Abstract
1. In cats anaesthetized with Saffan, which does not block afferent activation of the brain stem defence areas, we have analysed the cardiovascular changes induced by 3 min periods of graded systemic hypoxia (fraction of O2 in inspirate, Fi,O2, 0.15, 0.12, 0.08, 0.06). 2. At light levels of Saffan anaesthesia, hypoxia (particularly Fi, O2 0.08 and 0.06) or selective stimulation of carotid chemoreceptors evoked the pattern of tachycardia, decrease in renal and mesenteric vascular conductance (RVC, MVC), but increase in femoral vascular conductance (FVC) which is characteristic of the alerting-defence response. This supports our view that activation of the defence areas is an integral part of the response to systemic hypoxia. 3. Hypoxia also induced an increase in frequency of augmented breaths which was graded with the level of hypoxia: 0.6 min-1 at Fi, O2 0.21 to 1.1 min-1 at Fi, O2 0.06; in some cats each of these was accompanied by a transient fall in arterial pressure (ABP) and increase in FVC. It is proposed that these responses were all part of a reflex elicited by lung irritant receptors and facilitated by peripheral chemoreceptors. However, their low rate of occurrence and the liability of the vasodilatation suggests they do not make major contributions to the overall response. 4. The above short-lasting responses were superimposed upon gradual changes whose magnitudes were graded with the level of hypoxia: hyperventilation, slight tachycardia, but bradycardia at Fi, O2 0.6, small increases in ABP, FVC and MVC allowing femoral and mesenteric blood flow to increase, but decreases in RVC which maintained renal blood flow constant. 5. Vagotomy had no significant effect on these changes. Further, hyperinflation of the lungs with pressures of 10 mmHg evoked the Breuer-Hering reflex but had no noticeable cardiovascular effect. It is proposed that, in the cat, reflex tachycardia and vasodilatation elicited by lung stretch receptors play no significant part in the response to hypoxia. 6. By contrast, after pneumothorax, with ventilation and thereby arterial PCO2 (Pa, CO2) maintained constant, graded hypoxia produced graded bradycardia, decrease in MVC and RVC and no change in FVC. Taken together, these results suggest that in the spontaneously breathing cat, the response to hypoxia is dominated by the effects of hypocapnia secondary to hyperventilation, which by inhibiting peripheral and central chemoreceptor activity effectively counteracts the primary bradycardia and peripheral vasoconstriction elicited by hypoxic stimulation of peripheral chemoreceptors. 7. These proposals are compared with those drawn for other species.
Full text
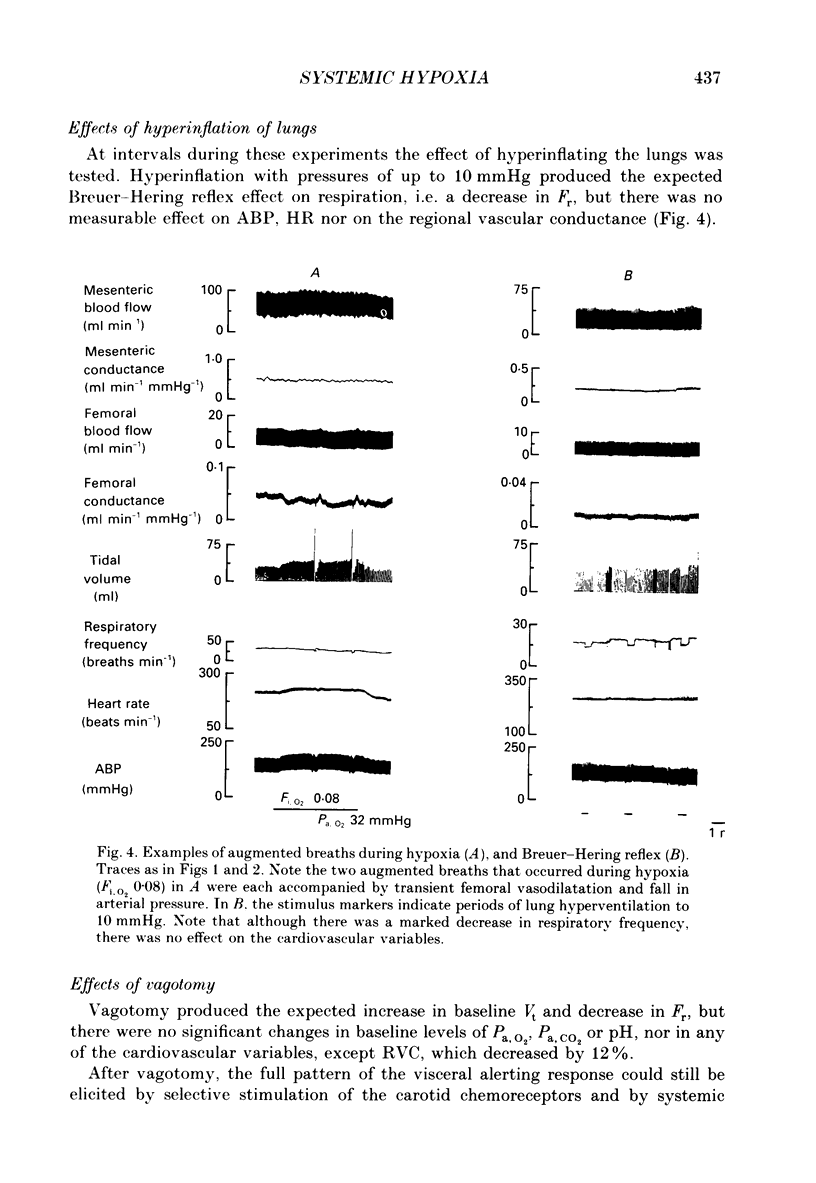
PDF



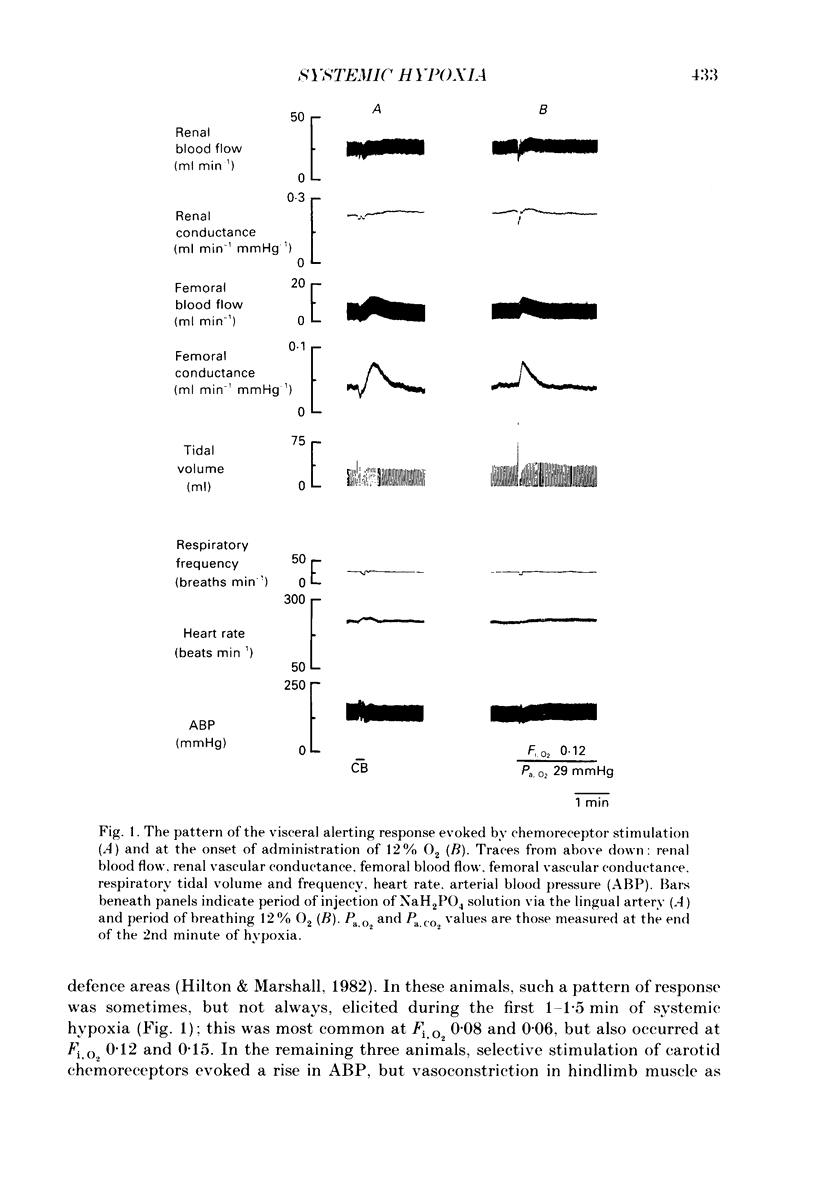

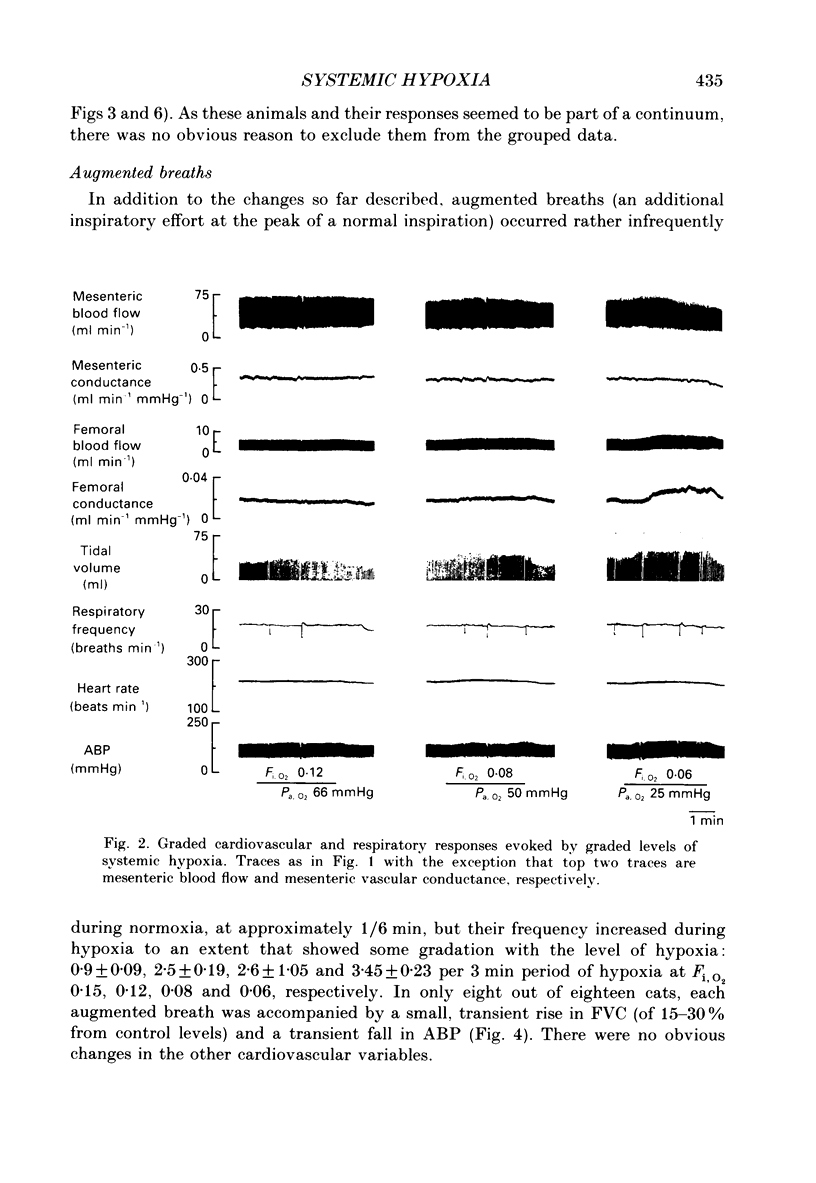
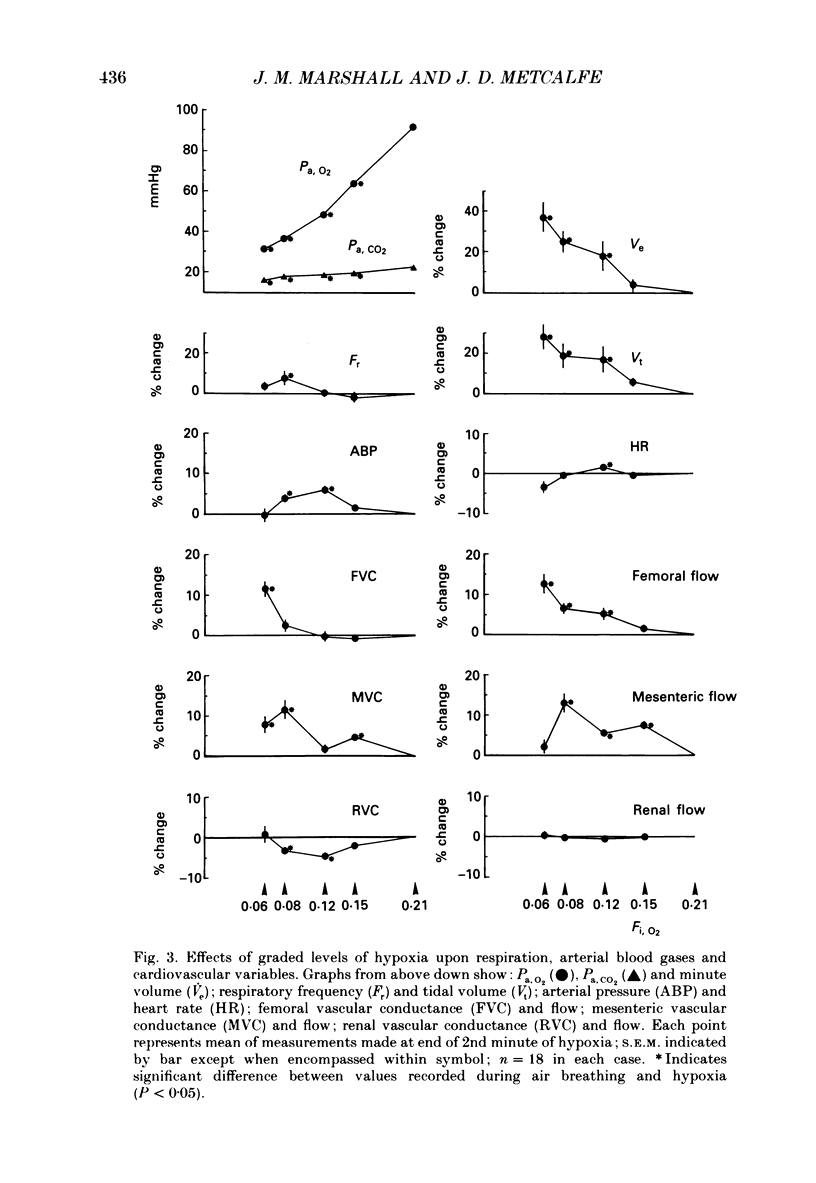
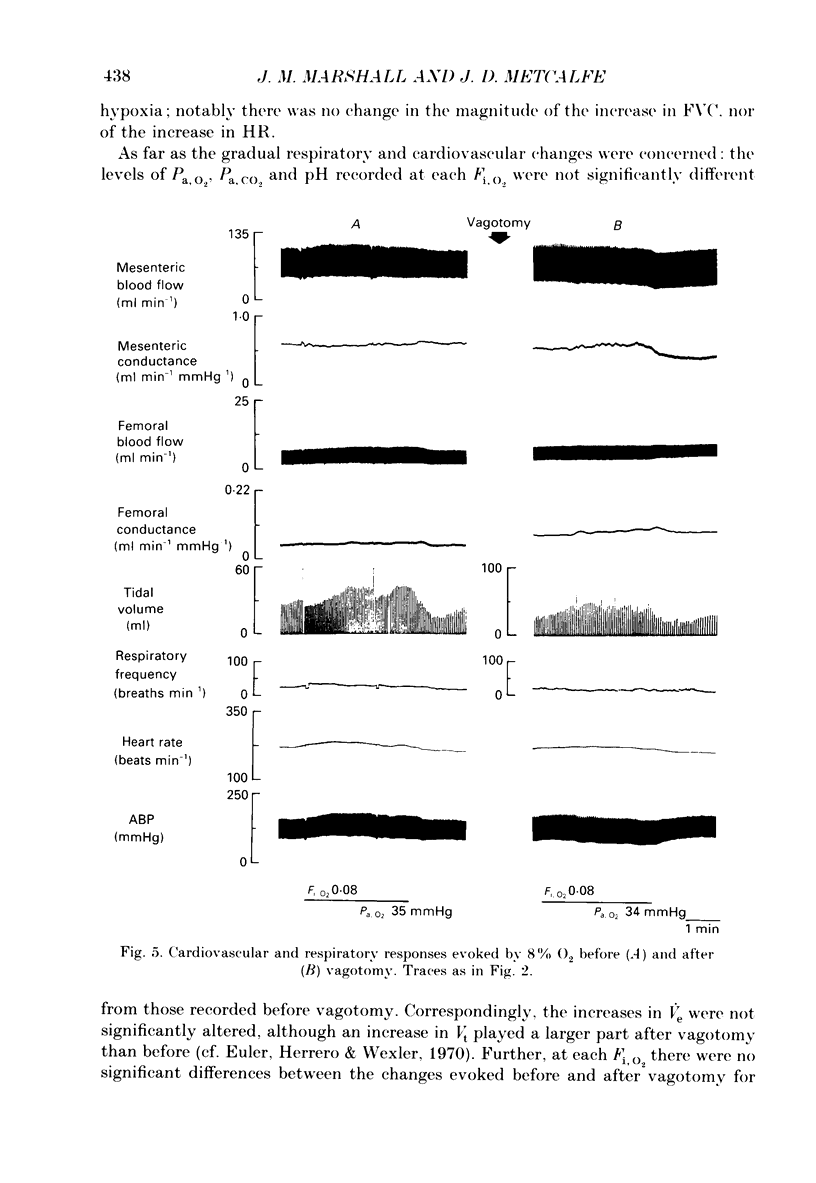
