Abstract
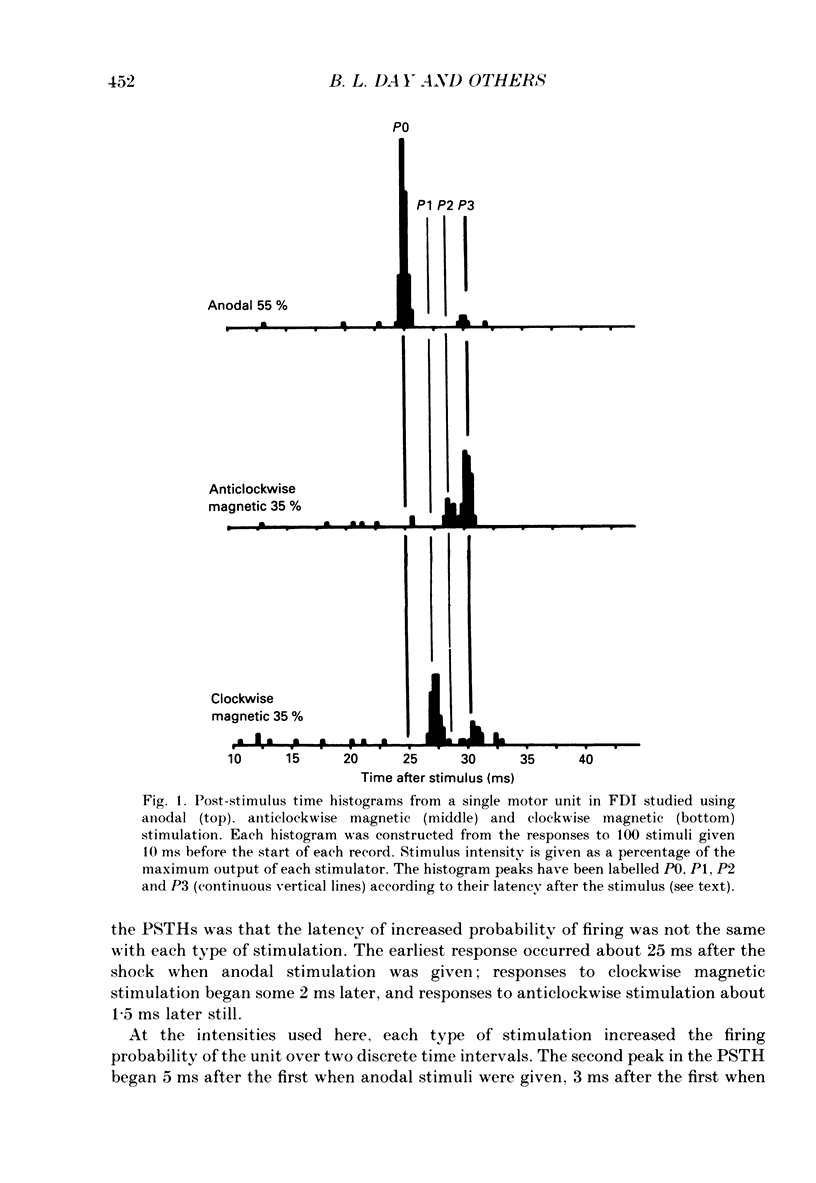
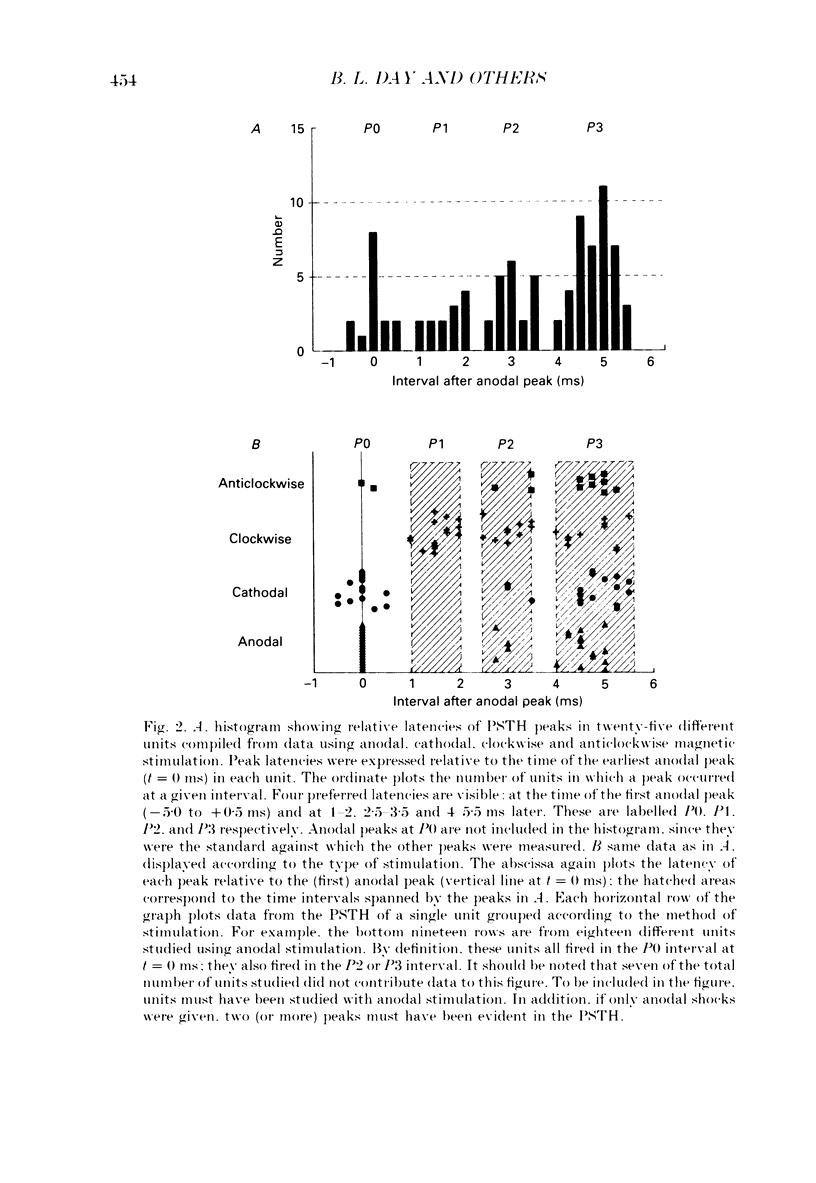
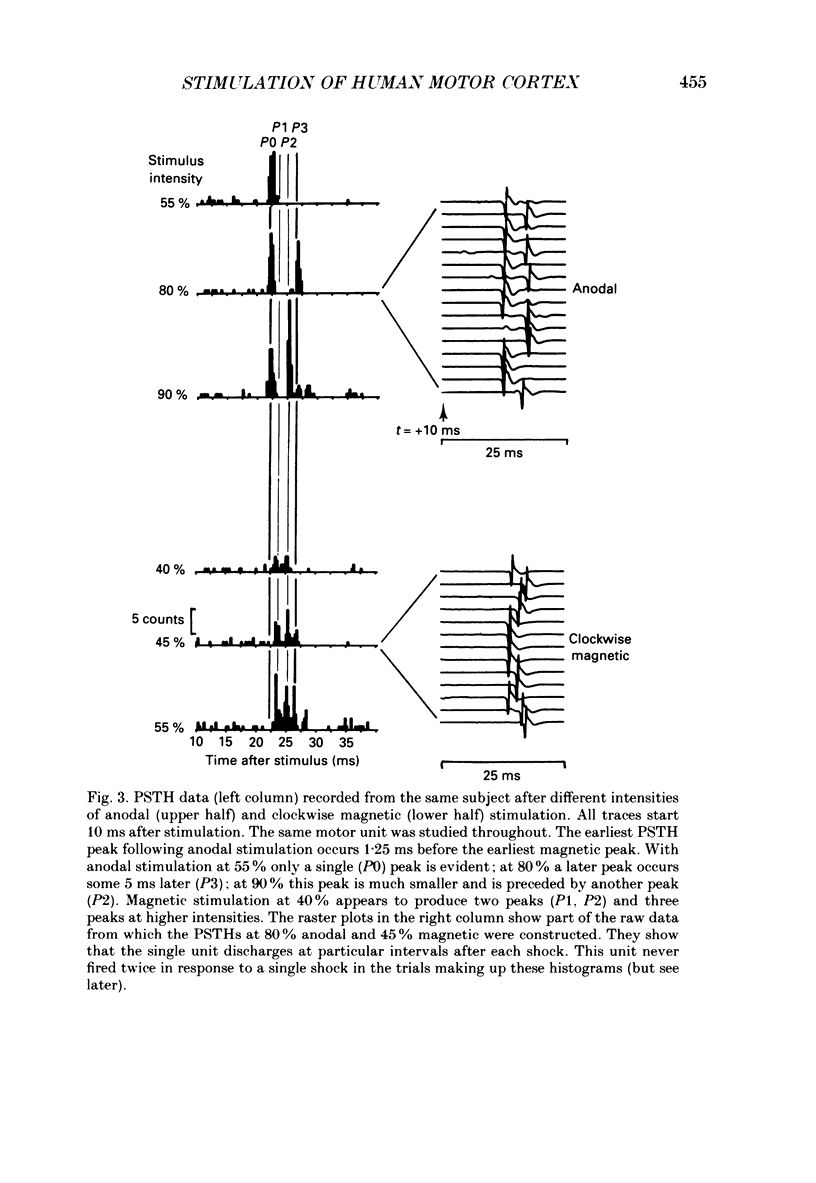
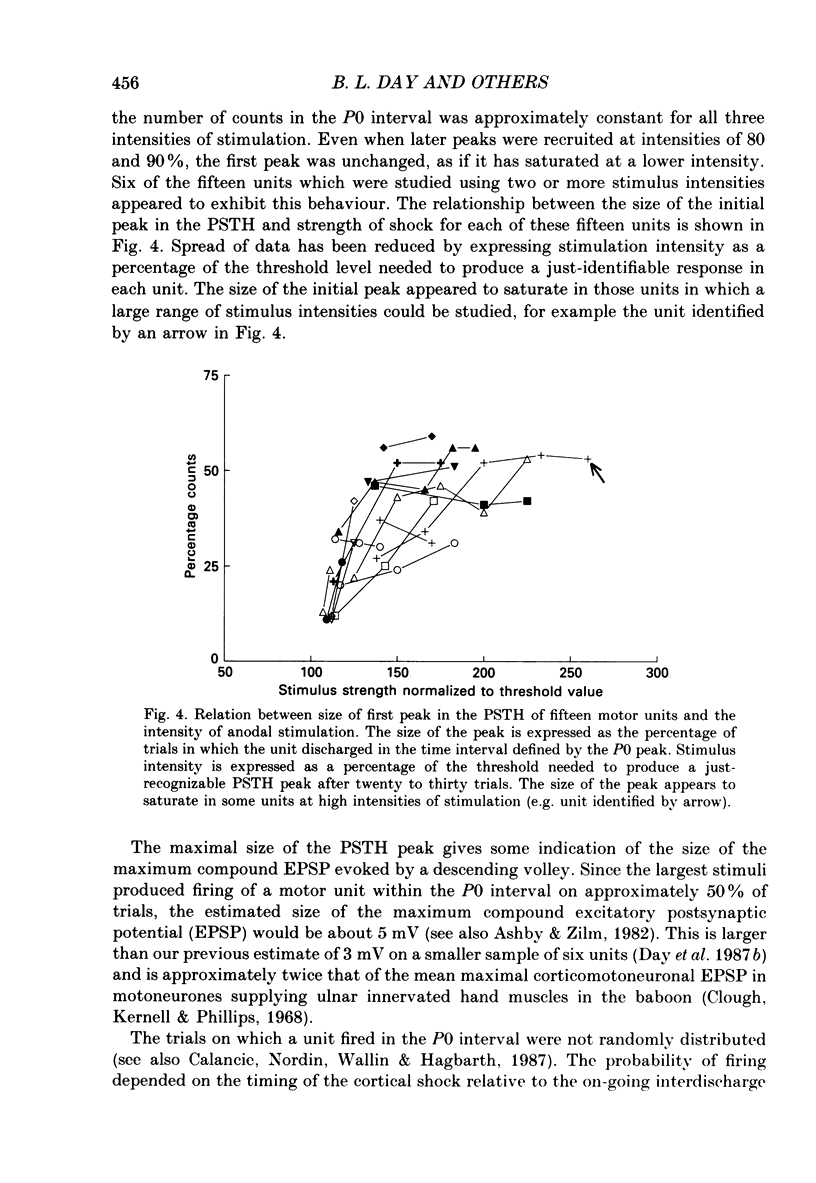
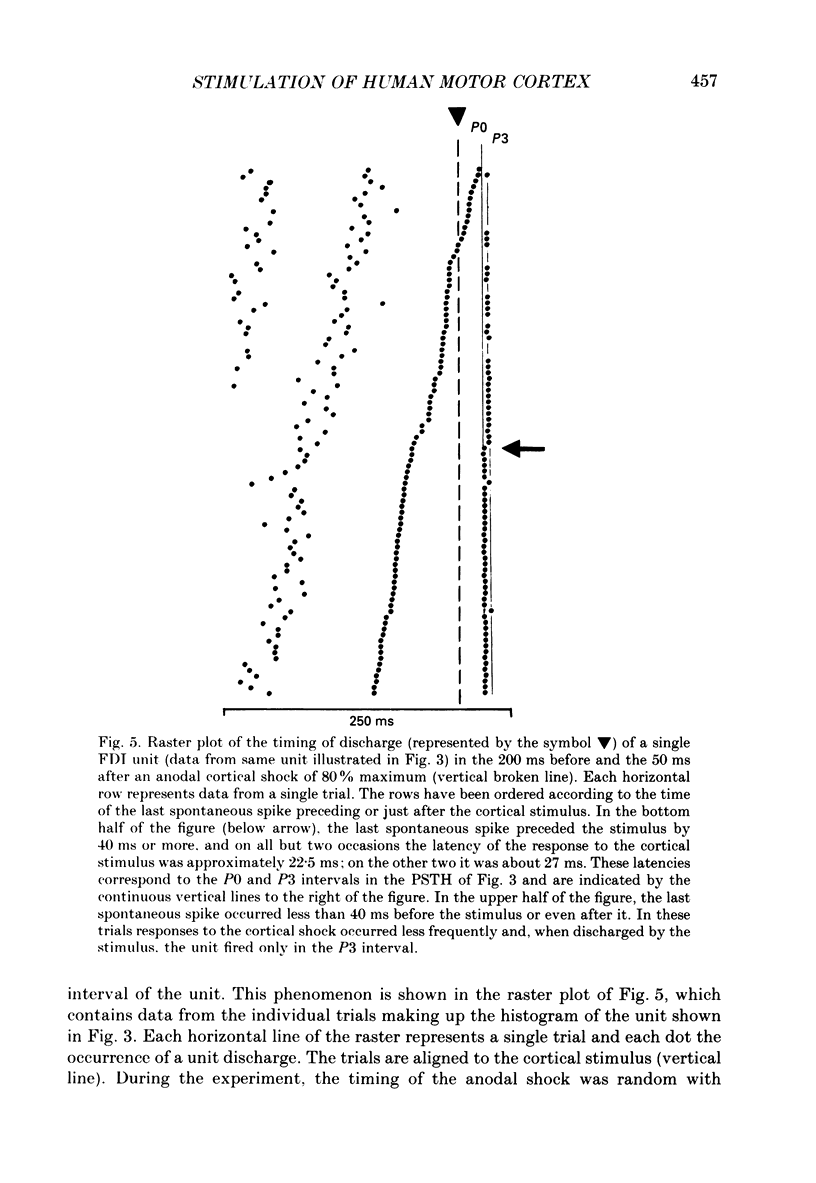
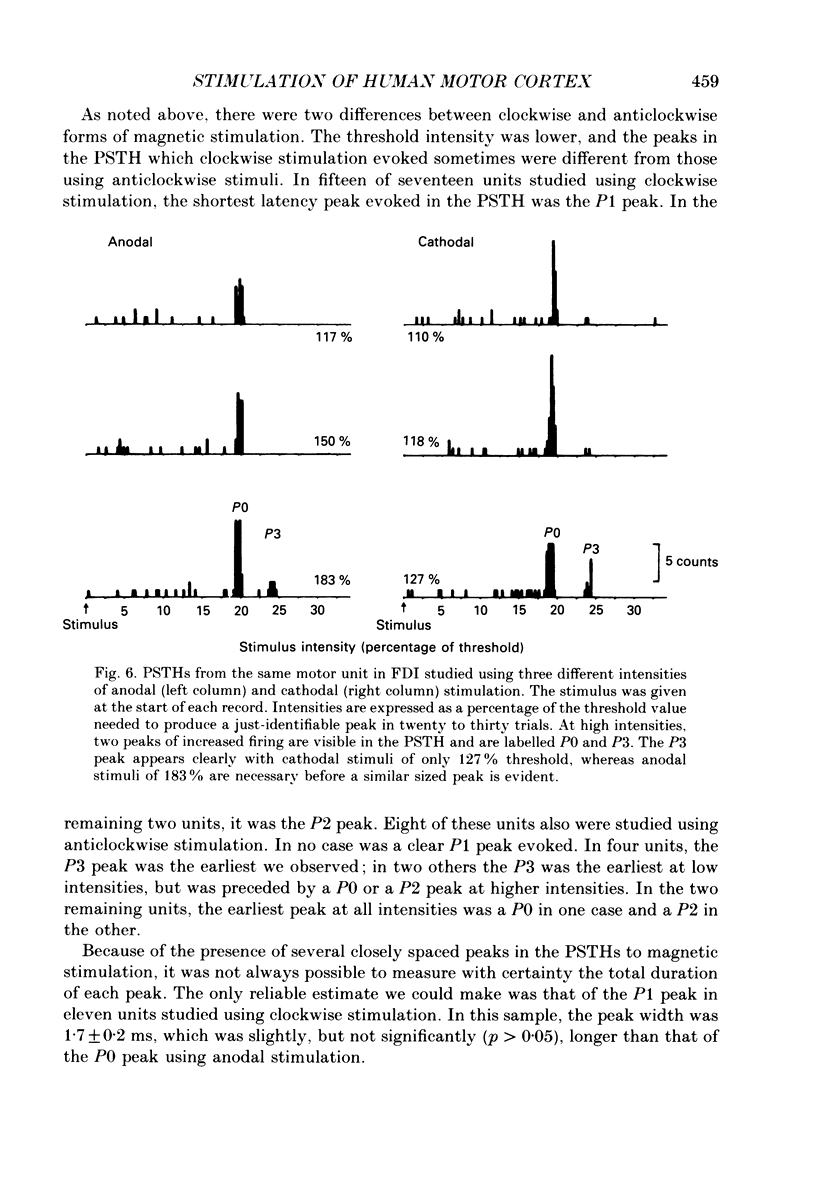
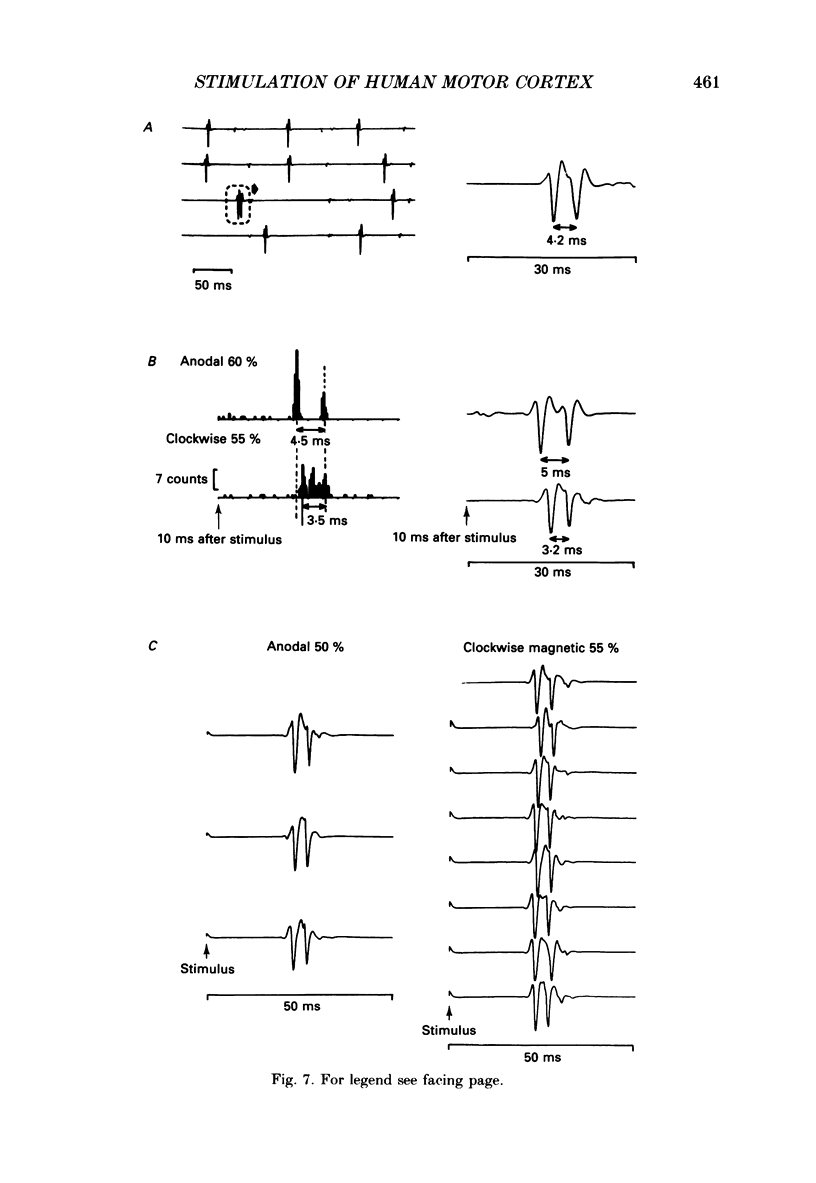
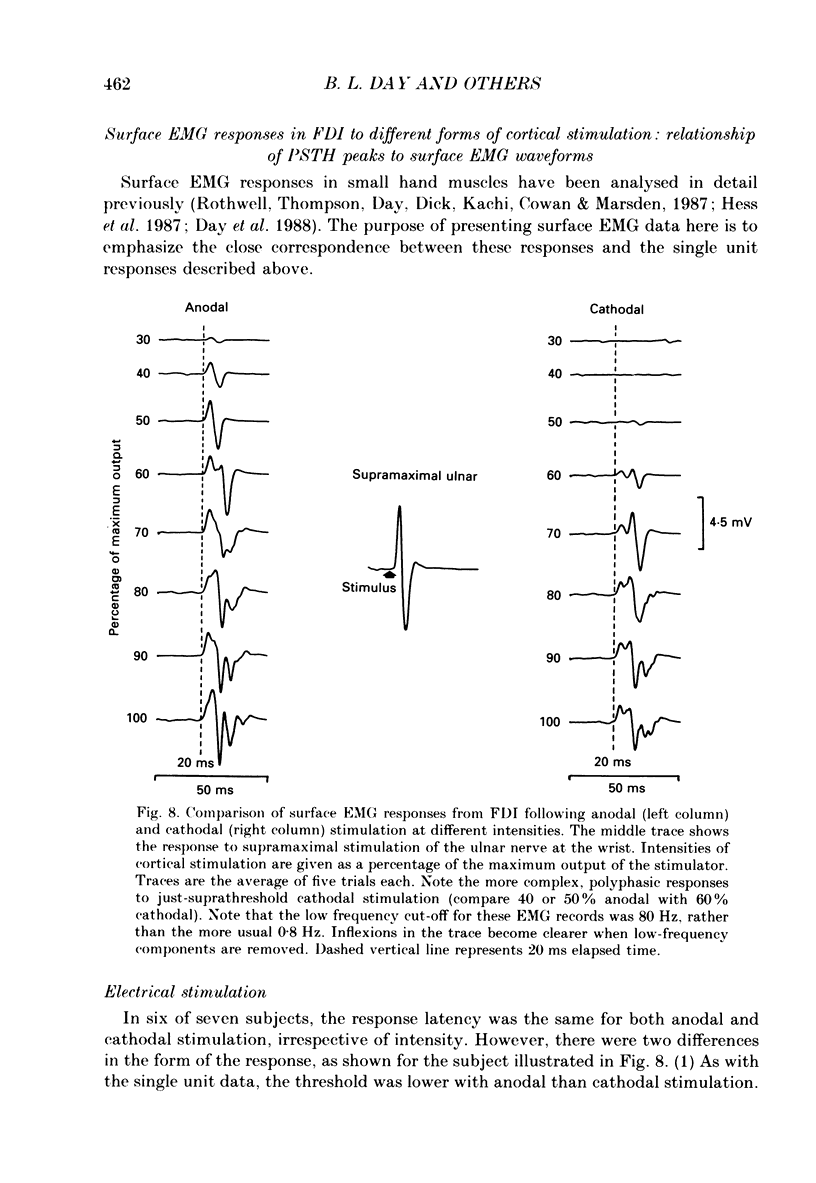
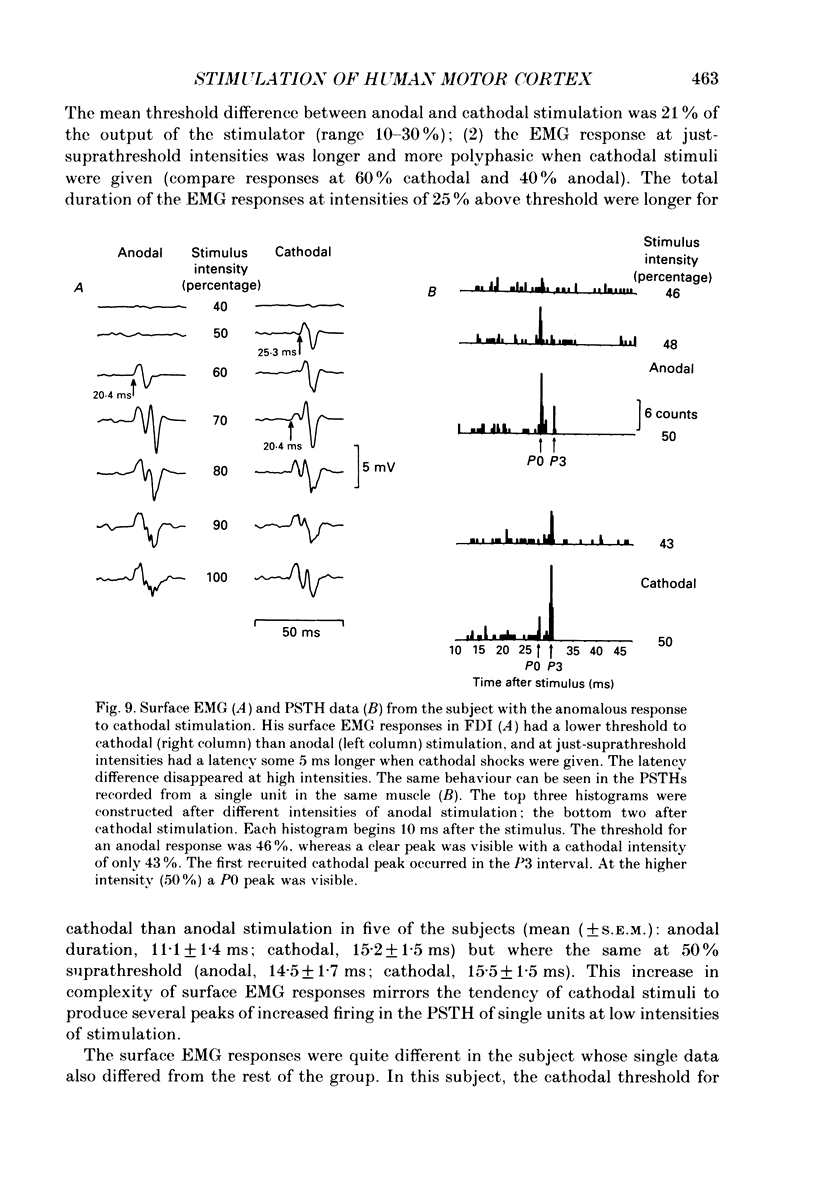
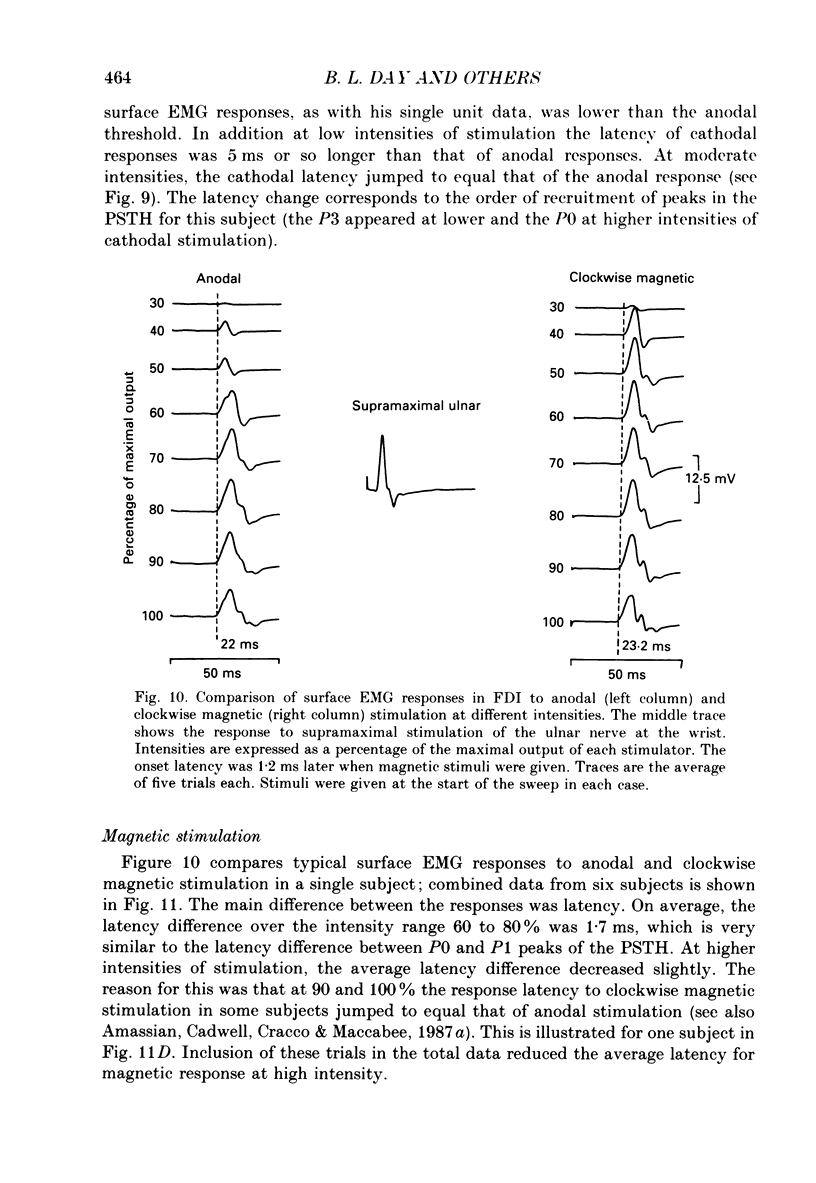
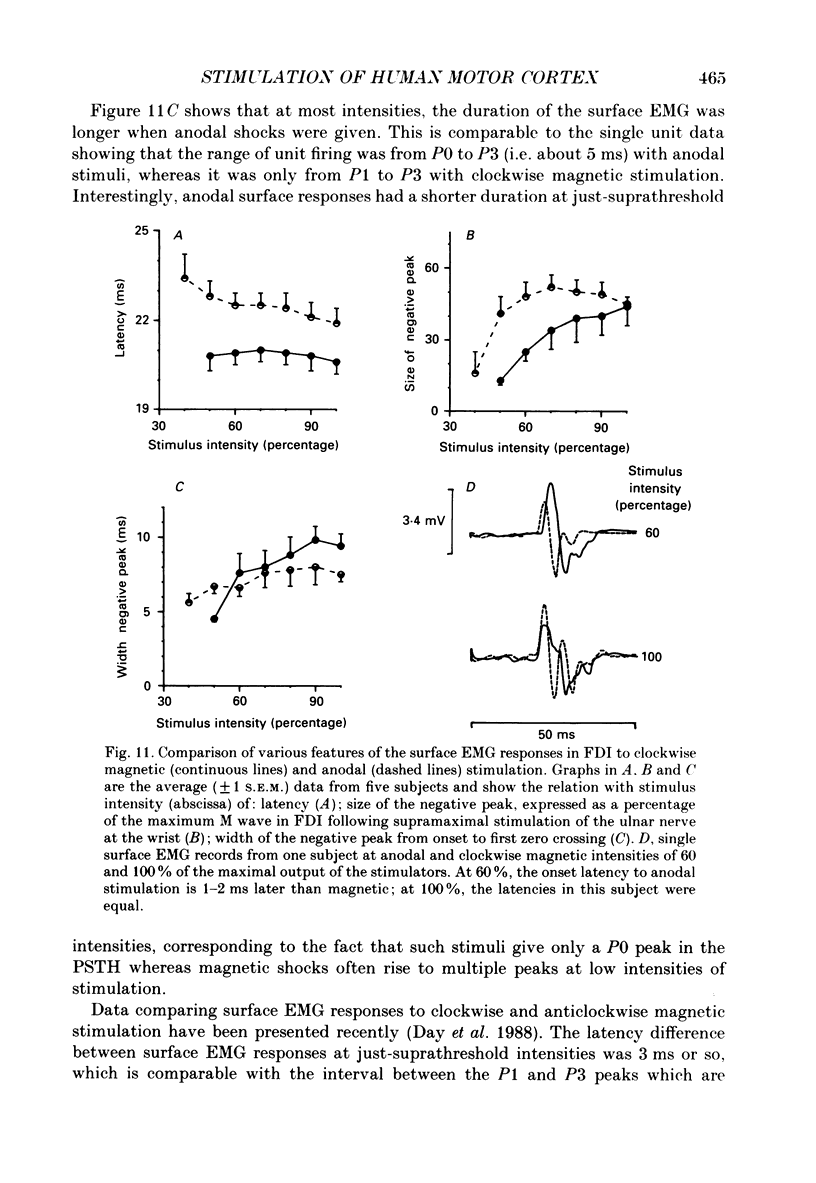
1. The effects of different forms of brain stimulation on the discharge pattern of single motor units were examined using the post-stimulus time histogram (PSTH) technique and by recording the compound surface electromyographic (EMG) responses in the first dorsal interosseous (FDI) muscle. Electrical and magnetic methods were used to stimulate the brain through the intact scalp of seven normal subjects. Electrical stimuli were applied either with the anode over the lateral central scalp and the cathode at the vertex (anodal stimulation) or with the anode at the vertex