Abstract
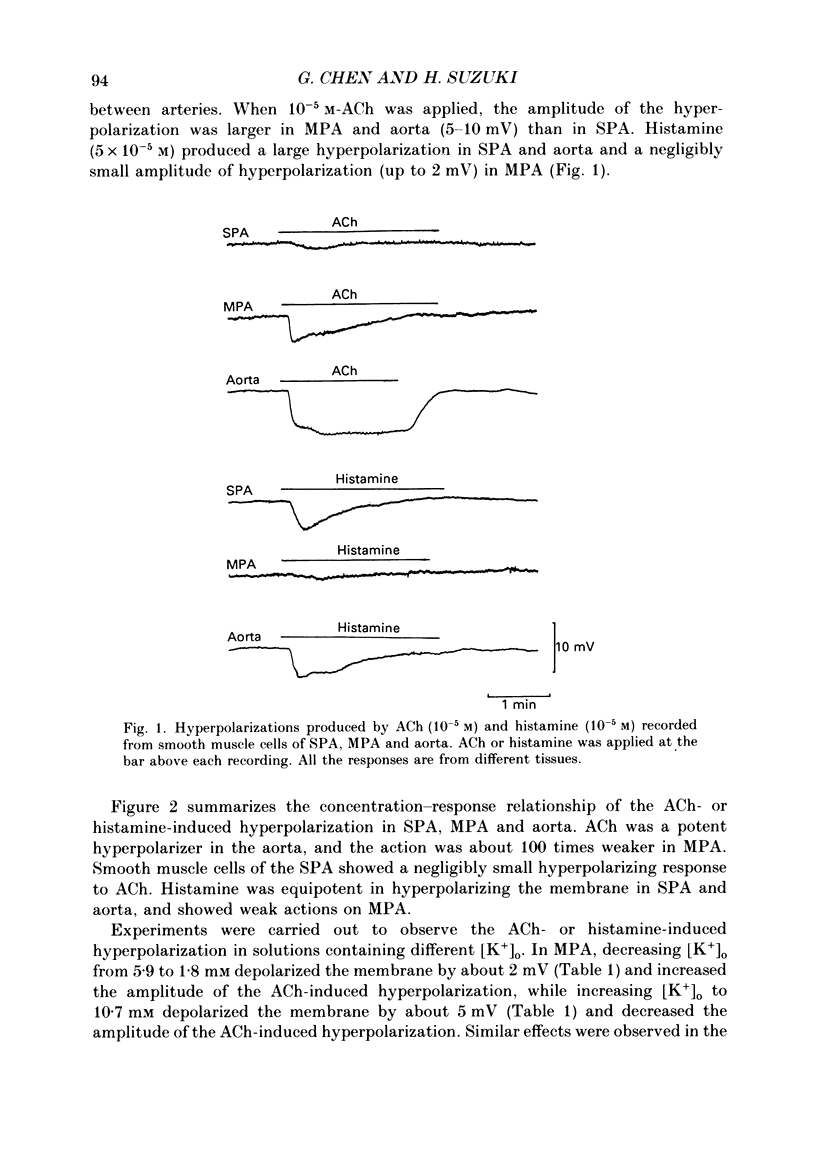
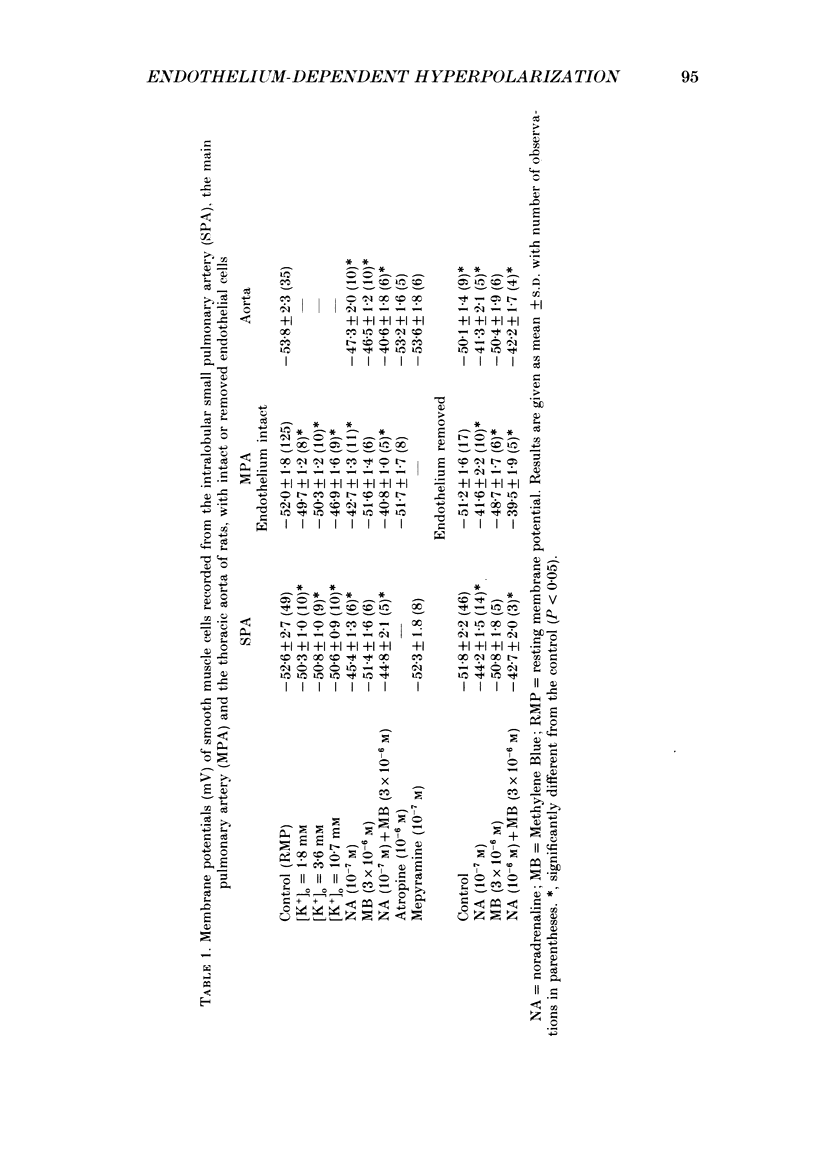
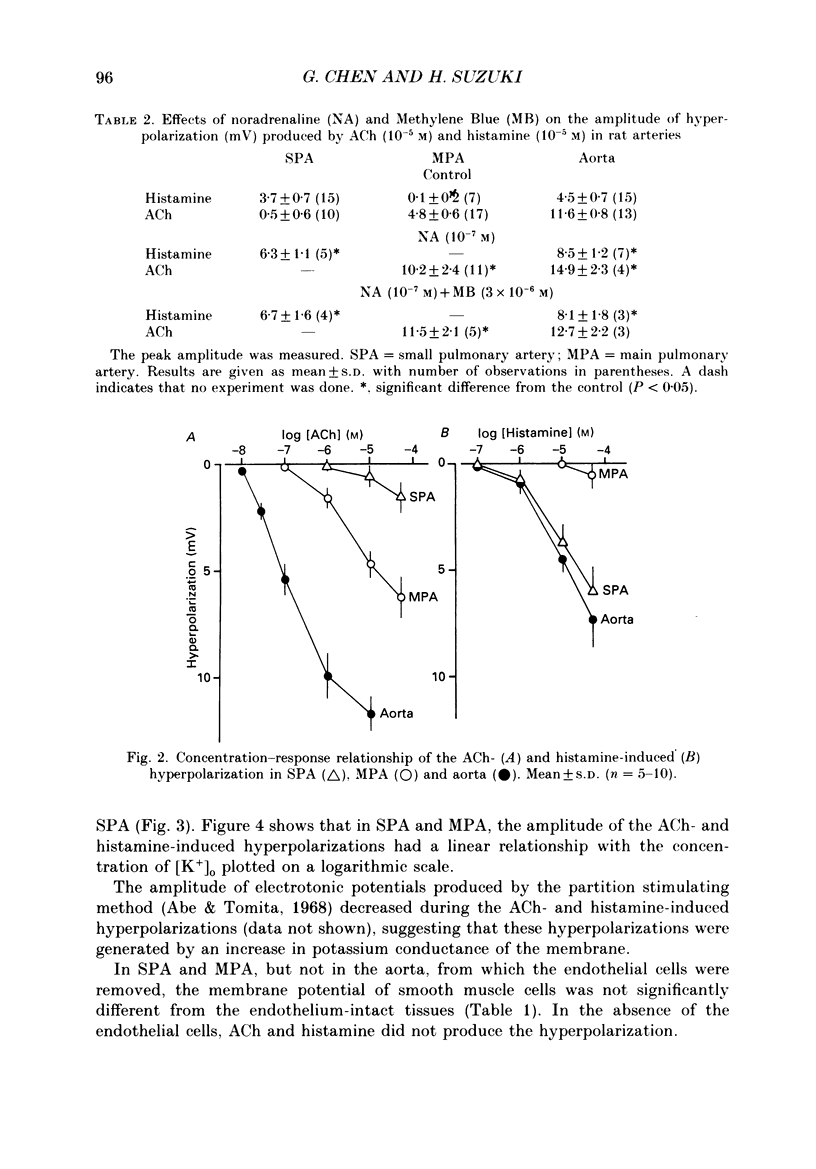
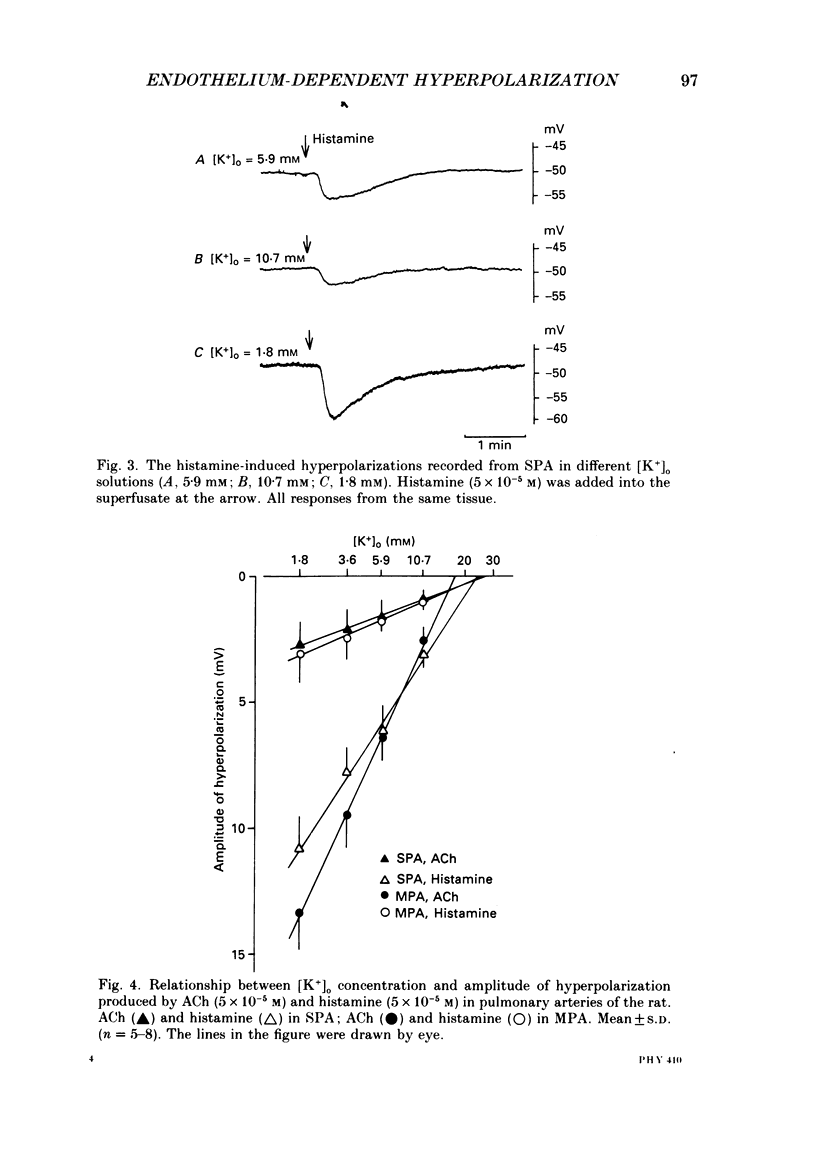
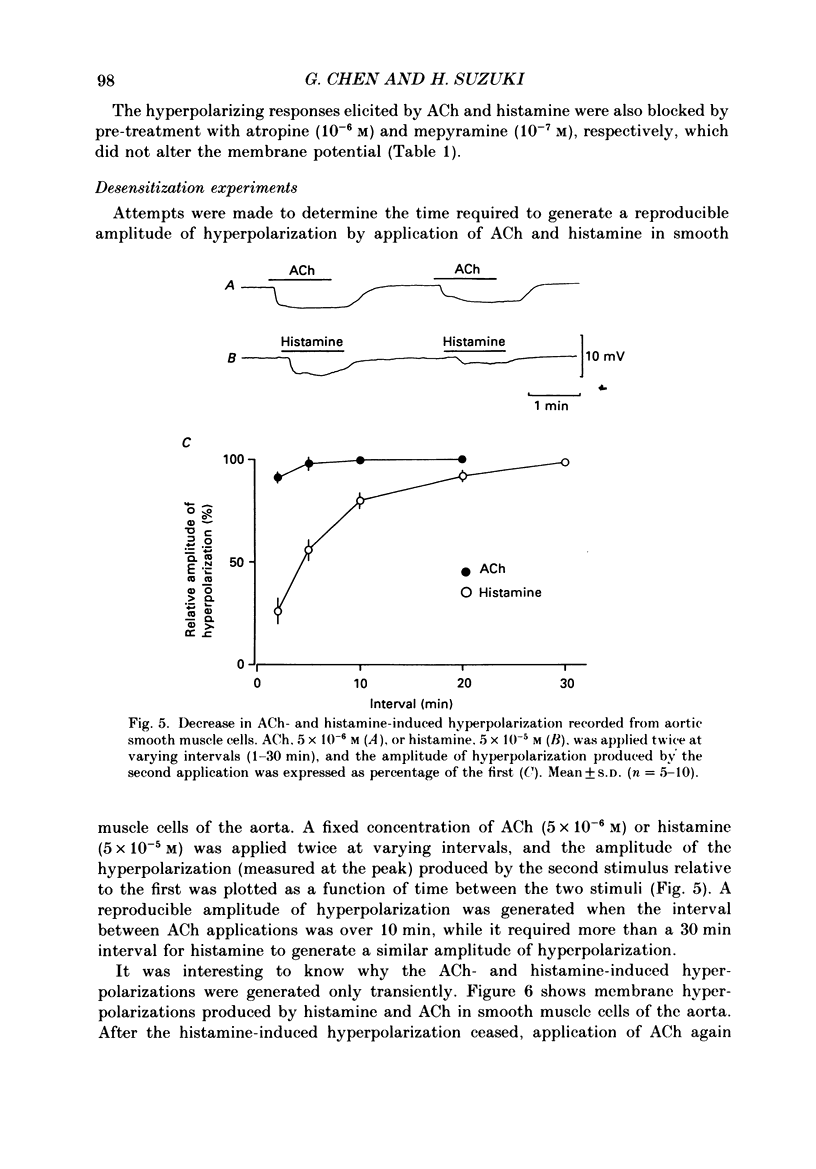
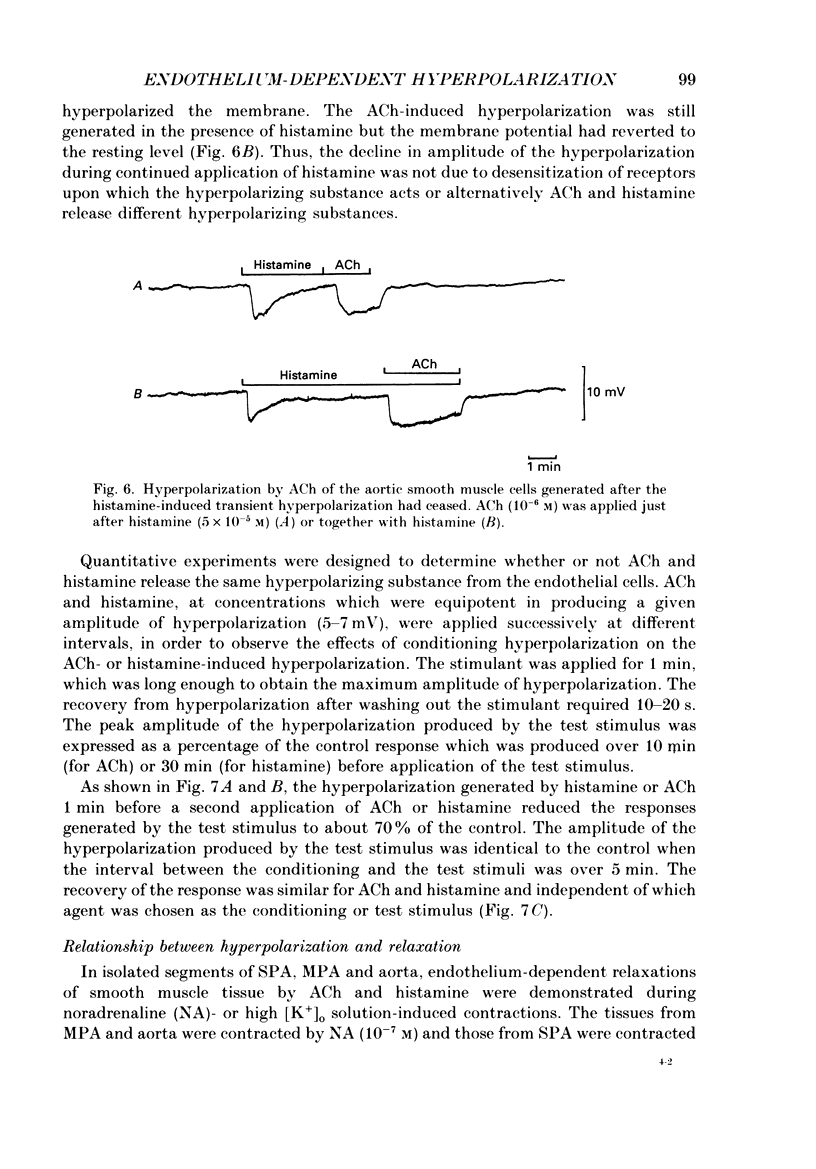
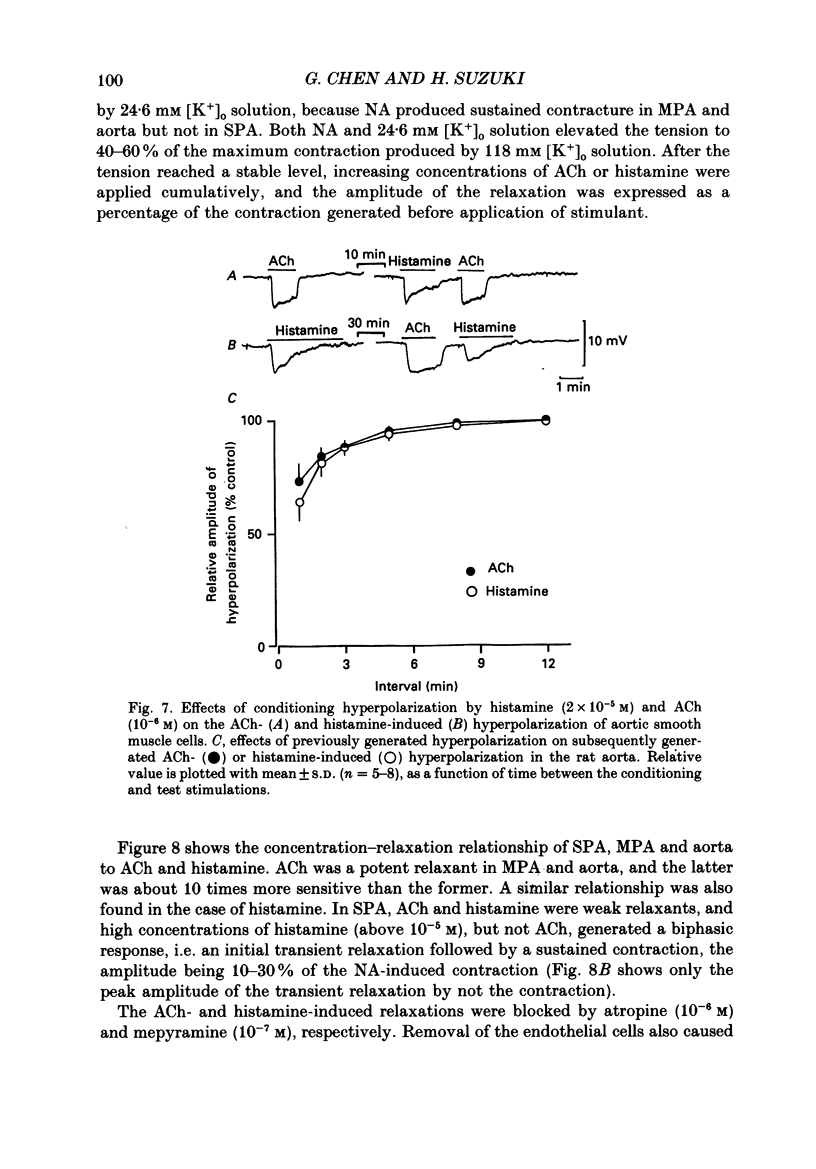
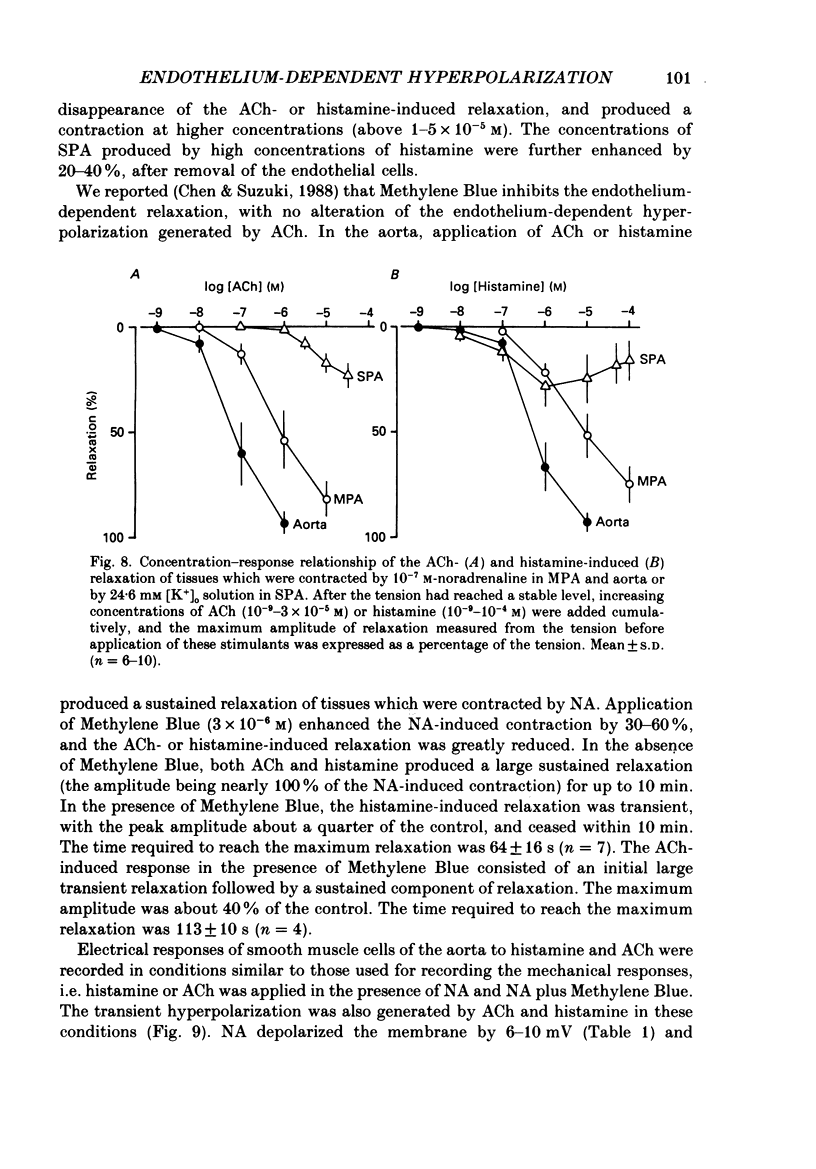
1. Electrical responses produced by acetylcholine (ACh) and histamine were recorded from smooth muscle cells of the intralobular small pulmonary artery (SPA), main pulmonary artery (MPA) and thoracic aorta of rats. 2. In MPA and SPA, ACh and histamine produced a transient hyperpolarization of the membrane, and the potential decayed exponentially with a time constant of 2-3 min. In aorta, ACh produced a sustained and histamine produced a transient hyperpolarization. 