195. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 23.Grimble G.K. Adverse gastrointestinal effects of arginine and related amino acids. J Nutr. 2007;137(6 Suppl 2):1693S–1701S. doi: 10.1093/jn/137.6.1693S. [DOI] [PubMed] [Google Scholar]
- 24.Tangphao O., Grossmann M., Chalon S., Hoffman B.B., Blaschke T.F. Pharmacokinetics of intravenous and oral L-arginine in normal volunteers. Br J Clin Pharmacol. 1999;47(3):261–266. doi: 10.1046/j.1365-2125.1999.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breuillard C., Cynober L., Moinard C. Citrulline and nitrogen homeostasis: an overview. Amino Acids. 2015;47(4):685–691. doi: 10.1007/s00726-015-1932-2. [DOI] [PubMed] [Google Scholar]
- 26.Moinard C., Nicolis I., Neveux N., Darquy S., Bénazeth S., Cynober L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: the Citrudose pharmacokinetic study. Br J Nutr. 2008;99(4):855–862. doi: 10.1017/S0007114507841110. [DOI] [PubMed] [Google Scholar]
- 27.Wijnands K.A.P., Castermans T.M.R., Hommen M.P.J., Meesters D.M., Poeze M. Arginine and citrulline and the immune response in sepsis. Nutrients. 2015;7(3):1426–1463. doi: 10.3390/nu7031426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwedhelm E., Maas R., Freese R., et al. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol. 2008;65(1):51–59. doi: 10.1111/j.1365-2125.2007.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nogales C., Mamdouh Z.M., List M., Kiel C., Casas A.I., Schmidt H.H.H.W. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022;43(2):136–150. doi: 10.1016/j.tips.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Lim C.Y., In J. Considerations for crossover design in clinical study. Korean J Anesthesiol. 2021;74(4):293–299. doi: 10.4097/kja.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontaine R., Kim M., Kieny R. [Surgical treatment of peripheral circulation disorders] Helv Chir Acta. 1954;21(5-6):499–533. [PubMed] [Google Scholar]
- 32.Rand Corporation . The Free Press; 1966. A Million Random Digits with 100,000 Normal Deviates.https://books.google.com/books/about/A_Million_Random_Digits_with_100_000_Nor.html?hl=&id=WaVEAAAAIAAJ [Google Scholar]
- 33.Müllner M. Springer-Verlag; 2013. Erfolgreich wissenschaftlich Arbeiten in der Klinik: Evidence Based Medicine.https://play.google.com/store/books/details?id=Qm0GBgAAQBAJ [Google Scholar]
- 34.Gardner A.W., Skinner J.S., Cantwell B.W., Smith L.K. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23(4):402–408. [PubMed] [Google Scholar]
- 35.Corretti M.C., Anderson T.J., Benjamin E.J., et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 36.Bonetti P.O., Pumper G.M., Higano S.T., Holmes D.R., Jr., Kuvin J.T., Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 37.Horowitz J.D., Heresztyn T. An overview of plasma concentrations of asymmetric dimethylarginine (ADMA) in health and disease and in clinical studies: methodological considerations. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851(1-2):42–50. doi: 10.1016/j.jchromb.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 38.Khan J., Brennan D.M., Bradley N., Gao B., Bruckdorfer R., Jacobs M. 3-Nitrotyrosine in the proteins of human plasma determined by an ELISA method. Biochem J. 1998;330(2):795–801. doi: 10.1042/bj3300795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robless P., Mikhailidis D.P., Stansby G.P. Cilostazol for peripheral arterial disease. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD003748.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Ashley J., Kim Y., Gonzales J.U. Impact of l-citrulline supplementation on oxygen uptake kinetics during walking. Appl Physiol Nutr Metab. 2018;43(6):631–637. doi: 10.1139/apnm-2017-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monti L.D., Galluccio E., Villa V., Fontana B., Spadoni S., Piatti P.M. Decreased diabetes risk over 9 year after 18-month oral L-arginine treatment in middle-aged subjects with impaired glucose tolerance and metabolic syndrome (extension evaluation of L-arginine study) Eur J Nutr. 2018;57(8):2805–2817. doi: 10.1007/s00394-017-1548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castillo L., Chapman T.E., Yu Y.M., Ajami A., Burke J.F., Young V.R. Dietary arginine uptake by the splanchnic region in adult humans. Am J Physiol. 1993;265(4 Pt 1):E532–E539. doi: 10.1152/ajpendo.1993.265.4.E532. [DOI] [PubMed] [Google Scholar]