and the cathode lateral (cathodal stimulation). Magnetic stimulation used a 9 cm diameter coil centred at the vertex; current in the coil flowed either clockwise or anticlockwise when viewed from above. 2. Supramotor threshold stimuli produced one or more narrow (less than 2 ms) peaks of increased firing in the PSTH of all thirty-two units studied. Anodal stimulation always produced an early peak. The latencies of the peaks produced by other forms of stimulation, or by high intensities of anodal stimulation, were grouped into four time bands relative to this early peak, at intervals of -0.5 to 0.5, 1-2, 2.5-3.5 and 4-5.5 ms later. Peaks occurring within these intervals are referred to as P0 (the earliest anodal), P1, P2 and P3 respectively. 3. At threshold, anodal stimulation evoked only the P0 peak; at higher intensities, the P2 or more commonly the P3 peak also was recruited. The size of the P0 peak appeared to saturate at high intensities. 4. In five of six subjects, cathodal stimulation behaved like anodal stimulation, except that there was a lower threshold for recruitment of the P2 or P3 peak relative to that of the P0 peak. In the other subject, the P3 peak was recruited before the P0 peak. 5. Anticlockwise magnetic [corrected] stimulation, at threshold, often produced several peaks. These always included a P1 peak, and usually a P3 peak. A P0 peak in the PSTH was never produced by an anticlockwise stimulation [corrected] at intensities which we could explore with the technique. 6. Clockwise magnetic [corrected] stimulation never recruited a P1 peak; in most subjects a P3 peak was recruited first and at higher intensities was accompanied by P0 or P2 peaks. 7. On most occasions when more than one peak was observed in a PSTH, the unit fired in only one of the preferred intervals after each shock. However, double firing was seen in five units when high intensities of stimulation were used.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvin W. H., Schwindt P. C. Steps in production of motoneuron spikes during rhythmic firing. J Neurophysiol. 