Abstract
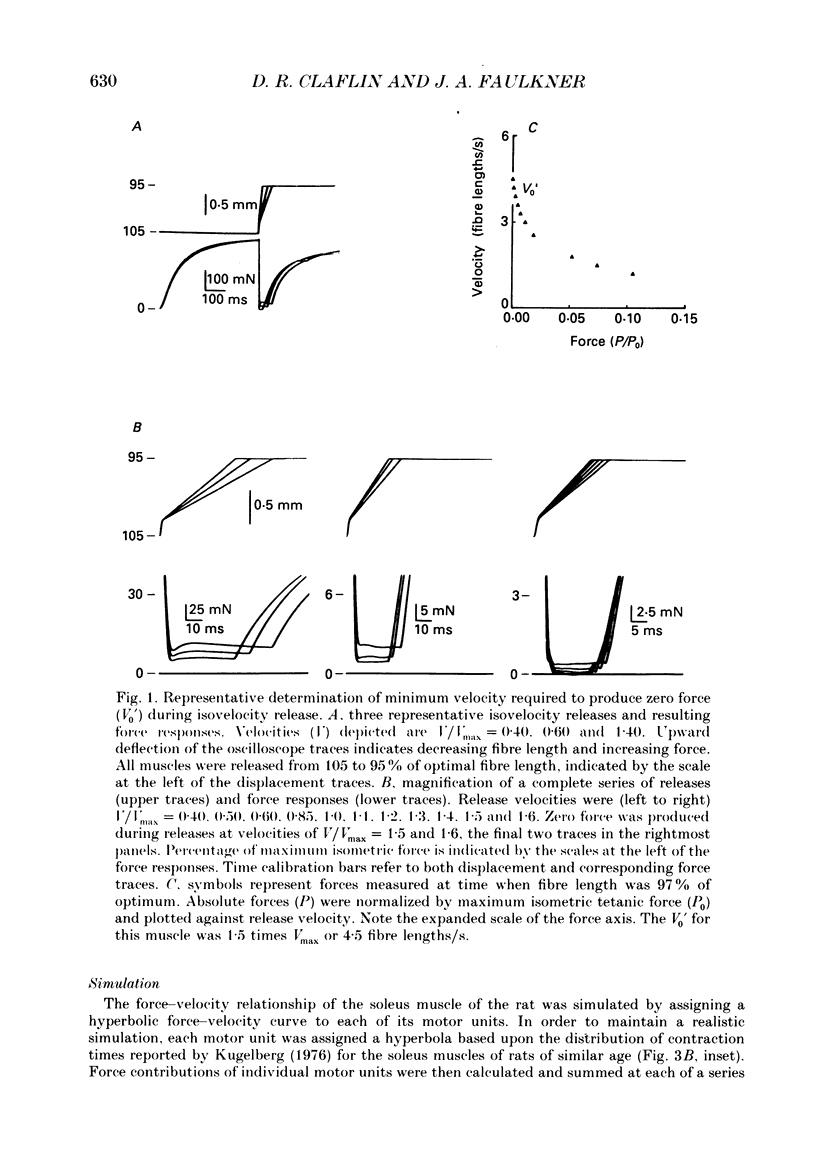
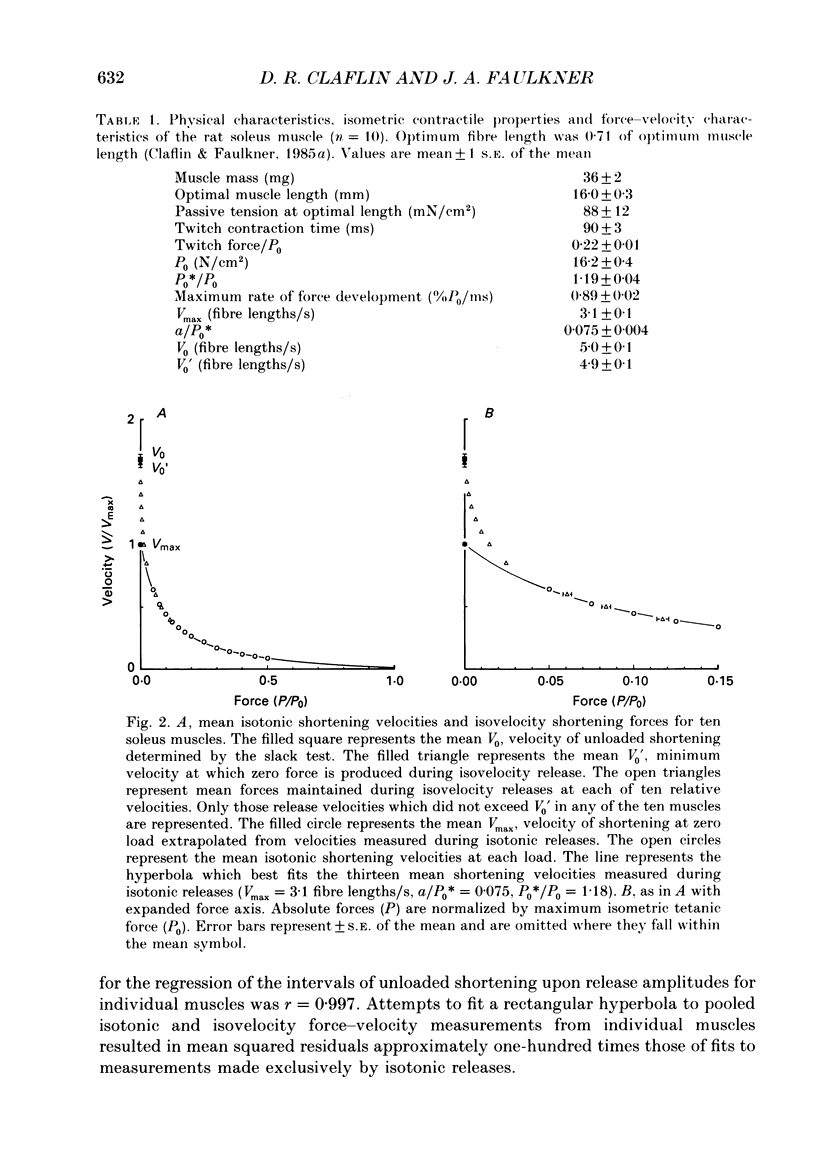
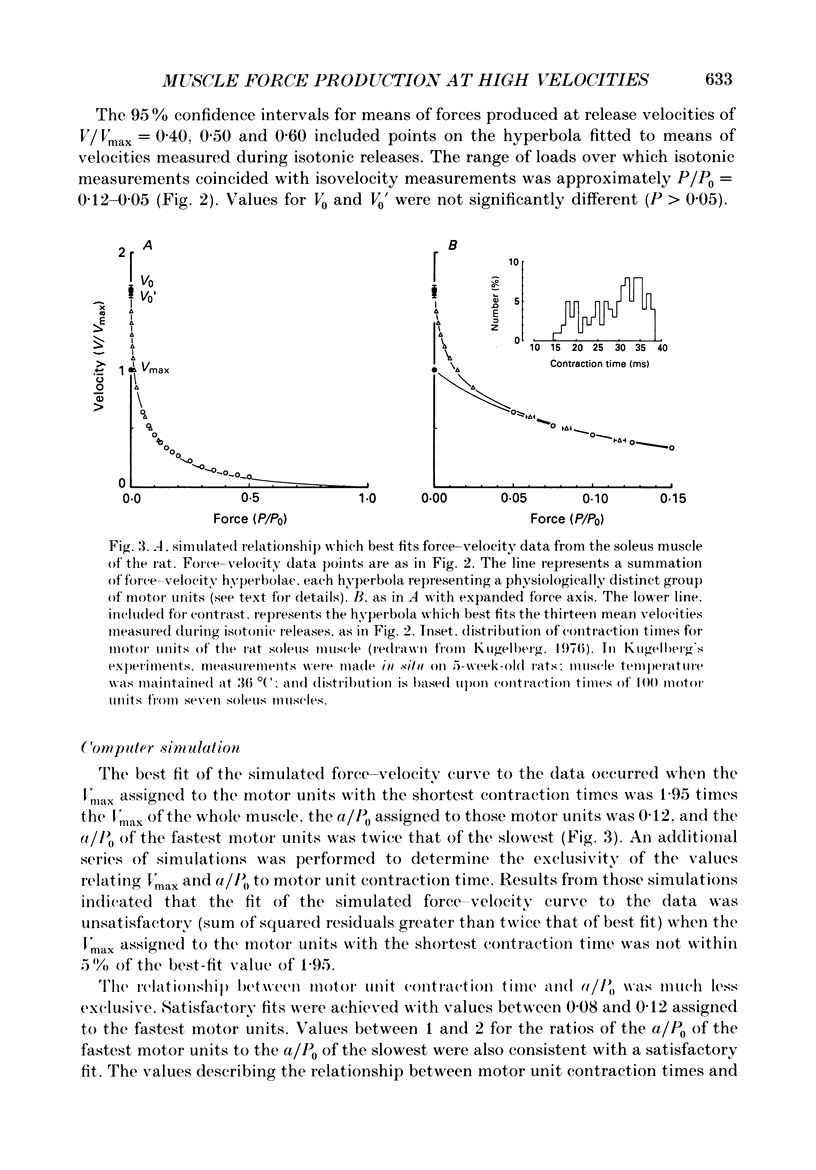
1. In intact skeletal muscle fibres, estimates of unloaded shortening velocity obtained from slack test measurements (V0) have been shown to exceed, by approximately 7%, estimates obtained from extrapolation of velocities measured during isotonic releases (Vmax). In contrast, published values for the V0 of whole soleus muscles of rats exceed Vmax by 56%. In the present study, we tested the hypothesis that this difference between whole muscles and single fibres is due to a difference in their respective force-velocity relationships at loads less than 5% of maximum isometric tetanic force (P0). In addition, we examined, by computer simulation, the effect of inter-fibre heterogeneity on the force-velocity characteristics of a whole muscle. 2. The force-velocity relationship of soleus muscles of rats was determined at low loads, in vitro at 20 degrees C, by recording force maintained during controlled shortening at constant velocities. The relationship was simulated by assigning a hyperbolic force-velocity curve to each motor unit and summing the force contributions of individual units at each of a series of velocities. 