3. The ACh- and histamine-induced hyperpolarizations were blocked by atropine and mepyramine, respectively, or by removing the endothelial cells. 4. The amplitude of the hyperpolarization was increased in low [K+]o solutions and decreased in high [K+]o solutions. The ionic conductance of the membrane was increased during the hyperpolarization, suggesting an involvement of the increased potassium conductance. 5. A reproducible amplitude of hyperpolarization was generated when ACh or histamine was applied at intervals of over 10 or 30 min, respectively. 6. In aorta, after the transient hyperpolarization had ceased during continued application of histamine, ACh again produced a hyperpolarization, i.e. the transient nature of the hyperpolarization was not due to desensitization of the receptor upon which the hyperpolarizing substance acted, assuming histamine and ACh release the same hyperpolarizing substance. 7. ACh and histamine relaxed the tissues from SPA, MPA and aorta during the noradrenaline (NA)- or high [K+]o solution-induced contraction, in a concentration-dependent manner, only when the endothelial cells were intact. Both ACh and histamine were potent relaxants in MPA and aorta, but showed weak relaxing actions in SPA. 8. In aorta, ACh and histamine produced a sustained relaxation for up to 10 min, and Methylene Blue diminished and altered it to a transient relaxation (for histamine) or an initial large, followed by a small sustained (for ACh), relaxation. 9. In the presence of NA and NA plus Methylene Blue, ACh and histamine also produced a hyperpolarization similar to that seen in the control. 