1972 May;35(3):297–310. doi: 10.1152/jn.1972.35.3.297. [DOI] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan J. M., Day B. L., Marsden C., Rothwell J. C. The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and leg in intact man. J Physiol. 1986 Aug;377:333–347. doi: 10.1113/jphysiol.1986.sp016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Rothwell J. C., Thompson P. D., Dick J. P., Cowan J. M., Berardelli A., Marsden C. D. Motor cortex stimulation in intact man. 2. Multiple descending volleys. Brain. 1987 Oct;110(Pt 5):1191–1209. doi: 10.1093/brain/110.5.1191. [DOI] [PubMed] [Google Scholar]
- Day B. L., Thompson P. D., Dick J. P., Nakashima K., Marsden C. D. Different sites of action of electrical and magnetic stimulation of the human brain. Neurosci Lett. 1987 Mar 20;75(1):101–106. doi: 10.1016/0304-3940(87)90083-8. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia S. C., Rothwell J. C. Knowledge of motor commands and the recruitment of human motoneurons. Brain. 1987 Oct;110(Pt 5):1117–1130. doi: 10.1093/brain/110.5.1117. [DOI] [PubMed] [Google Scholar]
- HERN J. E., LANDGREN S., PHILLIPS C. G., PORTER R. Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon's motor cortex. J Physiol. 1962 Apr;161:73–90. doi: 10.1113/jphysiol.1962.sp006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess C. W., Mills K. R., Murray N. M. Responses in small hand muscles from magnetic stimulation of the human brain. J Physiol. 1987 Jul;388:397–419. doi: 10.1113/jphysiol.1987.sp016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Tanaka R. Projections of pyramidal tract cells to alpha-motoneurones innervating hind-limb muscles in the monkey. J Physiol. 1975 Aug;249(3):637–667. doi: 10.1113/jphysiol.1975.sp011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol. 1975 Aug;249(3):617–636. doi: 10.1113/jphysiol.1975.sp011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERNELL D. THE DELAYED DEPOLARIZATION IN CAT AND RAT MOTONEURONES. Prog Brain Res. 1964;12:42–55. doi: 10.1016/s0079-6123(08)60616-0. [DOI] [PubMed] [Google Scholar]
