Abstract
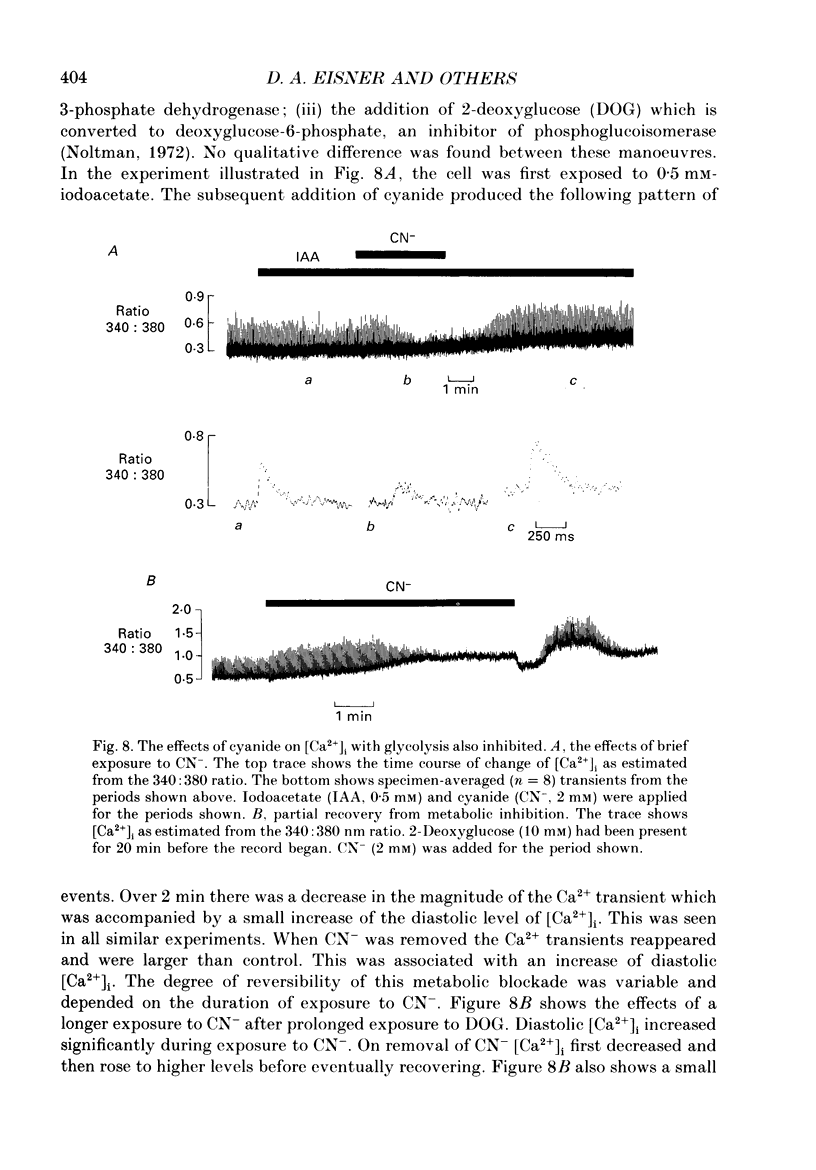
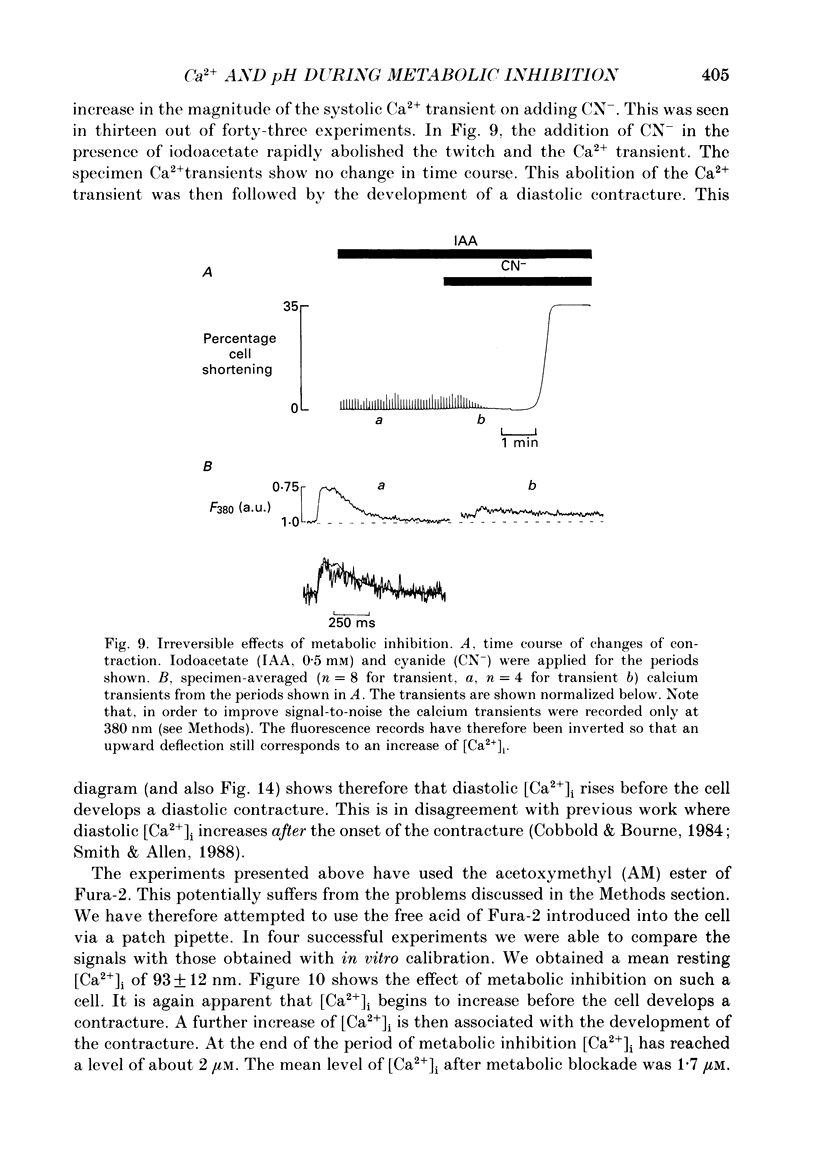
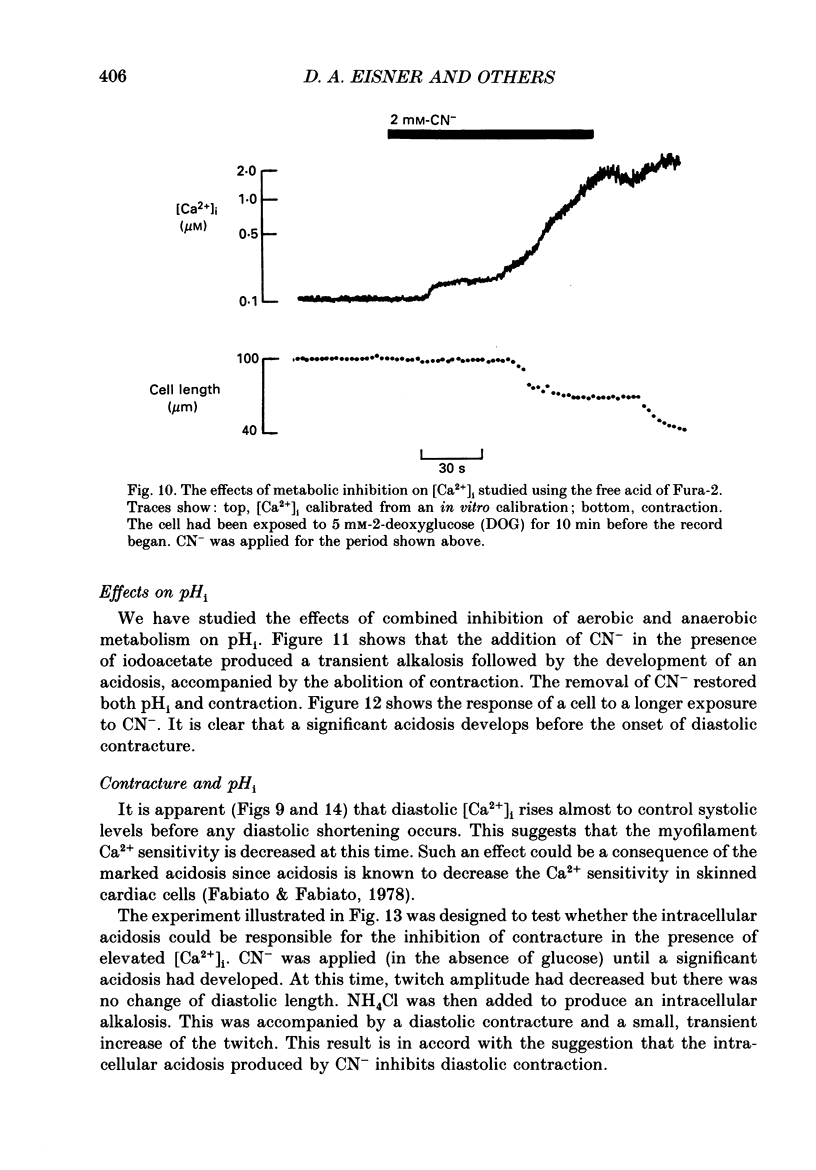
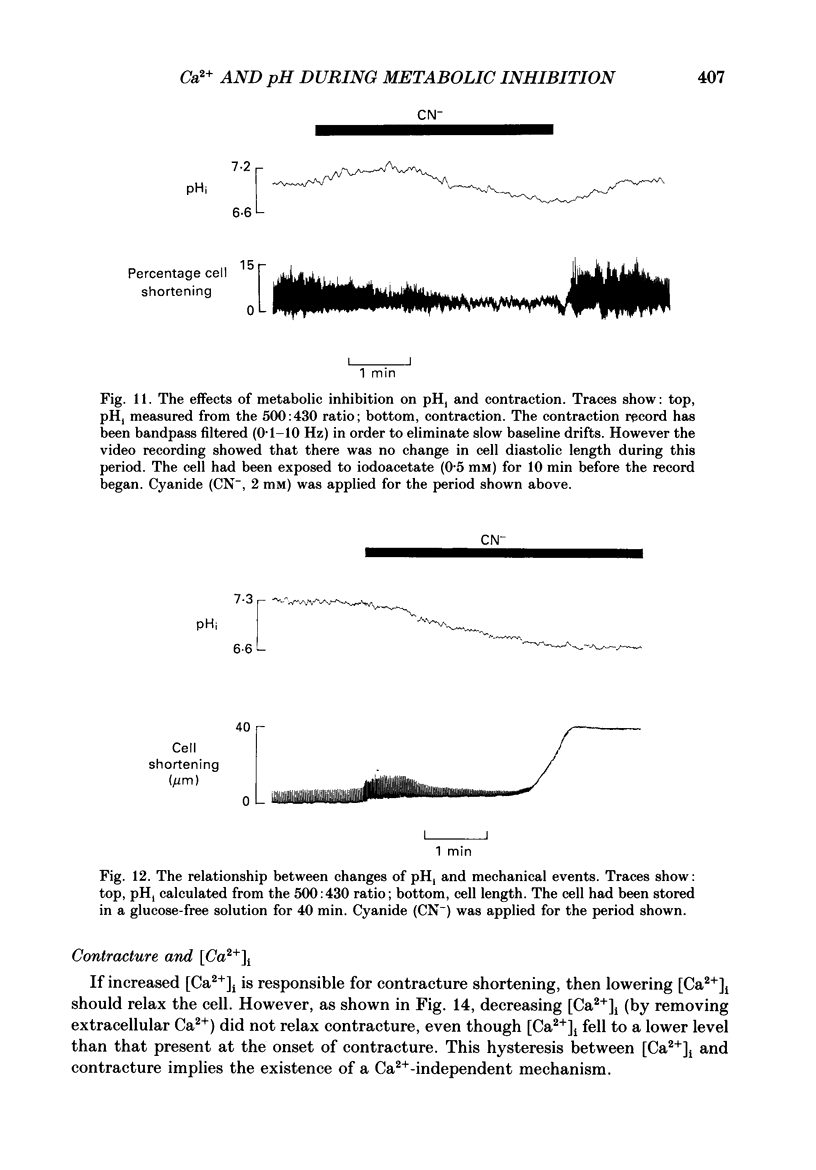
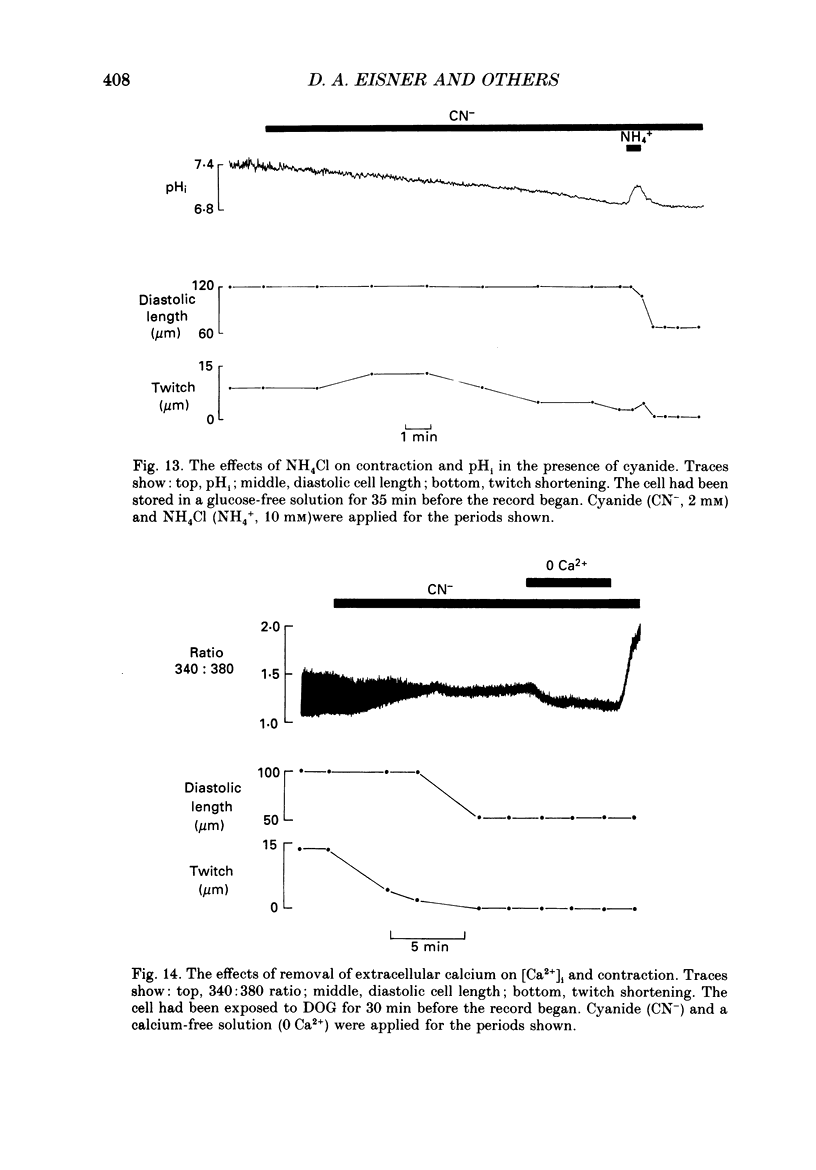
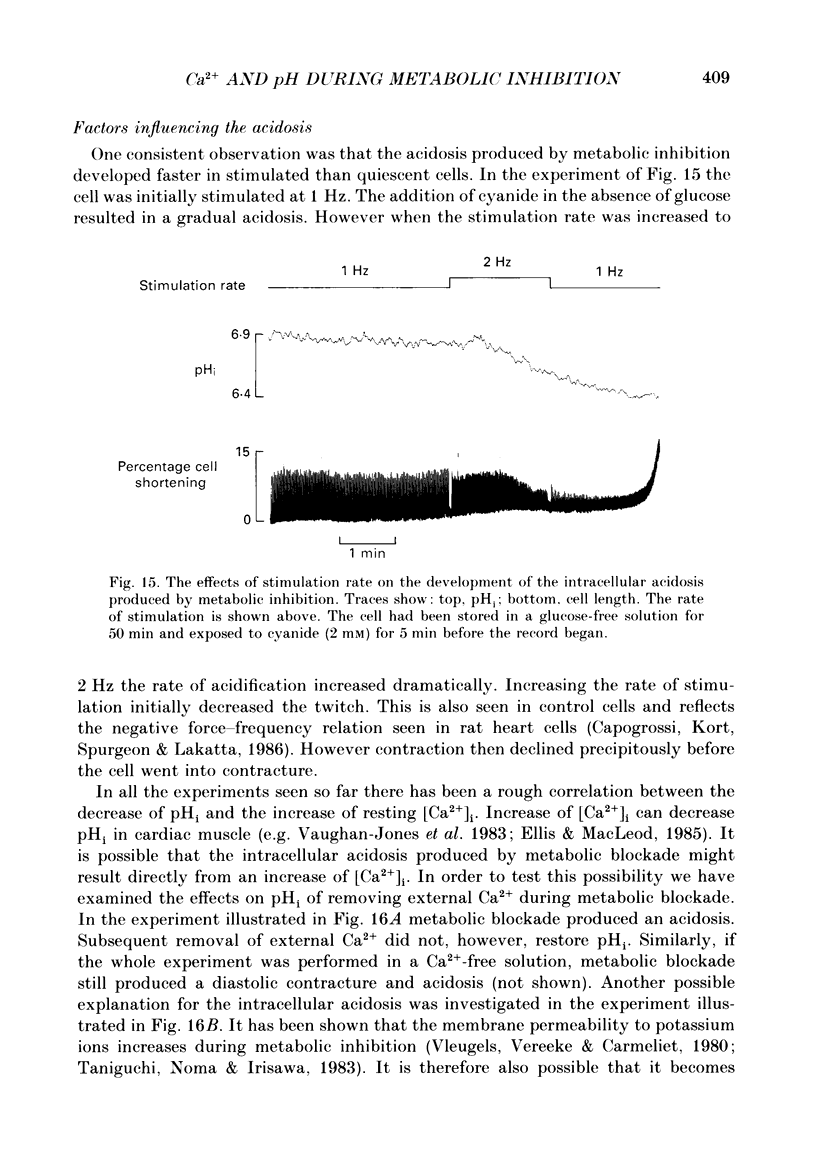
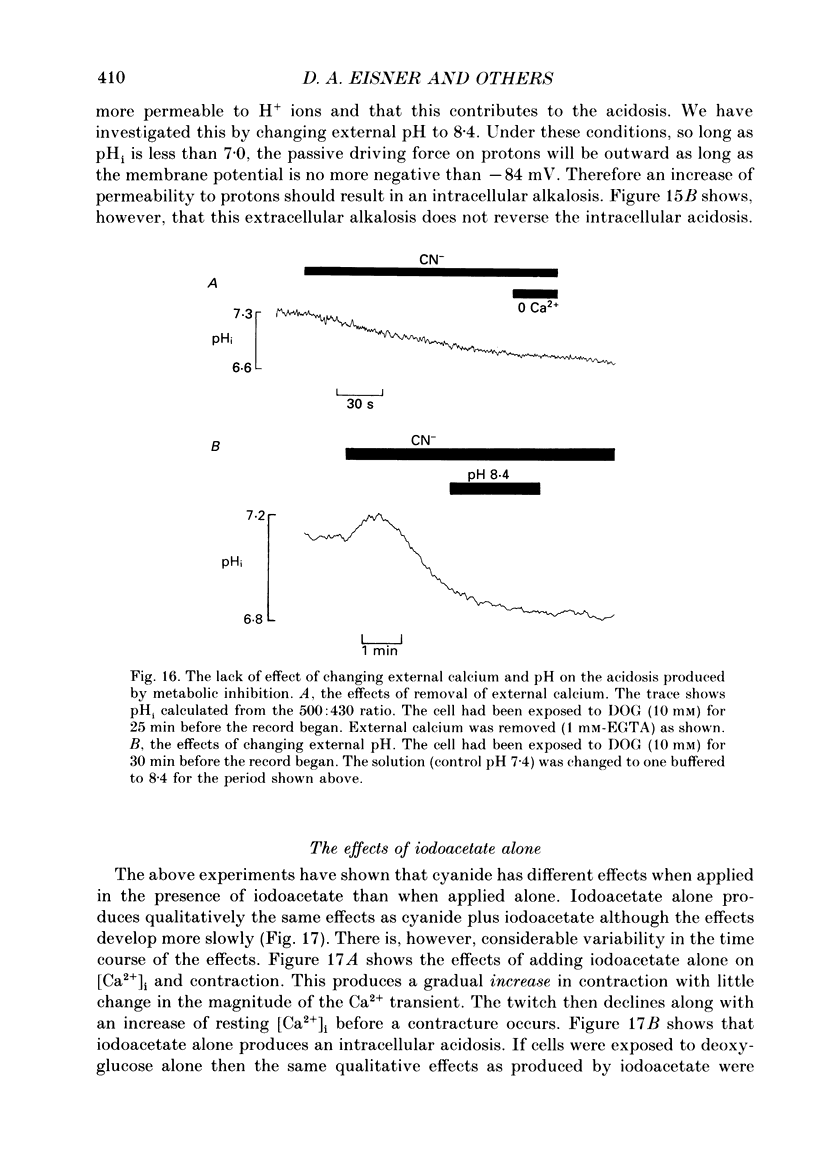
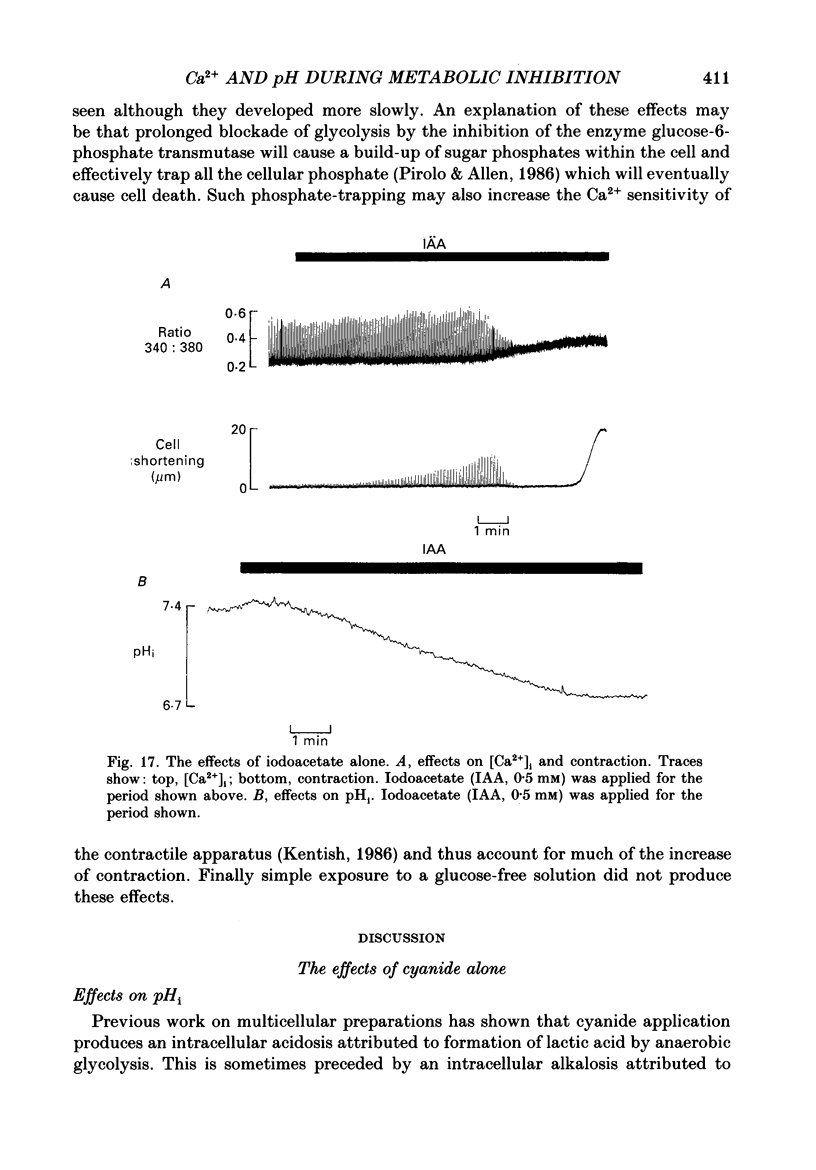
1. Intracellular calcium concentration [( Ca2+]i) and pH (pHi) were measured in single, isolated rat ventricular myocytes