10. It is concluded that in arteries of the rat, ACh and histamine release a hyperpolarizing substance from the endothelial cells. This substance may be different from the endothelium-derived relaxing factor (EDRF), and is released mainly transiently. The hyperpolarization is generated by an increase in potassium conductance of the membrane, and this has some contribution to the endothelium-dependent relaxation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beny J. L., Brunet P. C., Huggel H. Effect of mechanical stimulation, substance P and vasoactive intestinal polypeptide on the electrical and mechanical activities of circular smooth muscles from pig coronary arteries contracted with acetylcholine: role of endothelium. Pharmacology. 1986;33(2):61–68. doi: 10.1159/000138202. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Clapp L. H. Endothelial-dependent relaxant actions of carbachol and substance P in arterial smooth muscle. Br J Pharmacol. 1986 Apr;87(4):713–723. doi: 10.1111/j.1476-5381.1986.tb14589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E., Large W. A. Electrophysiological analysis of neurogenic vasodilatation in the isolated lingual artery of the rabbit. Br J Pharmacol. 1986 Sep;89(1):163–171. doi: 10.1111/j.1476-5381.1986.tb11132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Abel P. W., Hermsmeyer K. Membrane electrical mechanism of basilar artery constriction and pial artery dilation by norepinephrine. Circ Res. 1981 Dec;49(6):1237–1242. doi: 10.1161/01.res.49.6.1237. [DOI] [PubMed] [Google Scholar]
