Abstract
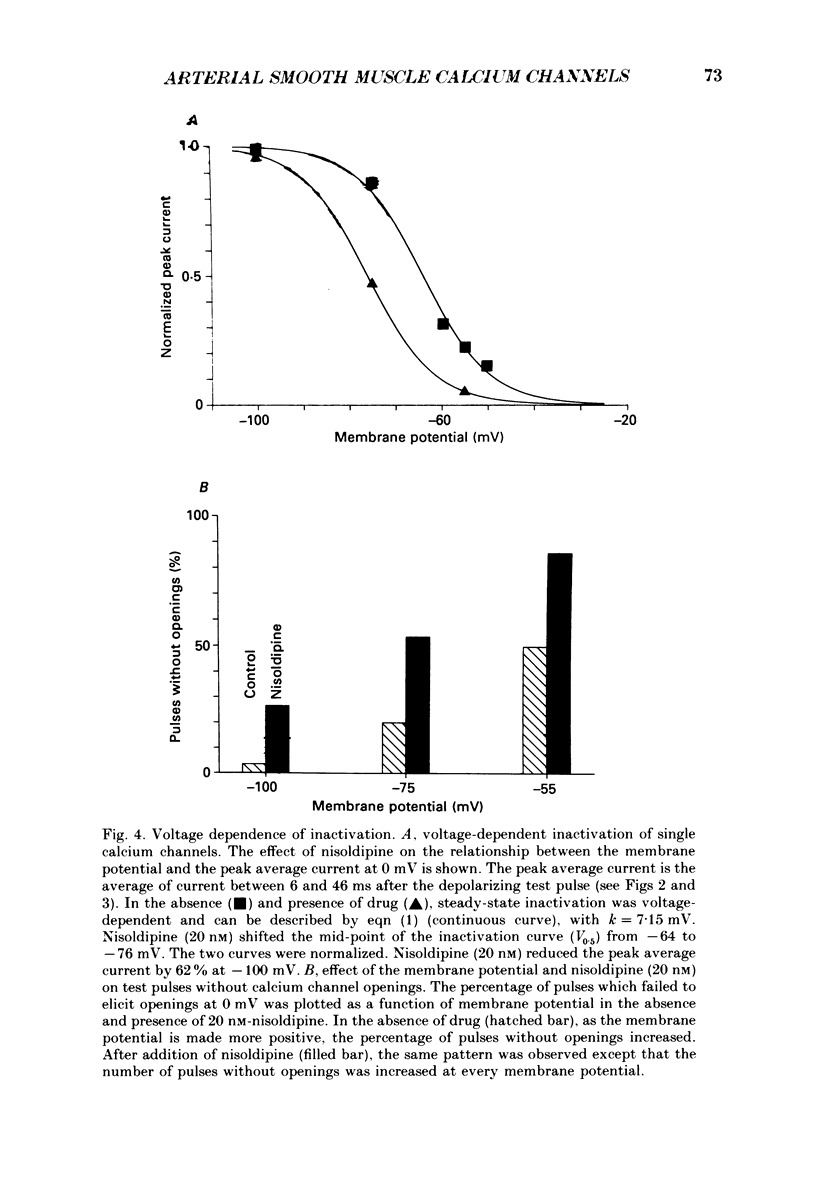
1. The effects of membrane potential and the dihydropyridine calcium channel inhibitor, nisoldipine, on single calcium channels in the presence of Bay K 8644 and contraction in the presence and absence of Bay K 8644 were examined in the rabbit mesenteric artery. 2. Membrane depolarization decreased the peak average single calcium channel current that could be elicited by a test pulse to 0 mV. The steady-state inactivation relationship could be described by the Boltzmann equation, [1 + exp[Vm-V0.5)/k)]-1, with a steepness factor, k, of 7.1 mV. Nisoldipine shifted the