Abstract
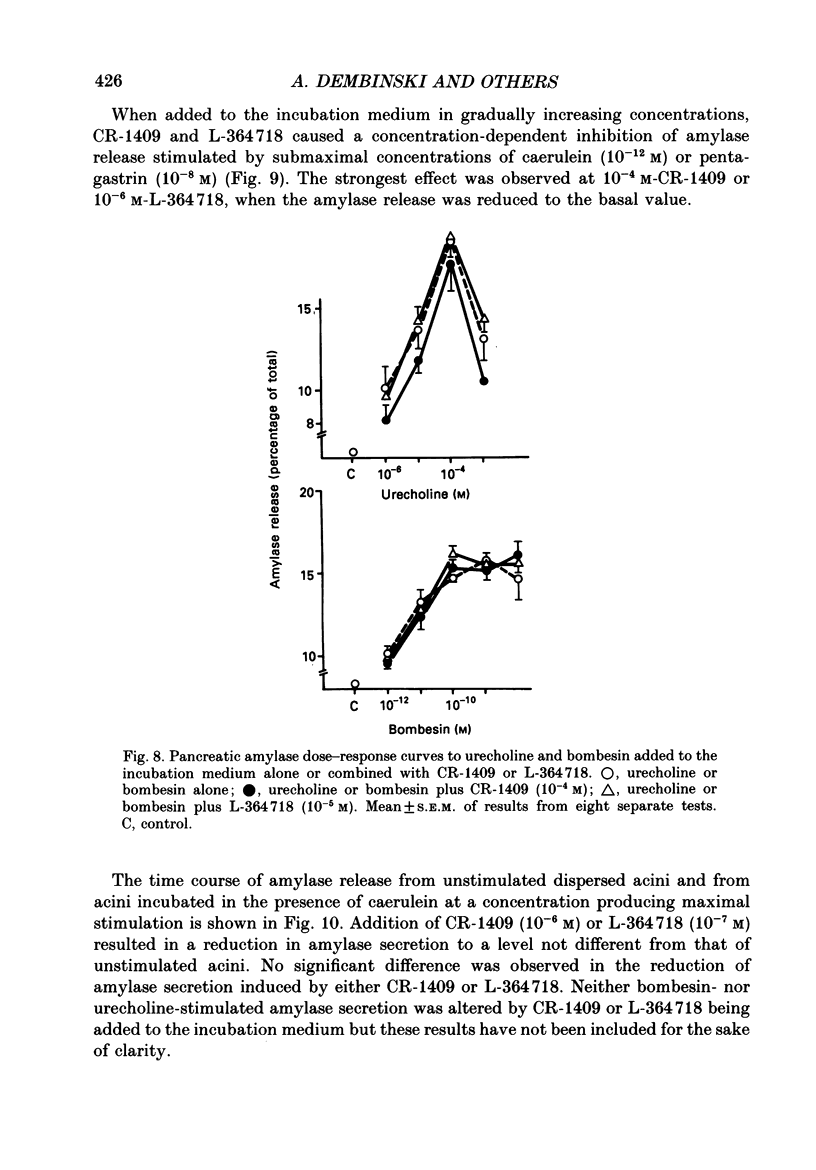
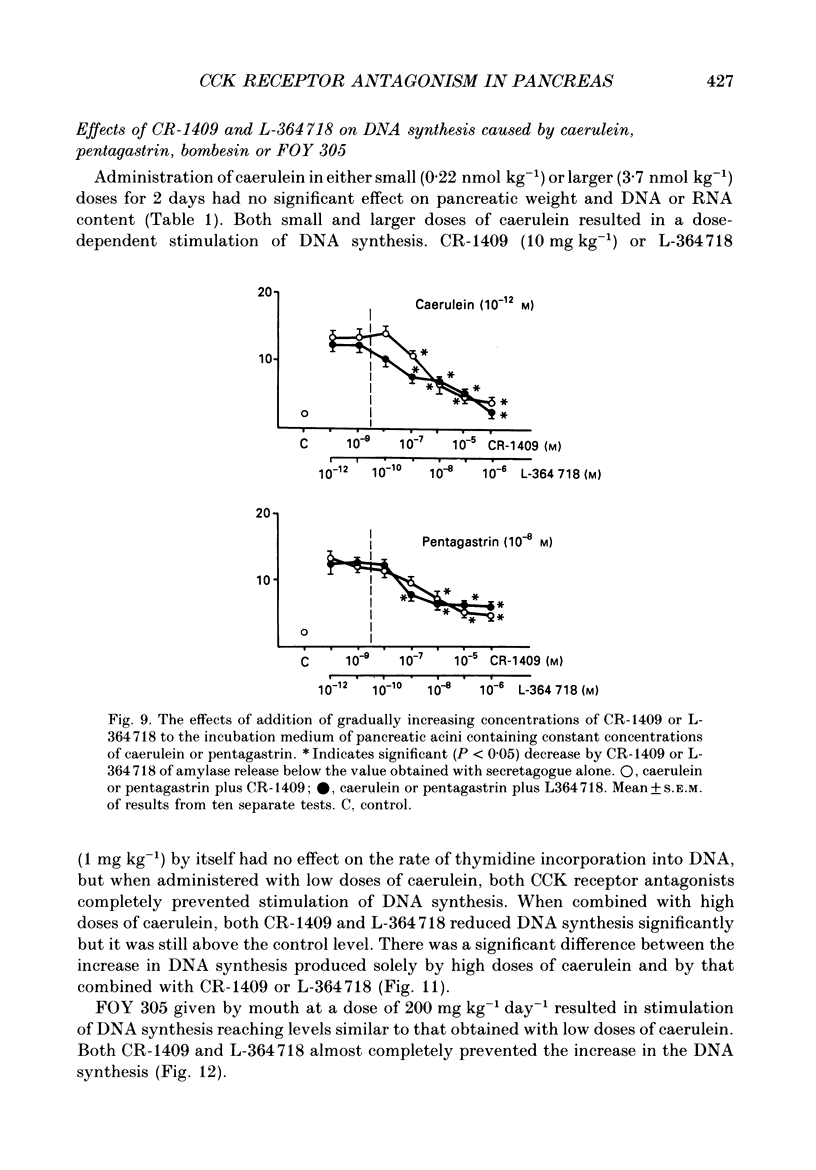
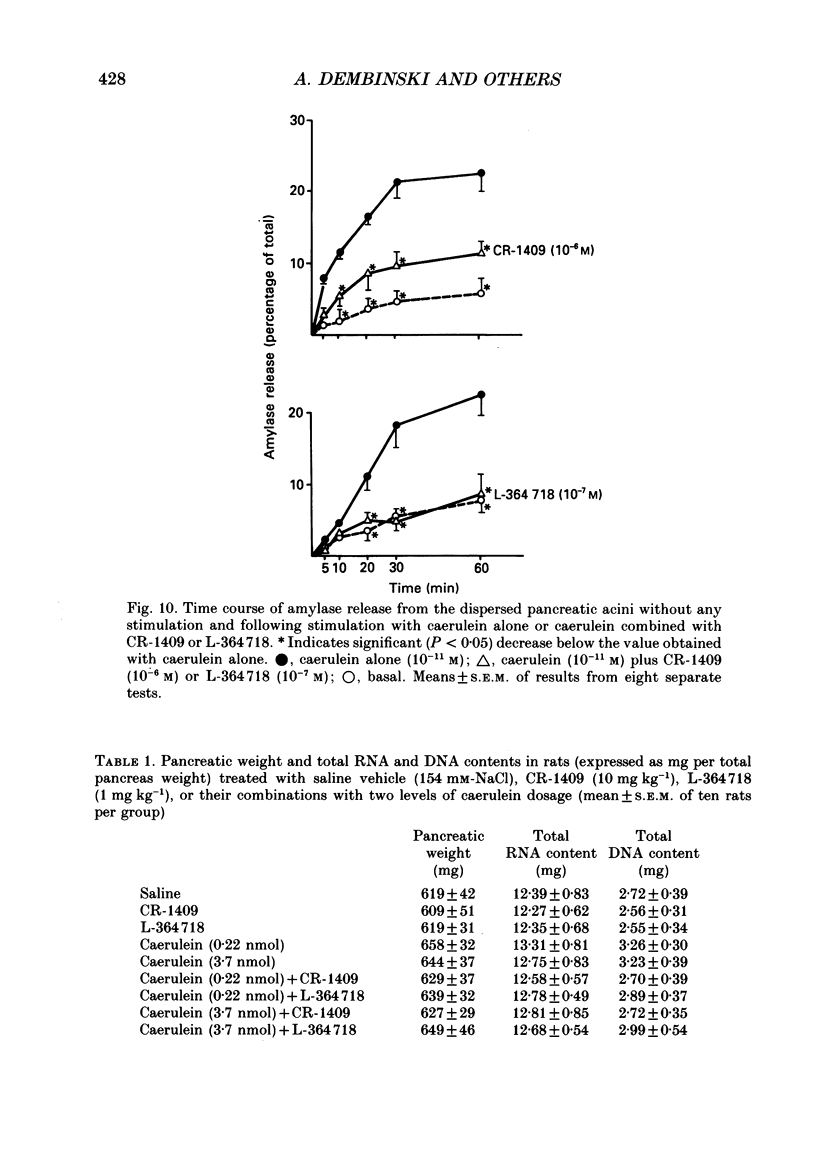
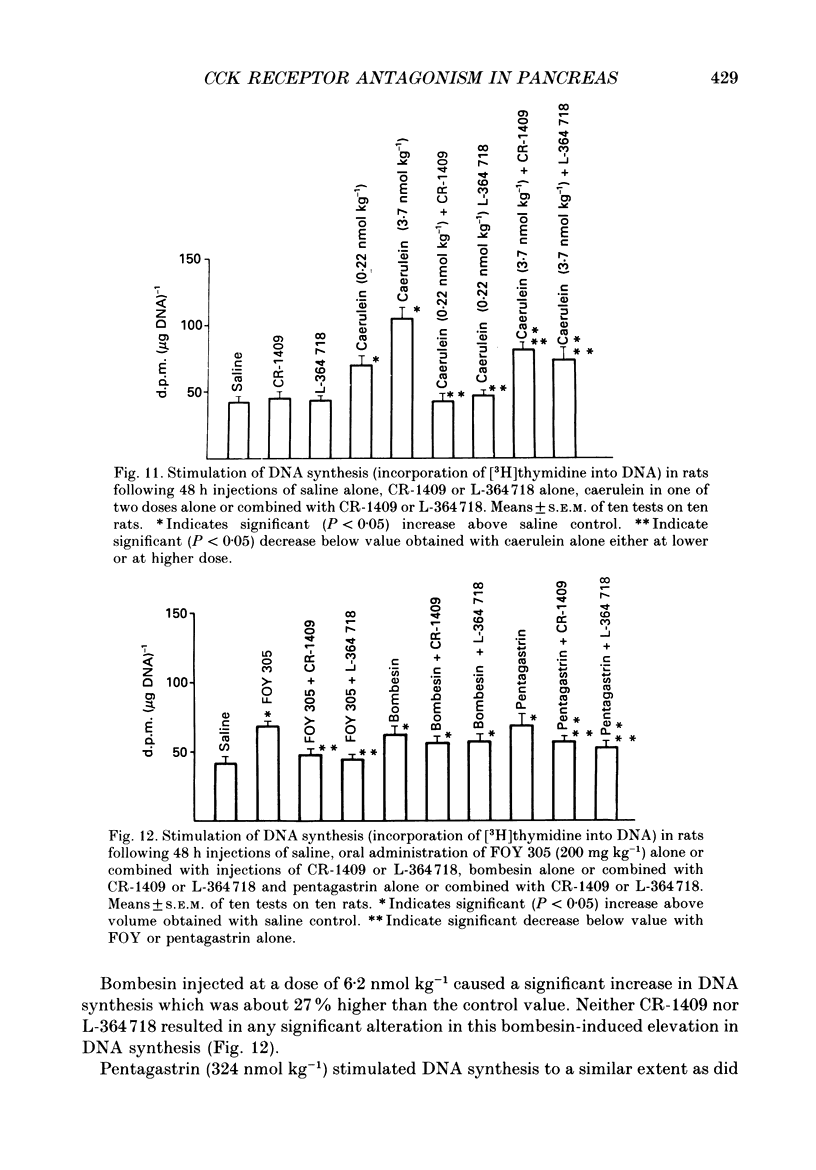
1. Graded doses of bombesin infused I.V. into conscious rats with chronic pancreatic fistulae induced a dose-dependent stimulation of protein secretion, similar to that obtained with caerulein. This stimulation does not appear to be mediated by cholecystokinin (CCK) receptors because peptidergic (CR-1409) and non-peptidergic (L-364718) CCK antagonists failed to affect protein secretion at a dose range which caused almost complete suppression of caerulein-induced pancreatic secretion. 2. Studies in vitro on isolated rat pancreatic acini revealed that caerulein, pentagastrin and bombesin all showed the same efficacy in their ability to stimulate amylase release. In contrast, CCK antagonists competitively inhibited amylase release induced by caerulein and pentagastrin but not by bombesin or urecholine, indicating that the latter two agents act directly on acinar cells via receptors which are separate from those involved in stimulation induced by caerulein and pentagastrin. 3. DNA synthesis, measured by the incorporation of [3H]thymidine into DNA, was significantly stimulated by caerulein, soybean trypsin inhibitor (FOY 305), pentagastrin and by bombesin in a dose-dependent manner. CCK receptor antagonists prevented stimulation of DNA synthesis induced by caerulein, FOY 305 and pentagastrin but not by bombesin. 4. This study indicates that bombesin strongly stimulates pancreatic enzyme secretion, with an efficacy similar to that of caerulein, and also exerts a potent growth-promoting action on the pancreas, both effects appearing to be mediated by mechanisms independent of the CCK receptors.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Solomon T. E., Jamieson J. D. Sequential dissociation of the exocrine pancreas into lobules, acini, and individual cells. Methods Cell Biol. 1978;20:361–378. doi: 10.1016/s0091-679x(08)62028-2. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S. J., Morgan R. G. The release of rat intestinal cholecystokinin after oral trypsin inhibitor measured by bio-assay. J Physiol. 1981;319:325–343. doi: 10.1113/jphysiol.1981.sp013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Monaghan R. L., Birnbaum J., Stapley E. O., Goetz M. A., Albers-Schönberg G., Patchett A. A., Liesch J. M., Hensens O. D. A potent nonpeptide cholecystokinin antagonist selective for peripheral tissues isolated from Aspergillus alliaceus. Science. 1985 Oct 11;230(4722):177–179. doi: 10.1126/science.2994227. [DOI] [PubMed] [Google Scholar]
- Dembinski A. B., Johnson L. R. Stimulation of pancreatic growth by secretin, caerulein, and pentagastrin. Endocrinology. 1980 Jan;106(1):323–328. doi: 10.1210/endo-106-1-323. [DOI] [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., De Neef P., Lammens M., Christophe J. In vitro action of bombesin and bombesin-like peptides on amylase secretion, calcium efflux, and adenylate cyclase activity in the rat pancreas: a comparison with other secretagogues. J Clin Invest. 1976 Oct;58(4):891–898. doi: 10.1172/JCI108542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., De Neef P., Lammens M., Christophe J. In vitro action of bombesin and bombesin-like peptides on amylase secretion, calcium efflux, and adenylate cyclase activity in the rat pancreas: a comparison with other secretagogues. J Clin Invest. 1976 Oct;58(4):891–898. doi: 10.1172/JCI108542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fender H. R., Curtis D. J., Rayford P. L., Thompson J. C. Effect of bombesin on serum gastrin and cholecystokinin in dogs. Surg Forum. 1976;27(62):414–416. [PubMed] [Google Scholar]
