Abstract
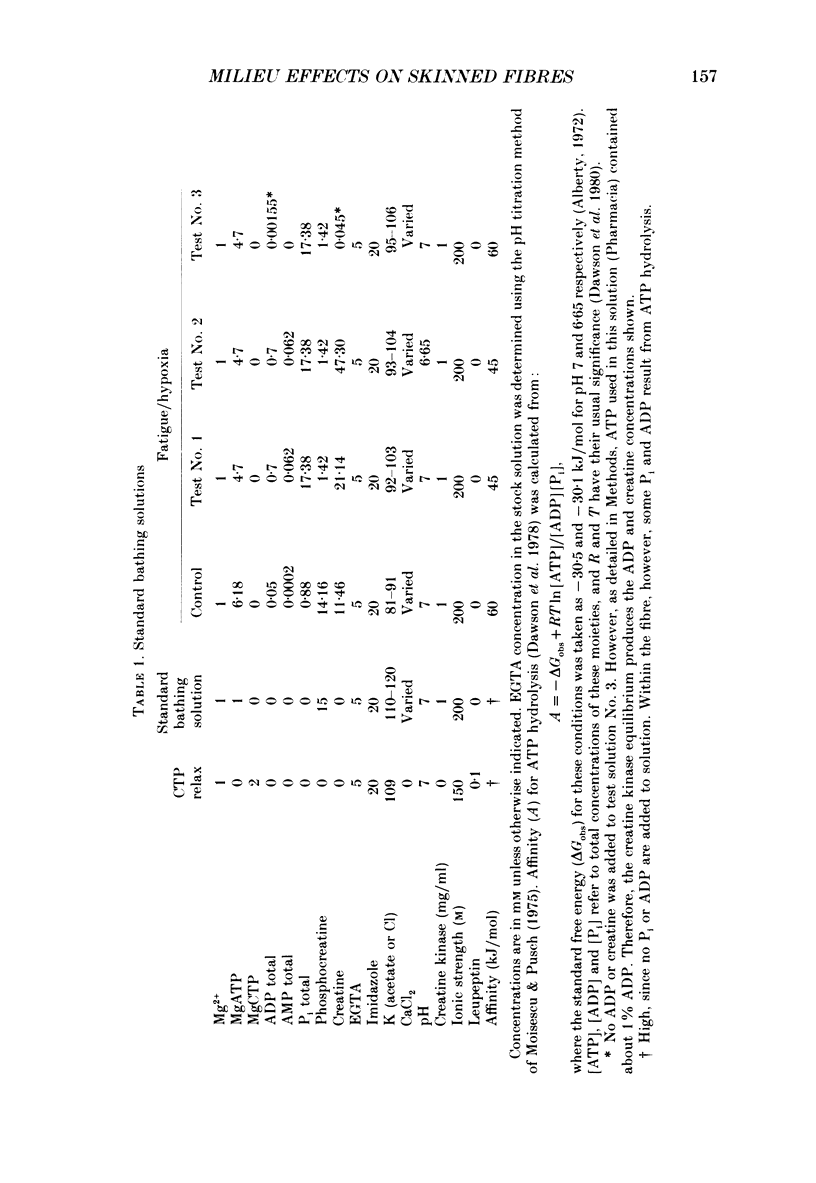
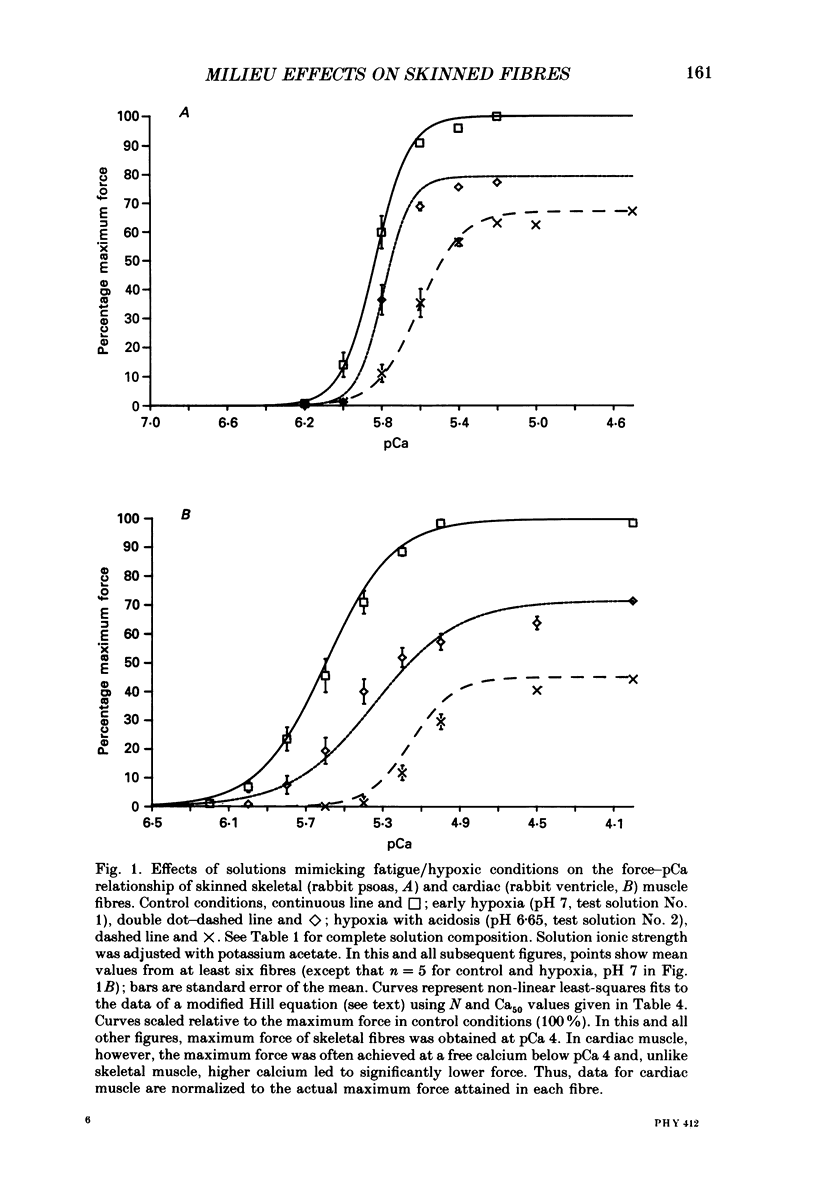
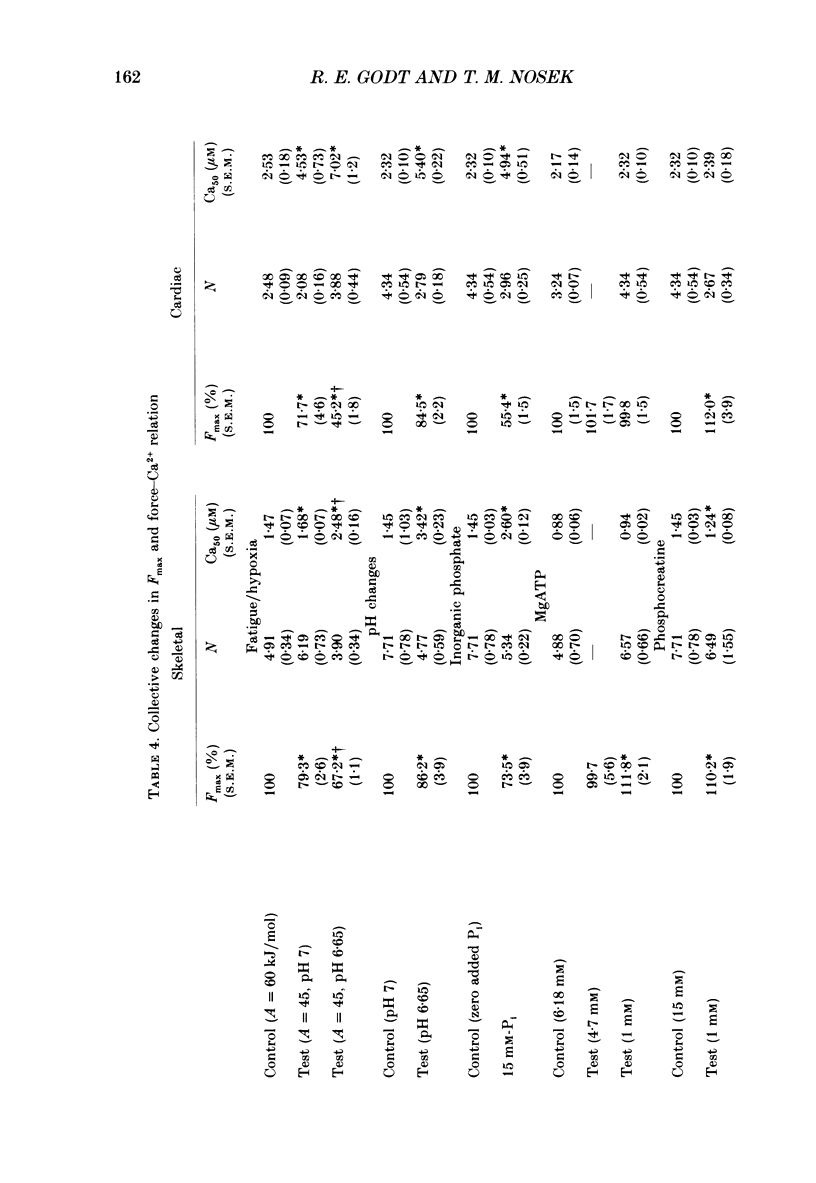
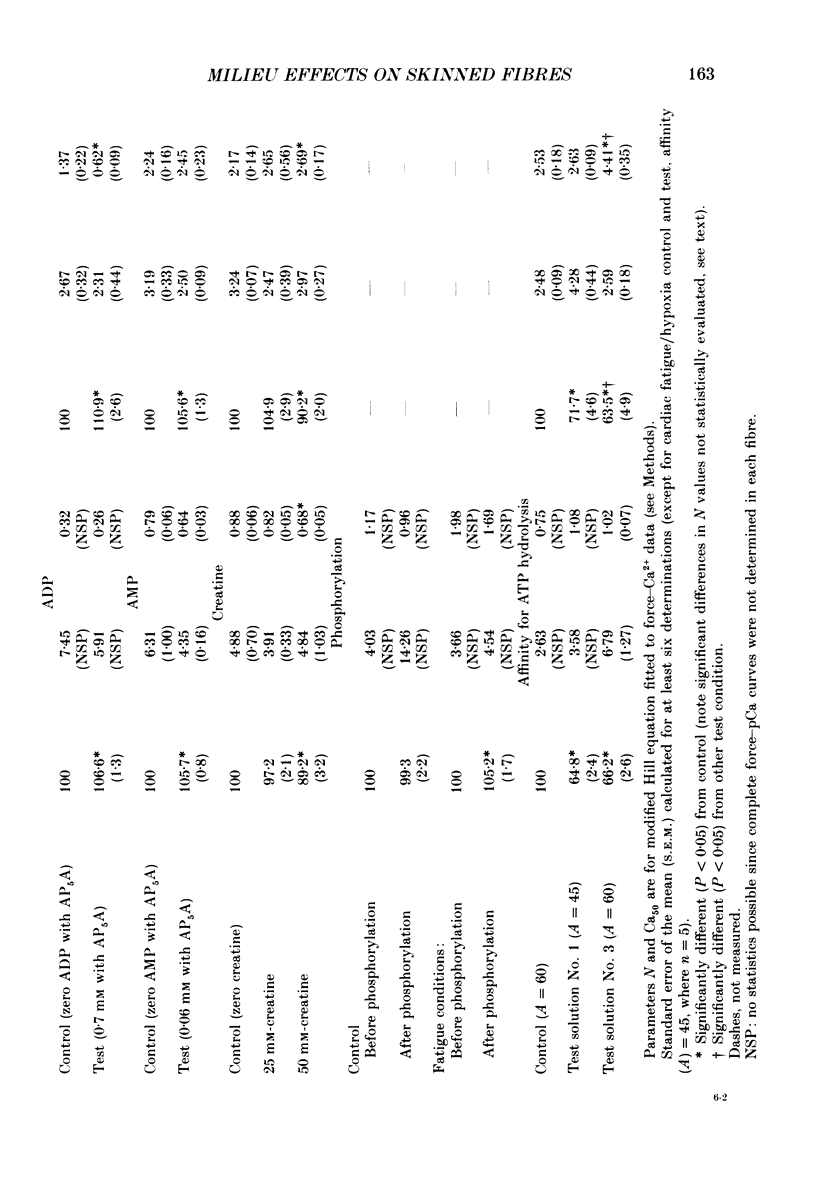
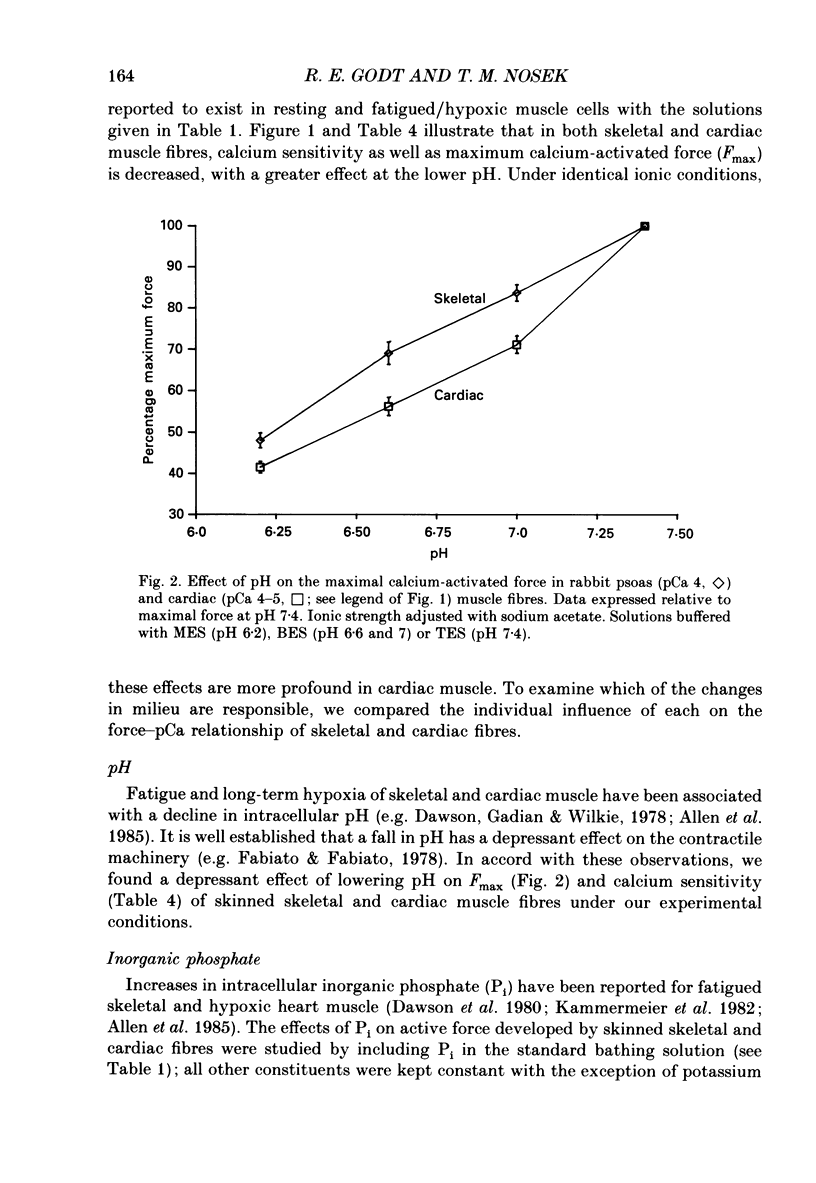
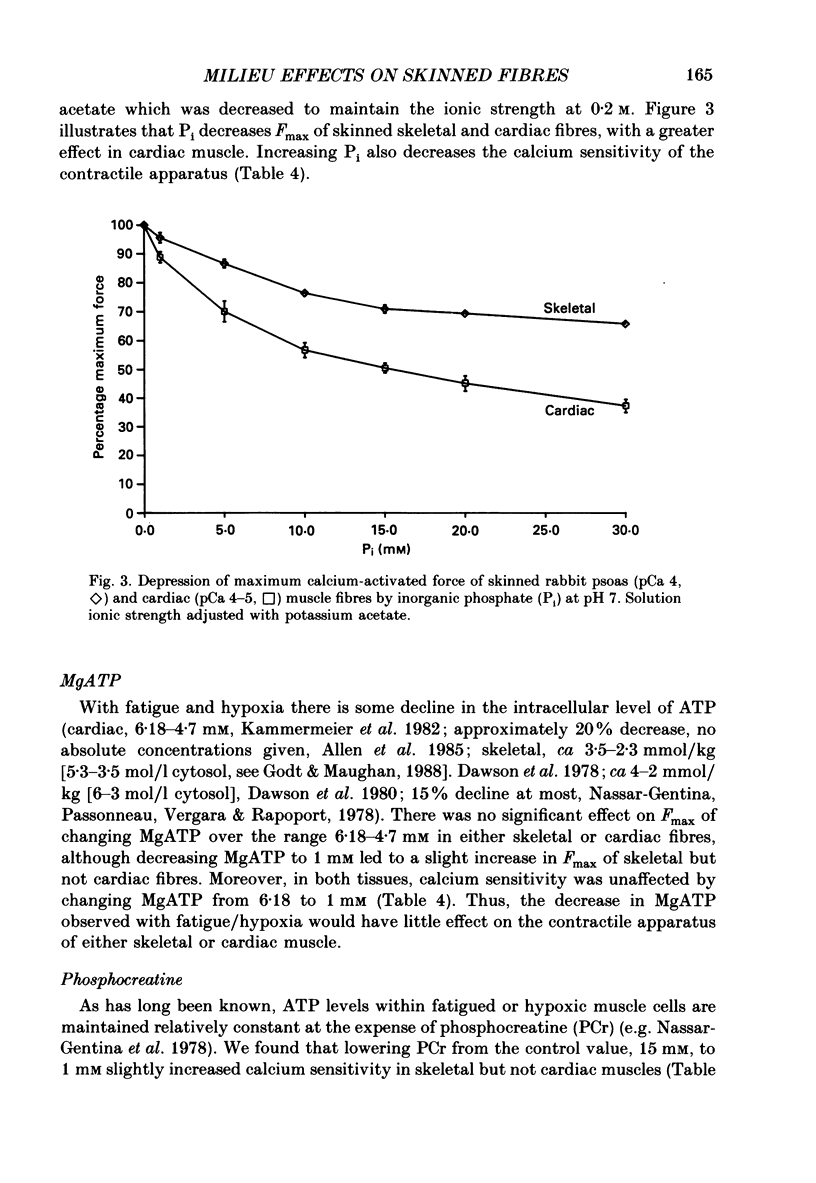
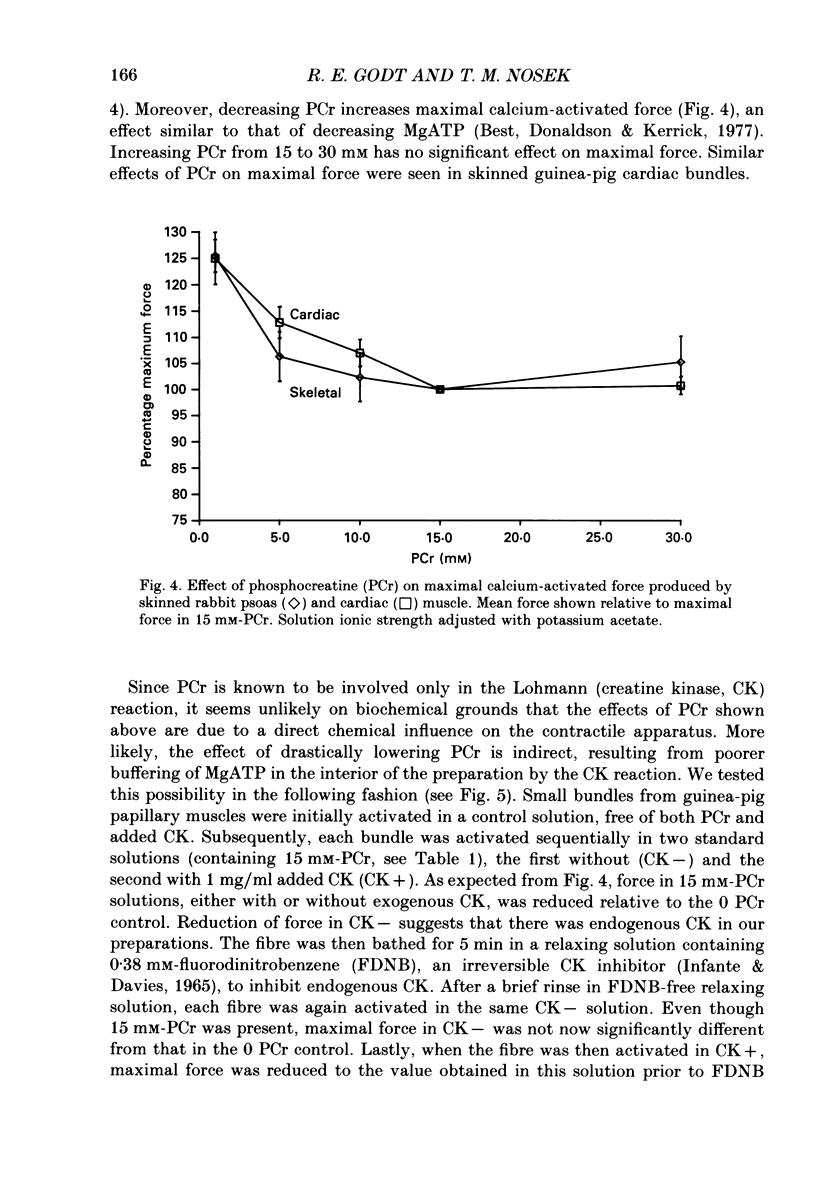
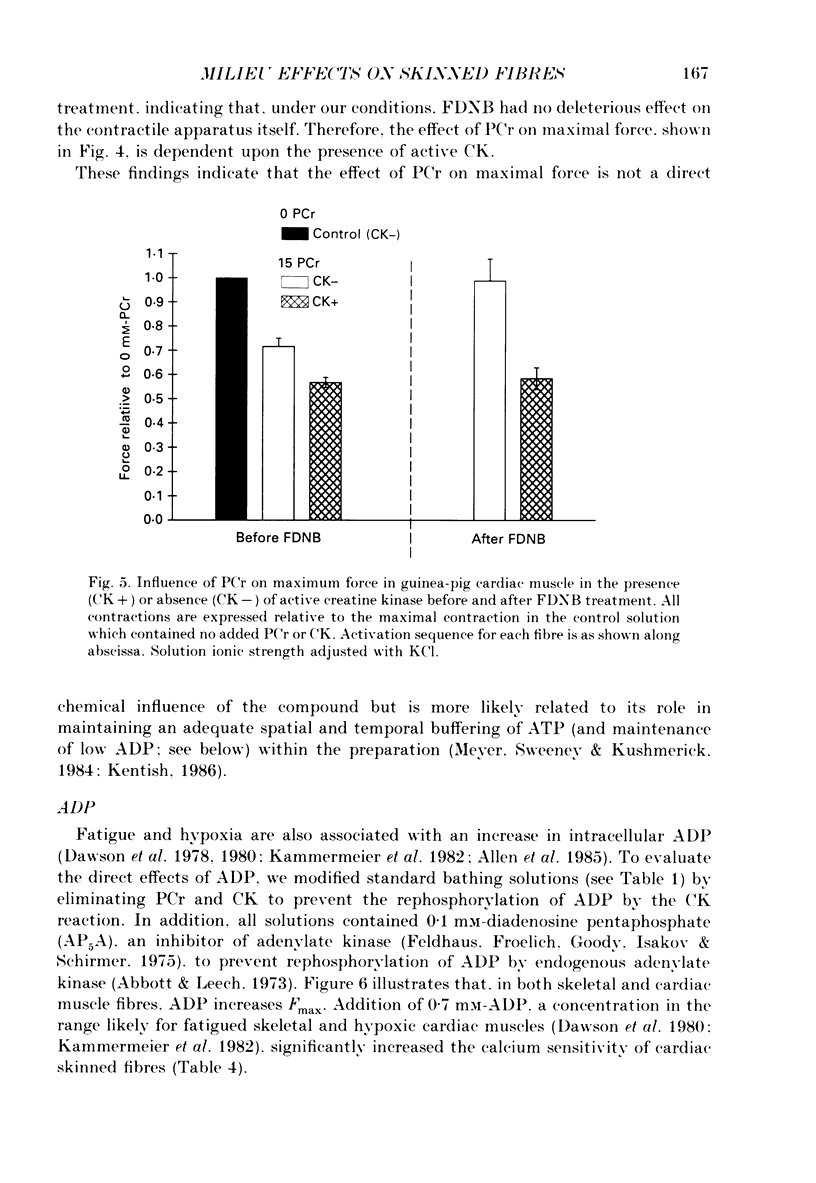
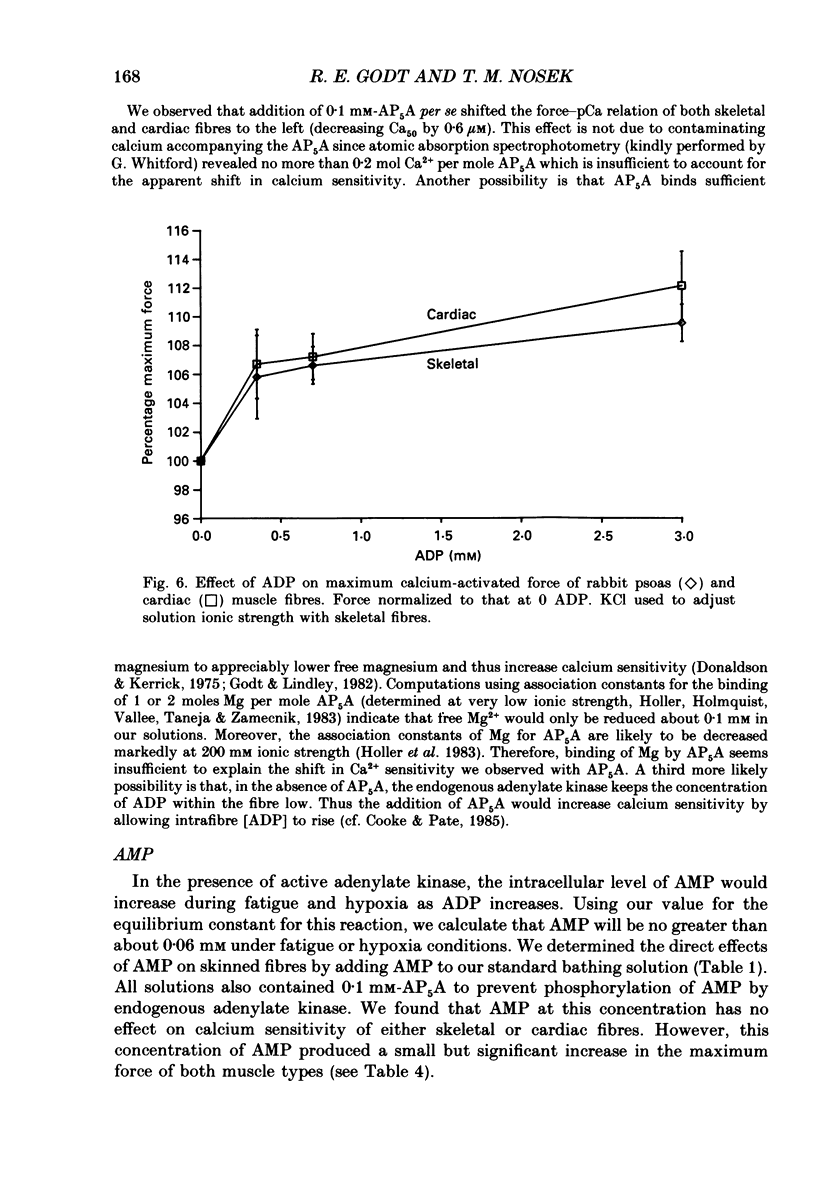
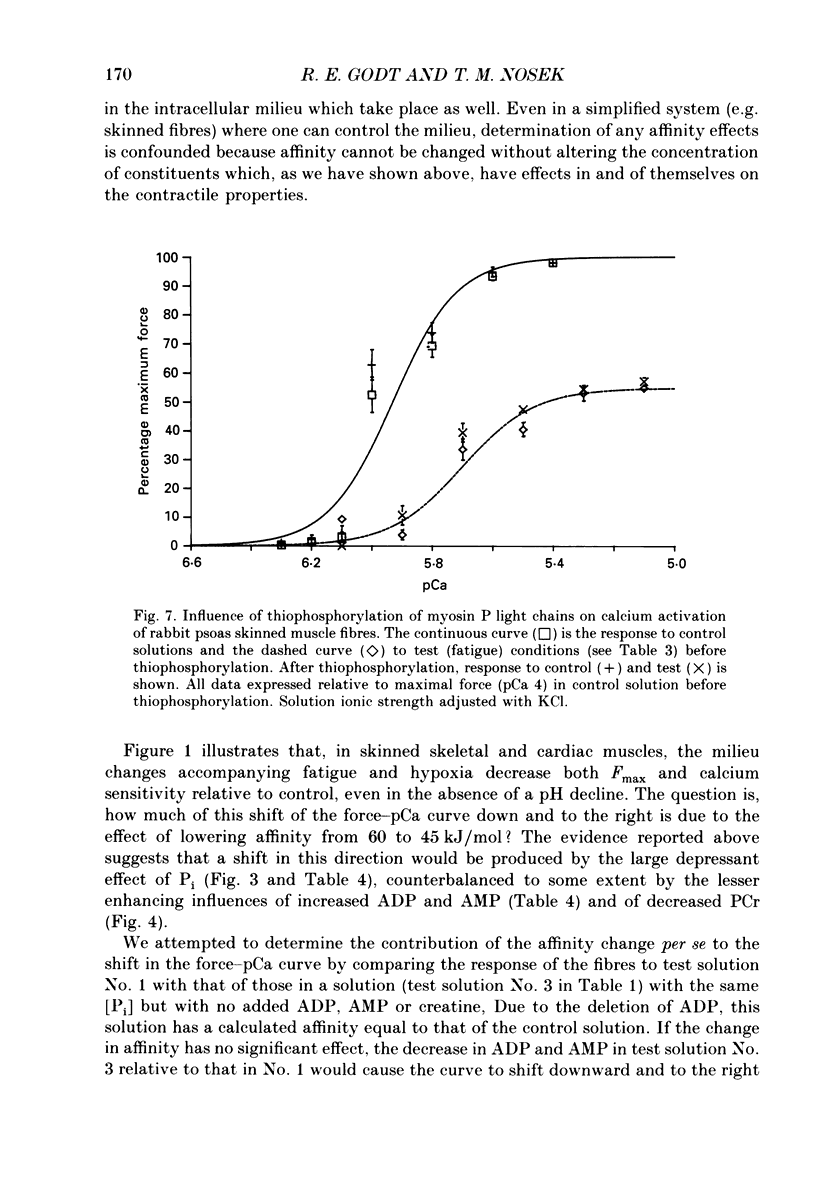
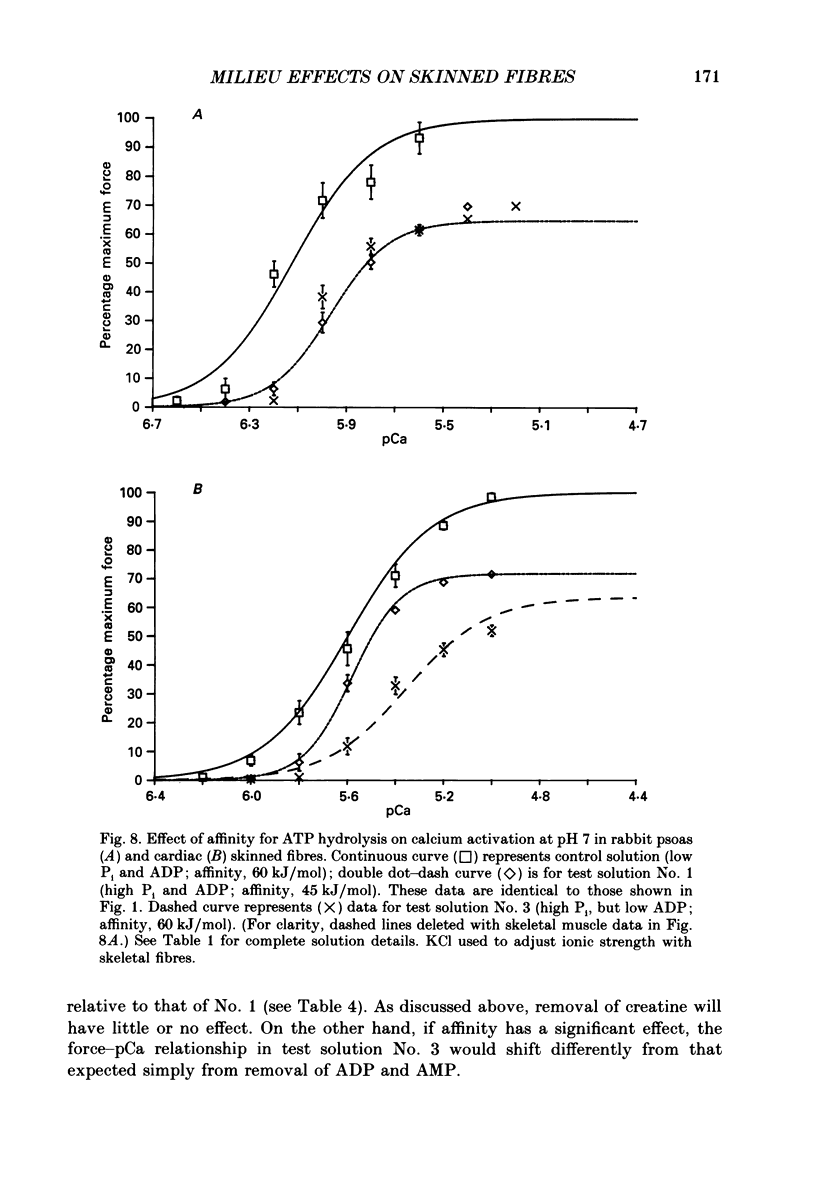
1. Maximal calcium-activated force (Fmax) and calcium sensitivity were markedly decreased in detergent-skinned fibres from skeletal and cardiac muscle by solutions that mimicked the total milieu changes associated with fatigue and hypoxia. Further experiments determined the relative contribution of each of the individual changes in milieu. 2. Both Ca2+ sensitivity and Fmax of skeletal and cardiac fibres were decreased with increased [H+] or inorganic phosphate (Pi). These effects were greater in cardiac muscle. 3. Decreasing MgATP over the range observed with fatigue and hypoxia (6.8-4.7 mM) had no effect on Fmax or Ca2+ sensitivity of either muscle type. 4. Decreasing phosphocreatine (PCr: 15-1 mM) increased Fmax but had little effect on Ca2+ sensitivity in both muscle types. In cardiac fibres, the effect on Fmax could be mimicked by inhibition of endogenous creatine kinase. 5. ADP (0.7 mM) increased Fmax and Ca2+ sensitivity, while AMP (0.06 mM) slightly increased Fmax but had no effect on Ca2+ sensitivity of either skeletal or cardiac fibres. 