Abstract
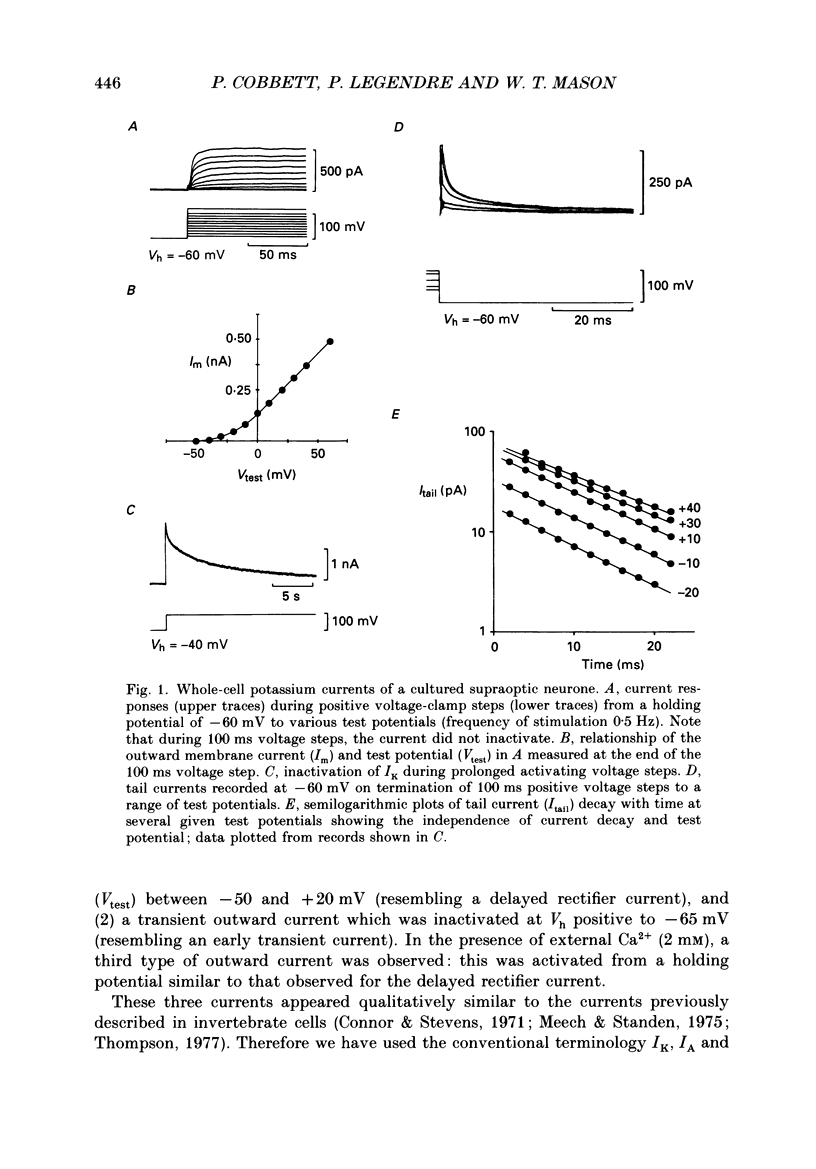
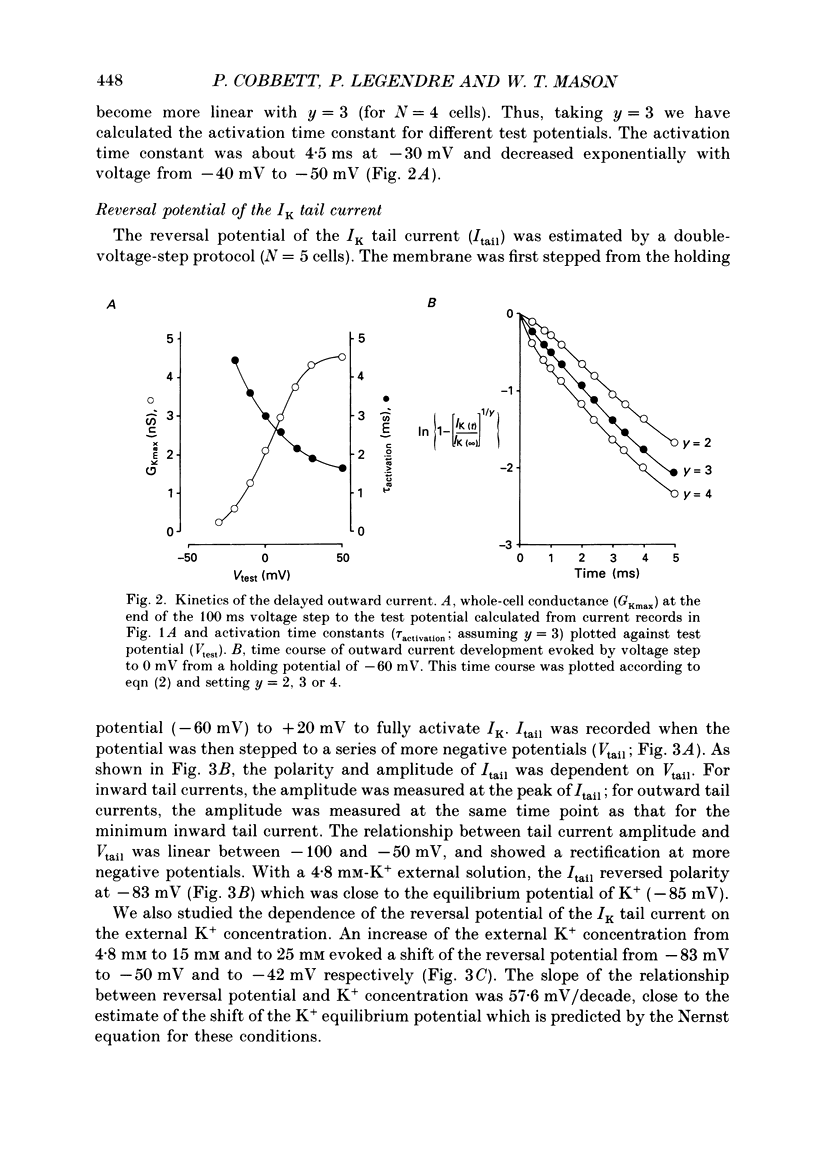
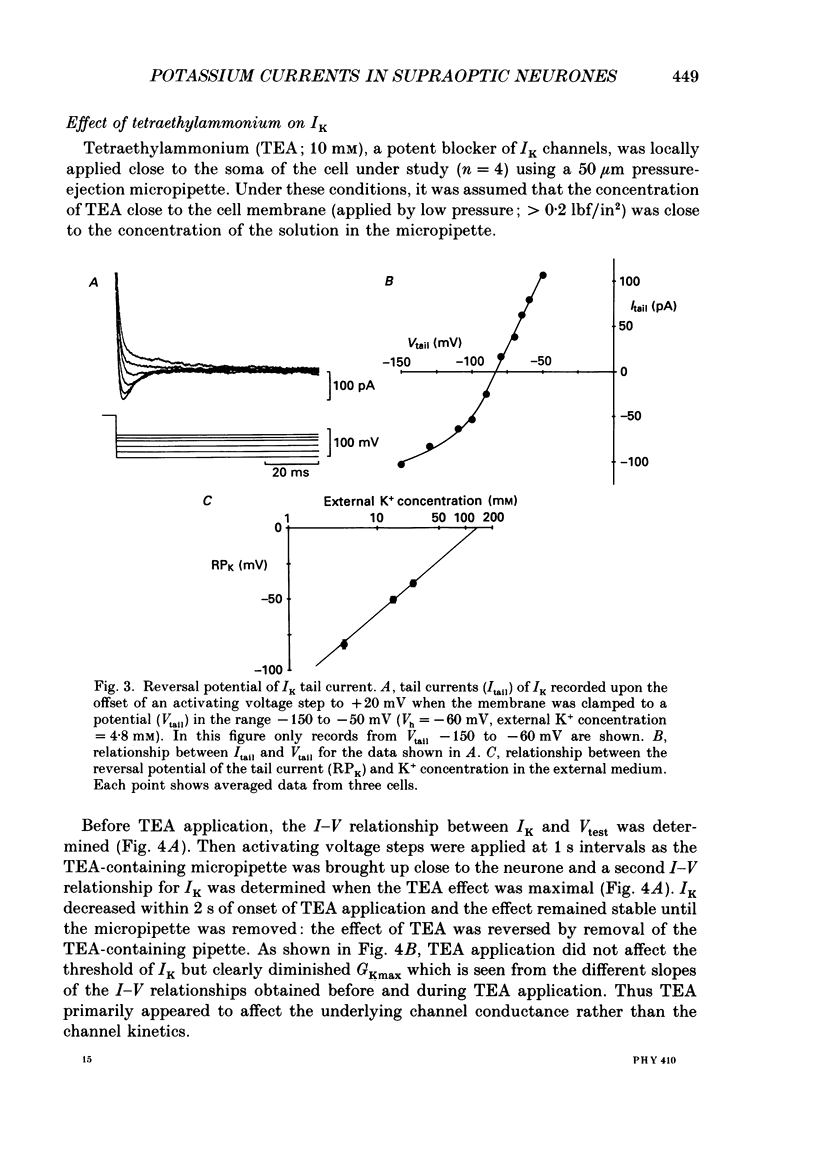
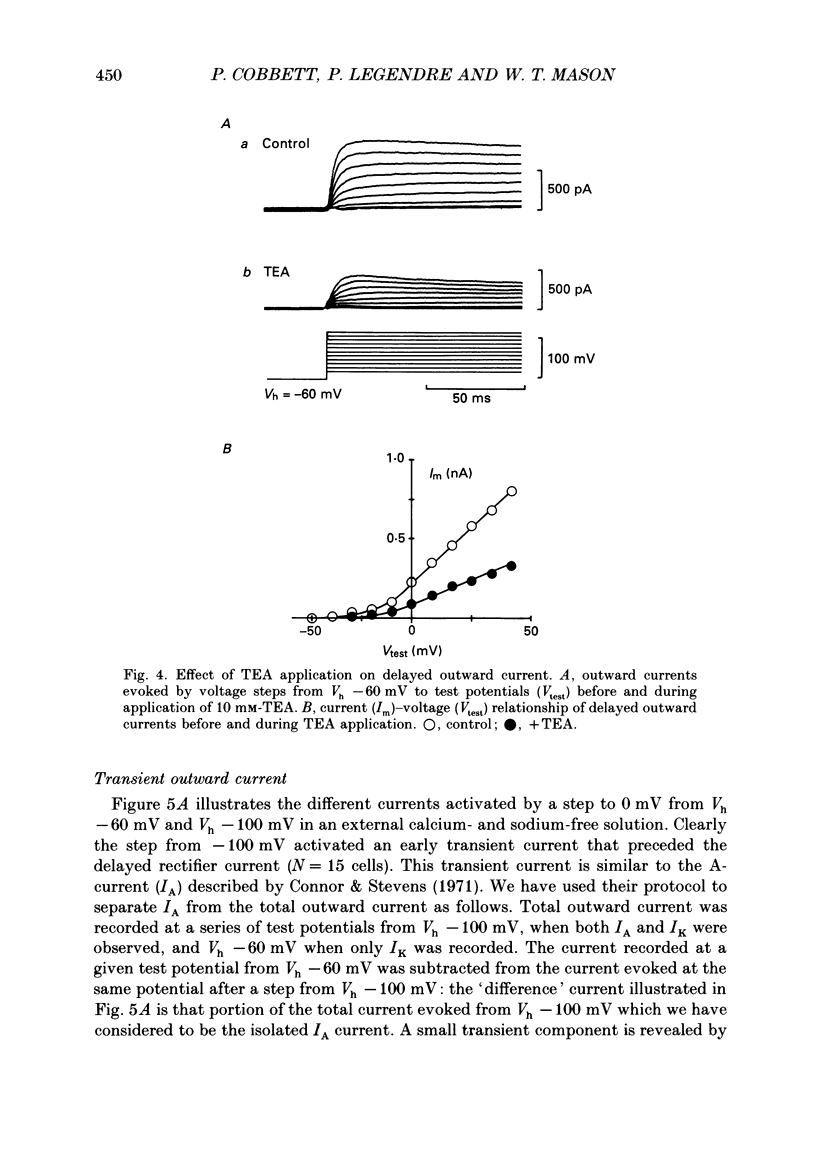
1. Whole-cell, voltage-clamp recordings were obtained from neurones of the supraoptic area of neonatal rats in dissociated cell culture. Recordings were made from neurones having the same morphology as those which were vasopressin or oxytocin immunoreactive. 2. Three types of voltage-activated K+ current were identified on the basis of their kinetics, voltage sensitivities, Ca2+ dependence and pharmacology. The currents corresponded to the delayed rectifier current (IK), the A-current (IA), and the Ca2+-dependent current (IK(Ca] described in other neurones. 