3. When measurements from low loads were included, the force-velocity relationship intersected the velocity axis at V0 (5.0 +/- 0.1 fibre lengths/s, mean +/- S.E.M., n = 10), not Vmax (3.1 +/- 0.1 fibre lengths/s). The simulated and measured force-velocity relationships agreed at all loads, supporting the premise that the deviation from hyperbolic form responsible for the large disparity between V0 and Vmax of whole muscles is a consequence of heterogeneity in shortening velocity among fibres.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. Force-velocity relation in normal and nitrate-treated frog single muscle fibres during rise of tension in an isometric tetanus. J Physiol. 1978 Dec;285:257–273. doi: 10.1113/jphysiol.1978.sp012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin D. R., Faulkner J. A. Shortening velocity extrapolated to zero load and unloaded shortening velocity of whole rat skeletal muscle. J Physiol. 1985 Feb;359:357–363. doi: 10.1113/jphysiol.1985.sp015589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967 Nov;193(1):45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. The relation between intrinsic speed of shortening and duration of the active state of muscle. J Physiol. 1965 Oct;180(3):542–559. doi: 10.1113/jphysiol.1965.sp007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edström L., Larsson L. Effects of age on contractile and enzyme-histochemical properties of fast- and slow-twitch single motor units in the rat. J Physiol. 1987 Nov;392:129–145. doi: 10.1113/jphysiol.1987.sp016773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett R. A., O'Donovan M. J., Stephens J. A., Taylor A. Motor unit organization of human medial gastrocnemius. J Physiol. 