steady-state inactivation curve to more negative potentials by increasing the fraction of test pulses without openings. 3. The degree of nisoldipine inhibition of average single calcium channel currents increased with membrane depolarization. Depolarization of the holding potential from -100 to -55 mV decreased the concentration of nisoldipine needed for 50% inhibition (Kapp) from 12.1 to 1.9 nM in the presence of 1 microM-Bay K 8644. 4. Membrane depolarization by external potassium (K+) of the intact artery in the presence of nisoldipine decreased contractions evoked by depolarizing test pulses. The relationship between membrane potential and contraction could be empirically described by the Boltzmann equation, with a steepness factor, k, of 7.1 mV. Increasing the nisoldipine concentration from 0.25 to 2.0 nM shifted the mid-point of this relationship from -20.5 to -33.0 mV, without affecting the steepness factor. 5. Nisoldipine inhibition of contraction increased with membrane depolarization. Membrane depolarization from -68.6 to -30.0 mV decreased the Kapp of nisoldipine for contractions from 3.02 to 0.69 nM. Bay K 8644 (1 microM) elevated Kapp about 9.3-fold at 5 mM-K+. In the presence of Bay K 8644, membrane depolarization from -68.6 to -30.0 mV reduced Kapp from 28.4 to 4.0 nM. 6. In the presence of nisoldipine, the effect of membrane depolarization on the time course of development of inhibition was examined. In 3 nM-nisoldipine, after membrane depolarization with 20 mM-K+, the time course of development of inhibition of force could be described by a single exponential with a time constant of 16.5 min. Membrane depolarization to a more positive potential accelerated the development of inhibition. 7. The results were interpreted by a model in which nisoldipine binds with higher affinity to the inactivated state than to the resting