Abstract
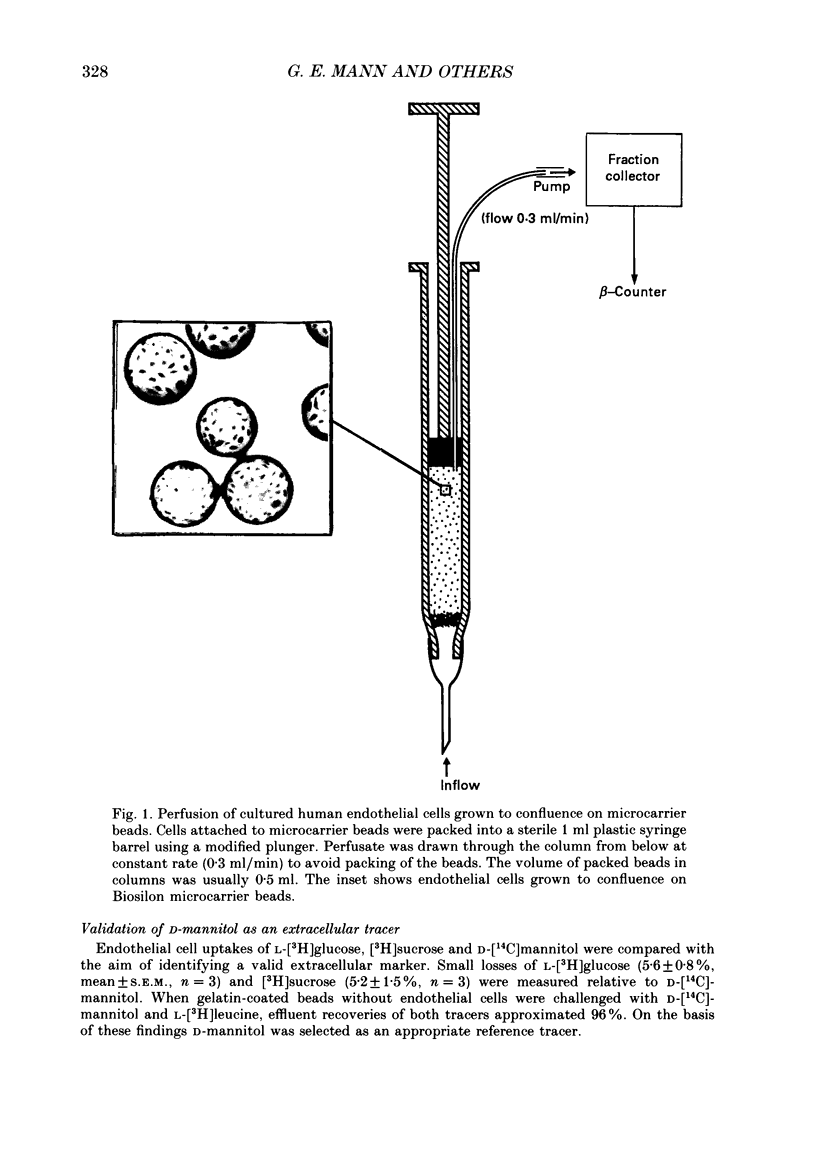
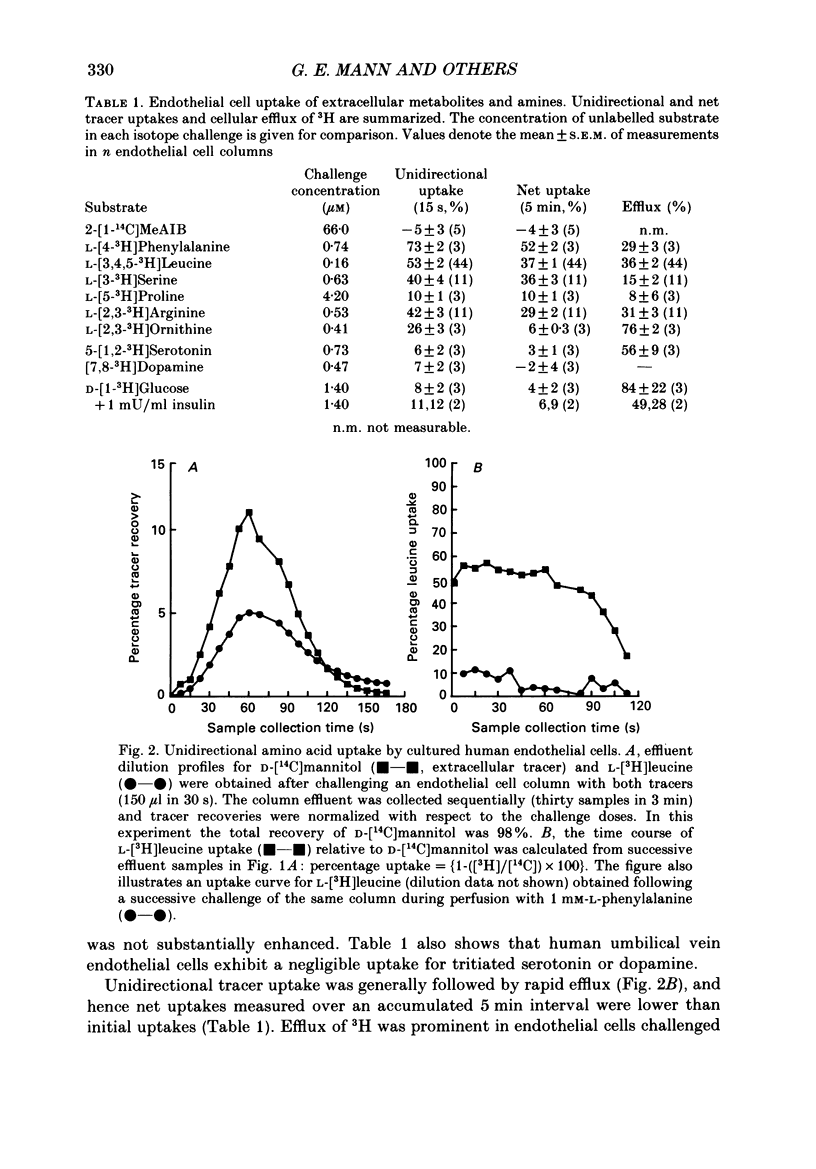
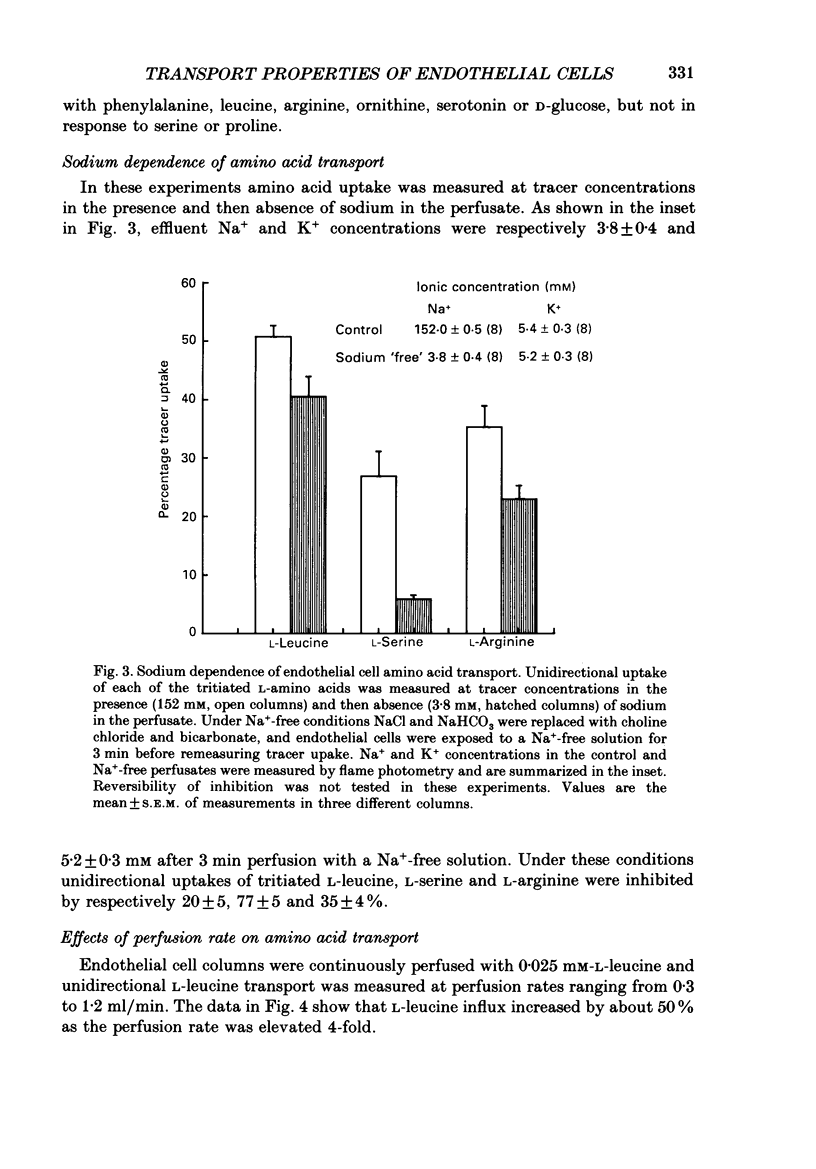
1. Nutrient transport in cultured human umbilical vein endothelial cells was characterized using a rapid dual-isotope dilution technique. Microcarrier beads with confluent endothelial cells were perfused in small columns, and uptake and efflux were assessed relative to D-mannitol (extracellular tracer) during a single transit through the column. 2. At tracer concentrations significant unidirectional uptakes were measured for L-leucine (53 +/- 2%), L-phenylalanine (73 +/- 2%), L-serine (40 +/- 4%), L-arginine (42 +/- 3%) and L-ornithine (26 +/- 3%). Uptake for L-proline, D-glucose, dopamine and serotonin was lower (6-10%), whereas uptake for the system A analogue 2-methylaminoisobutyric acid (2-MeAIB) was negligible. Uptakes rapidly decreased with time due to tracer efflux. 3. Endothelial cell transport of L-leucine was markedly inhibited during perfusion with 1 mM-BCH (beta-2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid, system L analogue), L-leucine, D-leucine, L-phenylalanine, L-methionine and L-DOPA. 2-MeAIB, L-cysteine, glycine, L-proline, hydroxy-L-proline, L-aspartate and beta-alanine were poor inhibitors, while L-serine and the cationic substrates L-lysine and L-arginine inhibited uptake by 10-35%. 4. When the kinetics of L-leucine transport were examined over a wide range of substrate concentrations (0.025-1 mM) transport was saturable. A single entry site analysis gave a half-maximal saturation constant Kt = 0.24 +/- 0.08 mM (mean +/- S.E.M., n = 5) and a Vmax = 27.8 +/- 4.6 nmol/min per column (approximately 3 x 10(6) cells). 5. Removal of sodium from the perfusate inhibited tracer uptake of L-leucine, L-serine and L-arginine by respectively 20 +/- 5% (n = 3), 77 +/- 5% (n = 3) and 35 +/- 4% (n = 3). 