6. Creatine (25 mM) had no significant effect on either Ca2+ sensitivity or Fmax of skeletal and cardiac muscle fibres. At higher levels (50 mM), however, creatine depressed Fmax and slightly altered Ca2+ sensitivity. 7. Thiophosphorylation of myosin P light chains (phosphorylatable light chains of myosin) in rabbit psoas fibres had no effect on Ca2+ sensitivity, yet slightly but significantly increased Fmax under fatigue conditions. 8. Reducing the affinity for ATP hydrolysis (by adding ADP, AMP and creatine) over the range calculated for fatigue/hypoxia (60-45 kJ/mol) produced the enhancement in Fmax expected from added ADP and AMP in cardiac but not skeletal muscle, indicating that changes in affinity influence Fmax of skeletal muscle. Reducing affinity produced little change in Ca2+ sensitivity of skeletal muscle. In contrast, the change produced in cardiac muscle was greater than that expected from addition of ADP and AMP; i.e. decreasing affinity increases calcium sensitivity of the heart. 9. Simple summation of all significant changes expected from each constituent altered by fatigue/hypoxia adequately predicted the observed changes in Fmax and Ca2+ sensitivity in both cardiac and skeletal muscle fibres with but one exception (the change in Ca2+ sensitivity of skeletal muscle at pH 7 was slightly overestimated).
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. H., Leech A. R. Persistence of adenylate kinase and other enzymes in glycerol extracted muscle. Pflugers Arch. 1973 Nov 28;344(3):233–243. doi: 10.1007/BF00588463. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. Intracellular calcium concentration during hypoxia and metabolic inhibition in mammalian ventricular muscle. J Physiol. 1983 Jun;339:107–122. doi: 10.1113/jphysiol.1983.sp014706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Moisescu D. G. Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils. J Physiol. 1977 Sep;270(3):627–652. doi: 10.1113/jphysiol.1977.sp011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best P. M., Donaldson S. K., Kerrick W. G. Tension in mechanically disrupted mammalian cardiac cells: effects of magnesium adenosine triphosphate. J Physiol. 1977 Feb;265(1):1–17. doi: 10.1113/jphysiol.1977.sp011702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt P. W., Cox R. N., Kawai M., Robinson T. Effect of cross-bridge kinetics on apparent Ca2+ sensitivity. J Gen Physiol. 1982 Jun;79(6):997–1016. doi: 10.1085/jgp.79.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Brozovich F. V., Yates L. D., Gordon A. M. Muscle force and stiffness during activation and relaxation. Implications for the actomyosin ATPase. J Gen Physiol. 1988 Mar;91(3):399–420. doi: 10.1085/jgp.91.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988 Jun;53(6):935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K., Stull J. T. Myosin phosphorylation regulates the ATPase activity of permeable skeletal muscle fibers. FEBS Lett. 1982 Jul 19;144(1):33–37. doi: 10.1016/0014-5793(82)80563-2. [DOI] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol. 1980 Feb;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Donaldson