state of calcium channels in the mesenteric artery. The approach presented here can be used to estimate the properties of steady-state calcium channel inactivation and dihydropyridine interactions in smooth muscle cells in the intact artery under physiological conditions.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






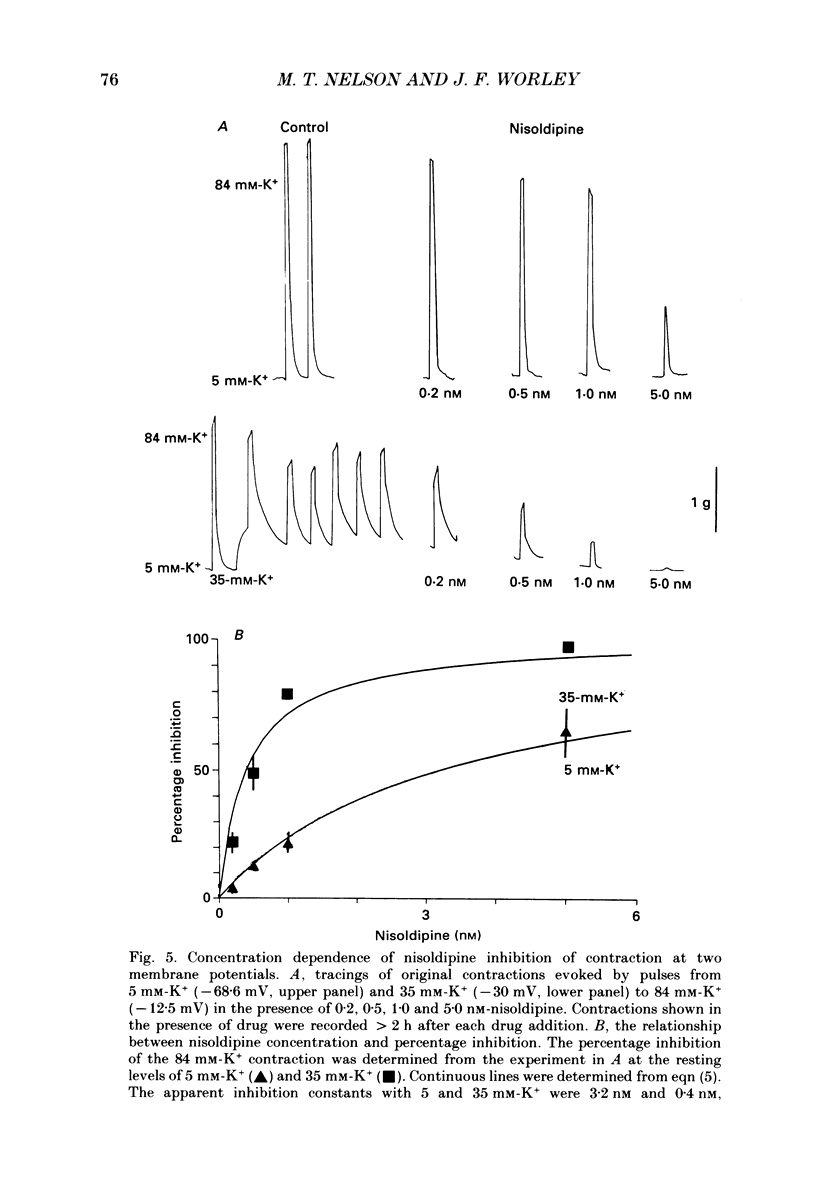

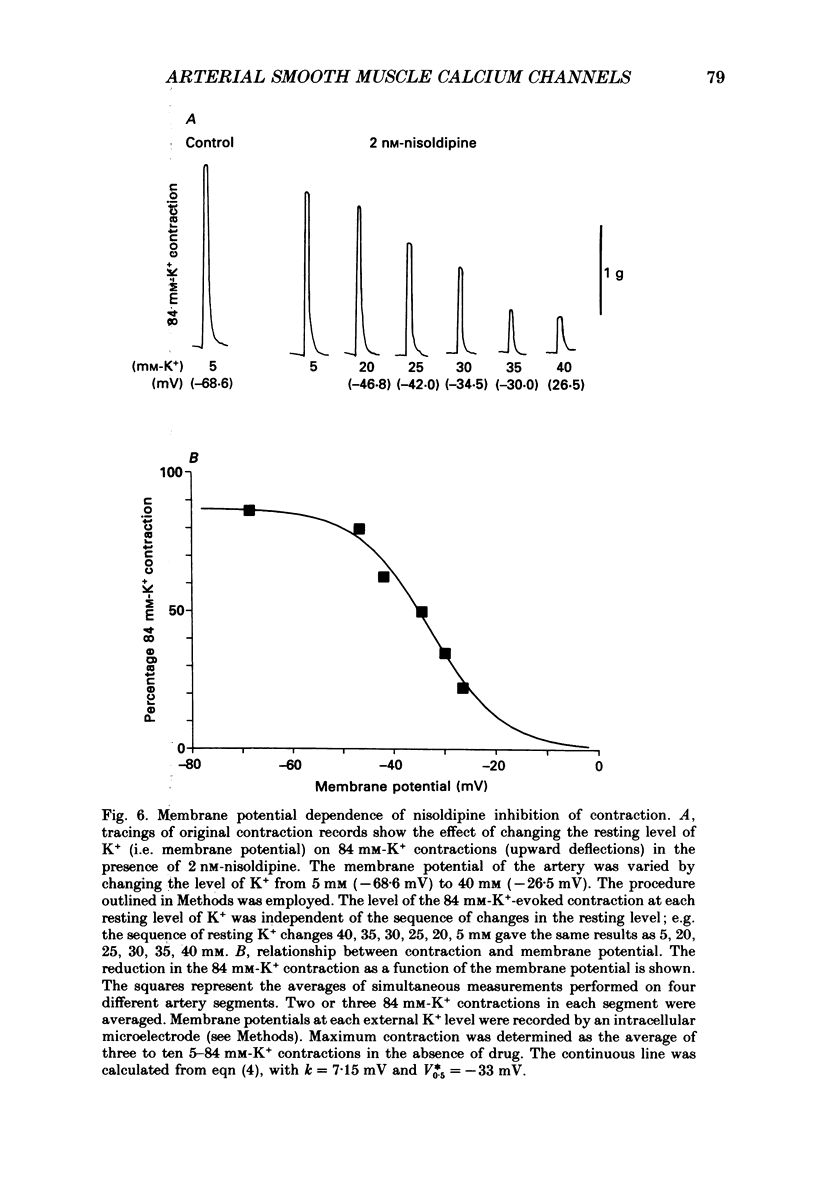
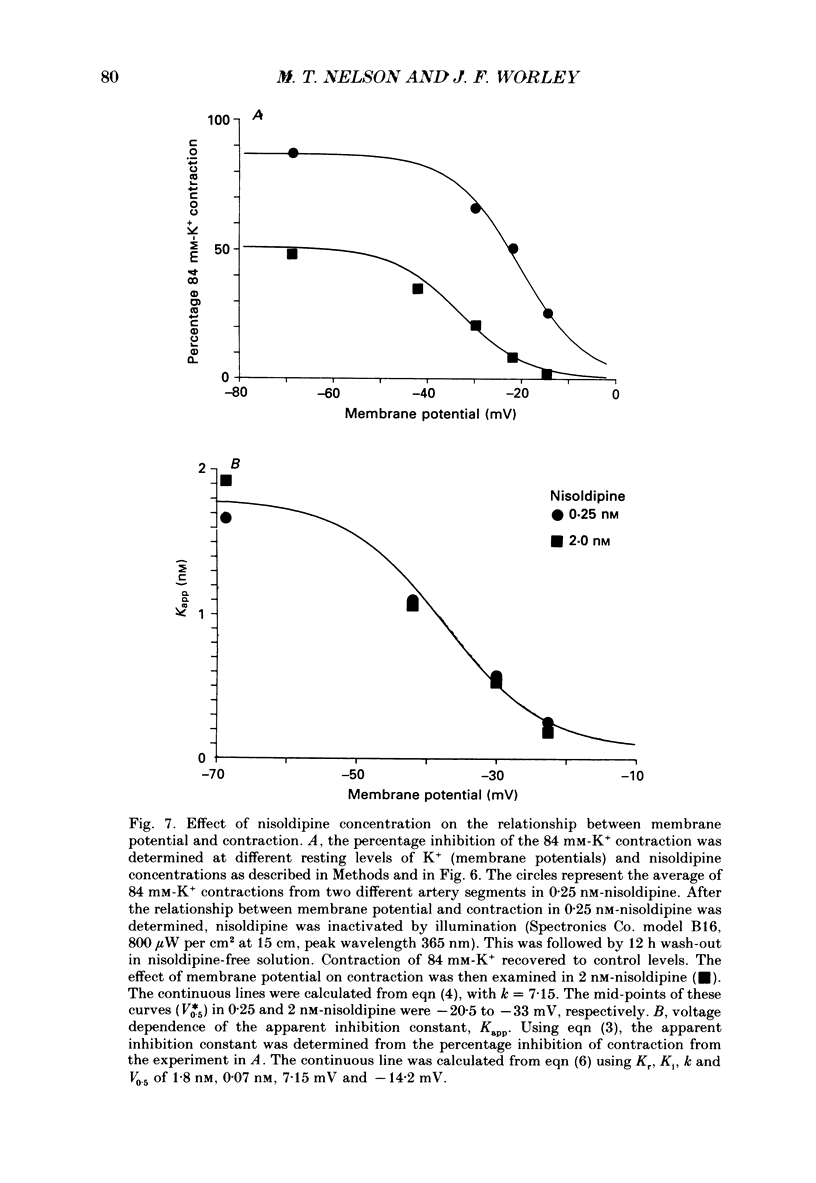









Selected References
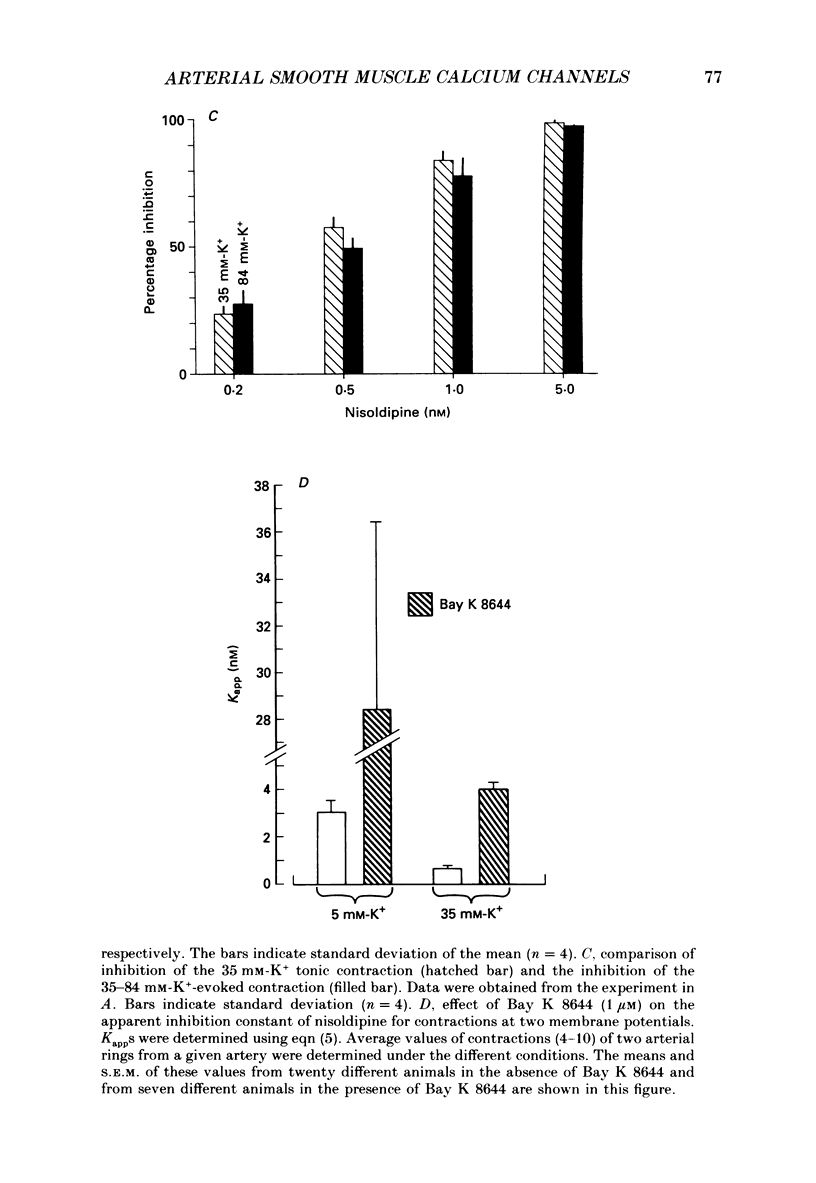
These references are in PubMed. This may not be the complete list of references from this article.
