Abstract
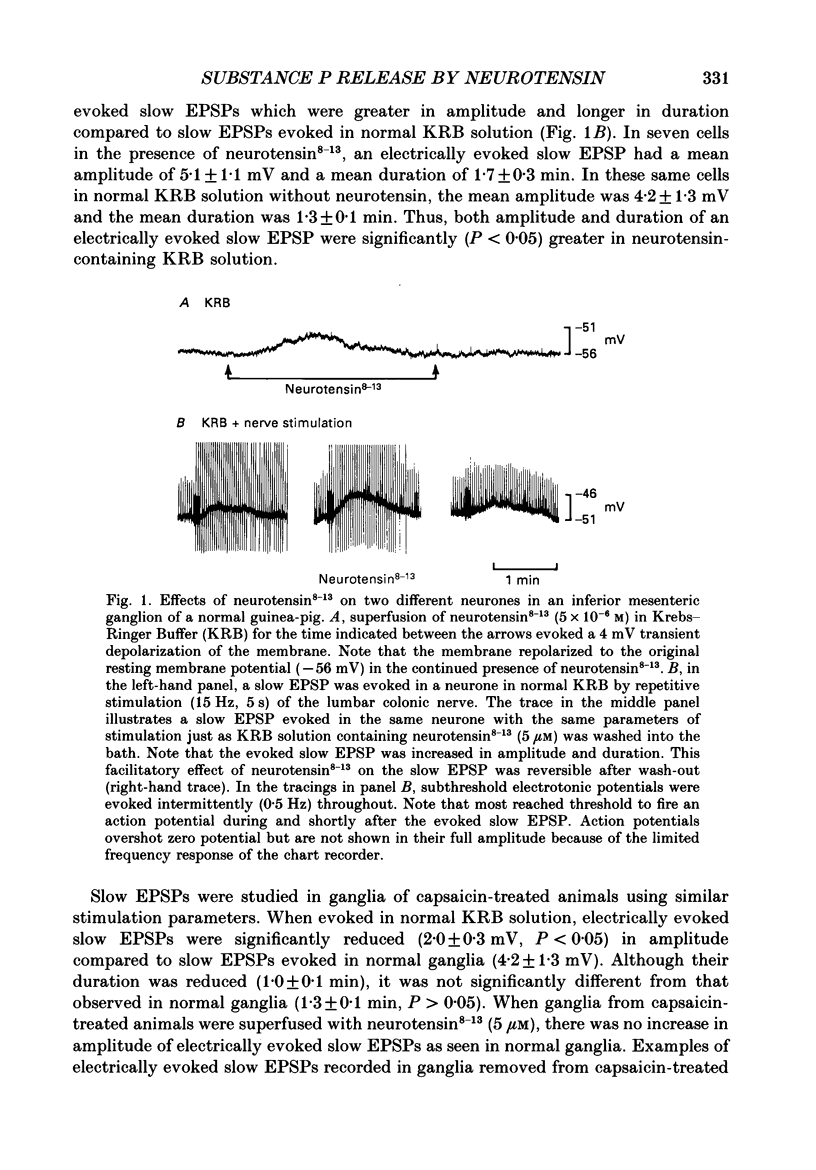
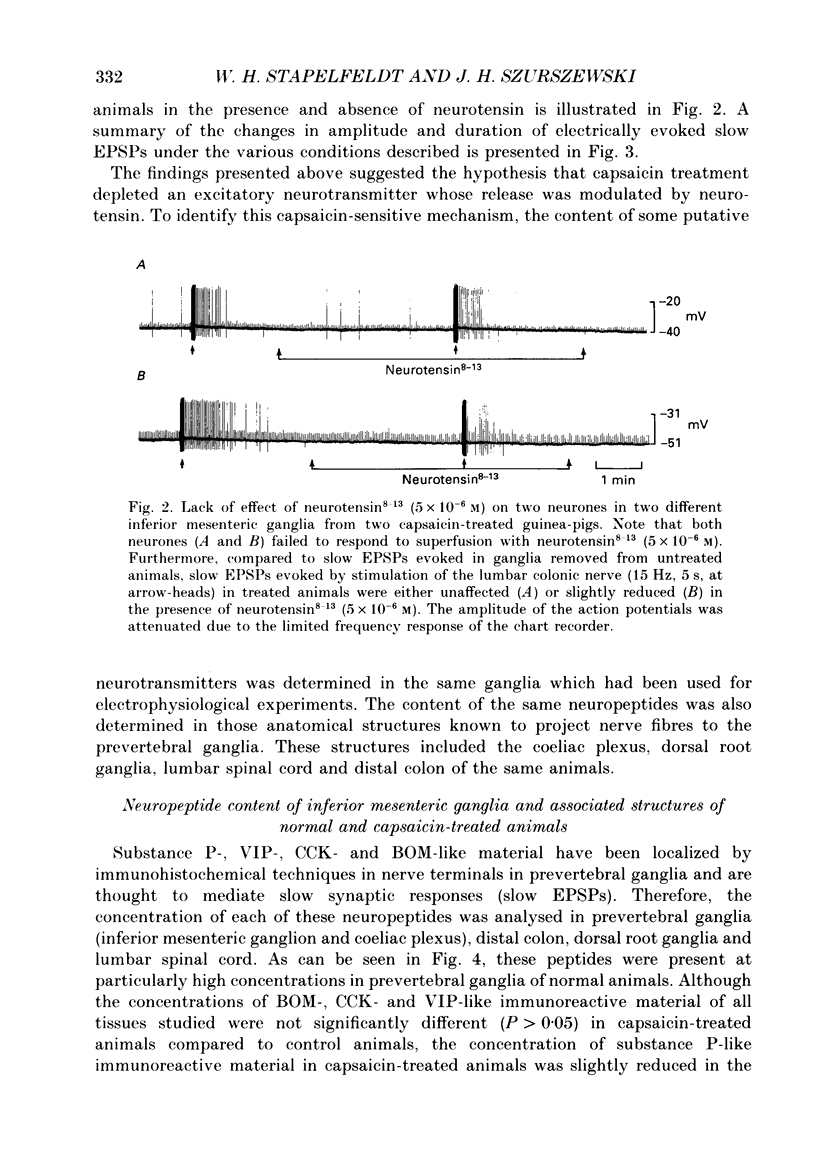
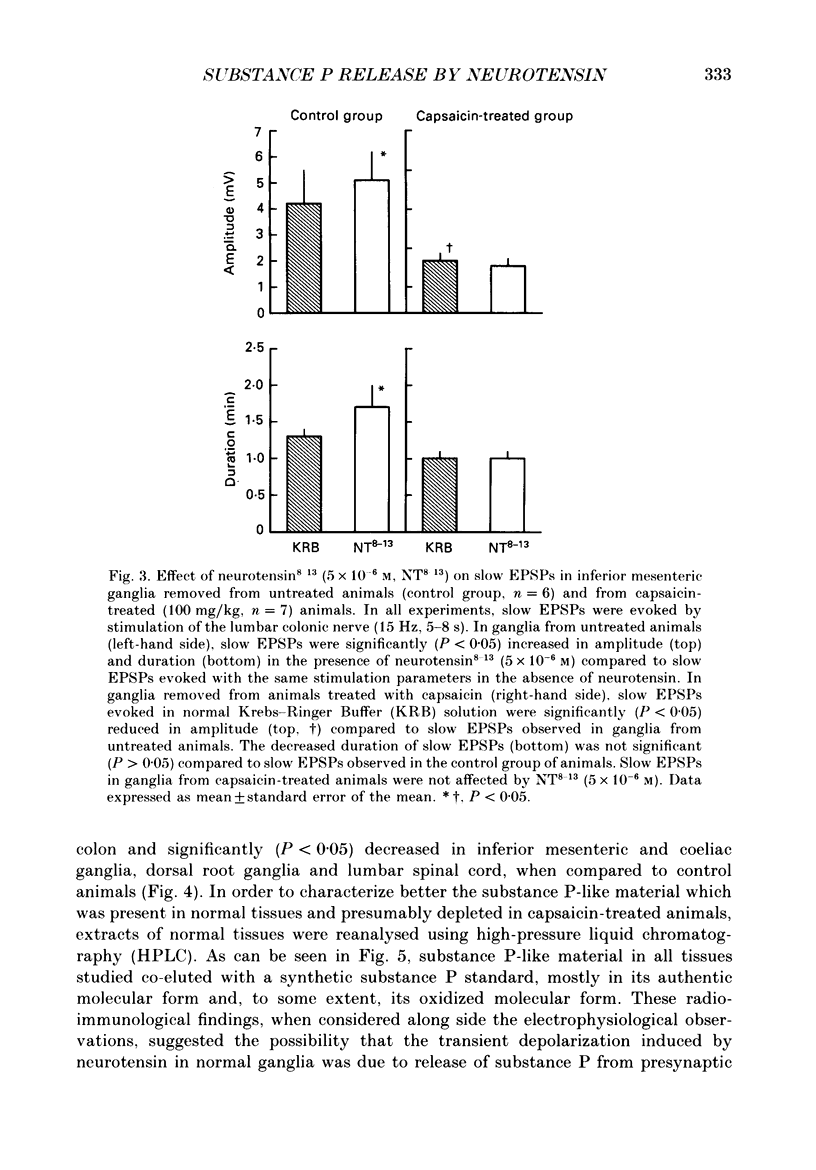
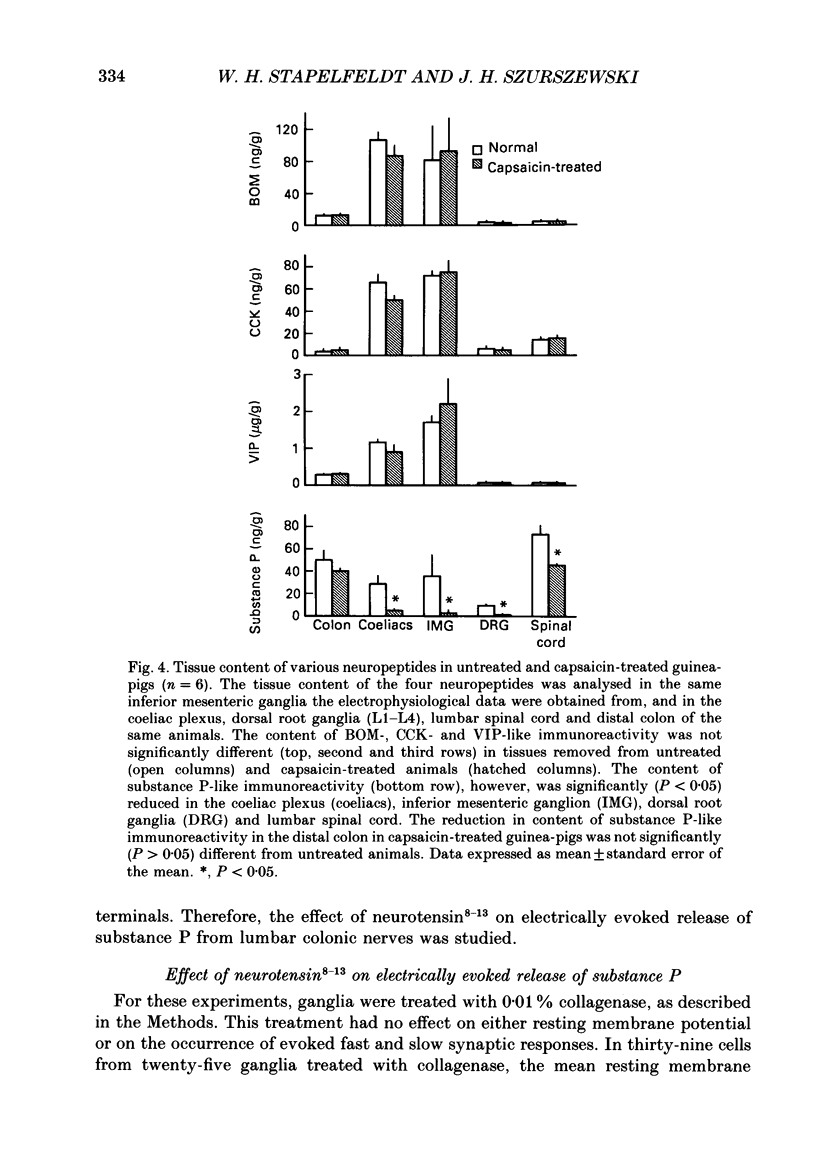
1. Intracellular, electrophysiological techniques were combined with radio-immunological, chromatographic and pharmacological techniques to determine if nerve terminals containing substance P mediated transient depolarizing responses of principal ganglion cells induced by neurotensin. Experiments were performed in vitro on guinea-pig inferior mesenteric ganglia. 2. In 61% of principal ganglion cells tested in normal ganglia, neurotensin caused a transient membrane depolarization. In ganglia which were removed from animals which had been pre-treated with capsaicin, transient responses to neurotensin were virtually abolished. 3. In normal ganglia, neurotensin increased the amplitude and duration of noncholinergic slow EPSPs evoked by electrical stimulation of the lumbar colonic nerve. Such increases were absent in ganglia obtained from animals pre-treated with capsaicin. 