6. Our results provide the first evidence that cultured human endothelial cells of venous origin express a saturable transport system for large neutral amino acids resembling system L described in brain microvascular endothelium. Detection of Na+-dependent and Na+-independent L-arginine uptake is of interest in view of recent reports that this cationic amino acid may be the physiological precursor for nitric oxide released by endothelium.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. A., Gerritsen M. E. Regulation of hexose transport in cultured bovine retinal microvessel endothelium by insulin. Exp Eye Res. 1986 Oct;43(4):679–686. doi: 10.1016/s0014-4835(86)80034-3. [DOI] [PubMed] [Google Scholar]
- Bassingthwaighte J. B., Sparks H. V. Indicator dilution estimation of capillary endothelial transport. Annu Rev Physiol. 1986;48:321–334. doi: 10.1146/annurev.ph.48.030186.001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A. L., Gilboe D. D., Drewes L. R. Kinetics of unidirectional leucine transport into brain: effects of isoleucine, valine, and anoxia. Am J Physiol. 1975 Mar;228(3):895–900. doi: 10.1152/ajplegacy.1975.228.3.895. [DOI] [PubMed] [Google Scholar]
- Betz A. L., Gilboe D. D., Yudilevich D. L., Drewes L. R. Kinetics of unidirectional glucose transport into the isolated dog brain. Am J Physiol. 1973 Sep;225(3):586–592. doi: 10.1152/ajplegacy.1973.225.3.586. [DOI] [PubMed] [Google Scholar]
- Betz A. L., Goldstein G. W. Specialized properties and solute transport in brain capillaries. Annu Rev Physiol. 1986;48:241–250. doi: 10.1146/annurev.ph.48.030186.001325. [DOI] [PubMed] [Google Scholar]
- Cancilla P. A., DeBault L. E. Neutral amino acid transport properties of cerebral endothelial cells in vitro. J Neuropathol Exp Neurol. 1983 Mar;42(2):191–199. doi: 10.1097/00005072-198303000-00008. [DOI] [PubMed] [Google Scholar]