- 43.Shearer J.D., Richards J.R., Mills C.D., Caldwell M.D. Differential regulation of macrophage arginine metabolism: a proposed role in wound healing. Am J Physiol. 1997;272(2 Pt 1):E181–E190. doi: 10.1152/ajpendo.1997.272.2.E181. [DOI] [PubMed] [Google Scholar]
- 44.Romero M.J., Platt D.H., Caldwell R.B., Caldwell R.W. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc Drug Rev. 2006;24(3-4):275–290. doi: 10.1111/j.1527-3466.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- 45.Closs E.I., Ostad M.A., Simon A., et al. Impairment of the extrusion transporter for asymmetric dimethyl-L-arginine: a novel mechanism underlying vasospastic angina. Biochem Biophys Res Commun. 2012;423(2):218–223. doi: 10.1016/j.bbrc.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 46.Speranza L., Franceschelli S., D'Orazio N., et al. The biological effect of pharmacological treatment on dimethylaminohydrolases (DDAH-1) and cationic amino acid transporter-1 (CAT-1) expression in patients with acute congestive heart failure. Microvasc Res. 2011;82(3):391–396. doi: 10.1016/j.mvr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Leiper J., Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov. 2011;10(4):277–291. doi: 10.1038/nrd3358. [DOI] [PubMed] [Google Scholar]
- 48.Channon K.M. Tetrahydrobiopterin and nitric oxide synthase recouplers. Handb Exp Pharmacol. 2021;264:339–352. doi: 10.1007/164_2020_390. [DOI] [PubMed] [Google Scholar]
- 49.Li H., Horke S., Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237(1):208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Schulz E., Gori T., Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34(6):665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 51.Cardounel A.J., Xia Y., Zweier J.L. Endogenous methylarginines modulate superoxide as well as nitric oxide generation from neuronal nitric-oxide synthase: differences in the effects of monomethyl- and dimethylarginines in the presence and absence of tetrahydrobiopterin. J Biol Chem. 2005;280(9):7540–7549. doi: 10.1074/jbc.M410241200. [DOI] [PubMed] [Google Scholar]
- 52.Gebhart V., Reiß K., Kollau A., Mayer B., Gorren A.C.F. Site and mechanism of uncoupling of nitric-oxide synthase uncoupling by monomerization and other misconceptions. Nitric Oxide. 2019;89:14–21. doi: 10.1016/j.niox.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Reif A., Fröhlich L.G., Kotsonis P., et al. Tetrahydrobiopterin inhibits monomerization and is consumed during catalysis in neuronal NO synthase. J Biol Chem. 1999;274(35):24921–24929. doi: 10.1074/jbc.274.35.24921. [DOI] [PubMed] [Google Scholar]
- 54.Porkert M., Sher S., Reddy U., et al. Tetrahydrobiopterin: a novel antihypertensive therapy. J Hum Hypertens. 2008;22(6):401–407. doi: 10.1038/sj.jhh.1002329. [DOI] [PubMed] [Google Scholar]