4. In guinea-pigs pre-treated with capsaicin, the content of substance P-like material was significantly reduced in inferior mesenteric and coeliac ganglia, dorsal root ganglia and lumbar spinal cord, compared to control animals. The content of substance P-like material in segments of distal colon was slightly reduced. The content of vasoactive intestinal polypeptide-, cholecystokinin- and bombesin-like material in the same tissues from animals pre-treated with capsaicin was not significantly different from control animals. 5. Chromatographic analysis using HPLC (high-performance liquid chromatography) techniques revealed that the material depleted from inferior mesenteric and coeliac ganglia, dorsal root ganglia and lumbar spinal cord by capsaicin pre-treatment co-eluted with synthetic substance P. 6. Electrical stimulation of the lumbar colonic nerve released substance P-like material from isolated inferior mesenteric ganglia as determined by radioimmunoassay of samples of superfusate. Exogenous administration of neurotensin caused a significant increase in the amount of substance P-like material released during nerve stimulation. 7. Transient depolarizing responses evoked by neurotensin were markedly attenuated when ganglion cells were postsynaptically desensitized to exogenously administered substance P. 8. Taken together, these findings suggest that transient depolarizations mediated by an indirect action of neurotensin and facilitation of electrically evoked non-cholinergic slow EPSPs by neurotensin involved presynaptic release of substance P from collateral nerve terminals of primary afferent nerve fibres in the inferior mesenteric ganglion. 9. It was suggested that under normal in vivo conditions, neurotensin or a C-terminal-related peptide contained in central preganglionic nerve endings might function as an excitatory neuromodulator to enhance the release of substance P from primary afferent nerve terminals thereby facilitating non-cholinergic peripheral afferent synaptic input to prevertebral ganglion cells.