using, respectively, the fluorescent indicators Fura-2 and BCECF (2',7'-bis(carboxyethyl-5(6)-carboxyfluorescein). Contraction was measured simultaneously. The intracellular calibration of BCECF is demonstrated. In a HEPES-buffered bathing solution of pH 7.4, pHi had a mean value of 7.16 +/- 0.05 (mean +/- S.E.M.). 2. Addition of NH4Cl (5-20 mM) produced an intracellular alkalosis that was associated with an increase of contraction amplitude. Removal of NH4Cl produced an acidosis and decrease of contraction. 3. The addition of 2 mM-cyanide (CN-) to inhibit oxidative phosphorylation had variable effects on contraction amplitude. Changes of contraction amplitude could largely be accounted for by changes in the systolic Ca2+ transient. 4. CN- addition increased lactic acid production. However, in the majority of experiments, this was not accompanied by an intracellular acidosis. 5. Anaerobic glycolysis was inhibited by either removal of glucose, addition of deoxyglucose, or addition of iodoacetate. Under these conditions the application of CN- decreased systolic [Ca2+]i and contraction amplitude. This was sometimes preceded by a transient increase of systolic [Ca2+]i and contraction amplitude. 6. When glycolysis was inhibited, the subsequent addition of CN- always increased diastolic [Ca2+]i and produced a contracture. The increase of [Ca2+]i occurred before the contracture. However, once the contracture had developed, decreasing [Ca2+]i (by removal of external Ca2+) did not cause relaxation. 7. With glycolysis inhibited, addition of CN- resulted in a large (0.51 +/- 0.05 pH unit) acidosis that was sometimes preceded by an alkalosis. This acidosis was unaffected by removal of external Ca2+ or external alkalinization. Calculations show that some of this acidosis may result from protons released by ATP hydrolysis. 8. If the acidosis produced by metabolic blockade was partly reversed by adding NH4Cl then a contracture immediately developed. This suggests that the acidosis delays the onset of the contracture. 9. We conclude that metabolic inhibition increases diastolic [Ca2+]i. The accompanying acidosis prevents contraction. Once the contracture has developed it is maintained by factors other than increased [Ca2+]i, possibly by a fall of [ATP].