- Cardelli-Cangiano P., Fiori A., Cangiano C., Barberini F., Allegra P., Peresempio V., Strom R. Isolated brain microvessels as in vitro equivalents of the blood-brain barrier: selective removal by collagenase of the A-system of neutral amino acid transport. J Neurochem. 1987 Dec;49(6):1667–1675. doi: 10.1111/j.1471-4159.1987.tb02424.x. [DOI] [PubMed] [Google Scholar]
- Corkey R. F., Corkey B. E., Gimbrone M. A., Jr Hexose transport in normal and SV40-transformed human endothelial cells in culture. J Cell Physiol. 1981 Mar;106(3):425–434. doi: 10.1002/jcp.1041060312. [DOI] [PubMed] [Google Scholar]
- Fincham D. A., Mason D. K., Paterson J. Y., Young J. D. Heterogeneity of amino acid transport in horse erythrocytes: a detailed kinetic analysis of inherited transport variation. J Physiol. 1987 Aug;389:385–409. doi: 10.1113/jphysiol.1987.sp016662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. L., Atkins G. L. Kinetic analysis of transport processes in the intestine and other tissues. Clin Sci (Lond) 1982 Nov;63(5):405–414. doi: 10.1042/cs0630405. [DOI] [PubMed] [Google Scholar]
- Gerritsen M. E., Burke T. M., Allen L. A. Glucose starvation is required for insulin stimulation of glucose uptake and metabolism in cultured microvascular endothelial cells. Microvasc Res. 1988 Mar;35(2):153–166. doi: 10.1016/0026-2862(88)90059-3. [DOI] [PubMed] [Google Scholar]
- Gerritsen M. E., Cheli C. D. Arachidonic acid and prostaglandin endoperoxide metabolism in isolated rabbit and coronary microvessels and isolated and cultivated coronary microvessel endothelial cells. J Clin Invest. 1983 Nov;72(5):1658–1671. doi: 10.1172/JCI111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis C. N. Pharmacological aspects of metabolic processes in the pulmonary microcirculation. Annu Rev Pharmacol Toxicol. 1986;26:183–200. doi: 10.1146/annurev.pa.26.040186.001151. [DOI] [PubMed] [Google Scholar]
