Abstract
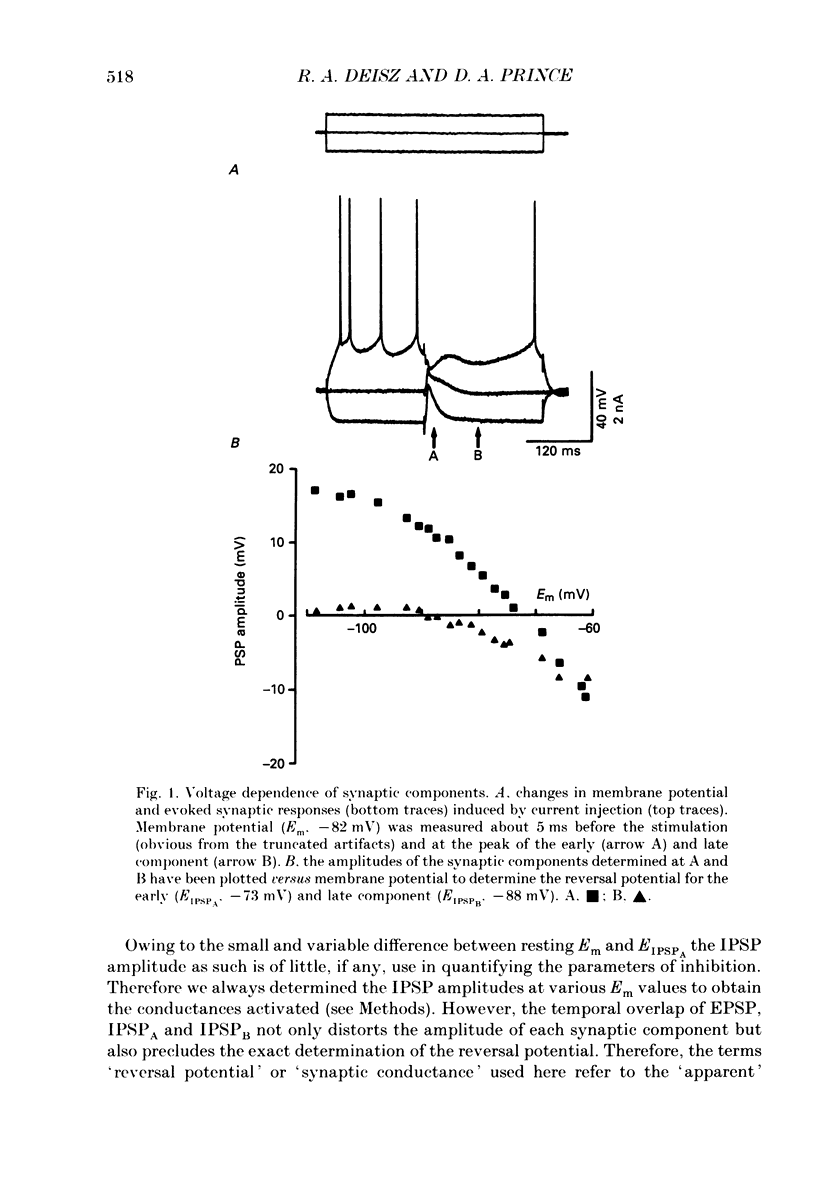