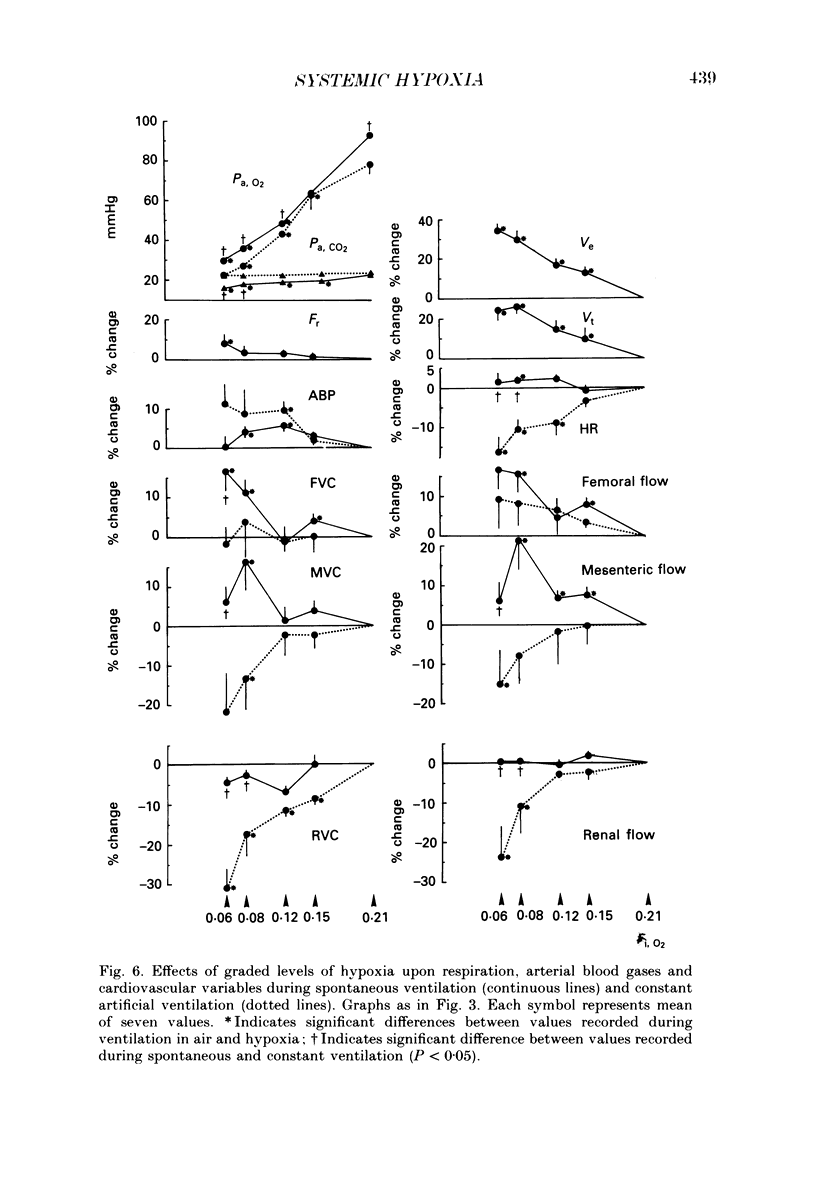









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett D., Jr Origin and regulation of spontaneous deep breaths. Respir Physiol. 1971 Jun;12(2):230–238. doi: 10.1016/0034-5687(71)90055-7. [DOI] [PubMed] [Google Scholar]
- Child K. J., Davis B., Dodds M. G., Twissell D. J. Anaesthetic, cardiovascular and respiratory effects of a new steroidal agent CT 1341: a comparison with other intravenous anaesthetic drugs in the unrestrained cat. Br J Pharmacol. 1972 Oct;46(2):189–200. doi: 10.1111/j.1476-5381.1972.tb06864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALYMDE B., HAZZLEDINE J. L., HOWE A. REFLEX RESPIRATORY AND PERIPHERAL VASCULAR RESPONSES TO STIMULATION OF THE ISOLATED PERFUSED AORTIC ARCH CHEMORECEPTORS OF THE DOG. J Physiol. 1965 Mar;177:300–322. doi: 10.1113/jphysiol.1965.sp007593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty R. M., Jr, Scott J. B., Dabney J. M., Haddy F. J. Local effects of O2 and CO2 on limb, renal, and coronary vascular resistances. Am J Physiol. 1967 Nov;213(5):1102–1110. doi: 10.1152/ajplegacy.1967.213.5.1102. [DOI] [PubMed] [Google Scholar]
- Glogowska M., Richardson P. S., Widdicombe J. G., Winning A. J. The role of the vagus nerves, peripheral chemoreceptors and other afferent pathways in the genesis of augmented breaths in cats and rabbits. Respir Physiol. 1972 Oct;16(2):179–196. doi: 10.1016/0034-5687(72)90050-3. [DOI] [PubMed] [Google Scholar]