Full text
PDF











Selected References
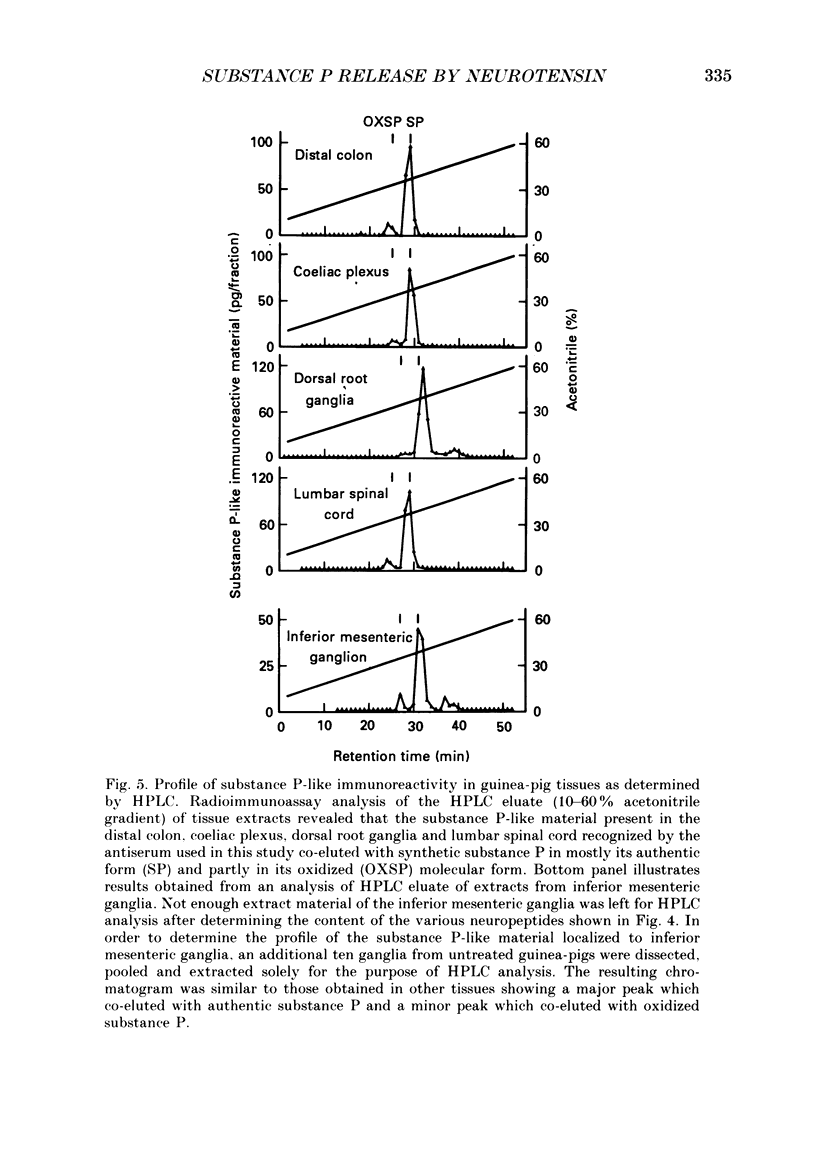
These references are in PubMed. This may not be the complete list of references from this article.