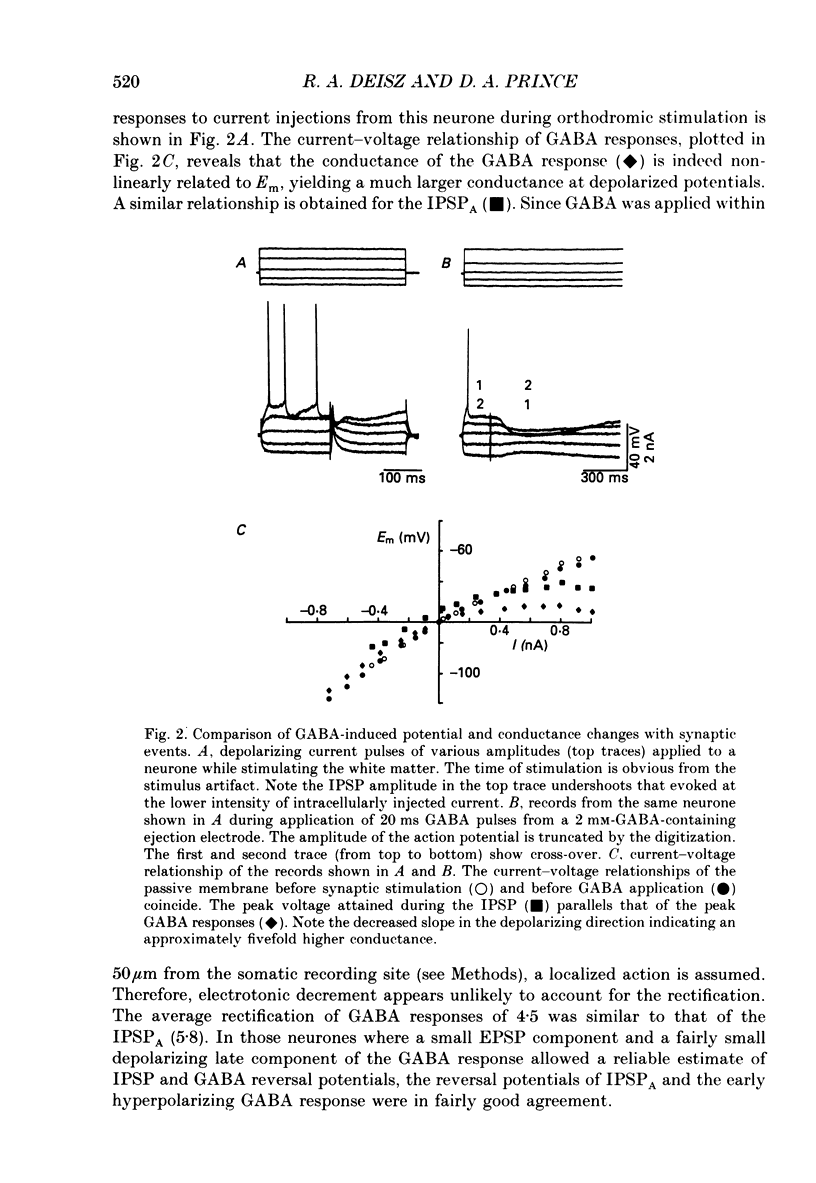
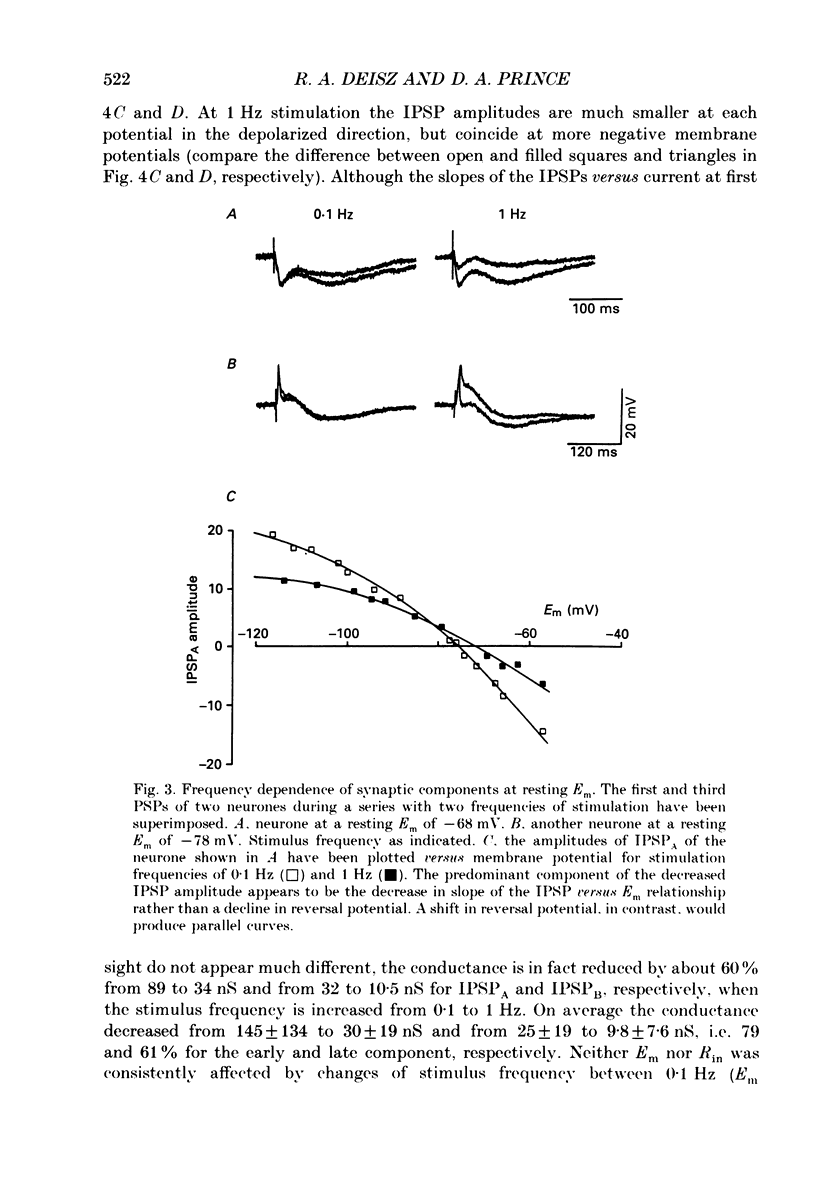