S. K., Hermansen L., Bolles L. Differential, direct effects of H+ on Ca2+ -activated force of skinned fibers from the soleus, cardiac and adductor magnus muscles of rabbits. Pflugers Arch. 1978 Aug 25;376(1):55–65. doi: 10.1007/BF00585248. [DOI] [PubMed] [Google Scholar]
- Donaldson S. K., Kerrick W. G. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers. J Gen Physiol. 1975 Oct;66(4):427–444. doi: 10.1085/jgp.66.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H. Human muscle function and fatigue. Ciba Found Symp. 1981;82:1–18. doi: 10.1002/9780470715420.ch1. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhau P., Fröhlich T., Goody R. S., Isakov M., Schirmer R. H. Synthetic inhibitors of adenylate kinases in the assays for ATPases and phosphokinases. Eur J Biochem. 1975 Sep 1;57(1):197–204. doi: 10.1111/j.1432-1033.1975.tb02291.x. [DOI] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E. A simple electrostatic model can explain the effect of pH upon the force-pCa relation of skinned frog skeletal muscle fibers. Biophys J. 1981 Aug;35(2):385–392. doi: 10.1016/S0006-3495(81)84797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. On the composition of the cytosol of relaxed skeletal muscle of the frog. Am J Physiol. 1988 May;254(5 Pt 1):C591–C604. doi: 10.1152/ajpcell.1988.254.5.C591. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Grabowski W., Lobsiger E. A., Lüttgau H. C. The effect of repetitive stimulation at low frequencies upon the electrical and mechanical activity of single muscle fibres. Pflugers Arch. 1972;334(3):222–239. doi: 10.1007/BF00626225. [DOI] [PubMed] [Google Scholar]