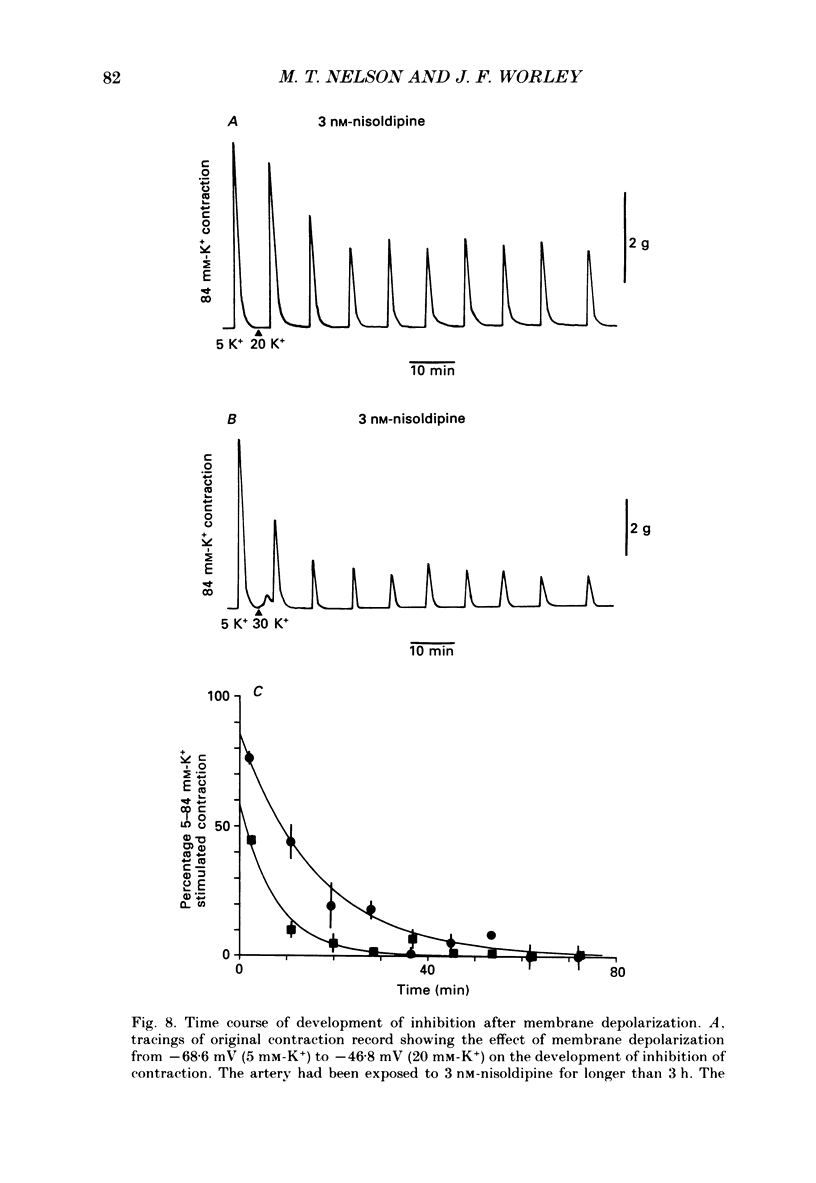
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
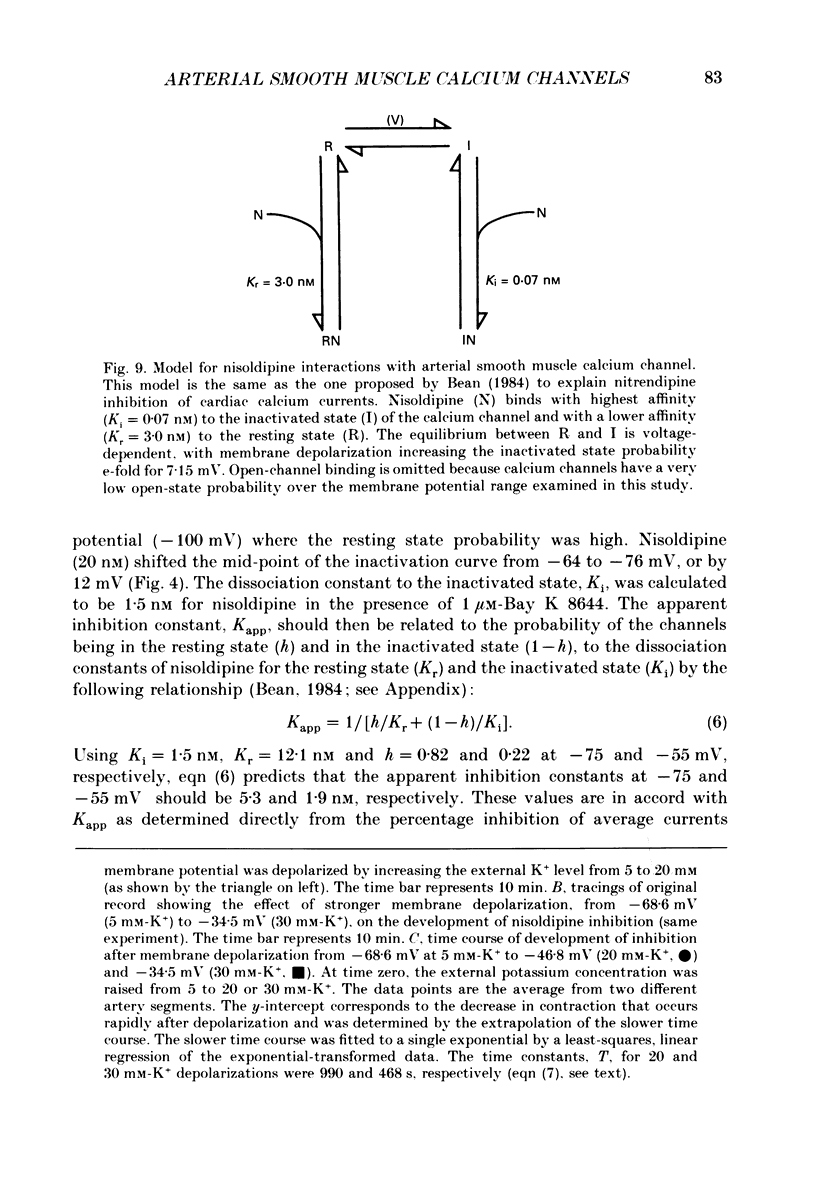
- Bean B. P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res. 1986 Aug;59(2):229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. Calcium-permeable channels in vascular smooth muscle: voltage-activated, receptor-operated, and leak channels. Soc Gen Physiol Ser. 1987;42:45–64. [PubMed] [Google Scholar]
- Bevan J. A., Osher J. V. A direct method for recording tension changes in the wall of small blood vessels in vitro. Agents Actions. 1972;2(5):257–260. doi: 10.1007/BF02087051. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Large W. A. Electrophysiological analysis of neurogenic vasodilatation in the isolated lingual artery of the rabbit. Br J Pharmacol. 1986 Sep;89(1):163–171. doi: 10.1111/j.1476-5381.1986.tb11132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Catecholamine action on smooth muscle. Pharmacol Rev. 1987 Mar;39(1):49–96. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Brann L., Halpern W. Altered membrane electrical properties of smooth muscle cells from small cerebral arteries of hypertensive rats. Blood Vessels. 1983;20(3):154–160. doi: 10.1159/000158469. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Smeda J., Lombard J. Enhanced myogenic depolarization in hypertensive cerebral arterial muscle. Circ Res. 1985 Aug;57(2):319–322. doi: 10.1161/01.res.57.2.319. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Kazda S., Garthoff B., Meyer H., Schlossmann K., Stoepel K., Towart R., Vater W., Wehinger E. Pharmacology of a new calcium antagonistic compound, isobutyl methyl 1,4-dihydro-2,6-dimethyl-4(2-nitrophenyl)-3,5-pyridinedicarboxylate (Nisoldipine, Bay k 5552). Arzneimittelforschung. 1980;30(12):2144–2162. [PubMed] [Google Scholar]