- Hanna B. D., Lioy F., Polosa C. The effect of cold blockade of the medullary chemoreceptors on the CO2 modulation of vascular tone and heart rate. Can J Physiol Pharmacol. 1979 May;57(5):461–468. doi: 10.1139/y79-070. [DOI] [PubMed] [Google Scholar]
- Hilton S. M., Marshall J. M. The pattern of cardiovascular response to carotid chemoreceptor stimulation in the cat. J Physiol. 1982 May;326:495–513. doi: 10.1113/jphysiol.1982.sp014208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton S. M. The defence-arousal system and its relevance for circulatory and respiratory control. J Exp Biol. 1982 Oct;100:159–174. doi: 10.1242/jeb.100.1.159. [DOI] [PubMed] [Google Scholar]
- KONTOS H. A., MAUCK H. P., Jr, RICHARDSON D. W., PATTERSON J. L., Jr MECHANISM OF CIRCULATORY RESPONSES TO SYSTEMIC HYPOXIA IN THE ANESTHETIZED DOG. Am J Physiol. 1965 Aug;209:397–403. doi: 10.1152/ajplegacy.1965.209.2.397. [DOI] [PubMed] [Google Scholar]
- Koehler R. C., McDonald B. W., Krasney J. A. Influence of CO2 on cardiovascular response to hypoxia in conscious dogs. Am J Physiol. 1980 Oct;239(4):H545–H558. doi: 10.1152/ajpheart.1980.239.4.H545. [DOI] [PubMed] [Google Scholar]
- Korner P. I. Operation of the central nervous system in reflex circulatory control. Fed Proc. 1980 Jun;39(8):2504–2512. [PubMed] [Google Scholar]
- Korner P. I., Uther J. B., White S. W. Central nervous integration of the circulatory and respiratory responses to arterial hypoxemia in the rabbit. Circ Res. 1969 Jun;24(6):757–776. doi: 10.1161/01.res.24.6.757. [DOI] [PubMed] [Google Scholar]