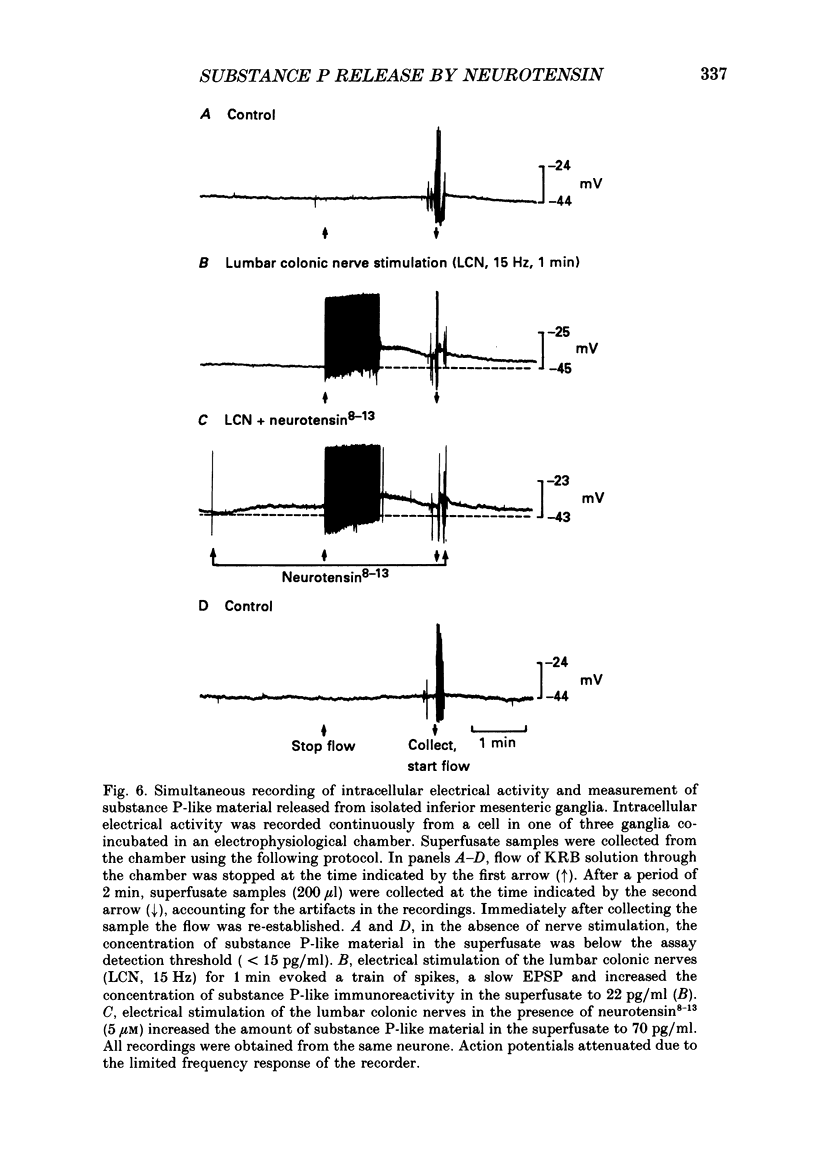
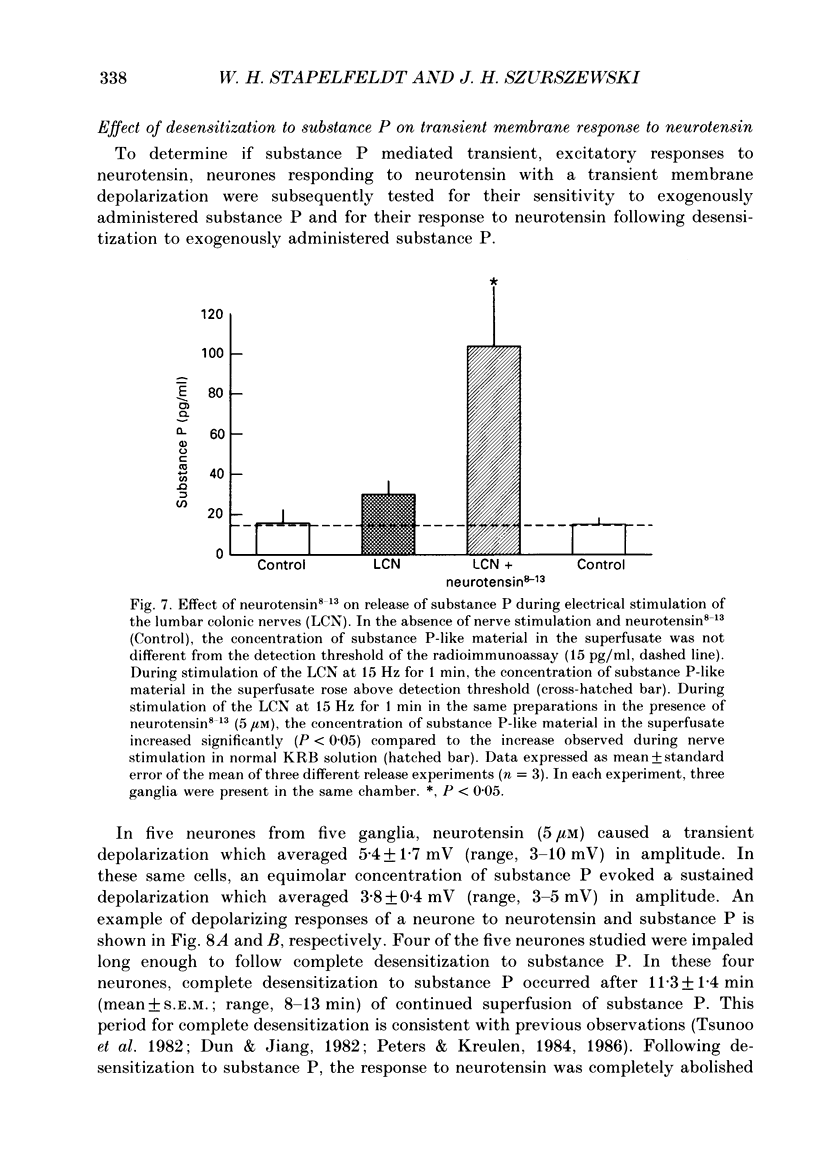
- Angel F., Go V. L., Szurszewski J. H. Innervation of the muscularis mucosae of canine proximal colon. J Physiol. 1984 Dec;357:93–108. doi: 10.1113/jphysiol.1984.sp015491. [DOI] [PMC free article] [PubMed] [Google Scholar]
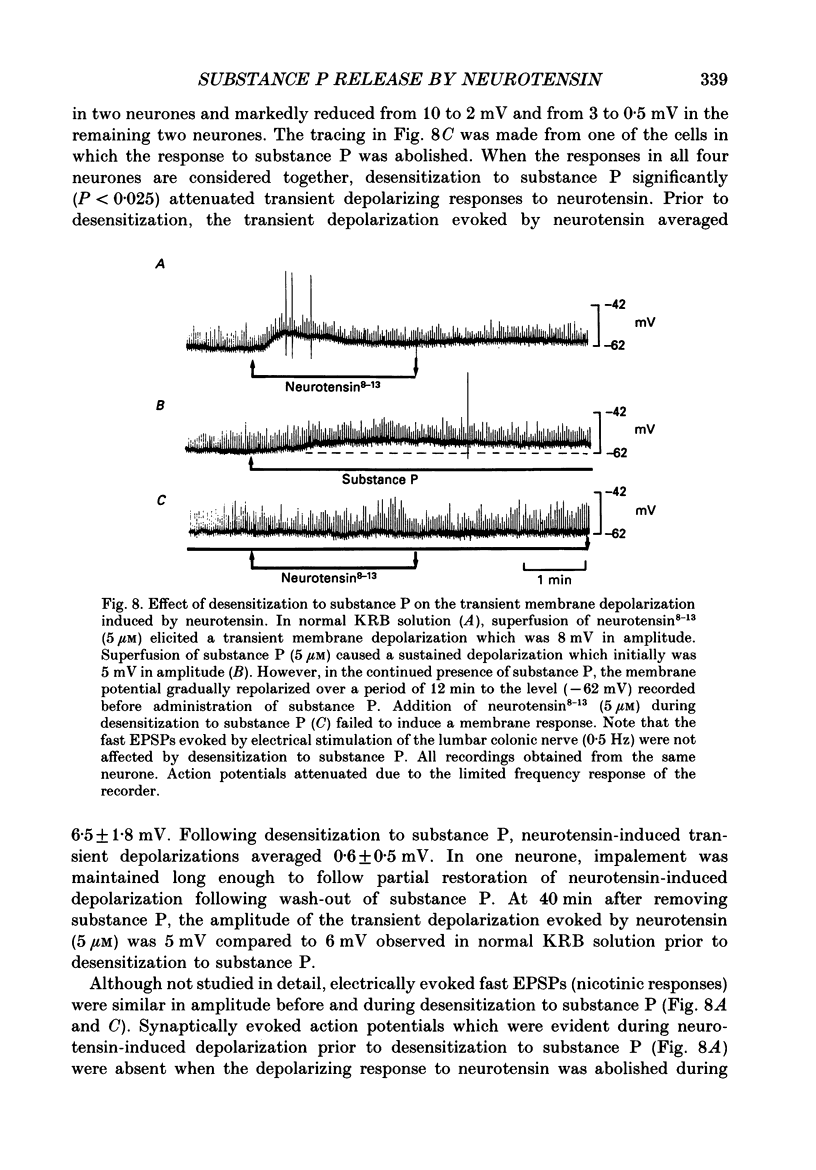
- Cervero F. Afferent activity evoked by natural stimulation of the biliary system in the ferret. Pain. 1982 Jun;13(2):137–151. doi: 10.1016/0304-3959(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Hökfelt T., Elfvin L. G., Skirboll L., Emson P. Substance P-containing primary sensory neurons projecting to the inferior mesenteric ganglion: evidence from combined retrograde tracing and immunohistochemistry. Neuroscience. 1982 Mar;7(3):647–654. doi: 10.1016/0306-4522(82)90070-7. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Hökfelt T., Schultzberg M., Lundberg J. M., Terenius L., Dockray G. J., Goldstein M. Origin of peptide-containing fibers in the inferior mesenteric ganglion of the guinea-pig: immunohistochemical studies with antisera to substance P, enkephalin, vasoactive intestinal polypeptide, cholecystokinin and bombesin. Neuroscience. 1983 May;9(1):191–211. doi: 10.1016/0306-4522(83)90056-8. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Vincent S. R., Hökfelt V. T., Christensson I., Terenius L. Separate origins for the dynorphin and enkephalin immunoreactive fibers in the inferior mesenteric ganglion of the guinea pig. J Comp Neurol. 1983 Dec 20;221(4):482–489. doi: 10.1002/cne.902210410. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Vincent S. R., Schultzberg M., Hökfelt T., Elfvin L. G., Terenius L., Dockray G. J. Capsaicin-induced depletion of substance P-like immunoreactivity in guinea pig sympathetic ganglia. J Auton Nerv Syst. 1983 Dec;9(4):595–606. doi: 10.1016/0165-1838(83)90116-9. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Jiang Z. G. Non-cholinergic excitatory transmission in inferior mesenteric ganglia of the guinea-pig: possible mediation by substance P. J Physiol. 