- Kokubun S., Prod'hom B., Becker C., Porzig H., Reuter H. Studies on Ca channels in intact cardiac cells: voltage-dependent effects and cooperative interactions of dihydropyridine enantiomers. Mol Pharmacol. 1986 Dec;30(6):571–584. [PubMed] [Google Scholar]
- Lederballe Pedersen O., Mikkelsen E., Andersson K. E. Effects of extracellular calcium on potassium and noradrenaline induced contractions in the aorta of spontaneously hypertensive rats--increased sensitivity to nifedipine. Acta Pharmacol Toxicol (Copenh) 1978 Aug;43(2):137–144. doi: 10.1111/j.1600-0773.1978.tb02247.x. [DOI] [PubMed] [Google Scholar]
- Ma J., Coronado R. Heterogeneity of conductance states in calcium channels of skeletal muscle. Biophys J. 1988 Mar;53(3):387–395. doi: 10.1016/S0006-3495(88)83115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita Y., Kanmura Y., Itoh T., Suzuki H., Kuriyama H. Effects of nifedipine derivatives on smooth muscle cells and neuromuscular transmission in the rabbit mesenteric artery. Naunyn Schmiedebergs Arch Pharmacol. 1983 Dec;324(4):302–312. doi: 10.1007/BF00502628. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Nilsson H., Flatman J. A. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J Physiol. 1982 Nov;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O., Keef K. Measurements of the membrane potential of arterial smooth muscle in anesthetized animals and its relationship to changes in artery diameter. Microvasc Res. 1985 Jul;30(1):19–28. doi: 10.1016/0026-2862(85)90034-2. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Standen N. B., Brayden J. E., Worley J. F., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988 Nov 24;336(6197):382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Robinson B. F., Dobbs R. J., Bayley S. Response of forearm resistance vessels to verapamil and sodium nitroprusside in normotensive and hypertensive men: evidence for a functional abnormality of vascular smooth muscle in primary hypertension. Clin Sci (Lond) 1982 Jul;63(1):33–42. doi: 10.1042/cs0630033. [DOI] [PubMed] [Google Scholar]