- Fölsch U. R., Cantor P., Wilms H. M., Schafmayer A., Becker H. D., Creutzfeldt W. Role of cholecystokinin in the negative feedback control of pancreatic enzyme secretion in conscious rats. Gastroenterology. 1987 Feb;92(2):449–458. doi: 10.1016/0016-5085(87)90141-7. [DOI] [PubMed] [Google Scholar]
- Fölsch U. R., Winckler K., Wormsley K. G. Influence of repeated administration of cholecystokinin and secretin on the pancreas of the rat. Scand J Gastroenterol. 1978;13(6):663–671. doi: 10.3109/00365527809181779. [DOI] [PubMed] [Google Scholar]
- Göke B., Printz H., Koop I., Rausch U., Richter G., Arnold R., Adler G. Endogenous CCK release and pancreatic growth in rats after feeding a proteinase inhibitor (camostate). Pancreas. 1986;1(6):509–515. doi: 10.1097/00006676-198611000-00008. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Raper H. S. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol. 1943 Jun 30;102(1):115–125. doi: 10.1113/jphysiol.1943.sp004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Gardner J. D. Identification and characterization of receptors for secretagogues on pancreatic acinar cells. Fed Proc. 1981 Aug;40(10):2486–2496. [PubMed] [Google Scholar]
- Jensen R. T., Moody T., Pert C., Rivier J. E., Gardner J. D. Interaction of bombesin and litorin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6139–6143. doi: 10.1073/pnas.75.12.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Zhou Z. C., Murphy R. B., Jones S. W., Setnikar I., Rovati L. A., Gardner J. D. Structural features of various proglumide-related cholecystokinin receptor antagonists. Am J Physiol. 1986 Dec;251(6 Pt 1):G839–G846. doi: 10.1152/ajpgi.1986.251.6.G839. [DOI] [PubMed] [Google Scholar]
