Abstract
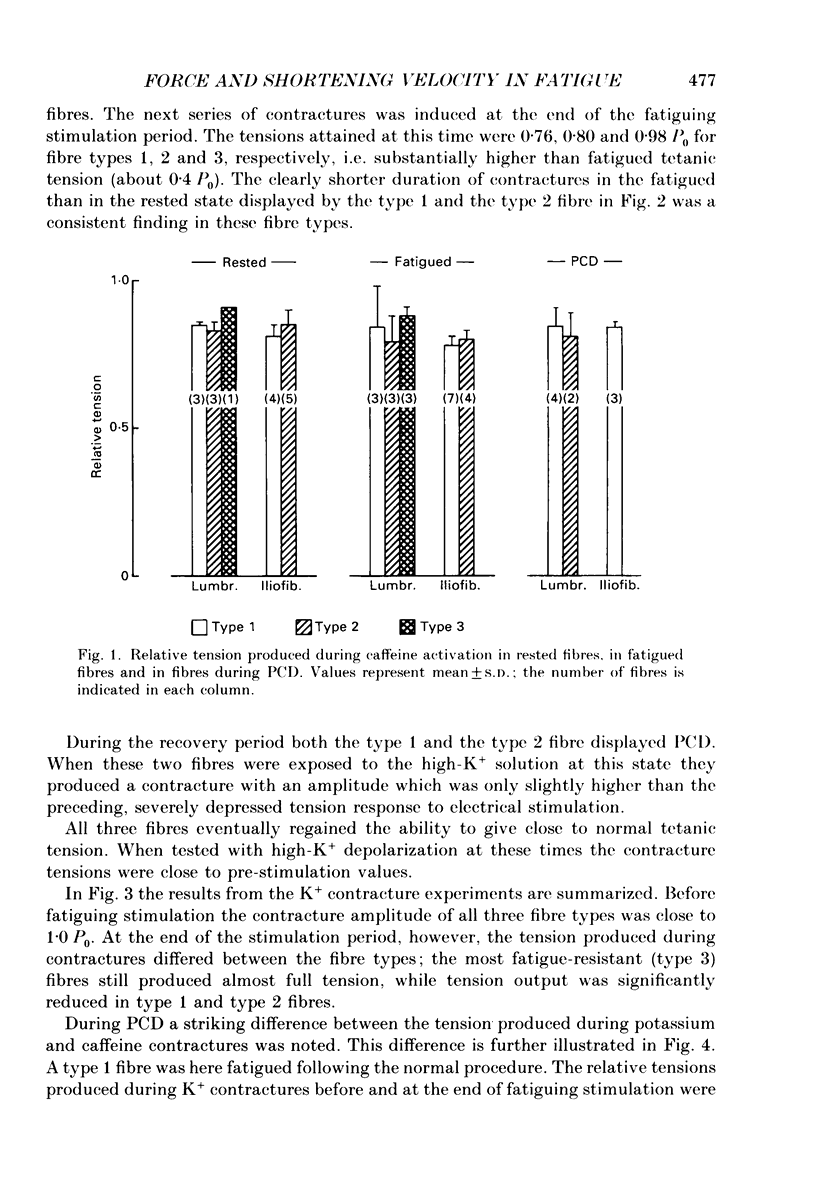
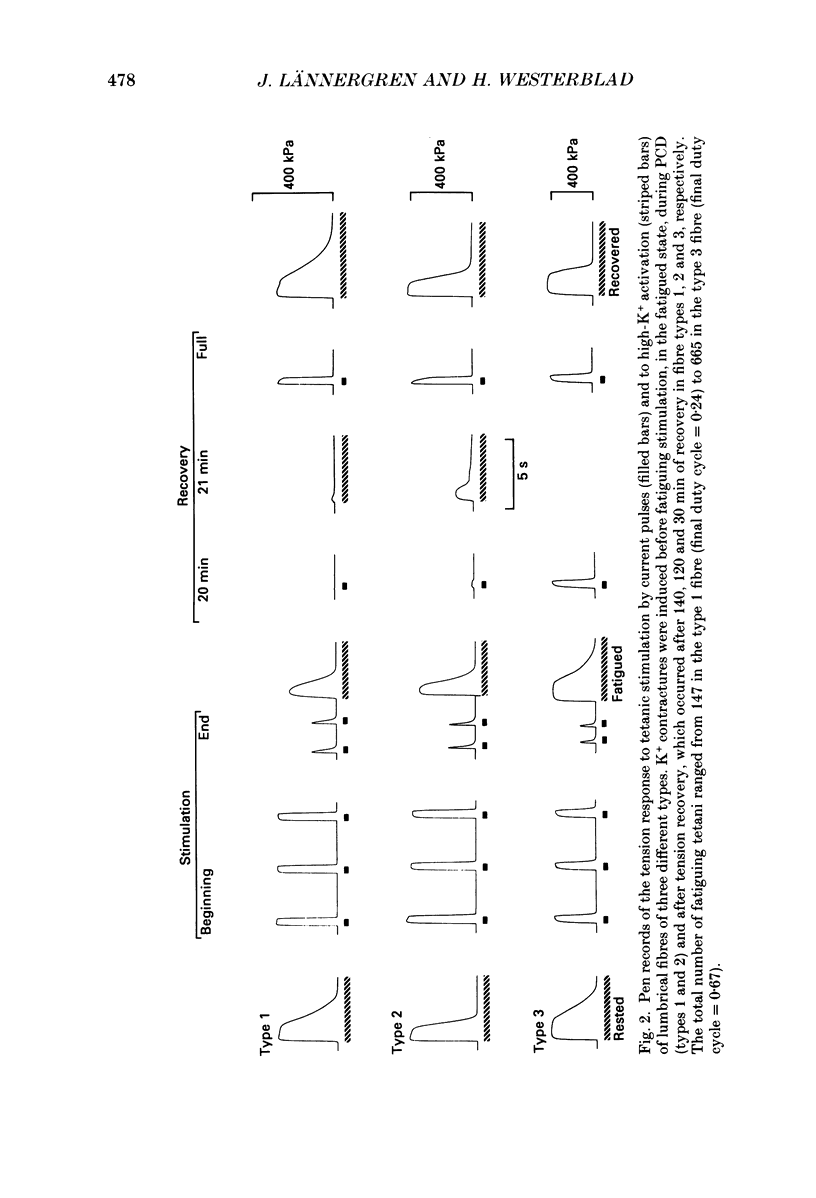
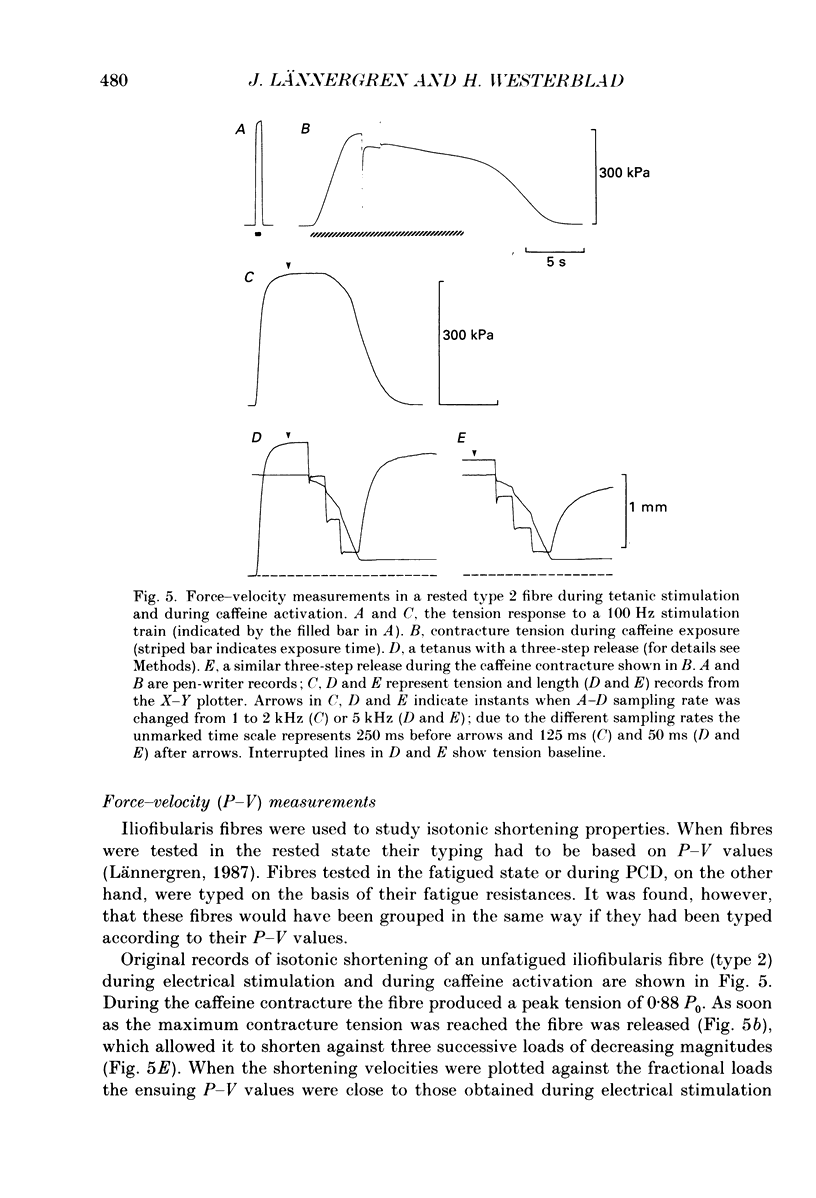
1. The importance of reduced maximum force-generating capacity in the development of skeletal muscle fatigue has been studied using potassium and caffeine contractures as tools. 2. Single, intact fibres isolated from the lumbrical and iliofibularis muscles of Xenopus were fatigued by repeated tetanic stimulations until they produced close to 40% of the original tetanic tension (P0). Using this stimulation scheme three major types of fibres can be distinguished: easily fatigued (type 1), fatigue resistant (type 2), and very fatigue-resistant (type 3) fibres (Westerblad & Lännergren, 1986). 3. When activated by 8-15 mM-caffeine-Ringer solutions fatigued fibres of all three types developed tensions similar to those of controls (81.0 +/- 6.6 vs. 83.9 +/- 4.2% of P0, respectively; means +/- S.D.). 