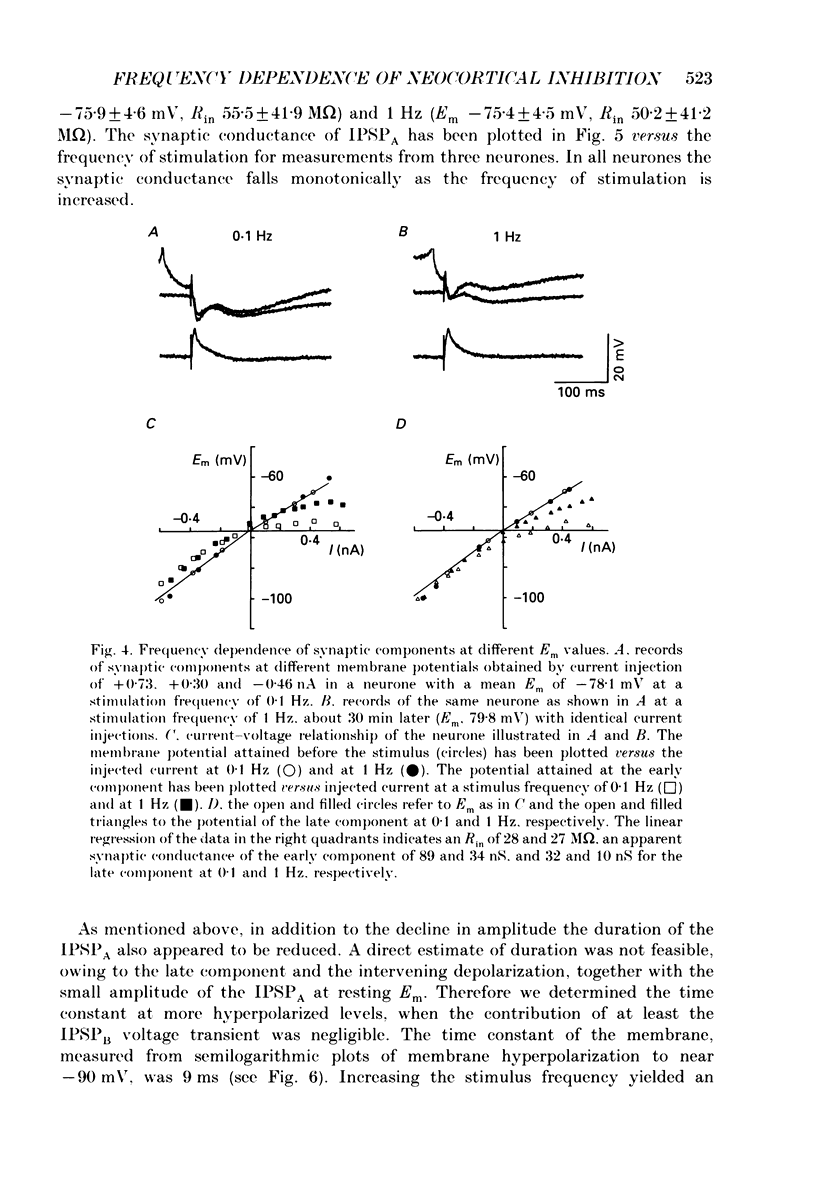