- Khayambashi H., Lyman R. L. Secretion of rat pancreas perfused with plasma from rats fed soybean trypsin inhibitor. Am J Physiol. 1969 Sep;217(3):646–651. doi: 10.1152/ajplegacy.1969.217.3.646. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Król R., Tasler J. Effect of bombesin and related peptides on the release and action of intestinal hormones on pancreatic secretion. J Physiol. 1976 Jun;257(3):663–672. doi: 10.1113/jphysiol.1976.sp011390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek S. J., Tasler J., Cieszkowski M., Szewczyk K., Hladij M. Effect of cholecystokinin receptor antagonist on pancreatic responses to exogenous gastrin and cholecystokinin and to meal stimuli. Gastroenterology. 1988 Apr;94(4):1014–1023. doi: 10.1016/0016-5085(88)90561-6. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Newman B. M., Praissman M., Cooney D. R., Lebenthal E. Cholecystokinin: a factor responsible for the enteral feedback control of pancreatic hypertrophyphy. Pancreas. 1986;1(4):335–340. [PubMed] [Google Scholar]
- Lehy T., Puccio F., Chariot J., Labeille D. Stimulating effect of bombesin on the growth of gastrointestinal tract and pancreas in suckling rats. Gastroenterology. 1986 Jun;90(6):1942–1949. doi: 10.1016/0016-5085(86)90265-9. [DOI] [PubMed] [Google Scholar]
- Lhoste E., Aprahamian M., Pousse A., Hoeltzel A., Stock-Damge C. Combined effect of chronic bombesin and secretin or cholecystokinin on the rat pancreas. Peptides. 1985;6 (Suppl 3):83–87. doi: 10.1016/0196-9781(85)90355-9. [DOI] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Williams J. A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- Lotti V. J., Pendleton R. G., Gould R. J., Hanson H. M., Chang R. S., Clineschmidt B. V. In vivo pharmacology of L-364,718, a new potent nonpeptide peripheral cholecystokinin antagonist. J Pharmacol Exp Ther. 1987 Apr;241(1):103–109. [PubMed] [Google Scholar]
- Louie D. S., Williams J. A., Owyang C. Action of pancreatic polypeptide on rat pancreatic secretion: in vivo and in vitro. Am J Physiol. 1985 Oct;249(4 Pt 1):G489–G495. doi: 10.1152/ajpgi.1985.249.4.G489. [DOI] [PubMed] [Google Scholar]