- Herzig J. W., Peterson J. W., Rüegg J. C., Solaro R. J. Vanadate and phosphate ions reduce tension and increase cross-bridge kinetics in chemically skinned heart muscle. Biochim Biophys Acta. 1981 Jan 21;672(2):191–196. doi: 10.1016/0304-4165(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. Phosphate release and force generation in skeletal muscle fibers. Science. 1985 Jun 14;228(4705):1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Holler E., Holmquist B., Vallee B. L., Taneja K., Zamecnik P. Circular dichroism and ordered structure of bisnucleoside oligophosphates and their Zn2+ and Mg2+ complexes. Biochemistry. 1983 Oct 11;22(21):4924–4933. doi: 10.1021/bi00290a008. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Davies R. E. The effect of 2,4-dinitrofluorobenzene on the activity of striated muscle. J Biol Chem. 1965 Oct;240(10):3996–4001. [PubMed] [Google Scholar]
- Kammermeier H., Schmidt P., Jüngling E. Free energy change of ATP-hydrolysis: a causal factor of early hypoxic failure of the myocardium? J Mol Cell Cardiol. 1982 May;14(5):267–277. doi: 10.1016/0022-2828(82)90205-x. [DOI] [PubMed] [Google Scholar]
- Kentish J. C., Allen D. G. Is force production in the myocardium directly dependent upon the free energy change of ATP hydrolysis? J Mol Cell Cardiol. 1986 Sep;18(9):879–884. doi: 10.1016/s0022-2828(86)80001-3. [DOI] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Meyer R. A. Chemical changes in rat leg muscle by phosphorus nuclear magnetic resonance. Am J Physiol. 1985 May;248(5 Pt 1):C542–C549. doi: 10.1152/ajpcell.1985.248.5.C542. [DOI] [PubMed] [Google Scholar]