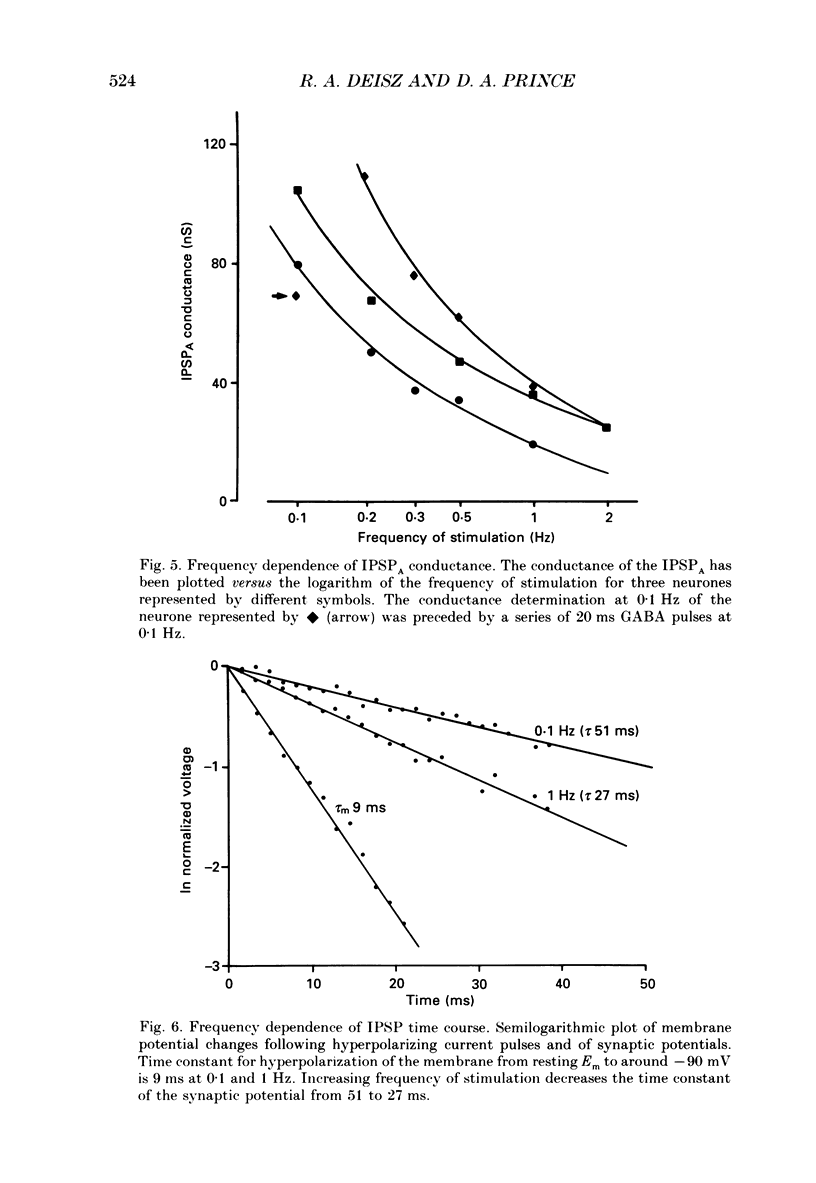
