Abstract
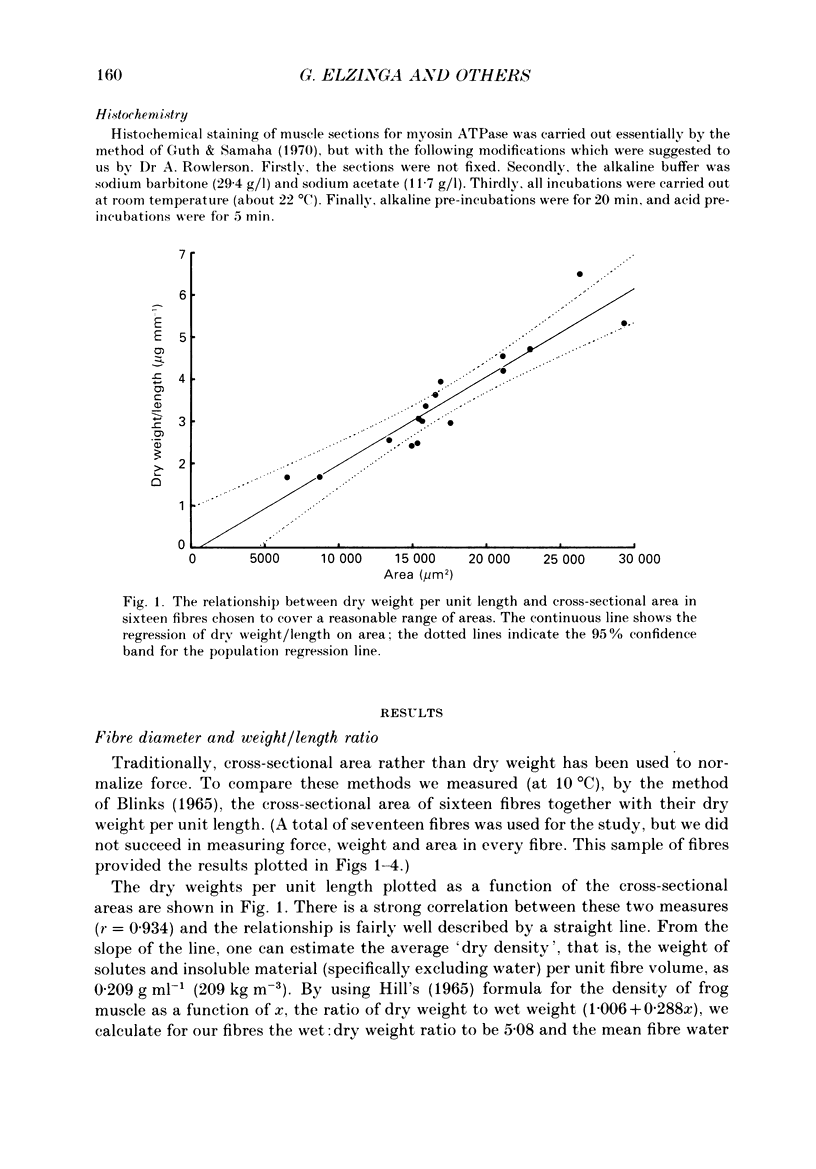
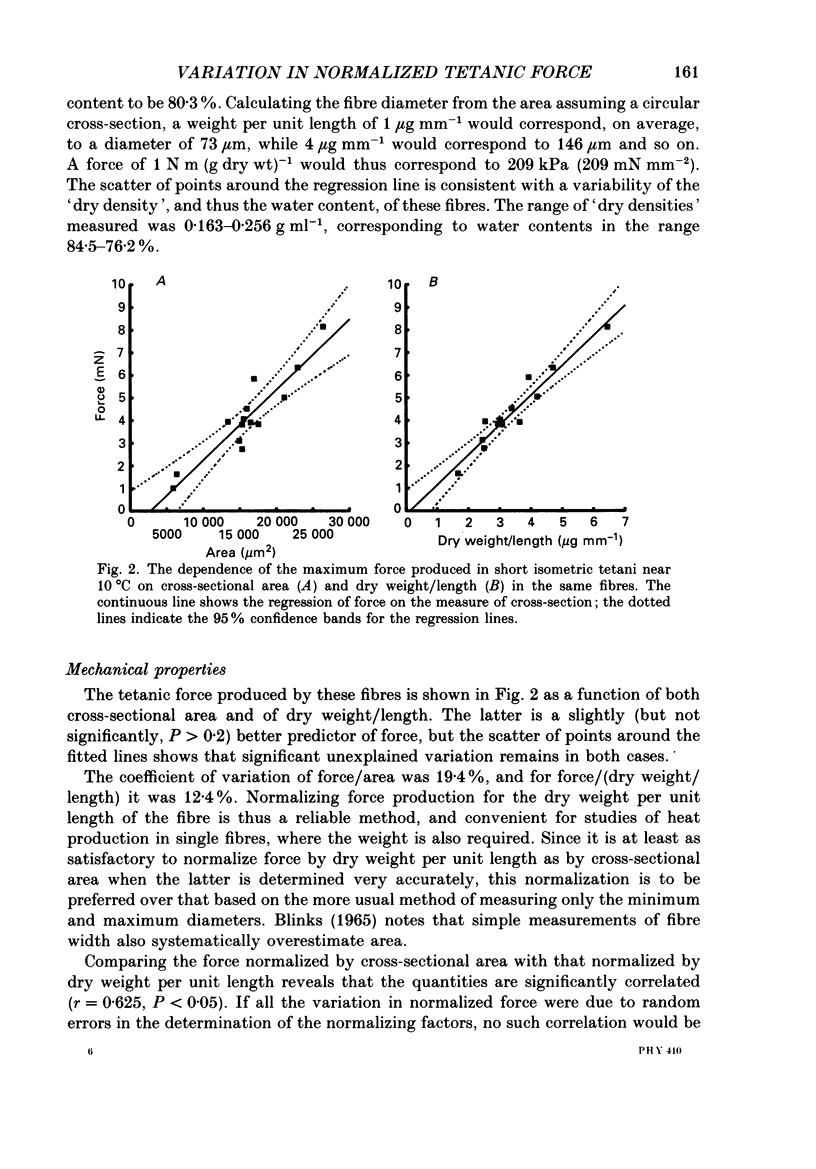
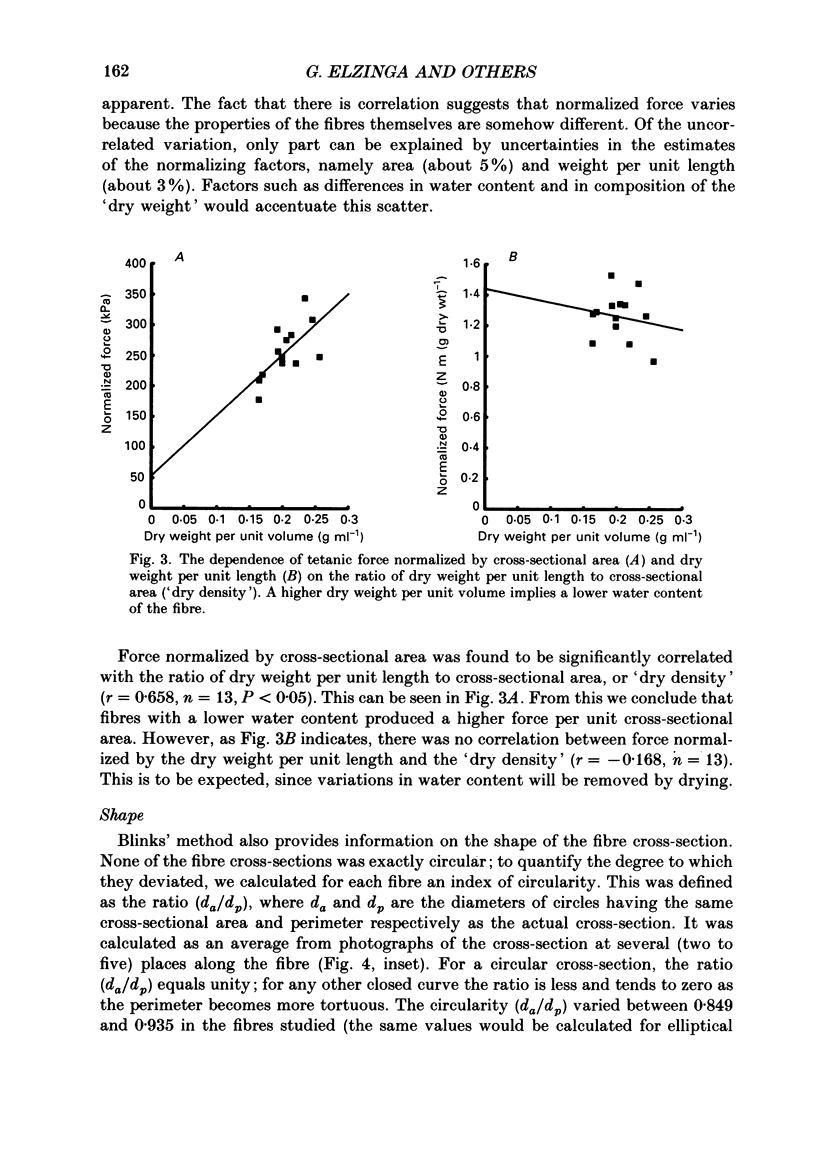
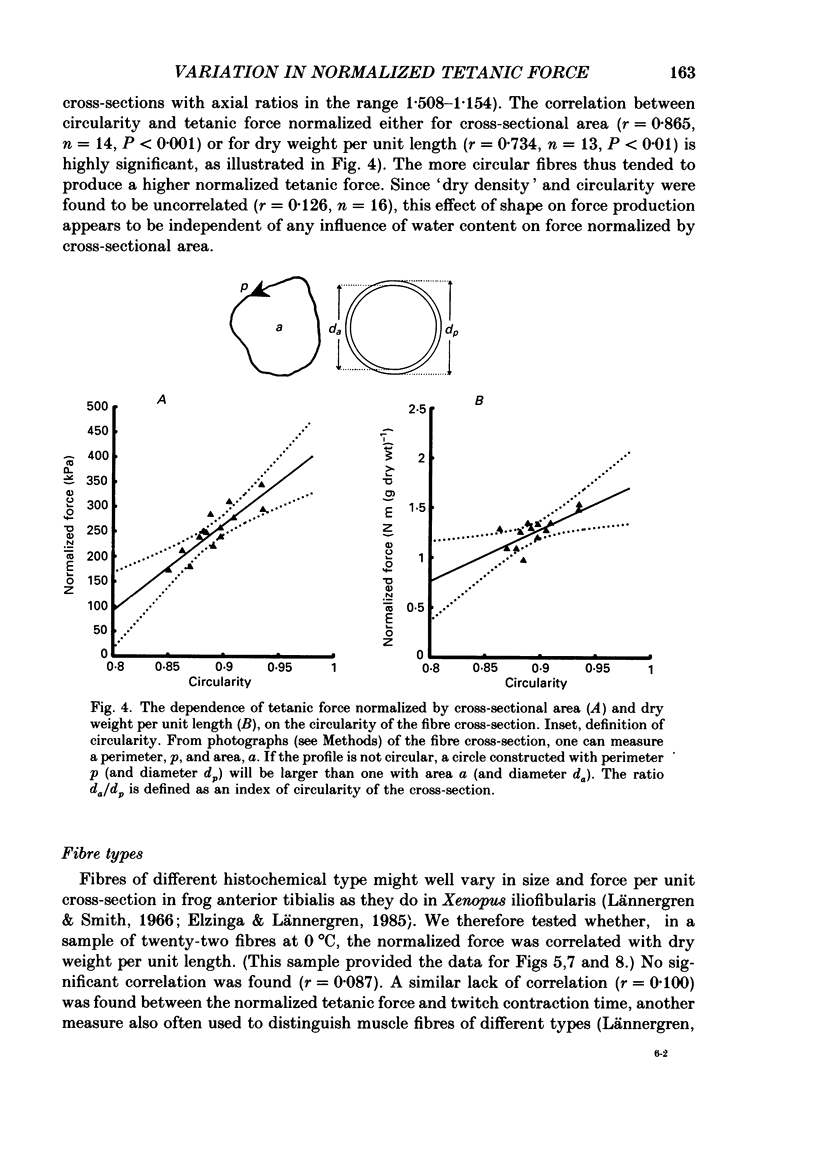
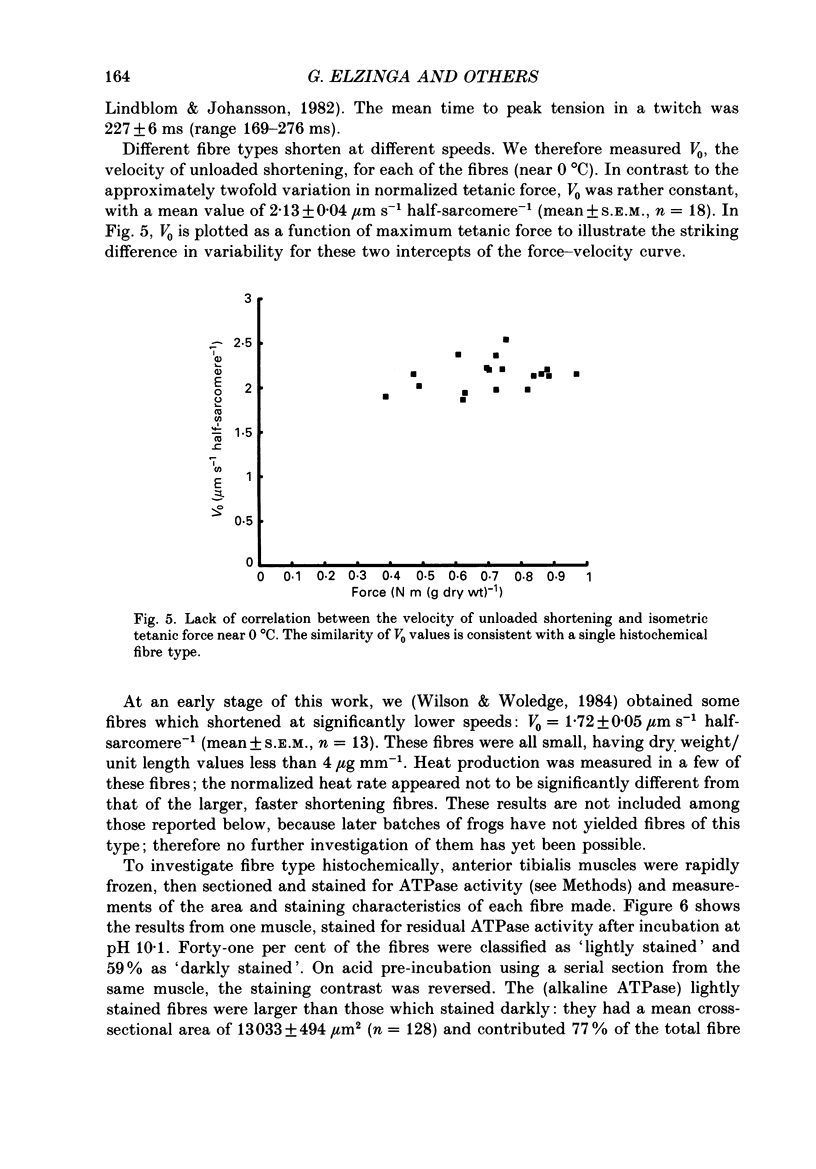
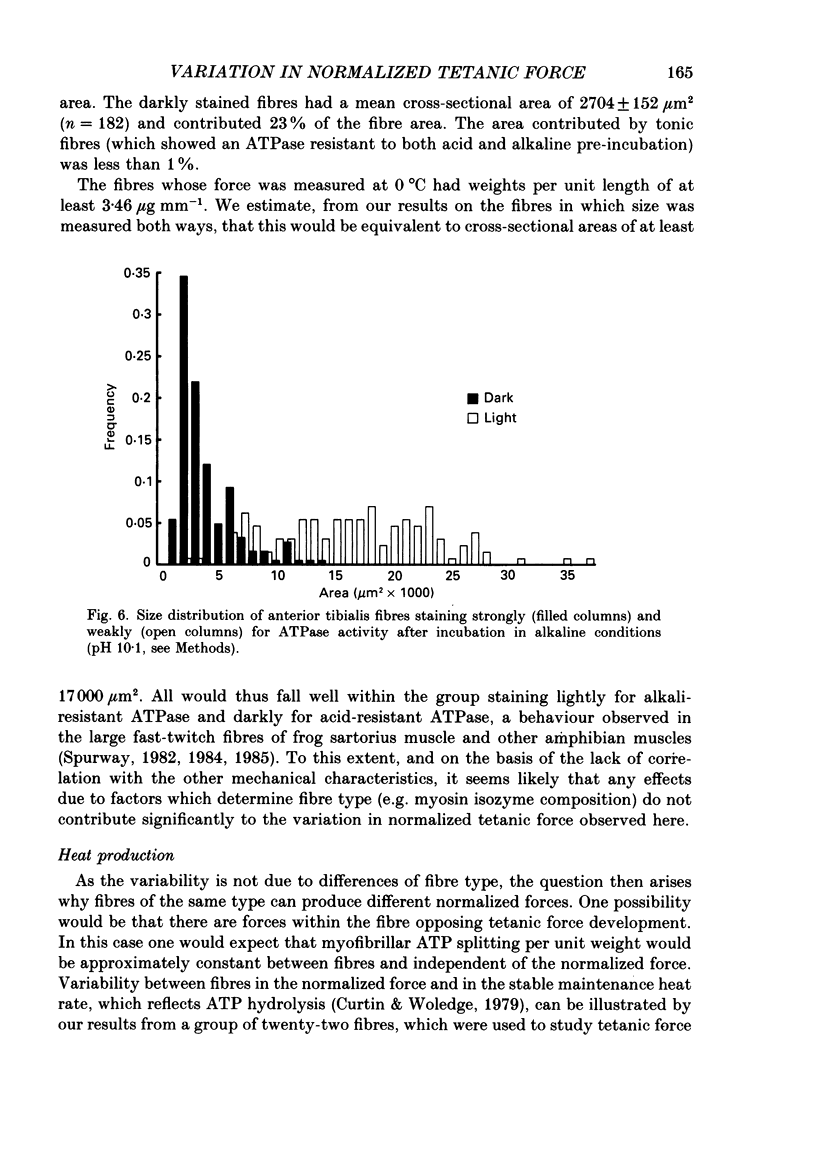
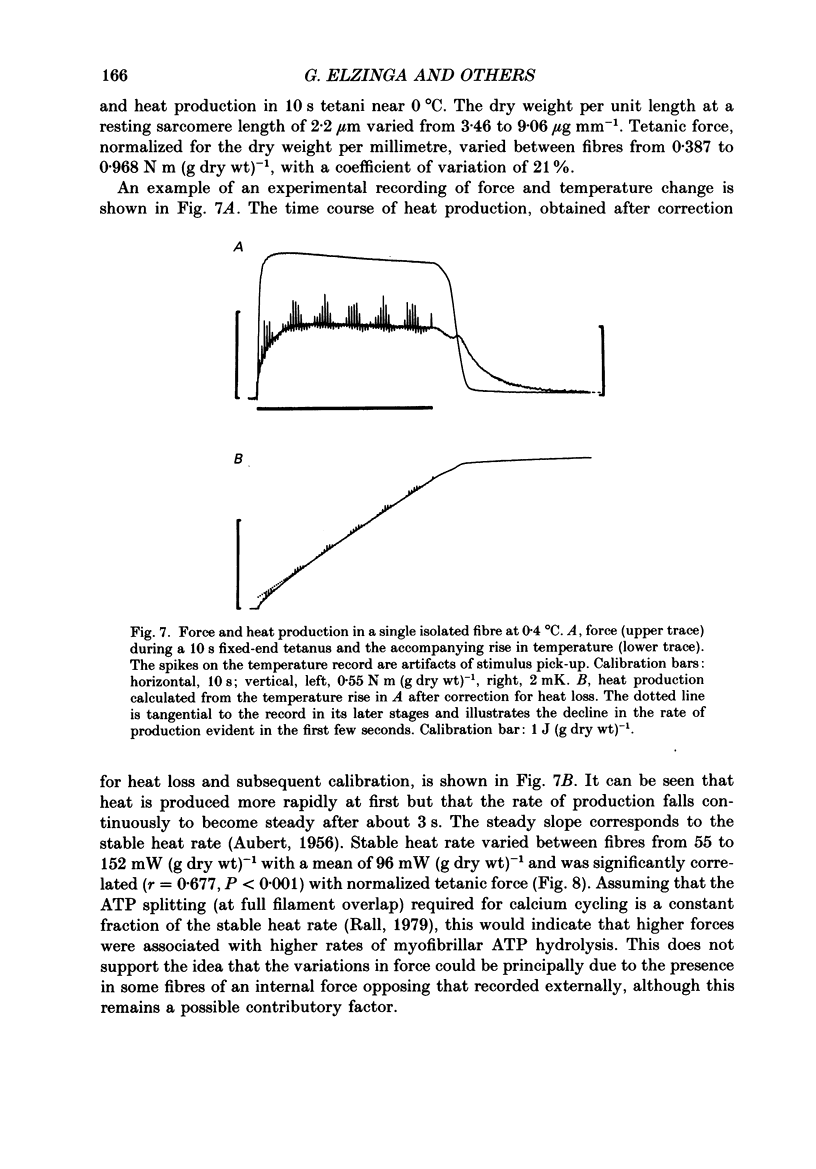
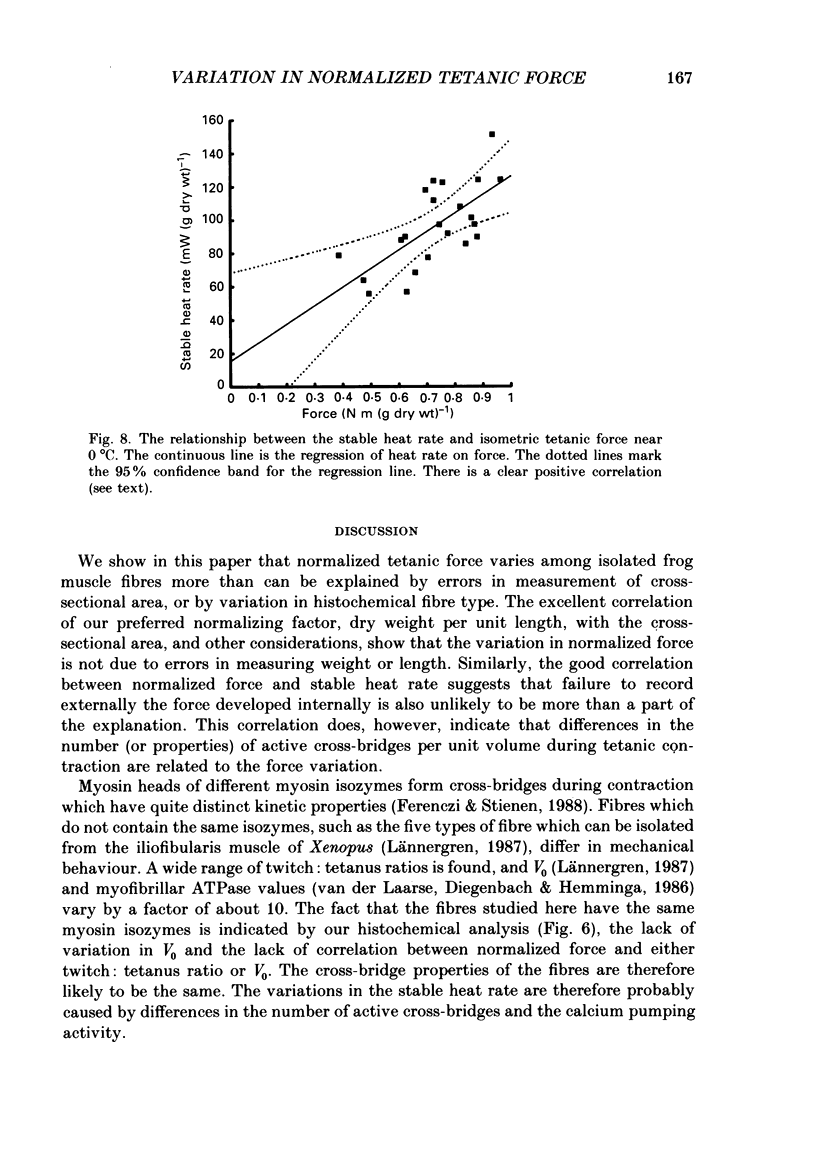
1. The forces produced in maximal fixed-end tetani of single fibres isolated from the anterior tibialis muscle of the frog Rana temporaria have been measured at sarcomere lengths of 2.2 microns and temperatures near 0 and 10 degrees C. 2. When normalized by either cross-sectional area or dry weight per unit length at a sarcomere length of 2.2 microns, the forces vary over a twofold range. 