1982 Apr;325:145–159. doi: 10.1113/jphysiol.1982.sp014141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Kiraly M. Capsaicin causes release of a substance P-like peptide in guinea-pig inferior mesenteric ganglia. J Physiol. 1983 Jul;340:107–120. doi: 10.1113/jphysiol.1983.sp014752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse R., Wax A., Zigmond R. E., Leeman S. E. Immunoreactive substance P in sympathetic ganglia: distribution and sensitivity towards capsaicin. Neuroscience. 1981;6(3):437–441. doi: 10.1016/0306-4522(81)90136-6. [DOI] [PubMed] [Google Scholar]
- Gibbins I. L., Furness J. B., Costa M., MacIntyre I., Hillyard C. J., Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett. 1985 Jun 12;57(2):125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- Gibbins I. L., Furness J. B., Costa M. Pathway-specific patterns of the co-existence of substance P, calcitonin gene-related peptide, cholecystokinin and dynorphin in neurons of the dorsal root ganglia of the guinea-pig. Cell Tissue Res. 1987 May;248(2):417–437. doi: 10.1007/BF00218210. [DOI] [PubMed] [Google Scholar]
- Heym C., Reinecke M., Weihe E., Forssmann W. G. Dopamine-beta-hydroxylase-, neurotensin-, substance P-, vasoactive intestinal polypeptide- and enkephalin-immunohistochemistry of paravertebral and prevertebral ganglia in the cat. Cell Tissue Res. 1984;235(2):411–418. doi: 10.1007/BF00217867. [DOI] [PubMed] [Google Scholar]
- Hua X. Y., Theodorsson-Norheim E., Brodin E., Lundberg J. M., Hökfelt T. Multiple tachykinins (neurokinin A, neuropeptide K and substance P) in capsaicin-sensitive sensory neurons in the guinea-pig. Regul Pept. 1985 Dec;13(1):1–19. doi: 10.1016/0167-0115(85)90082-5. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Elfvin L. G., Schultzberg M., Goldstein M., Nilsson G. On the occurrence of substance P-containing fibers in sympathetic ganglia: immunohistochemical evidence. Brain Res. 1977 Aug 19;132(1):29–41. doi: 10.1016/0006-8993(77)90704-1. [DOI] [PubMed] [Google Scholar]