- Mainz D. L., Black O., Webster P. D. Hormonal control of pancreatic growth. J Clin Invest. 1973 Sep;52(9):2300–2304. doi: 10.1172/JCI107418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J. Effect of caerulein on protein synthesis and secretion in the guinea-pig pancreas. Br J Pharmacol. 1970 Dec;40(4):721–731. doi: 10.1111/j.1476-5381.1970.tb10649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon W. H., Beauchamp R. D., Townsend C. M., Jr, Thompson J. C. Role of cholecystokinin in canine pancreatic exocrine response to bombesin stimulation. Am J Surg. 1987 Jan;153(1):96–101. doi: 10.1016/0002-9610(87)90208-x. [DOI] [PubMed] [Google Scholar]
- Pendleton R. G., Bendesky R. J., Schaffer L., Nolan T. E., Gould R. J., Clineschmidt B. V. Roles of endogenous cholecystokinin in biliary, pancreatic and gastric function: studies with L-364,718, a specific cholecystokinin receptor antagonist. J Pharmacol Exp Ther. 1987 Apr;241(1):110–116. [PubMed] [Google Scholar]
- Petersen H., Solomon T., Grossman M. I. Effect of chronic pentagastrin, cholecystokinin, and secretin on pancreas of rats. Am J Physiol. 1978 Mar;234(3):E286–E293. doi: 10.1152/ajpendo.1978.234.3.E286. [DOI] [PubMed] [Google Scholar]
- Schneeman B. O., Chang I., Smith L. B., Lyman R. L. Effect of dietary amino acids, casein, and soybean trypsin inhibitor on pancreatic protein secretion in rats. J Nutr. 1977 Feb;107(2):281–288. doi: 10.1093/jn/107.2.281. [DOI] [PubMed] [Google Scholar]
- Wisner J. R., Jr, McLaughlin R. E., Rich K. A., Ozawa S., Renner I. G. Effects of L-364,718, a new cholecystokinin receptor antagonist, on camostate-induced growth of the rat pancreas. Gastroenterology. 1988 Jan;94(1):109–113. doi: 10.1016/0016-5085(88)90617-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Tabata K., Johnson L. R. Effect of proglumide on rat pancreatic growth. Am J Physiol. 1985 Aug;249(2 Pt 1):G294–G298. doi: 10.1152/ajpgi.1985.249.2.G294. [DOI] [PubMed] [Google Scholar]


