Abstract
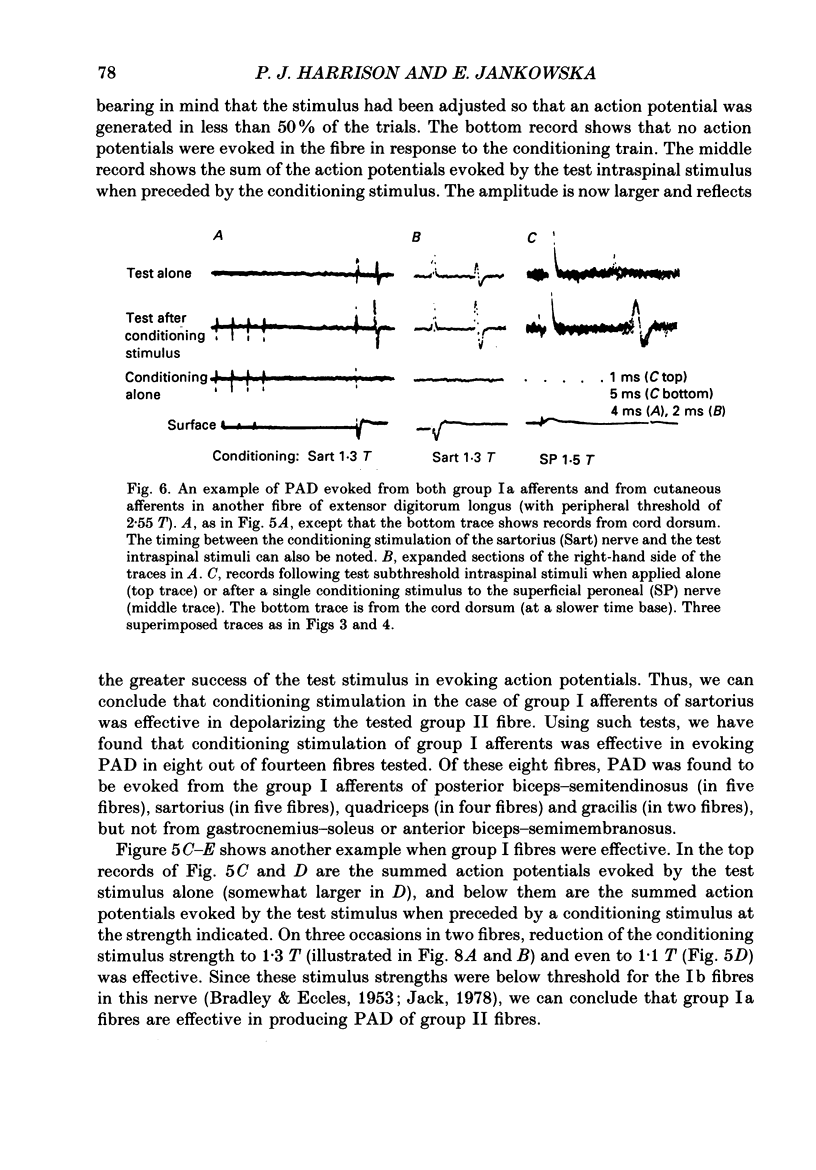
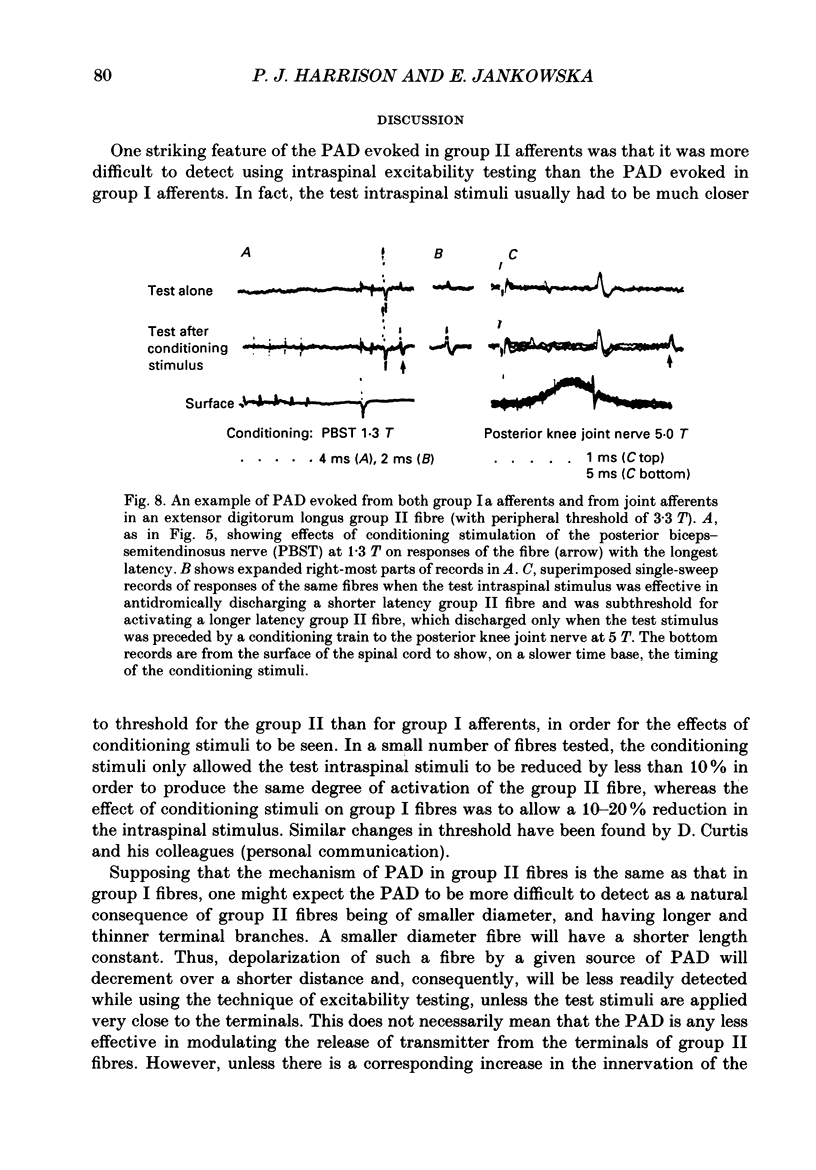
1. The origin of primary afferent depolarization (PAD) of the central terminals of group II afferent fibres of tibialis anterior and extensor digitorum longus muscles has been investigated in the cat. Changes in the excitability of the terminals to intraspinal stimuli, upon application of conditioning stimuli to muscle nerves (quadriceps, sartorius, gracilis, posterior biceps-semitendinosus, anterior biceps-semimembranosus, gastrocnemius-soleus, deep peroneal), cutaneous nerves (sural, superficial peroneal) and the posterior nerve to the knee joint, were used as a measure of PAD. 2. PAD was most readily evoked by conditioning stimuli which were maximal for group II muscle afferents. However, some PAD was also evoked from group I afferents and evidence is presented that group Ia afferents contributed. Afferents of posterior biceps-semitendinosus and sartorius muscles appeared to be most effective. PAD was also evoked by stimulation of cutaneous and joint nerves, often in the same fibres which were affected by group Ia afferents. 3. It is concluded that there are several common sources of PAD of group II and group Ia afferent terminals on the one hand, and group Ib afferent terminals on the other. 4. The properties of PAD of group II afferents are discussed in relation to the problem of how PAD affects transmission from fibres with long terminal branches of small diameter.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E., Jankowska E., Skoog B. Convergence onto interneurons subserving primary afferent depolarization of group I afferents. J Neurophysiol. 1984 Mar;51(3):432–449. doi: 10.1152/jn.1984.51.3.432. [DOI] [PubMed] [Google Scholar]
- CARPENTER D., LUNDBERG A., NORRSELL U. PRIMARY AFFERENT DEPOLARIZATION EVOKED FROM THE SENSORIMOTOR CORTEX. Acta Physiol Scand. 1963 Sep-Oct;59:126–142. doi: 10.1111/j.1748-1716.1963.tb02729.x. [DOI] [PubMed] [Google Scholar]
- Cavallari P., Edgley S. A., Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol. 1987 Aug;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987 Aug;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987 Apr;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R., Tripathi A. Closely coupled excitation of gamma-motoneurones by group III Muscle afferents with low mechanical threshold in the cat. J Physiol. 