- Lahiri S., DeLaney R. G. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respir Physiol. 1975 Sep;24(3):249–266. doi: 10.1016/0034-5687(75)90017-1. [DOI] [PubMed] [Google Scholar]
- Marshall J. M. Analysis of cardiovascular responses evoked following changes in peripheral chemoreceptor activity in the rat. J Physiol. 1987 Dec;394:393–414. doi: 10.1113/jphysiol.1987.sp016877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol. 1988 Dec;407:385–403. doi: 10.1113/jphysiol.1988.sp017422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Cardiovascular changes associated with augmented breaths in normoxia and hypoxia in the rat. J Physiol. 1988 Jun;400:15–27. doi: 10.1113/jphysiol.1988.sp017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Influences on the cardiovascular response to graded levels of systemic hypoxia of the accompanying hypocapnia in the rat. J Physiol. 1989 Mar;410:381–394. doi: 10.1113/jphysiol.1989.sp017539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M. Modulation of the centrally-evoked visceral alerting/defence response by changes in CSF pH at the ventral surface of the medulla oblongata and by systemic hypercapnia. Pflugers Arch. 1986 Jul;407(1):46–54. doi: 10.1007/BF00580719. [DOI] [PubMed] [Google Scholar]
- Marshall J. M. The role of the glycine sensitive area of the ventral medulla in cardiovascular responses to carotid chemoreceptor and peripheral nerve stimulation. Pflugers Arch. 1986 Feb;406(2):225–231. doi: 10.1007/BF00586687. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Tenney S. M. Hypoxia-induced tachypnea in carotid-deafferented cats. Respir Physiol. 1975 Jan;23(1):31–39. doi: 10.1016/0034-5687(75)90069-9. [DOI] [PubMed] [Google Scholar]
- Rose C. E., Jr, Anderson R. J., Carey R. M. Antidiuresis and vasopressin release with hypoxemia and hypercapnia in conscious dogs. Am J Physiol. 1984 Jul;247(1 Pt 2):R127–R134. doi: 10.1152/ajpregu.1984.247.1.R127. [DOI] [PubMed] [Google Scholar]
- Rosén K. G., Kjellmer I. Changes in the fetal heart rate and ECG during hypoxia. Acta Physiol Scand. 1975 Jan;93(1):59–66. doi: 10.1111/j.1748-1716.1975.tb05790.x. [DOI] [PubMed] [Google Scholar]
- Rowell L. B., Blackmon J. R. Lack of sympathetic vasoconstriction in hypoxemic humans at rest. Am J Physiol. 1986 Sep;251(3 Pt 2):H562–H570. doi: 10.1152/ajpheart.1986.251.3.H562. [DOI] [PubMed] [Google Scholar]
- Spyer K. M. Neural organisation and control of the baroreceptor reflex. Rev Physiol Biochem Pharmacol. 1981;88:24–124. [PubMed] [Google Scholar]
- Svanvik J., Tyllström J., Wallentin I. The effects of hypercapnia and hypoxia on the distribution of capillary blood flow in the denervated intestinal vascular bed. Acta Physiol Scand. 1968 Dec;74(4):543–551. doi: 10.1111/j.1748-1716.1968.tb04266.x. [DOI] [PubMed] [Google Scholar]