3. IK had a threshold of -40 mV, a sigmoidal time course of activation, and was sustained during voltage steps lasting less than 300 ms. The underlying conductance was voltage dependent reaching a maximum at +30 mV (mean maximum conductance 4.09 nS). The activation time constant was also voltage dependent declining exponentially from 4.5 ms at -30 mV to 1.8 ms at +50 mV. 4. IA was transient, and was activated from holding potentials negative to -70 mV; the maximum conductance (mean 5.9 nS) underlying the current was obtained at +10 mV. The activation and inactivation time constants were voltage dependent: the activation time constant declined exponentially between -40 mV (2.2 ms) and +40 mV (0.65 ms). 5. IK and IA were attenuated by the K+ channel blockers tetraethylammonium (TEA) and 4-aminopyridine (4-AP). TEA blocked the conductance underlying IK but appeared to alter the kinetics of IA. In contrast, 4-AP blocked the conductance underlying IA and, to a lesser extent, IK. 6. IK and IA were activated independently of external Ca2+ and the voltage activation of Ca2+ channels since these currents were recorded in the presence of Co2+, a Ca2+ channel blocker. 7. IK(Ca) was recorded only when Ca2+ (2 mM) was present in the external medium. From a holding potential of -30 mV, IK(Ca) had a threshold of -20 mV, was maximal at about +20 mV and declined at more positive potentials. This current was sustained during voltage steps lasting 100 ms and was abolished by addition of Co2+ (2 mM) to the medium. 8. The possible roles of the three K+ currents in regulating the characteristic firing behaviour of supraoptic neurones previously recorded in vivo and in vitro are discussed.