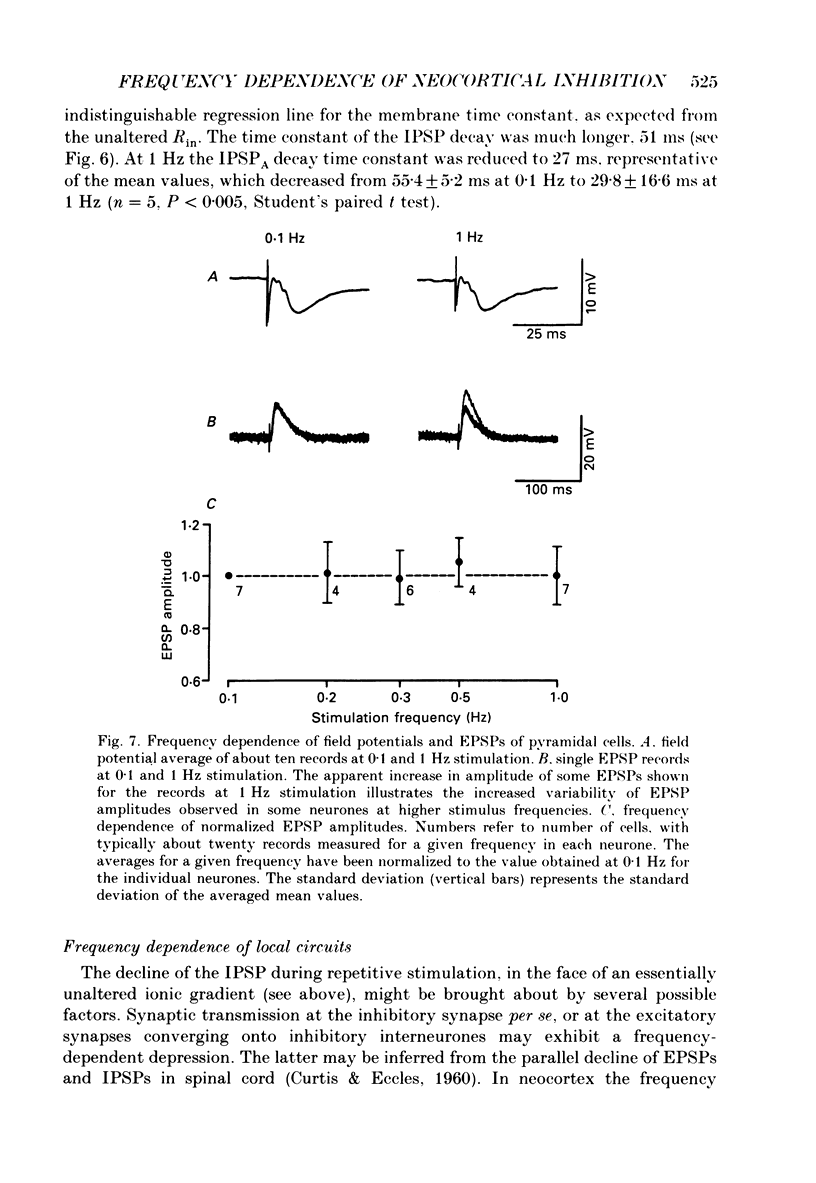
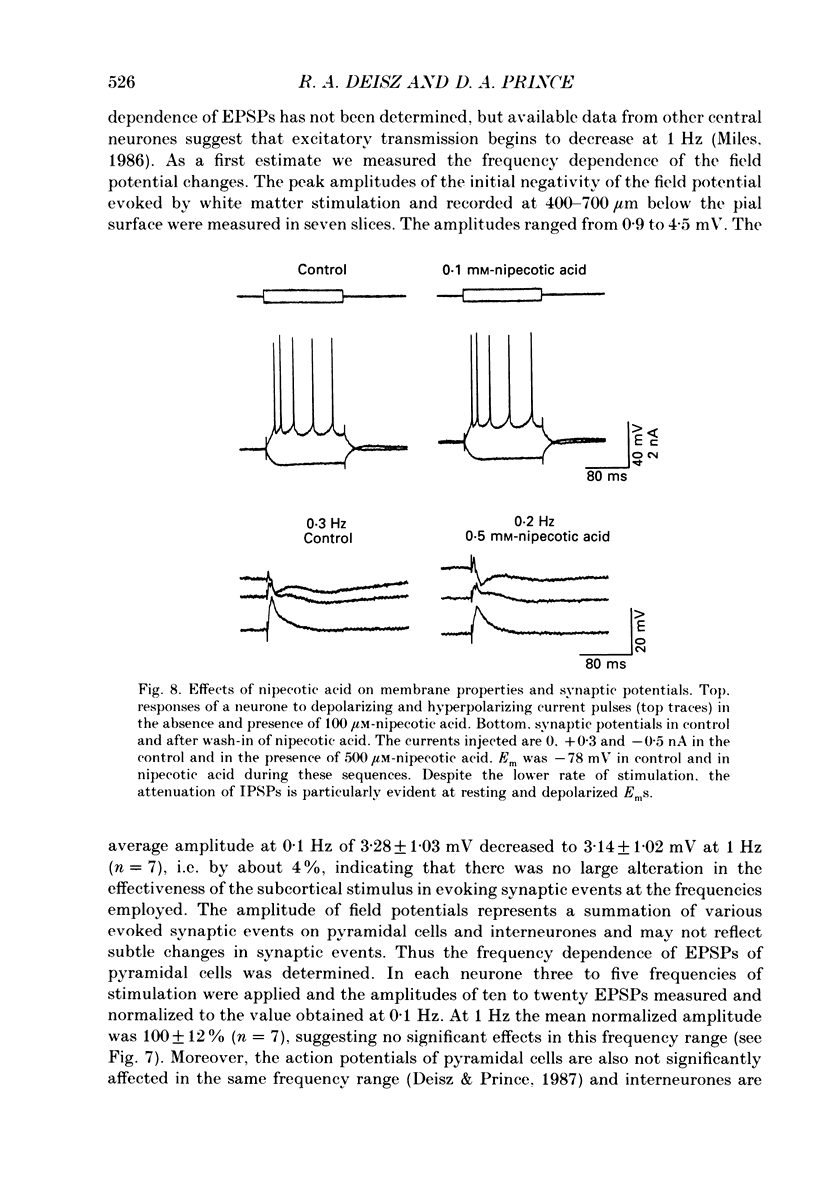