- Kusuoka H., Weisfeldt M. L., Zweier J. L., Jacobus W. E., Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circ Res. 1986 Sep;59(3):270–282. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- Kübler W., Katz A. M. Mechanism of early "pump" failure of the ischemic heart: possible role of adenosine triphosphate depletion and inorganic phosphate accumulation. Am J Cardiol. 1977 Sep;40(3):467–471. doi: 10.1016/0002-9149(77)90174-6. [DOI] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. The effect of temperature and stimulation scheme on fatigue and recovery in Xenopus muscle fibres. Acta Physiol Scand. 1988 May;133(1):73–82. doi: 10.1111/j.1748-1716.1988.tb08382.x. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Gwathmey J. K., Morgan J. P. Differential effects of reoxygenation on intracellular calcium and isometric tension. Pflugers Arch. 1987 Aug;409(4-5):448–453. doi: 10.1007/BF00583800. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Stull J. T. Myosin light chain phosphorylation and phosphorylase A activity in rat extensor digitorum longus muscle. Biochem Biophys Res Commun. 1979 Sep 12;90(1):164–170. doi: 10.1016/0006-291x(79)91604-8. [DOI] [PubMed] [Google Scholar]
- Marban E., Kusuoka H. Maximal Ca2+-activated force and myofilament Ca2+ sensitivity in intact mammalian hearts. Differential effects of inorganic phosphate and hydrogen ions. J Gen Physiol. 1987 Nov;90(5):609–623. doi: 10.1085/jgp.90.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhfi H., Ventura-Clapier R. Dependence upon high-energy phosphates of the effects of inorganic phosphate on contractile properties in chemically skinned rat cardiac fibres. Pflugers Arch. 1988 Apr;411(4):378–385. doi: 10.1007/BF00587716. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol. 1987 Dec;393:727–742. doi: 10.1113/jphysiol.1987.sp016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. A., Sweeney H. L., Kushmerick M. J. A simple analysis of the "phosphocreatine shuttle". Am J Physiol. 1984 May;246(5 Pt 1):C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Nassar-Gentina V., Passonneau J. V., Vergara J. L., Rapoport S. I. Metabolic correlates of fatigue and of recovery from fatigue in single frog muscle fibers. J Gen Physiol. 1978 Nov;72(5):593–606. doi: 10.1085/jgp.72.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely J. R., Morgan H. E. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- Nosek T. M., Fender K. Y., Godt R. E. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science. 1987 Apr 10;236(4798):191–193. doi: 10.1126/science.3563496. [DOI] [PubMed] [Google Scholar]
- Persechini A., Stull J. T., Cooke R. The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal muscle fibers. J Biol Chem. 1985 Jul 5;260(13):7951–7954. [PubMed] [Google Scholar]