Full text
PDF



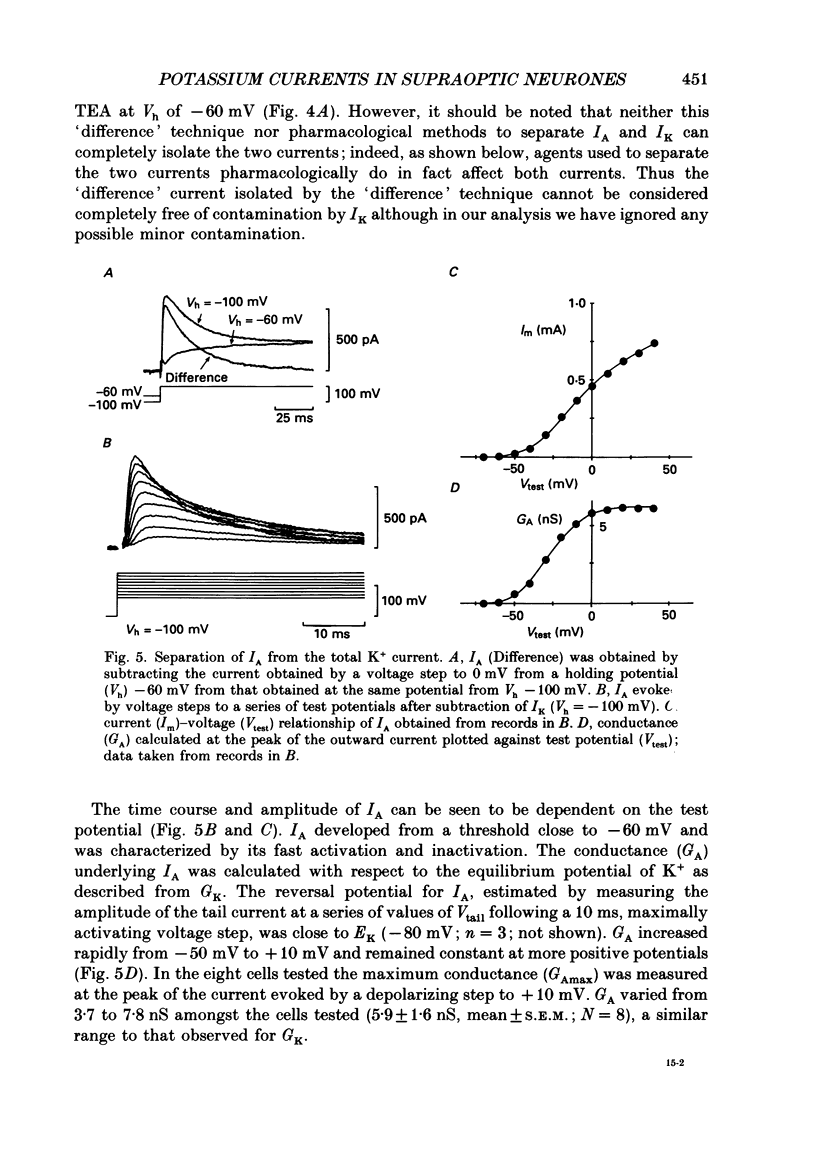

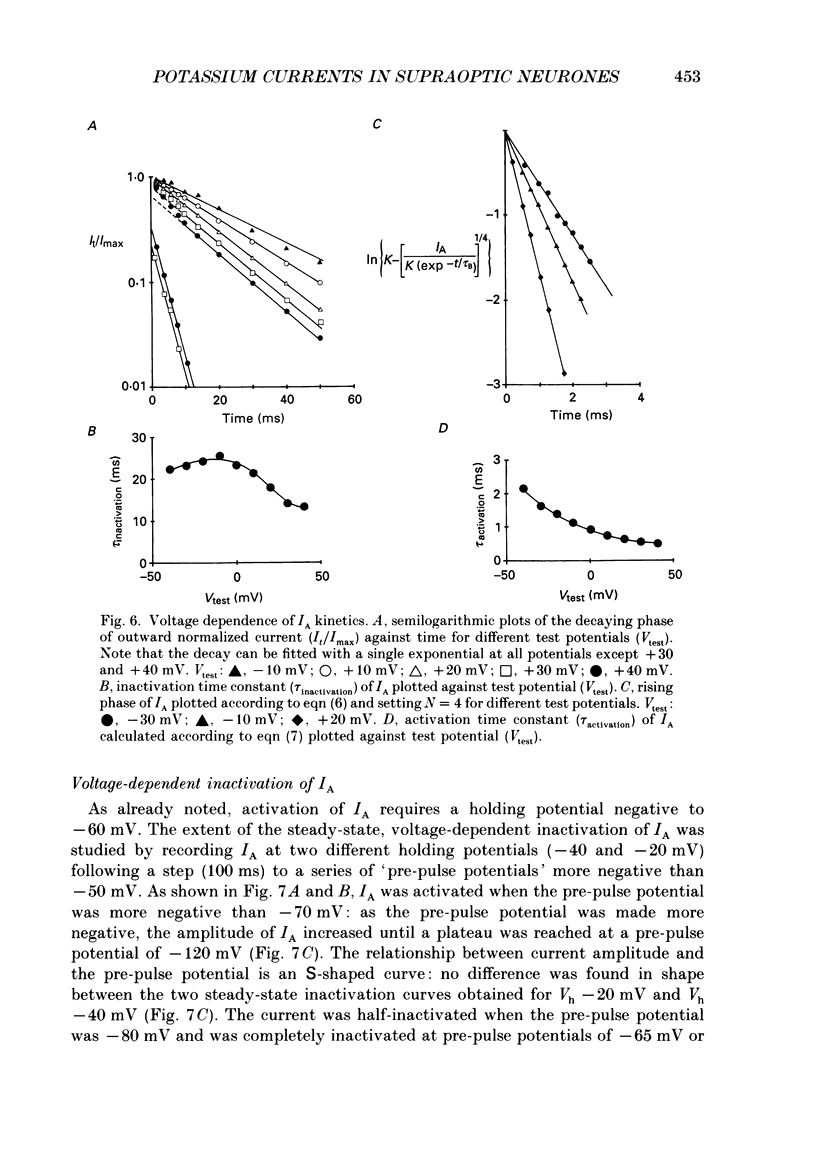
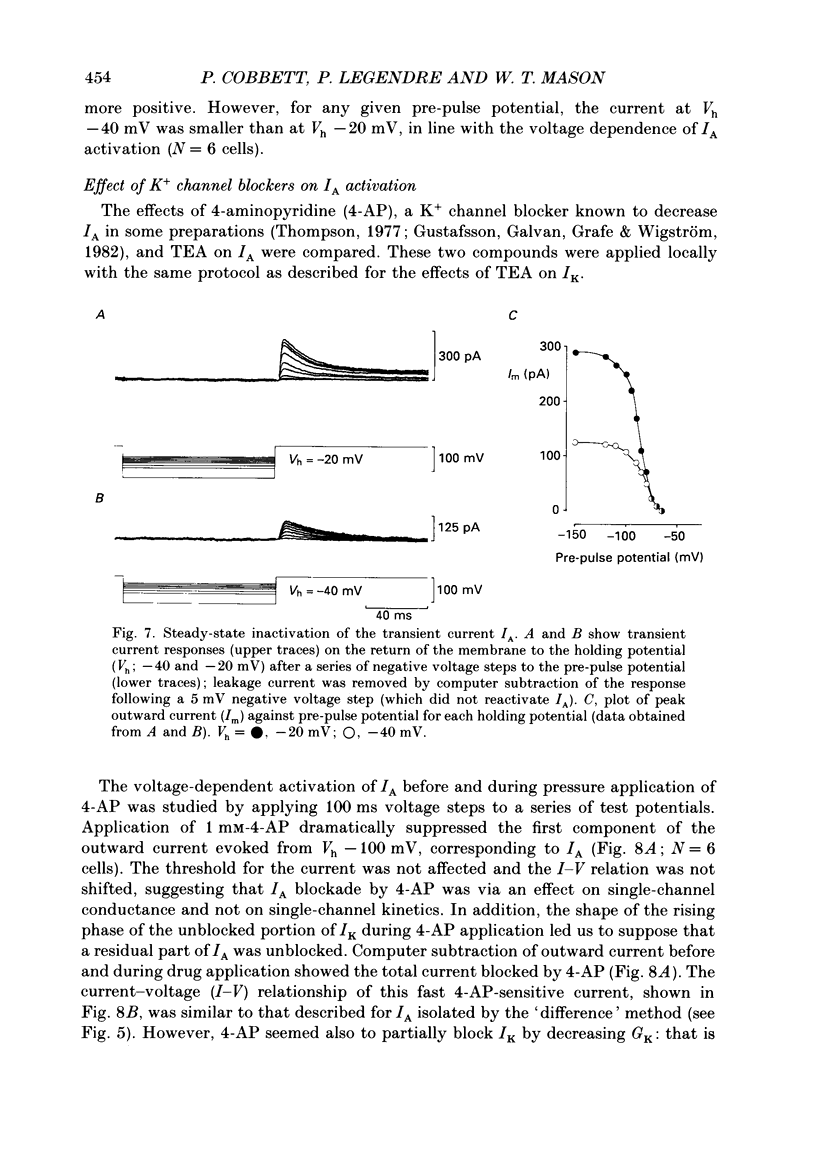
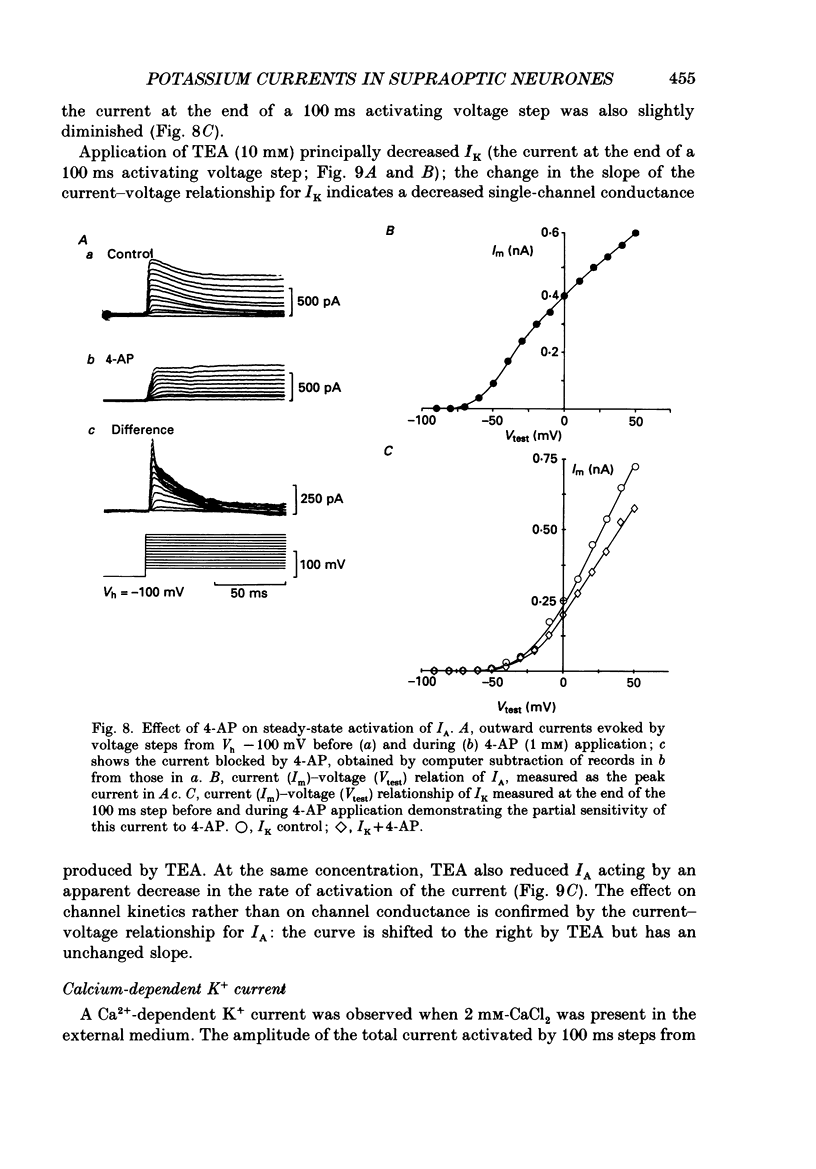
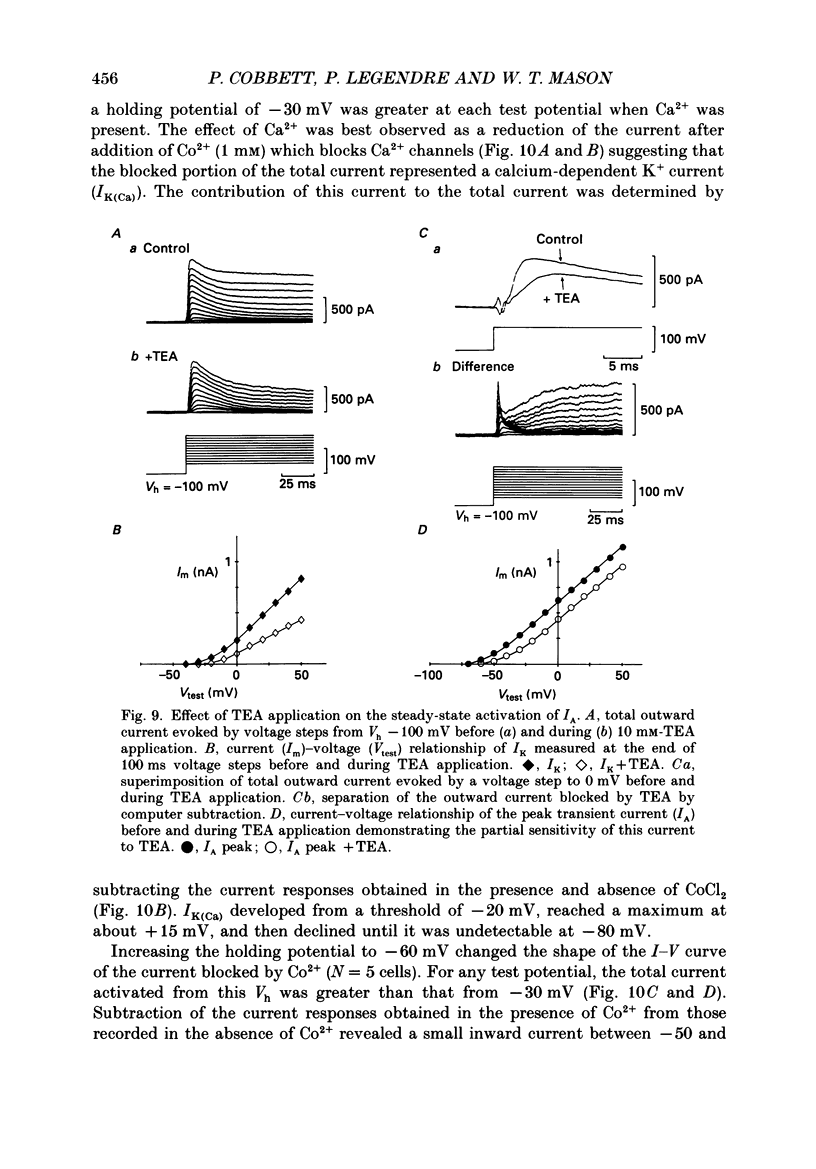
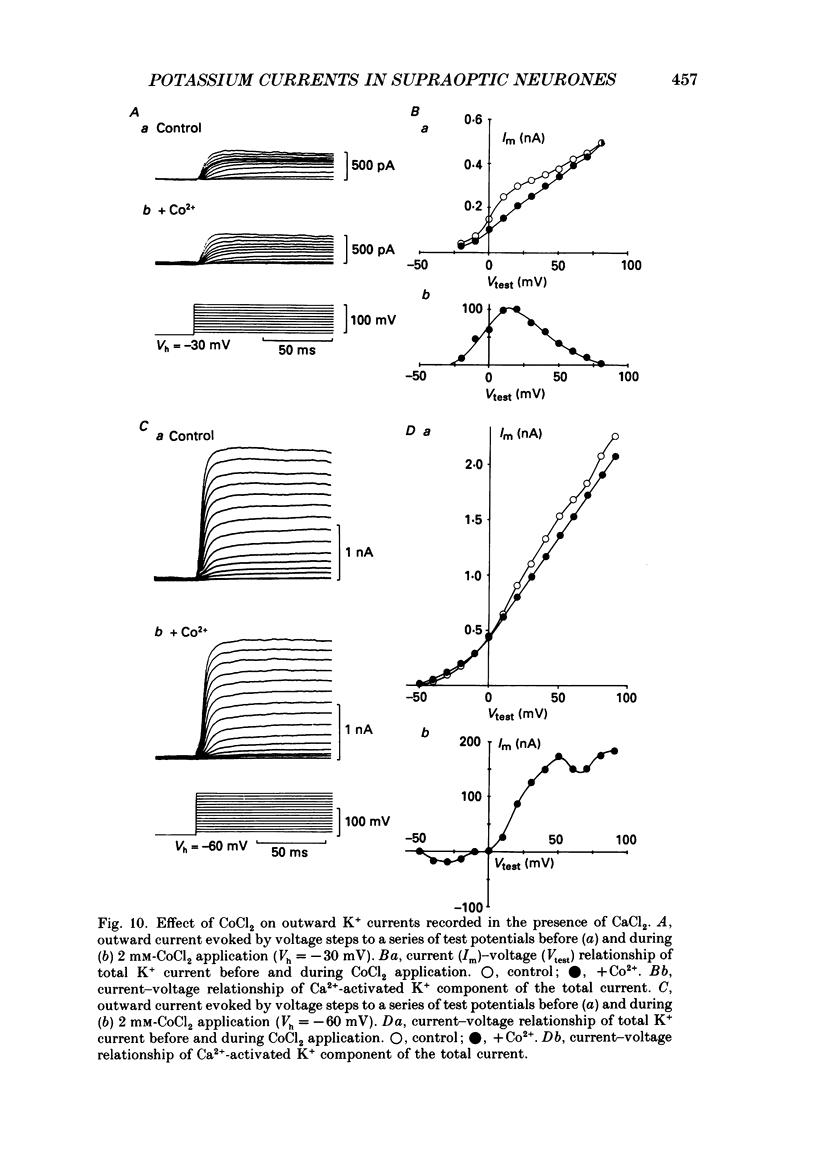





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich R. W., Jr, Getting P. A., Thompson S. H. Inactivation of delayed outward current in molluscan neurone somata. J Physiol. 1979 Jun;291:507–530. doi: 10.1113/jphysiol.1979.sp012828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol. 1984 Mar;51(3):552–566. doi: 10.1152/jn.1984.51.3.552. [DOI] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Intrinsic inhibition in magnocellular neuroendocrine cells of rat hypothalamus. J Physiol. 1984 Aug;353:171–185. doi: 10.1113/jphysiol.1984.sp015330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Spike broadening in magnocellular neuroendocrine cells of rat hypothalamic slices. Brain Res. 1985 May 13;334(1):176–179. doi: 10.1016/0006-8993(85)90583-9. [DOI] [PubMed] [Google Scholar]