- Gordon E. L., Pearson J. D., Slakey L. L. The hydrolysis of extracellular adenine nucleotides by cultured endothelial cells from pig aorta. Feed-forward inhibition of adenosine production at the cell surface. J Biol Chem. 1986 Nov 25;261(33):15496–15507. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. L., Buzney S. M., Kahn C. R., Hetu N., Buchwald S., Macdonald S. G., Rand L. I. Differential responsiveness to insulin of endothelial and support cells from micro- and macrovessels. J Clin Invest. 1983 Apr;71(4):974–979. doi: 10.1172/JCI110852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann G. E., Peran S. Basolateral amino acid transport systems in the perfused exocrine pancreas: sodium-dependency and kinetic interactions between influx and efflux mechanisms. Biochim Biophys Acta. 1986 Jun 26;858(2):263–274. doi: 10.1016/0005-2736(86)90331-7. [DOI] [PubMed] [Google Scholar]
- Mann G. E., Yudilevich D. L. Discrimination of parallel neutral amino acid transport systems in the basolateral membrane of cat salivary epithelium. J Physiol. 1984 Feb;347:111–127. doi: 10.1113/jphysiol.1984.sp015056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham L., Cusack N. J., Pearson J. D., Gordon J. L. Characteristics of the P2 purinoceptor that mediates prostacyclin production by pig aortic endothelial cells. Eur J Pharmacol. 1987 Feb 10;134(2):199–209. doi: 10.1016/0014-2999(87)90166-x. [DOI] [PubMed] [Google Scholar]
- Norman P. S., Mann G. E. Ionic dependence of amino-acid transport in the exocrine pancreatic epithelium: calcium dependence of insulin action. J Membr Biol. 1987;96(2):153–163. doi: 10.1007/BF01869241. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983 Oct;63(4):1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Peran S., McGee M. P. Unidirectional flux of phenylalanine into Vero cells. Measurement using paired tracers in perfused cultures. Biochim Biophys Acta. 1986 Apr 14;856(2):231–236. doi: 10.1016/0005-2736(86)90032-5. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Klein M. M., Niroomand F., Böhme E. Is arginine a physiological precursor of endothelium-derived nitric oxide? Eur J Pharmacol. 1988 Mar 29;148(2):293–295. doi: 10.1016/0014-2999(88)90578-x. [DOI] [PubMed] [Google Scholar]