3. The normalized force is not significantly correlated with the velocity of unloaded shortening or the twitch characteristics of the fibres. Lack of variability of these two quantities (together with histochemical evidence) suggest that only one fibre type is present in the experimental sample. 4. The steady rate of energy liberation (stable, heart rate) of the fibres during isometric tetani is positively correlated with the normalized force, indicating that extra ATP splitting is required to produce higher forces. 5. Fibres with a higher ratio of dry weight per unit length to cross-sectional area ('dry density') show a higher force when normalized by area, but not when normalized by dry weight per unit length. 6. Fibres with a more circular cross-sectional profile produce more force when normalized by either cross-sectional area or dry weight per unit length. The significance of this correlation is unclear. 7. The contribution of various sources to the total overall variation in normalized force is assessed. It is suggested that a diffusible substance or substances may be involved in modulating fibre force.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Howarth J. V., Rall J. A., Wilson M. G., Woledge R. C. Absolute values of myothermic measurements on single muscle fibres from frog. J Muscle Res Cell Motil. 1986 Aug;7(4):327–332. doi: 10.1007/BF01753653. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Howarth J. V., Rall J. A., Wilson M. G., Woledge R. C. Simultaneous heat and tension measurements from single muscle cells. Adv Exp Med Biol. 1984;170:887–899. doi: 10.1007/978-1-4684-4703-3_87. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Howarth J. V., Woledge R. C. Heat production by single fibres of frog muscle. J Muscle Res Cell Motil. 1983 Apr;4(2):207–222. doi: 10.1007/BF00712031. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Chemical change and energy production during contraction of frog muscle: how are their time courses related? J Physiol. 