- Ibengwe J. K., Suzuki H. Protective action of elastase on changes in mechanical properties of vascular smooth muscles during atherosclerogenesis in hypercholesterolemic rabbits. Arch Int Pharmacodyn Ther. 1987 May;287(1):48–64. [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987 Dec;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Electrical responses of smooth muscle cells during cholinergic vasodilation in the rabbit saphenous artery. Circ Res. 1987 Oct;61(4):586–593. doi: 10.1161/01.res.61.4.586. [DOI] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Heterogeneous distribution of muscarinic receptors in the rabbit saphenous artery. Br J Pharmacol. 1987 Nov;92(3):657–664. doi: 10.1111/j.1476-5381.1987.tb11369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. The effects of acetylcholine on the membrane and contractile properties of smooth muscle cells of the rabbit superior mesenteric artery. Br J Pharmacol. 1978 Dec;64(4):493–501. doi: 10.1111/j.1476-5381.1978.tb17310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Nagao T., Suzuki H. Non-neural electrical responses of smooth muscle cells of the rabbit basilar artery to electrical field stimulation. Jpn J Physiol. 1987;37(3):497–513. doi: 10.2170/jjphysiol.37.497. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Prehn J. L., Bevan J. A. Facial vein of the rabbit. Intracellularly recorded hyperpolarization of smooth muscle cells induced by beta-adrenergic receptor stimulation. Circ Res. 1983 Apr;52(4):465–470. doi: 10.1161/01.res.52.4.465. [DOI] [PubMed] [Google Scholar]
- Shikano K., Ohlstein E. H., Berkowitz B. A. Differential selectivity of endothelium-derived relaxing factor and nitric oxide in smooth muscle. Br J Pharmacol. 1987 Nov;92(3):483–485. doi: 10.1111/j.1476-5381.1987.tb11347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Twarog B. M. Membrane properties of smooth muscle cells in pulmonary arteries of the rat. Am J Physiol. 1982 May;242(5):H900–H906. doi: 10.1152/ajpheart.1982.242.5.H900. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Southerton J. S., Weston A. H., Baker J. R. Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br J Pharmacol. 1988 Jul;94(3):853–863. doi: 10.1111/j.1476-5381.1988.tb11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Weir S. W., Weston A. H. The effects of BRL 34915 and nicorandil on electrical and mechanical activity and on 86Rb efflux in rat blood vessels. Br J Pharmacol. 1986 May;88(1):121–128. doi: 10.1111/j.1476-5381.1986.tb09478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