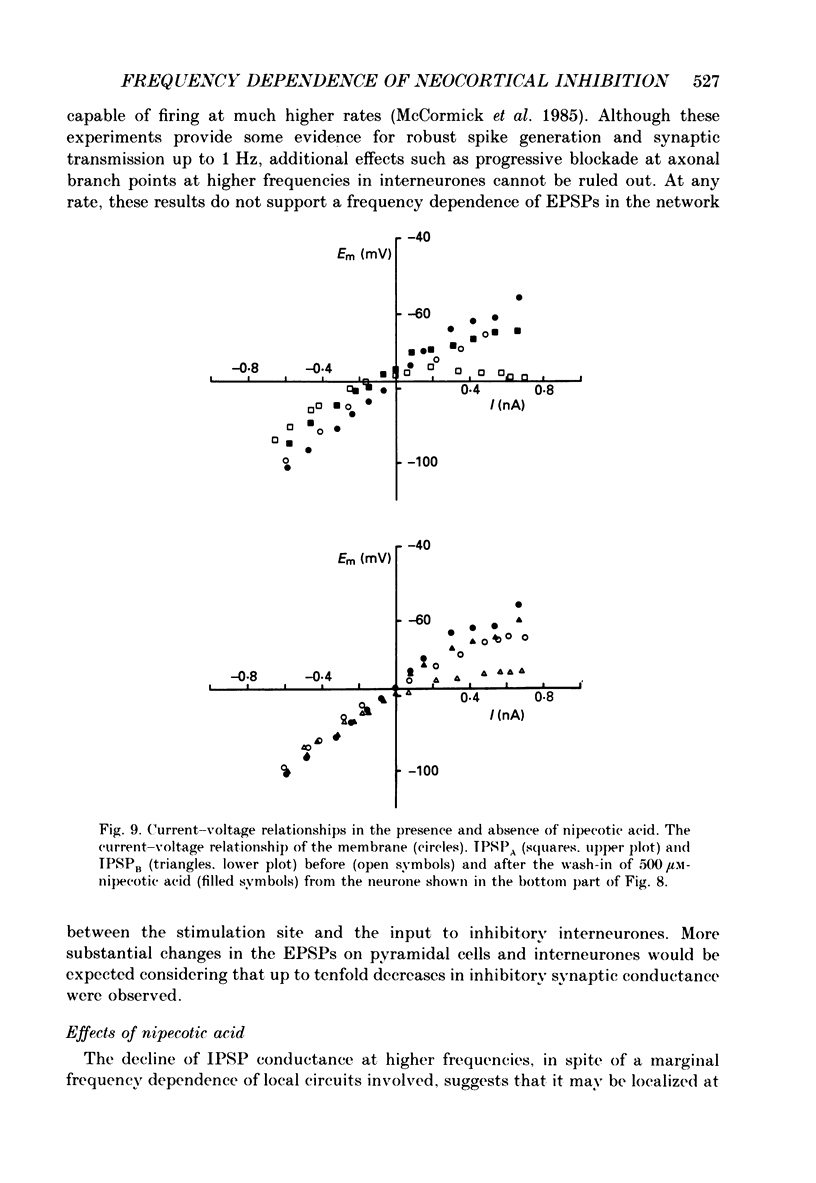