1982 Oct;331:481–498. doi: 10.1113/jphysiol.1982.sp014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fern R., Harrison P. J., Riddell J. S. The dorsal column projection of muscle afferent fibres from the cat hindlimb. J Physiol. 1988 Jul;401:97–113. doi: 10.1113/jphysiol.1988.sp017153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jami L., Jankowska E. Further evidence for synaptic actions of muscle spindle secondaries in the middle lumbar segments of the cat spinal cord. J Physiol. 1988 Aug;402:671–686. doi: 10.1113/jphysiol.1988.sp017228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Do interneurones in lower lumbar segments contribute to the presynaptic depolarization of group I muscle afferents in Clarke's column? Brain Res. 1984 Mar 19;295(2):203–210. doi: 10.1016/0006-8993(84)90968-5. [DOI] [PubMed] [Google Scholar]
- Jankowska E., McCrea D., Rudomín P., Sykova E. Observations on neuronal pathways subserving primary afferent depolarization. J Neurophysiol. 1981 Sep;46(3):506–516. doi: 10.1152/jn.1981.46.3.506. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Padel Y. On the origin of presynaptic depolarization of group I muscle afferents in Clarke's column in the cat. Brain Res. 1984 Mar 19;295(2):195–201. doi: 10.1016/0006-8993(84)90967-3. [DOI] [PubMed] [Google Scholar]
- Jiménez I., Rudomin P., Solodkin M. PAD patterns of physiologically identified afferent fibres from the medial gastrocnemius muscle. Exp Brain Res. 1988;71(3):643–657. doi: 10.1007/BF00248758. [DOI] [PubMed] [Google Scholar]
- MacLennan C. R. The behaviour of receptors of extramuscular and muscular origin with afferent fibres contributing to the group I and the group II of the cat tibialis anterior muscle nerve. J Physiol. 1972 Apr;222(1):90P–91P. [PubMed] [Google Scholar]
- Rudomin P., Solodkin M., Jiménez I. PAD and PAH response patterns of group Ia- and Ib-fibers to cutaneous and descending inputs in the cat spinal cord. J Neurophysiol. 1986 Oct;56(4):987–1006. doi: 10.1152/jn.1986.56.4.987. [DOI] [PubMed] [Google Scholar]
- Rudomín P., Jiménez I., Solodkin M., Dueñas S. Sites of action of segmental and descending control of transmission on pathways mediating PAD of Ia- and Ib-afferent fibers in cat spinal cord. J Neurophysiol. 1983 Oct;50(4):743–769. doi: 10.1152/jn.1983.50.4.743. [DOI] [PubMed] [Google Scholar]
- Schmidt R. F. Presynaptic inhibition in the vertebrate central nervous system. Ergeb Physiol. 1971;63:20–101. doi: 10.1007/BFb0047741. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]