1979 Mar;288:353–366. [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga G., Peckham M., Woledge R. C. The sarcomere length dependence of the rate of heat production during isometric tetanic contraction of frog muscles. J Physiol. 1984 Dec;357:495–504. doi: 10.1113/jphysiol.1984.sp015513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Hartmann P. E., Prosser C. G. Acute changes in the composition of milk during the ovulatory menstrual cycle in lactating women. J Physiol. 1982 Mar;324:21–30. doi: 10.1113/jphysiol.1982.sp014098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J. Contractile properties and myosin isoenzymes of various kinds of Xenopus twitch muscle fibres. J Muscle Res Cell Motil. 1987 Jun;8(3):260–273. doi: 10.1007/BF01574594. [DOI] [PubMed] [Google Scholar]
- Lännergren J., Hoh J. F. Myosin isoenzymes in single muscle fibres of Xenopus laevis: analysis of five different functional types. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):401–408. doi: 10.1098/rspb.1984.0072. [DOI] [PubMed] [Google Scholar]
- Lännergren J., Lindblom P., Johansson B. Contractile properties of two varieties of twitch muscle fibres in Xenopus laevis. Acta Physiol Scand. 1982 Apr;114(4):523–535. doi: 10.1111/j.1748-1716.1982.tb07020.x. [DOI] [PubMed] [Google Scholar]
- Rall J. A. Effects of temperature on tension, tension-dependent heat, and activation heat in twitches of frog skeletal muscle. J Physiol. 1979 Jun;291:265–275. doi: 10.1113/jphysiol.1979.sp012811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street S. F. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol. 1983 Mar;114(3):346–364. doi: 10.1002/jcp.1041140314. [DOI] [PubMed] [Google Scholar]
- Winegrad S., Weisberg A. Isozyme specific modification of myosin ATPase by cAMP in rat heart. Circ Res. 1987 Mar;60(3):384–392. doi: 10.1161/01.res.60.3.384. [DOI] [PubMed] [Google Scholar]
- van der Laarse W. J., Diegenbach P. C., Hemminga M. A. Calcium-stimulated myofibrillar ATPase activity correlates with shortening velocity of muscle fibres in Xenopus laevis. Histochem J. 1986 Sep;18(9):487–496. doi: 10.1007/BF01675616. [DOI] [PubMed] [Google Scholar]