- Bourque C. W. Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory cells. Neurosci Lett. 1986 Oct 8;70(2):204–209. doi: 10.1016/0304-3940(86)90464-7. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Randle J. C., Renaud L. P. Calcium-dependent potassium conductance in rat supraoptic nucleus neurosecretory neurons. J Neurophysiol. 1985 Dec;54(6):1375–1382. doi: 10.1152/jn.1985.54.6.1375. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. Calcium-dependent action potentials in rat supraoptic neurosecretory neurones recorded in vitro. J Physiol. 1985 Jun;363:419–428. doi: 10.1113/jphysiol.1985.sp015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1988 Mar;397:331–347. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett P., Ingram C. D., Mason W. T. Sodium and potassium currents involved in action potential propagation in normal bovine lactotrophs. J Physiol. 1987 Nov;392:273–299. doi: 10.1113/jphysiol.1987.sp016780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett P., Mason W. T. Whole cell voltage clamp recordings from cultured neurons of the supraoptic area of neonatal rat hypothalamus. Brain Res. 1987 Apr 14;409(1):175–180. doi: 10.1016/0006-8993(87)90756-6. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Slow repetitive activity from fast conductance changes in neurons. Fed Proc. 1978 Jun;37(8):2139–2145. [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M. Simultaneous changes in the equilibrium potential and potassium conductance in voltage clamped Ranvier node in the frog. J Physiol. 1981 Sep;318:279–295. doi: 10.1113/jphysiol.1981.sp013864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. A QUANTITATIVE DESCRIPTION OF POTASSIUM CURRENTS IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. J Physiol. 