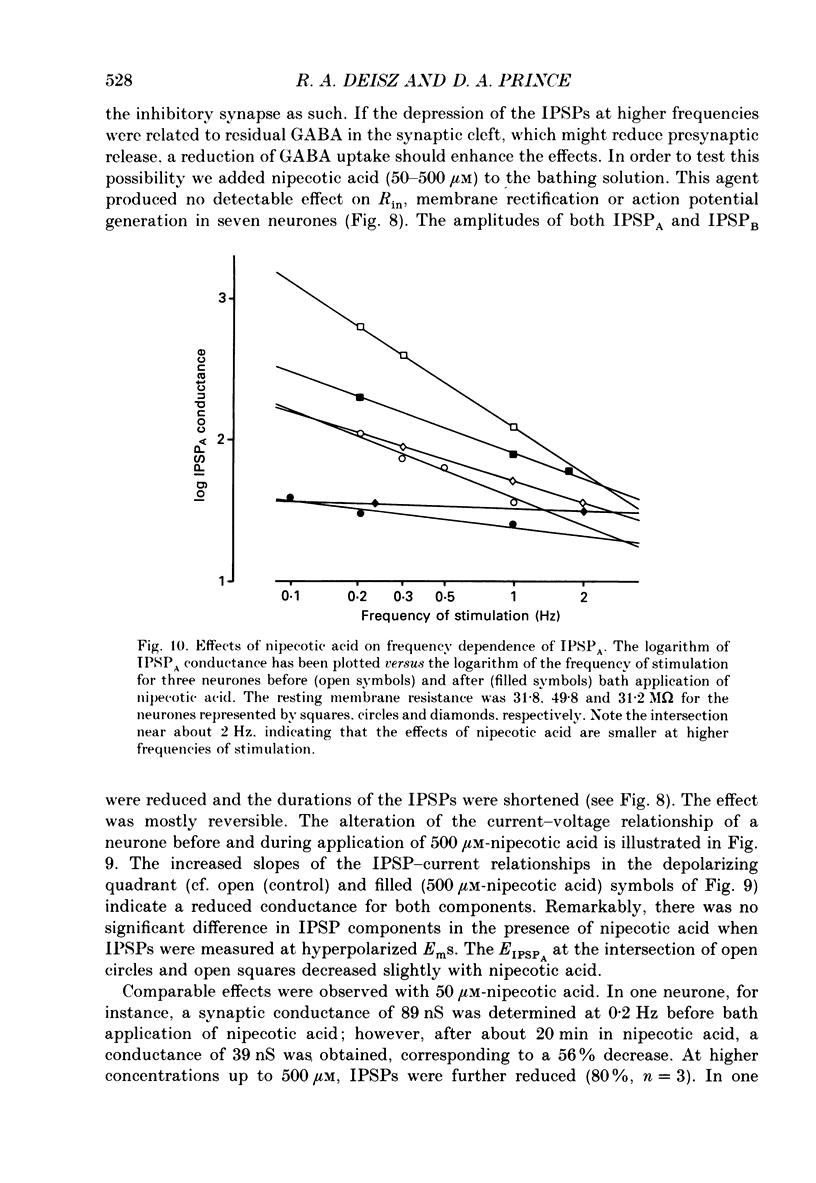
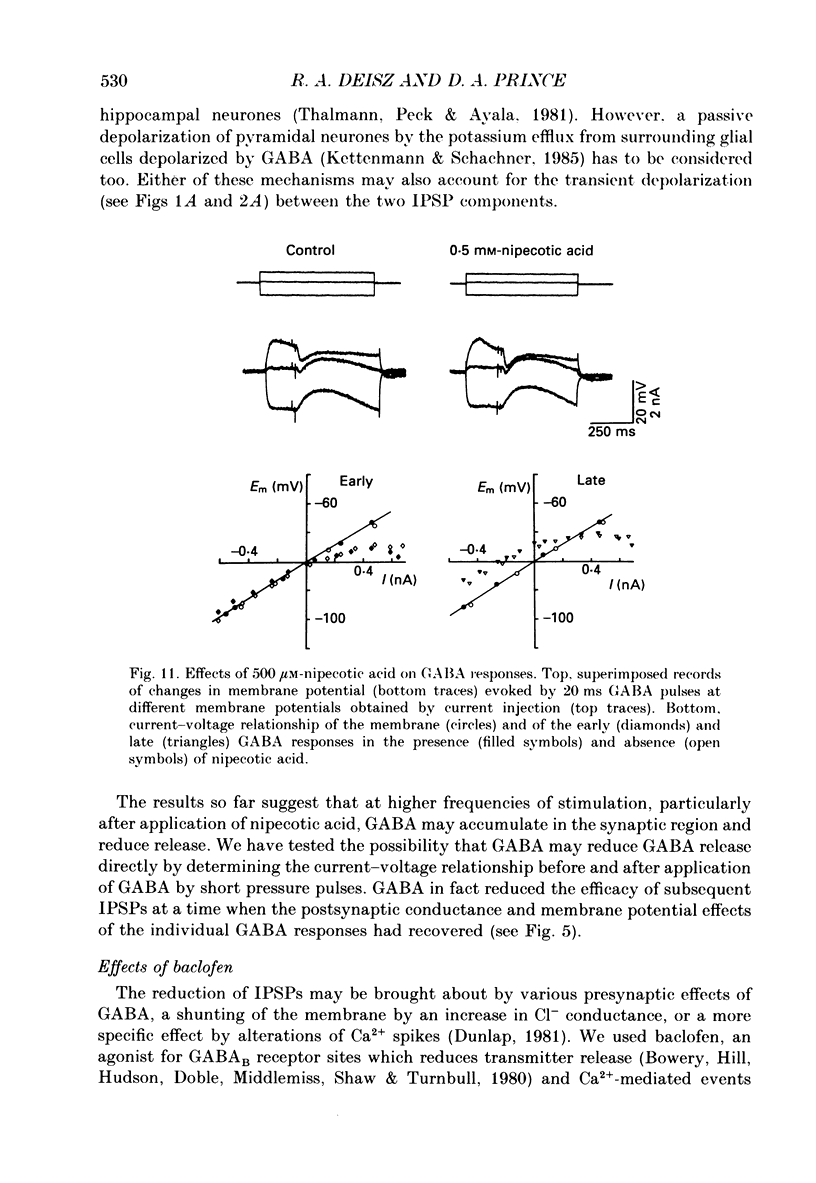