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Orchard C. H. Factors influencing free intracellular calcium concentration in quiescent ferret ventricular muscle. J Physiol. 1984 May;350:615–630. doi: 10.1113/jphysiol.1984.sp015221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. Intracellular calcium concentration during hypoxia and metabolic inhibition in mammalian ventricular muscle. J Physiol. 1983 Jun;339:107–122. doi: 10.1113/jphysiol.1983.sp014706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987 Feb;60(2):153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Allshire A., Piper H. M., Cuthbertson K. S., Cobbold P. H. Cytosolic free Ca2+ in single rat heart cells during anoxia and reoxygenation. Biochem J. 1987 Jun 1;244(2):381–385. doi: 10.1042/bj2440381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Eisner D., Cohen I. Voltage clamp and tracer flux data: effects of a restricted extra-cellular space. Q Rev Biophys. 1979 Aug;12(3):213–261. doi: 10.1017/s0033583500005448. [DOI] [PubMed] [Google Scholar]
- Bailey I. A., Radda G. K., Seymour A. M., Williams S. R. The effects of insulin on myocardial metabolism and acidosis in normoxia and ischaemia. A 31P-NMR study. Biochim Biophys Acta. 1982 Feb 10;720(1):17–27. doi: 10.1016/0167-4889(82)90034-9. [DOI] [PubMed] [Google Scholar]
- Barry W. H., Peeters G. A., Rasmussen C. A., Jr, Cunningham M. J. Role of changes in [Ca2+]i in energy deprivation contracture. Circ Res. 1987 Nov;61(5):726–734. doi: 10.1161/01.res.61.5.726. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982 Apr;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- Capogrossi M. C., Kort A. A., Spurgeon H. A., Lakatta E. G. Single adult rabbit and rat cardiac myocytes retain the Ca2+- and species-dependent systolic and diastolic contractile properties of intact muscle. J Gen Physiol. 1986 Nov;88(5):589–613. doi: 10.1085/jgp.88.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. The effects of temperature and metabolic inhibitors on the spontaneous relaxation of the potassium contracture of the heart of the frog Rana pipiens. J Physiol. 1973 Jun;231(2):233–249. doi: 10.1113/jphysiol.1973.sp010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold P. H., Bourne P. K. Aequorin measurements of free calcium in single heart cells. 1984 Nov 29-Dec 5Nature. 312(5993):444–446. doi: 10.1038/312444a0. [DOI] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuticke B. Monocarboxylate transport in erythrocytes. J Membr Biol. 1982;70(2):89–103. doi: 10.1007/BF01870219. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Elliott A. C., Smith G. L. The contribution of intracellular acidosis to the decline of developed pressure in ferret hearts exposed to cyanide. J Physiol. 1987 Oct;391:99–108. doi: 10.1113/jphysiol.1987.sp016728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The dependence of sodium pumping and tension on intracellular sodium activity in voltage-clamped sheep Purkinje fibres. J Physiol. 1981 Aug;317:163–187. doi: 10.1113/jphysiol.1981.sp013819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Orchard C. H., Allen D. G. Control of intracellular ionized calcium concentration by sarcolemmal and intracellular mechanisms. J Mol Cell Cardiol. 1984 Feb;16(2):137–146. doi: 10.1016/s0022-2828(84)80702-6. [DOI] [PubMed] [Google Scholar]