4. Tension output also increased markedly when fatigued fibres were depolarized by 190 mM-K+ solution. The tension produced was in this case fibre type dependent: 71.4 +/- 6.6, 81.3 +/- 2.5 and 95.0 +/- 4.4% of P0 in fibre types 1, 2 and 3, respectively. 5. Force-velocity measurements were performed during caffeine contractures in fatigued iliofibularis fibres (types 1 and 2) to obtain more information about the functional state of cross-bridges. 6. In fatigued type 1 fibres the shortening velocity was reduced to about 25% of that in controls, while it was not significantly depressed in type 2 fibres. 7. It is concluded that cross-bridges of fatigued fibres can produce nearly full tension, but they may work at a much slower rate in this state. 8. Fibre types 1 and 2 mostly display a long-lasting, reversible state of severely depressed tension production during the recovery period, which has been named post-contractile depression, PCD (Westerblad & Lännergren, 1986). Fibres tested in this state generated full caffeine-activated tension and the shortening velocity was not significantly reduced. The tension output during K+ contractures was, however, markedly depressed (12.4 +/- 4.1% of P0). 9. In conclusion, cross-bridges are able to produce close to full tension during PCD as well as in the fatigued state if they are fully activated. The form of functional impairment seems, however, not to be the same in the two cases.
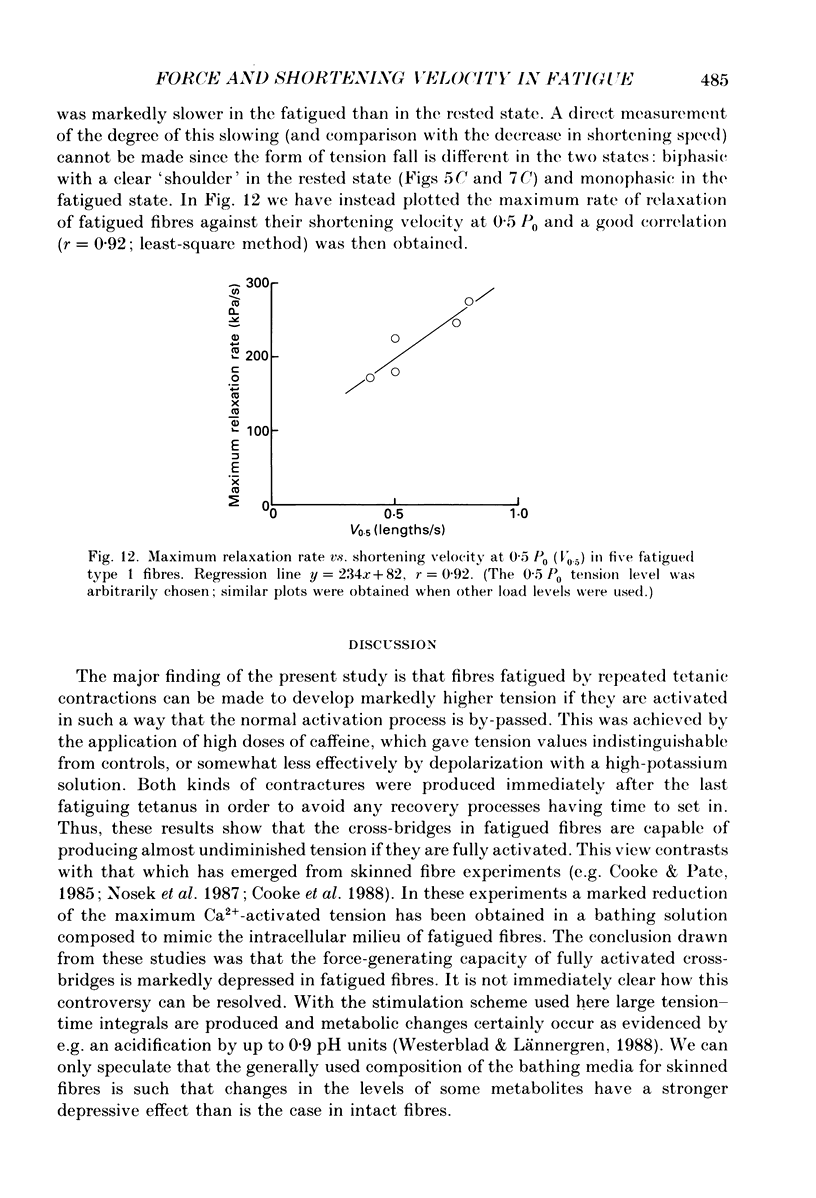
Full text
PDF



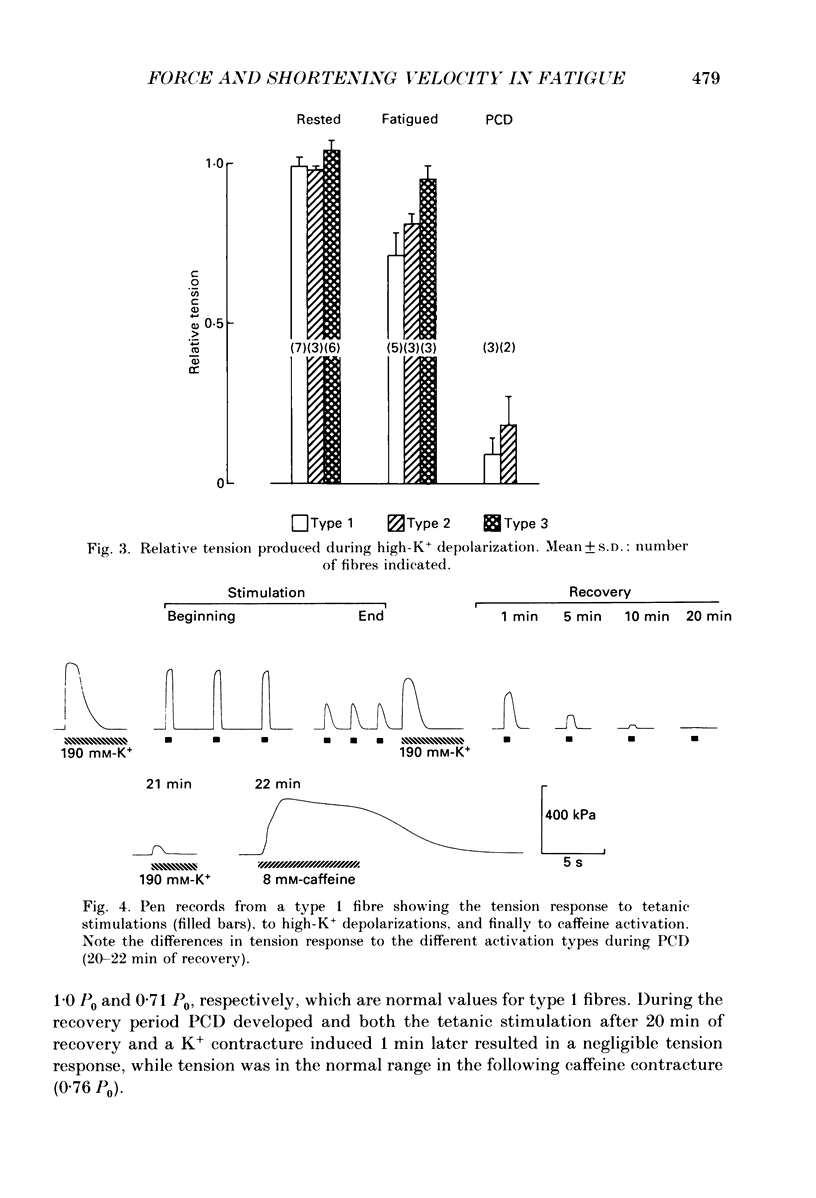
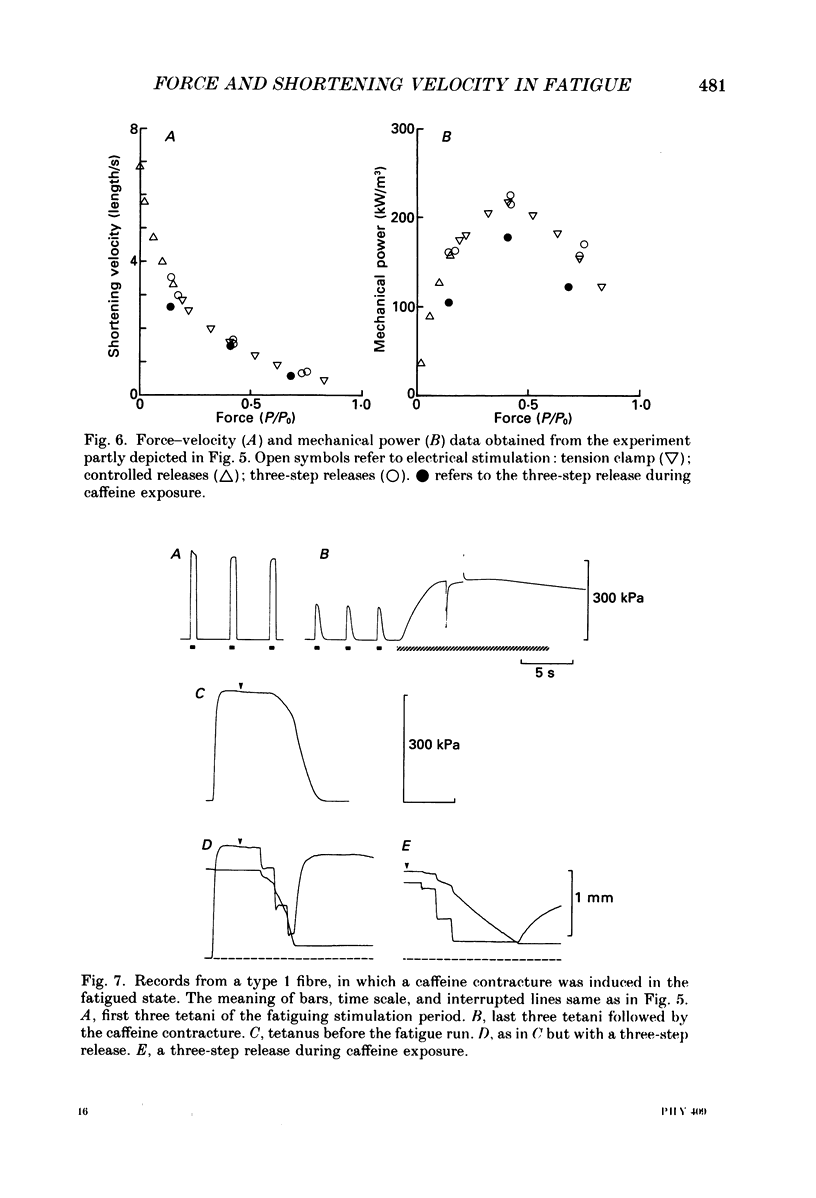
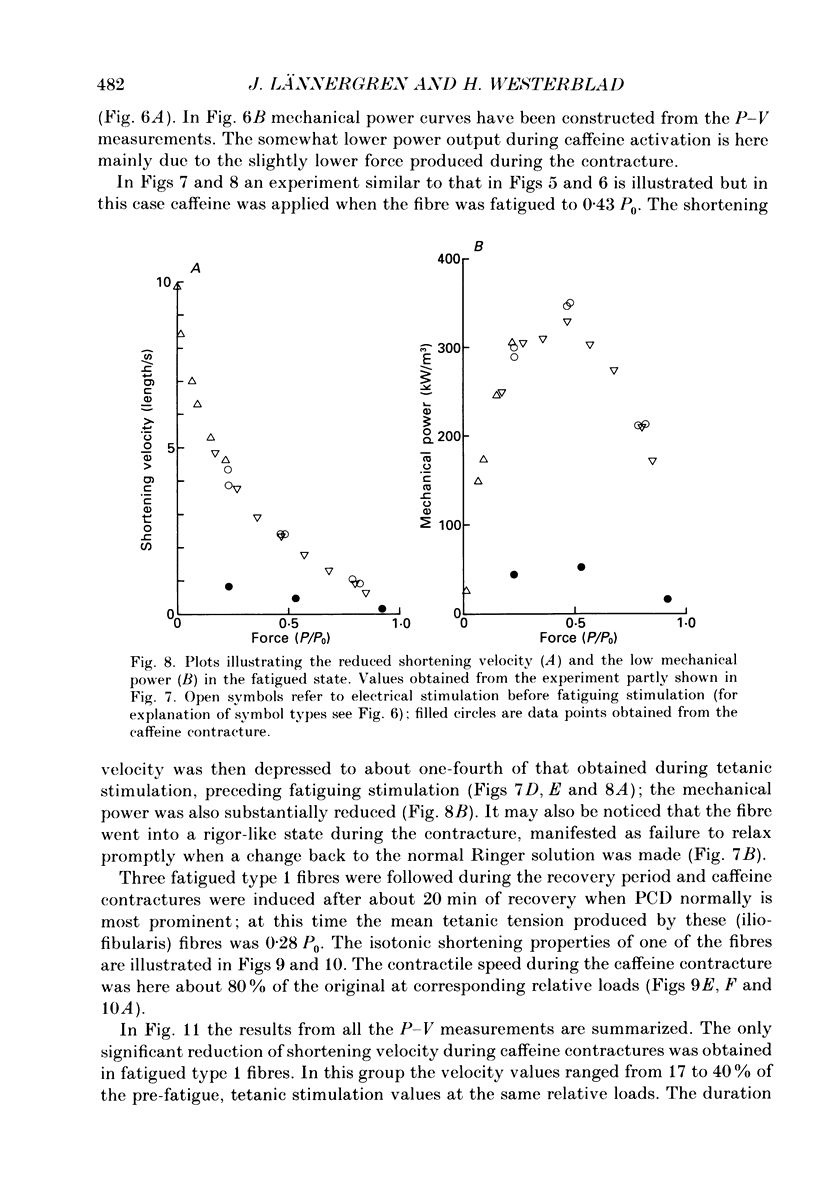