- 55.Arefin S., Löfgren L., Stenvinkel P., Granqvist A.B., Kublickiene K. Associations of biopterins and ADMA with vascular function in peripheral microcirculation from patients with chronic kidney disease. Int J Mol Sci. 2023;24(6):5582. doi: 10.3390/ijms24065582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guzik T.J., Chen W., Gongora M.C., et al. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol. 2008;52(22):1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Casas A.I., Kleikers P.W., Geuss E., et al. Calcium-dependent blood-brain barrier breakdown by NOX5 limits postreperfusion benefit in stroke. J Clin Invest. 2019;129(4):1772–1778. doi: 10.1172/JCI124283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Touyz R.M., Anagnostopoulou A., Camargo L.L., Rios F.J., Montezano A.C. Vascular biology of superoxide-generating NADPH oxidase 5 - implications in hypertension and cardiovascular disease. Antioxidants Redox Signal. 2019;30(7):1027–1040. doi: 10.1089/ars.2018.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jha J.C., Banal C., Okabe J., et al. NADPH oxidase Nox5 accelerates renal injury in diabetic nephropathy. Diabetes. 2017;66(10):2691–2703. doi: 10.2337/db16-1585. [DOI] [PubMed] [Google Scholar]
- 60.Khalaf D., Krüger M., Wehland M., Infanger M., Grimm D. The effects of oral l-arginine and l-citrulline supplementation on blood pressure. Nutrients. 2019;11(7):1679. doi: 10.3390/nu11071679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajapakse N.W., Mattson D.L. Role of L-arginine in nitric oxide production in health and hypertension. Clin Exp Pharmacol Physiol. 2009;36(3):249–255. doi: 10.1111/j.1440-1681.2008.05123.x. [DOI] [PubMed] [Google Scholar]
- 62.Dao V.T.V., Elbatreek M.H., Fuchß T., et al. Nitric oxide synthase inhibitors into the clinic at last. Handb Exp Pharmacol. 2021;264:169–204. doi: 10.1007/164_2020_382. [DOI] [PubMed] [Google Scholar]
- 63.Wilcox C.S. Asymmetric dimethylarginine and reactive oxygen species: unwelcome twin visitors to the cardiovascular and kidney disease tables. Hypertension. 2012;59(2):375–381. doi: 10.1161/HYPERTENSIONAHA.111.187310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Channon K.M. Tetrahydrobiopterin: a vascular redox target to improve endothelial function. Curr Vasc Pharmacol. 2012;10(6):705–708. doi: 10.2174/157016112803520819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landmesser U., Dikalov S., Price S.R., et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noguchi K., Hamadate N., Matsuzaki T., et al. Increasing dihydrobiopterin causes dysfunction of endothelial nitric oxide synthase in rats in vivo. Am J Physiol Heart Circ Physiol. 2011;301(3):H721–H729. doi: 10.1152/ajpheart.01089.2010. [DOI] [PubMed] [Google Scholar]
- 67.Schmidt H.H.H.W., Smith R.M., Nakane M., Murad F. Ca2+/Calmodulin-Dependent no synthase type I: a biopteroflavoprotein with Ca2+/calmodulin-independent diaphorase and reductase activities. Biochemistry. 1992;31(12):3243–3249. doi: 10.1021/bi00127a028. [DOI] [PubMed] [Google Scholar]
- 68.Cunnington C., Van Assche T., Shirodaria C., et al. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation. 2012;125(11):1356–1366. doi: 10.1161/CIRCULATIONAHA.111.038919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reverter E., Mesonero F., Seijo S., et al. Effects of sapropterin on portal and systemic hemodynamics in patients with cirrhosis and portal hypertension: a bicentric double-blind placebo-controlled study. Am J Gastroenterol. 2015;110(7):985–992. doi: 10.1038/ajg.2015.185. [DOI] [PubMed] [Google Scholar]
- 70.Werner E.R., Blau N., Thöny B. Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J. 2011;438(3):397–414. doi: 10.1042/BJ20110293. [DOI] [PubMed] [Google Scholar]
- 71.Crabtree M.J., Channon K.M. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide. 2011;25(2):81–88. doi: 10.1016/j.niox.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antoniades C., Shirodaria C., Warrick N., et al. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114(11):1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 73.Evgenov O.V., Pacher P., Schmidt P.M., Haskó G., Schmidt H.H.H.W., Stasch J.P. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goh K.I., Cusick M.E., Valle D., Childs B., Vidal M., Barabasi A.L. The human disease network. Proc Natl Acad Sci USA. 2007;104(21):8685–8690. doi: 10.1073/pnas.0701361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.