- Timms R. J. A study of the amygdaloid defence reaction showing the value of Althesin anaesthesia in studies of the functions of the fore-brain in cats. Pflugers Arch. 1981 Jul;391(1):49–56. doi: 10.1007/BF00580694. [DOI] [PubMed] [Google Scholar]
- Timms R. J. Central blocking action of ketamine anaesthesia on the visceral alerting and chemoreceptor reflex responses in the cat. Pflugers Arch. 1982 Jul;394(1):12–15. doi: 10.1007/BF01108301. [DOI] [PubMed] [Google Scholar]
- Trzebski A., Kubin L. Is the central inspiratory activity responsible for pCO2-dependent drive of the sympathetic discharge? J Auton Nerv Syst. 1981 Apr;3(2-4):401–420. doi: 10.1016/0165-1838(81)90078-3. [DOI] [PubMed] [Google Scholar]
- Uther J. B., Hunyor S. N., Shaw J., Korner P. I. Bulbar and suprabulbar control of the cardiovascular autonomic effects during arterial hypoxia in the rabbit. Circ Res. 1970 Apr;26(4):491–506. doi: 10.1161/01.res.26.4.491. [DOI] [PubMed] [Google Scholar]
- Weissman M. L., Rubinstein E. H., Sonnenschein R. R. Vascular response to short-term systemic hypoxia, hypercapnia, and asphyxia in the cat. Am J Physiol. 1976 Mar;230(3):595–601. doi: 10.1152/ajplegacy.1976.230.3.595. [DOI] [PubMed] [Google Scholar]
- von Euler C., Wexler I., Herrero F. Control mechanisms determining rate and depth of respiratory movements. Respir Physiol. 1970 Jul;10(1):93–108. doi: 10.1016/0034-5687(70)90030-7. [DOI] [PubMed] [Google Scholar]