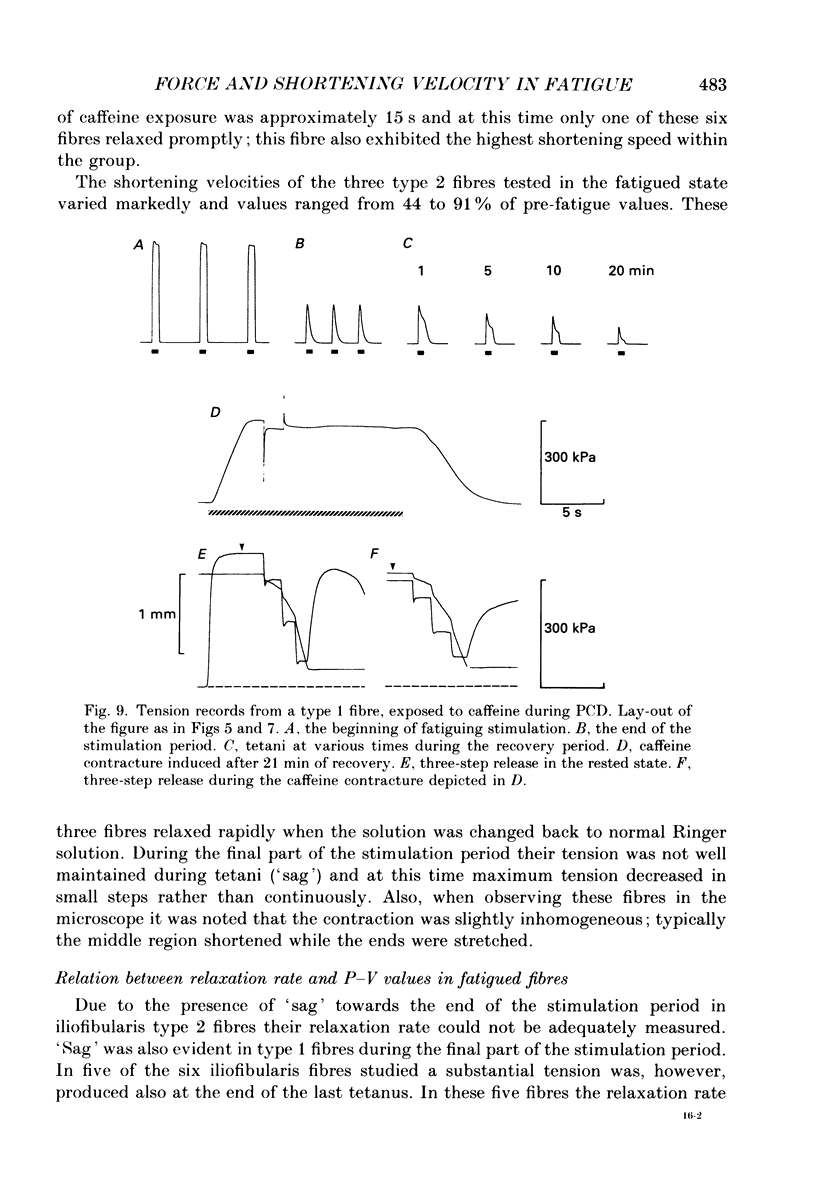
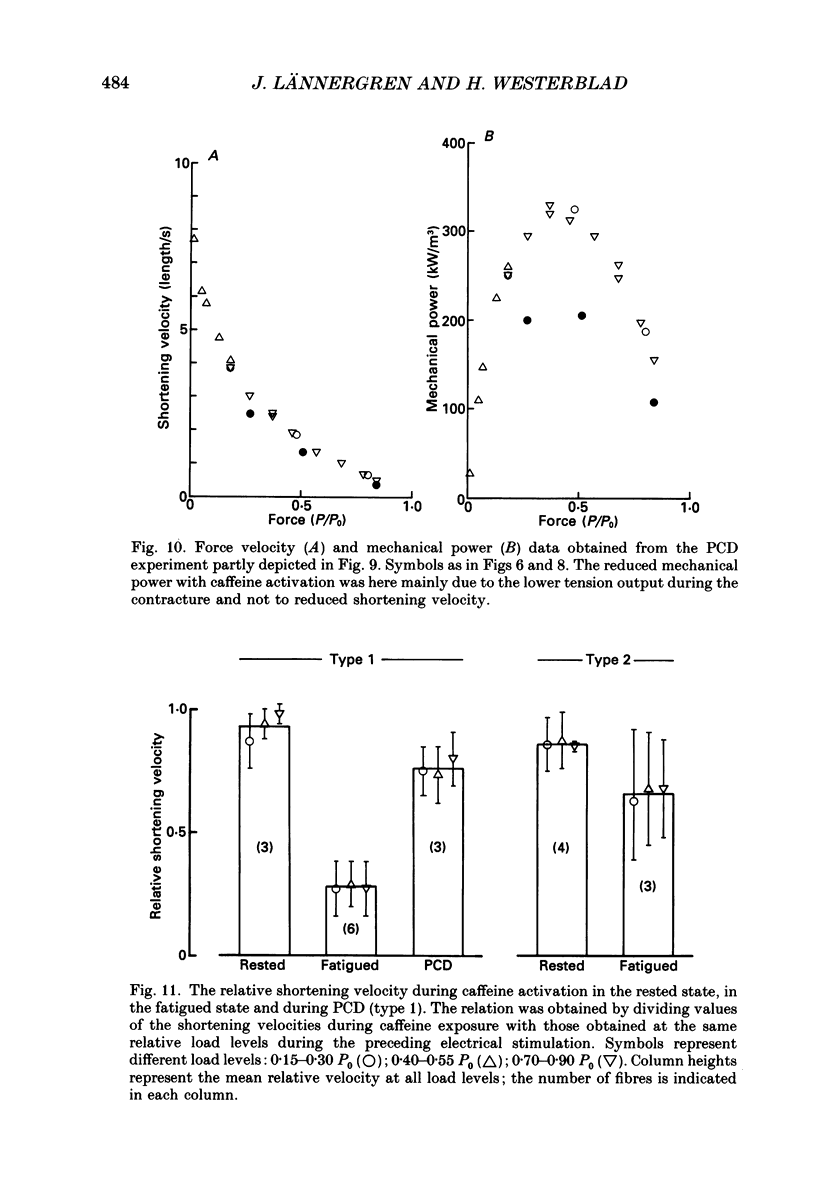





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Bialek W. Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophys J. 1979 Nov;28(2):241–258. doi: 10.1016/S0006-3495(79)85174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K., Luciani G. B., Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988 Jan;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. Biphasic potassium contractures in frog muscle fibers. J Gen Physiol. 1971 Aug;58(2):117–130. doi: 10.1085/jgp.58.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol. 1980 Feb;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Mattiazzi A. R. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil. 1981 Sep;2(3):321–334. doi: 10.1007/BF00713270. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A. Metabolic changes associated with the slowing of relaxation in fatigued mouse muscle. J Physiol. 1975 Oct;251(2):287–301. doi: 10.1113/jphysiol.1975.sp011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H. Human muscle function and fatigue. Ciba Found Symp. 1981;82:1–18. doi: 10.1002/9780470715420.ch1. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski W., Lobsiger E. A., Lüttgau H. C. The effect of repetitive stimulation at low frequencies upon the electrical and mechanical activity of single muscle fibres. Pflugers Arch. 1972;334(3):222–239. doi: 10.1007/BF00626225. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Kanaya H., Takauji M., Nagai T. Properties of caffeine- and potassium-contractures in fatigued frog single twitch muscle fibers. Jpn J Physiol. 1983;33(6):945–954. doi: 10.2170/jjphysiol.33.945. [DOI] [PubMed] [Google Scholar]
- Lännergren J. Contractile properties and myosin isoenzymes of various kinds of Xenopus twitch muscle fibres. J Muscle Res Cell Motil. 1987 Jun;8(3):260–273. doi: 10.1007/BF01574594. [DOI] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. The effect of temperature and stimulation scheme on fatigue and recovery in Xenopus muscle fibres. Acta Physiol Scand. 1988 May;133(1):73–82. doi: 10.1111/j.1748-1716.1988.tb08382.x. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol. 1987 Dec;393:727–742. doi: 10.1113/jphysiol.1987.sp016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Zhu P. H. Extracellular ions and excitation-contraction coupling in frog twitch muscle fibres. J Physiol. 1984 Jun;351:687–710. doi: 10.1113/jphysiol.1984.sp015271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar-Gentina V., Passonneau J. V., Rapoport S. I. Fatigue and metabolism of frog muscle fibers during stimulation and in response to caffeine. Am J Physiol. 