- Kreulen D. L., Peters S. Non-cholinergic transmission in a sympathetic ganglion of the guinea-pig elicited by colon distension. J Physiol. 1986 May;374:315–334. doi: 10.1113/jphysiol.1986.sp016081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J. A., Szurszewski J. H. The electrophysiological effects of vasoactive intestinal polypeptide in the guinea-pig inferior mesenteric ganglion. J Physiol. 1987 Dec;394:67–84. doi: 10.1113/jphysiol.1987.sp016860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Rökaeus A., Hökfelt T., Rosell S., Brown M., Goldstein M. Neurotensin-like immunoreactivity in the preganglionic sympathetic nerves and in the adrenal medulla of the cat. Acta Physiol Scand. 1982 Jan;114(1):153–155. doi: 10.1111/j.1748-1716.1982.tb06965.x. [DOI] [PubMed] [Google Scholar]
- Matthews M. R., Connaughton M., Cuello A. C. Ultrastructure and distribution of substance P-immunoreactive sensory collaterals in the guinea pig prevertebral sympathetic ganglia. J Comp Neurol. 1987 Apr 1;258(1):28–51. doi: 10.1002/cne.902580103. [DOI] [PubMed] [Google Scholar]
- Matthews M. R., Cuello A. C. Substance P-immunoreactive peripheral branches of sensory neurons innervate guinea pig sympathetic neurons. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1668–1672. doi: 10.1073/pnas.79.5.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P. E., Yaksh T. L., Go V. L. Opiate-mediated inhibition of the release of cholecystokinin and substance P, but not neurotensin from cat hypothalamic slices. Brain Res. 1982 Nov 4;250(2):283–289. doi: 10.1016/0006-8993(82)90422-x. [DOI] [PubMed] [Google Scholar]
- Mo N., Dun N. J. Cholecystokinin octapeptide depolarizes guinea pig inferior mesenteric ganglion cells and facilitates nicotinic transmission. Neurosci Lett. 1986 Mar 14;64(3):263–268. doi: 10.1016/0304-3940(86)90339-3. [DOI] [PubMed] [Google Scholar]
- Mo N., Dun N. J. Vasoactive intestinal polypeptide facilitates muscarinic transmission in mammalian sympathetic ganglia. Neurosci Lett. 1984 Nov 23;52(1-2):19–23. doi: 10.1016/0304-3940(84)90344-6. [DOI] [PubMed] [Google Scholar]
- Owyang C., Miller L. J., DiMagno E. P., Brennan L. A., Jr, Go V. L. Gastrointestinal hormone profile in renal insufficiency. Mayo Clin Proc. 1979 Dec;54(12):769–773. [PubMed] [Google Scholar]