- Rogart R. B., deBruyn Kops A., Dzau V. J. Identification of two calcium channel receptor sites for [3H]nitrendipine in mammalian cardiac and smooth muscle membrane. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7452–7456. doi: 10.1073/pnas.83.19.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Krafte D. S., Kass R. S. Voltage-dependent modulation of Ca channel current in heart cells by Bay K8644. J Gen Physiol. 1986 Sep;88(3):369–392. doi: 10.1085/jgp.88.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., McCleskey E. W., Almers W. Dihydropyridine receptors in muscle are voltage-dependent but most are not functional calcium channels. 1985 Apr 25-May 1Nature. 314(6013):747–751. doi: 10.1038/314747a0. [DOI] [PubMed] [Google Scholar]
- Sharma J. N., Fernandez P. G., Laher I., Triggle C. R. Differential sensitivity of Dahl salt-sensitive and Dahl salt-resistant rats to the hypotensive action of acute nifedipine administration. Can J Physiol Pharmacol. 1984 Feb;62(2):241–243. doi: 10.1139/y84-036. [DOI] [PubMed] [Google Scholar]
- Talvenheimo J. A., Worley J. F., 3rd, Nelson M. T. Heterogeneity of calcium channels from a purified dihydropyridine receptor preparation. Biophys J. 1987 Nov;52(5):891–899. doi: 10.1016/S0006-3495(87)83283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Worley J. F., 3rd, Deitmer J. W., Nelson M. T. Single nisoldipine-sensitive calcium channels in smooth muscle cells isolated from rabbit mesenteric artery. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5746–5750. doi: 10.1073/pnas.83.15.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Seidel C. L., Allen J., Brown A. M. Whole-cell and single-channel calcium currents of isolated smooth muscle cells from saphenous vein. Circ Res. 1987 Apr;60(4):523–533. doi: 10.1161/01.res.60.4.523. [DOI] [PubMed] [Google Scholar]