- Pires E. M., Perry S. V. Purification and properties of myosin light-chain kinase from fast skeletal muscle. Biochem J. 1977 Oct 1;167(1):137–146. doi: 10.1042/bj1670137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J. M., Allard Y., Mainwood G. W. Is the change in intracellular pH during fatigue large enough to be the main cause of fatigue? Can J Physiol Pharmacol. 1986 Jun;64(6):764–767. doi: 10.1139/y86-130. [DOI] [PubMed] [Google Scholar]
- Robertson S. P., Kerrick W. G. The effects of pH on Ca2+-activated force in frog skeletal muscle fibers. Pflugers Arch. 1979 May 15;380(1):41–45. doi: 10.1007/BF00582610. [DOI] [PubMed] [Google Scholar]
- Rüegg J. C., Schädler M., Steiger G. J., Müller G. Effects of inorganic phosphate on the contractile mechanism. Pflugers Arch. 1971;325(4):359–364. doi: 10.1007/BF00592176. [DOI] [PubMed] [Google Scholar]
- Siemankowski R. F., Wiseman M. O., White H. D. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci U S A. 1985 Feb;82(3):658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Hartshorne D. J. Conversion of a Ca2+-dependent myosin light chain kinase from skeletal muscle to a Ca2+-independent form. Biochem Biophys Res Commun. 1983 Jan 27;110(2):701–708. doi: 10.1016/0006-291x(83)91206-8. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R., Mekhfi H., Vassort G. Role of creatine kinase in force development in chemically skinned rat cardiac muscle. J Gen Physiol. 1987 May;89(5):815–837. doi: 10.1085/jgp.89.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. The relation between force and intracellular pH in fatigued, single Xenopus muscle fibres. Acta Physiol Scand. 1988 May;133(1):83–89. doi: 10.1111/j.1748-1716.1988.tb08383.x. [DOI] [PubMed] [Google Scholar]