1963 Nov;169:424–430. doi: 10.1113/jphysiol.1963.sp007268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A., Thomas M. V. Intracellular calcium and the control of neuronal pacemaker activity. Fed Proc. 1981 Jun;40(8):2233–2239. [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J Physiol. 1985 Jan;358:131–152. doi: 10.1113/jphysiol.1985.sp015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P., Cooke I. M., Vincent J. D. Regenerative responses of long duration recorded intracellularly from dispersed cell cultures of fetal mouse hypothalamus. J Neurophysiol. 1982 Nov;48(5):1121–1141. doi: 10.1152/jn.1982.48.5.1121. [DOI] [PubMed] [Google Scholar]
- Mason W. T. Electrical properties of neurons recorded from the rat supraoptic nucleus in vitro. Proc R Soc Lond B Biol Sci. 1983 Jan 22;217(1207):141–161. doi: 10.1098/rspb.1983.0003. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Leng G. Complex action potential waveform recorded from supraoptic and paraventricular neurones of the rat: evidence for sodium and calcium spike components at different membrane sites. Exp Brain Res. 1984;56(1):135–143. doi: 10.1007/BF00237449. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L., Wyman R. Facilitation of membrane electrical excitability in Drosophila. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6216–6220. doi: 10.1073/pnas.77.10.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASAKI I., HAGIWAR A. S. Demonstration of two stable potential states in the squid giant axon under tetraethylammonium chloride. J Gen Physiol. 1957 Jul 20;40(6):859–885. doi: 10.1085/jgp.40.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B. S., Binstock L. Inhibition of potassium conductance with external tetraethylammonium ion in Myxicola giant axons. Biophys J. 1980 Dec;32(3):1037–1042. doi: 10.1016/S0006-3495(80)85034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]