- Kernell D., Chien-Ping W. U. Responses of the pyramidal tract to stimulation of the baboon's motor cortex. J Physiol. 1967 Aug;191(3):653–672. doi: 10.1113/jphysiol.1967.sp008273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D., Chien-Ping W. Post-synaptic effects of cortical stimulation on forelimb motoneurones in the baboon. J Physiol. 1967 Aug;191(3):673–690. doi: 10.1113/jphysiol.1967.sp008274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDGREN S., PHILLIPS C. G., PORTER R. Minimal synaptic actions of pyramidal impulses on some alpha motoneurones of the baboon's hand and forearm. J Physiol. 1962 Apr;161:91–111. doi: 10.1113/jphysiol.1962.sp006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau W. M., Bishop G. H., Clare M. H. Site of excitation in stimulation of the motor cortex. J Neurophysiol. 1965 Nov;28(6):1206–1222. doi: 10.1152/jn.1965.28.6.1206. [DOI] [PubMed] [Google Scholar]
- Mao C. C., Ashby P., Wang M., McCrea D. Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Exp Brain Res. 1984;56(2):341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Direct electrical stimulation of corticospinal pathways through the intact scalp in human subjects. Adv Neurol. 1983;39:387–391. [PubMed] [Google Scholar]
- Merton P. A., Morton H. B. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980 May 22;285(5762):227–227. doi: 10.1038/285227a0. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Burke R. E. Delayed depolarization in cat spinal motoneurons. Exp Neurol. 1967 Jan;17(1):16–26. doi: 10.1016/0014-4886(67)90118-5. [DOI] [PubMed] [Google Scholar]
- PATTON H. D., AMASSIAN V. E. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954 Jul;17(4):345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G., PORTER R. THE PYRAMIDAL PROJECTION TO MOTONEURONES OF SOME MUSCLE GROUPS OF THE BABOON'S FORELIMB. Prog Brain Res. 1964;12:222–245. doi: 10.1016/s0079-6123(08)60625-1. [DOI] [PubMed] [Google Scholar]