1981 Sep;241(3):C160–C166. doi: 10.1152/ajpcell.1981.241.3.C160. [DOI] [PubMed] [Google Scholar]
- Nosek T. M., Fender K. Y., Godt R. E. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science. 1987 Apr 10;236(4798):191–193. doi: 10.1126/science.3563496. [DOI] [PubMed] [Google Scholar]
- Renaud J. M., Allard Y., Mainwood G. W. Is the change in intracellular pH during fatigue large enough to be the main cause of fatigue? Can J Physiol Pharmacol. 1986 Jun;64(6):764–767. doi: 10.1139/y86-130. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Simon B. J., Szucs G. Depletion of calcium from the sarcoplasmic reticulum during calcium release in frog skeletal muscle. J Physiol. 1987 Nov;392:167–192. doi: 10.1113/jphysiol.1987.sp016775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985 Aug 1;316(6027):446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- Stienen G. J., van der Laarse W. J., Elzinga G. Dependency of the force-velocity relationships on Mg ATP in different types of muscle fibers from Xenopus laevis. Biophys J. 1988 Jun;53(6):849–855. doi: 10.1016/S0006-3495(88)83165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. Force and membrane potential during and after fatiguing, intermittent tetanic stimulation of single Xenopus muscle fibres. Acta Physiol Scand. 1986 Nov;128(3):369–378. doi: 10.1111/j.1748-1716.1986.tb07990.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. The relation between force and intracellular pH in fatigued, single Xenopus muscle fibres. Acta Physiol Scand. 1988 May;133(1):83–89. doi: 10.1111/j.1748-1716.1988.tb08383.x. [DOI] [PubMed] [Google Scholar]