- Peters S., Kreulen D. L. A slow EPSP in mammalian inferior mesenteric ganglion persists after in vivo capsaicin. Brain Res. 1984 Jun 11;303(1):186–189. doi: 10.1016/0006-8993(84)90227-0. [DOI] [PubMed] [Google Scholar]
- Peters S., Kreulen D. L. Fast and slow synaptic potentials produced in a mammalian sympathetic ganglion by colon distension. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1941–1944. doi: 10.1073/pnas.83.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke M., Forssmann W. G., Thiekötter G., Triepel J. Localization of neurotensin-immunoreactivity in the spinal cord and peripheral nervous system of the guinea pig. Neurosci Lett. 1983 May 27;37(1):37–42. doi: 10.1016/0304-3940(83)90501-3. [DOI] [PubMed] [Google Scholar]
- Saria A., Ma R. C., Dun N. J. Neurokinin A depolarizes neurons of the guinea pig inferior mesenteric ganglia. Neurosci Lett. 1985 Sep 30;60(2):145–150. doi: 10.1016/0304-3940(85)90235-6. [DOI] [PubMed] [Google Scholar]
- Schumann M. A., Kreulen D. L. Action of cholecystokinin octapeptide and CCK-related peptides on neurons in inferior mesenteric ganglion of guinea pig. J Pharmacol Exp Ther. 1986 Nov;239(2):618–625. [PubMed] [Google Scholar]
- Stapelfeldt W. H., Szurszewski J. H. The electrophysiological effects of neurotensin on neurones of guinea-pig prevertebral sympathetic ganglia. J Physiol. 1989 Apr;411:301–323. doi: 10.1113/jphysiol.1989.sp017575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski J. H. Physiology of mammalian prevertebral ganglia. Annu Rev Physiol. 1981;43:53–68. doi: 10.1146/annurev.ph.43.030181.000413. [DOI] [PubMed] [Google Scholar]
- Tsunoo A., Konishi S., Otsuka M. Substance P as an excitatory transmitter of primary afferent neurons in guinea-pig sympathetic ganglia. Neuroscience. 1982;7(9):2025–2037. doi: 10.1016/0306-4522(82)90117-8. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Abay E. O., 2nd, Go V. L. Studies on the location and release of cholecystokinin and vasoactive intestinal peptide in rat and cat spinal cord. Brain Res. 1982 Jun 24;242(2):279–290. doi: 10.1016/0006-8993(82)90311-0. [DOI] [PubMed] [Google Scholar]