- Palmer S. S., Fetz E. E. Effects of single intracortical microstimuli in motor cortex on activity of identified forearm motor units in behaving monkeys. J Neurophysiol. 1985 Nov;54(5):1194–1212. doi: 10.1152/jn.1985.54.5.1194. [DOI] [PubMed] [Google Scholar]
- Phillips C. G. Epicortical electrical mapping of motor areas in primates. Ciba Found Symp. 1987;132:5–20. doi: 10.1002/9780470513545.ch2. [DOI] [PubMed] [Google Scholar]
- Ranck J. B., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975 Nov 21;98(3):417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Rothwell J. C., Thompson P. D., Day B. L., Dick J. P., Kachi T., Cowan J. M., Marsden C. D. Motor cortex stimulation in intact man. 1. General characteristics of EMG responses in different muscles. Brain. 1987 Oct;110(Pt 5):1173–1190. doi: 10.1093/brain/110.5.1173. [DOI] [PubMed] [Google Scholar]
- Zidar J., Trontelj J. V., Mihelin M. Percutaneous stimulation of human corticospinal tract: a single-fibre EMG study of individual motor unit responses. Brain Res. 1987 Sep 29;422(1):196–199. doi: 10.1016/0006-8993(87)90559-2. [DOI] [PubMed] [Google Scholar]