1979 Feb;287:33–43. doi: 10.1113/jphysiol.1979.sp012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson R. K., Edman K. A. The consequences of fibre heterogeneity on the force-velocity relation of skeletal muscle. Acta Physiol Scand. 1988 Mar;132(3):341–352. doi: 10.1111/j.1748-1716.1988.tb08338.x. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J. An intermediate type of muscle fibre in Xenopus laevis. Nature. 1979 May 17;279(5710):254–256. doi: 10.1038/279254a0. [DOI] [PubMed] [Google Scholar]
- Lännergren J., Lindblom P., Johansson B. Contractile properties of two varieties of twitch muscle fibres in Xenopus laevis. Acta Physiol Scand. 1982 Apr;114(4):523–535. doi: 10.1111/j.1748-1716.1982.tb07020.x. [DOI] [PubMed] [Google Scholar]
- WELLS J. B. COMPARISON OF MECHANICAL PROPERTIES BETWEEN SLOW AND FAST MAMMALIAN MUSCLES. J Physiol. 1965 May;178:252–269. doi: 10.1113/jphysiol.1965.sp007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt I. R., Gibbs C. L. Energy production of mammalian fast- and slow-twitch muscles during development. Am J Physiol. 1974 Mar;226(3):642–647. doi: 10.1152/ajplegacy.1974.226.3.642. [DOI] [PubMed] [Google Scholar]
- Woledge R. C. The energetics of tortoise muscle. J Physiol. 1968 Aug;197(3):685–707. doi: 10.1113/jphysiol.1968.sp008582. [DOI] [PMC free article] [PubMed] [Google Scholar]