- Shepro D., Dunham B. Endothelial cell metabolism of biogenic amines. Annu Rev Physiol. 1986;48:335–345. doi: 10.1146/annurev.ph.48.030186.002003. [DOI] [PubMed] [Google Scholar]
- Smith Q. R., Momma S., Aoyagi M., Rapoport S. I. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1987 Nov;49(5):1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Strum J. M., Junod A. F. Radioautographic demonstration of 5-hydroxytryptamine- 3 H uptake by pulmonary endothelial cells. J Cell Biol. 1972 Sep;54(3):456–467. doi: 10.1083/jcb.54.3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrota A., Girault M., Pocidalo J. J., Yudilevich D. L. Endothelial uptake of amino acids, sugars, lipids, and prostaglandins in rat lung. Am J Physiol. 1982 Jul;243(1):C20–C26. doi: 10.1152/ajpcell.1982.243.1.C20. [DOI] [PubMed] [Google Scholar]
- Vinters H. V., Beck D. W., Bready J. V., Maxwell K., Berliner J. A., Hart M. N., Cancilla P. A. Uptake of glucose analogues into cultured cerebral microvessel endothelium. J Neuropathol Exp Neurol. 1985 Sep;44(5):445–458. doi: 10.1097/00005072-198509000-00001. [DOI] [PubMed] [Google Scholar]
- Wade L. A., Katzman R. Synthetic amino acids and the nature of L-DOPA transport at the blood-brain barrier. J Neurochem. 1975 Dec;25(6):837–842. doi: 10.1111/j.1471-4159.1975.tb04415.x. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Mann G. E. Unidirectional uptake of substrates at the blood side of secretory epithelia: stomach, salivary gland, pancreas. Fed Proc. 1982 Dec;41(14):3045–3053. [PubMed] [Google Scholar]
- Zetter B. R. Migration of capillary endothelial cells is stimulated by tumour-derived factors. Nature. 1980 May 1;285(5759):41–43. doi: 10.1038/285041a0. [DOI] [PubMed] [Google Scholar]