- Ellis D., MacLeod K. T. Sodium-dependent control of intracellular pH in Purkinje fibres of sheep heart. J Physiol. 1985 Feb;359:81–105. doi: 10.1113/jphysiol.1985.sp015576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., Noireaud J. Intracellular pH in sheep Purkinje fibres and ferret papillary muscles during hypoxia and recovery. J Physiol. 1987 Feb;383:125–141. doi: 10.1113/jphysiol.1987.sp016400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C. H., Harding D. P., Mounsey J. P. The effects of cyanide on intracellular ionic exchange in ferret and rat ventricular myocardium. Proc R Soc Lond B Biol Sci. 1987 Feb 23;230(1258):53–75. doi: 10.1098/rspb.1987.0009. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R., Berkoff H. A., Moss R. L. Metabolic cost of the stimulated beating of isolated adult rat heart cells in suspension. Circ Res. 1983 Mar;52(3):342–351. doi: 10.1161/01.res.52.3.342. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Bloebaum P., Snowdowne K. W. Sarcoplasmic reticulum interacts with the Ca(2+) indicator precursor fura-2-am. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1153–1162. doi: 10.1016/s0006-291x(86)80403-x. [DOI] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Smith T. W. Cellular mechanisms underlying calcium-proton interactions in cultured chick ventricular cells. J Physiol. 1988 Apr;398:391–410. doi: 10.1113/jphysiol.1988.sp017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T. Effect of extracellular calcium on contractile activation in guinea-pig ventricular muscle. J Physiol. 1984 Oct;355:635–659. doi: 10.1113/jphysiol.1984.sp015443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Lukács G. L., Kapus A. Measurement of the matrix free Ca2+ concentration in heart mitochondria by entrapped fura-2 and quin2. Biochem J. 1987 Dec 1;248(2):609–613. doi: 10.1042/bj2480609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R., Gwathmey J. K., Morgan J. P. Differential effects of reoxygenation on intracellular calcium and isometric tension. Pflugers Arch. 1987 Aug;409(4-5):448–453. doi: 10.1007/BF00583800. [DOI] [PubMed] [Google Scholar]
- Paradiso A. M., Tsien R. Y., Machen T. E. Digital image processing of intracellular pH in gastric oxyntic and chief cells. 1987 Jan 29-Feb 4Nature. 325(6103):447–450. doi: 10.1038/325447a0. [DOI] [PubMed] [Google Scholar]
- Pirolo J. S., Allen D. G. Assessment of techniques for preventing glycolysis in cardiac muscle. Cardiovasc Res. 1986 Nov;20(11):837–844. doi: 10.1093/cvr/20.11.837. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safford R. E., Bassingthwaighte J. B. Calcium diffusion in transient and steady states in muscle. Biophys J. 1977 Oct;20(1):113–136. doi: 10.1016/S0006-3495(77)85539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Allen D. G. Effects of metabolic blockade on intracellular calcium concentration in isolated ferret ventricular muscle. Circ Res. 1988 Jun;62(6):1223–1236. doi: 10.1161/01.res.62.6.1223. [DOI] [PubMed] [Google Scholar]
- Taniguchi J., Noma A., Irisawa H. Modification of the cardiac action potential by intracellular injection of adenosine triphosphate and related substances in guinea pig single ventricular cells. Circ Res. 1983 Aug;53(2):131–139. doi: 10.1161/01.res.53.2.131. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Eisner D. A., Lederer W. J. Effects of changes of intracellular pH on contraction in sheep cardiac Purkinje fibers. J Gen Physiol. 1987 Jun;89(6):1015–1032. doi: 10.1085/jgp.89.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Vleugels A., Vereecke J., Carmeliet E. Ionic currents during hypoxia in voltage-clamped cat ventricular muscle. Circ Res. 1980 Oct;47(4):501–508. doi: 10.1161/01.res.47.4.501. [DOI] [PubMed] [Google Scholar]
- Wilkie D. R. Generation of protons by metabolic processes other than glycolysis in muscle cells: a critical view. J Mol Cell Cardiol. 1979 Mar;11(3):325–330. doi: 10.1016/0022-2828(79)90446-2. [DOI] [PubMed] [Google Scholar]
- Wolfe C. L., Gilbert H. F., Brindle K. M., Radda G. K. Determination of buffering capacity of rat myocardium during ischemia. Biochim Biophys Acta. 1988 Aug 19;971(1):9–20. doi: 10.1016/0167-4889(88)90156-5. [DOI] [PubMed] [Google Scholar]