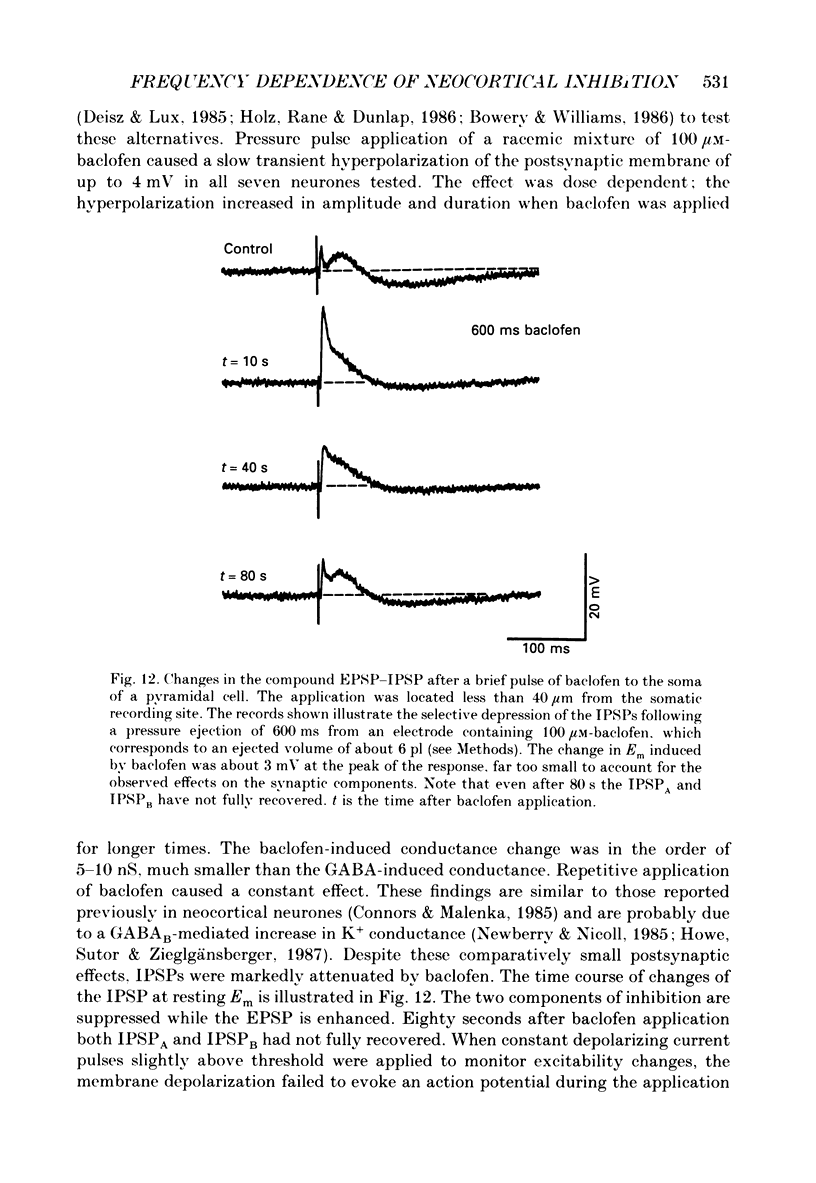
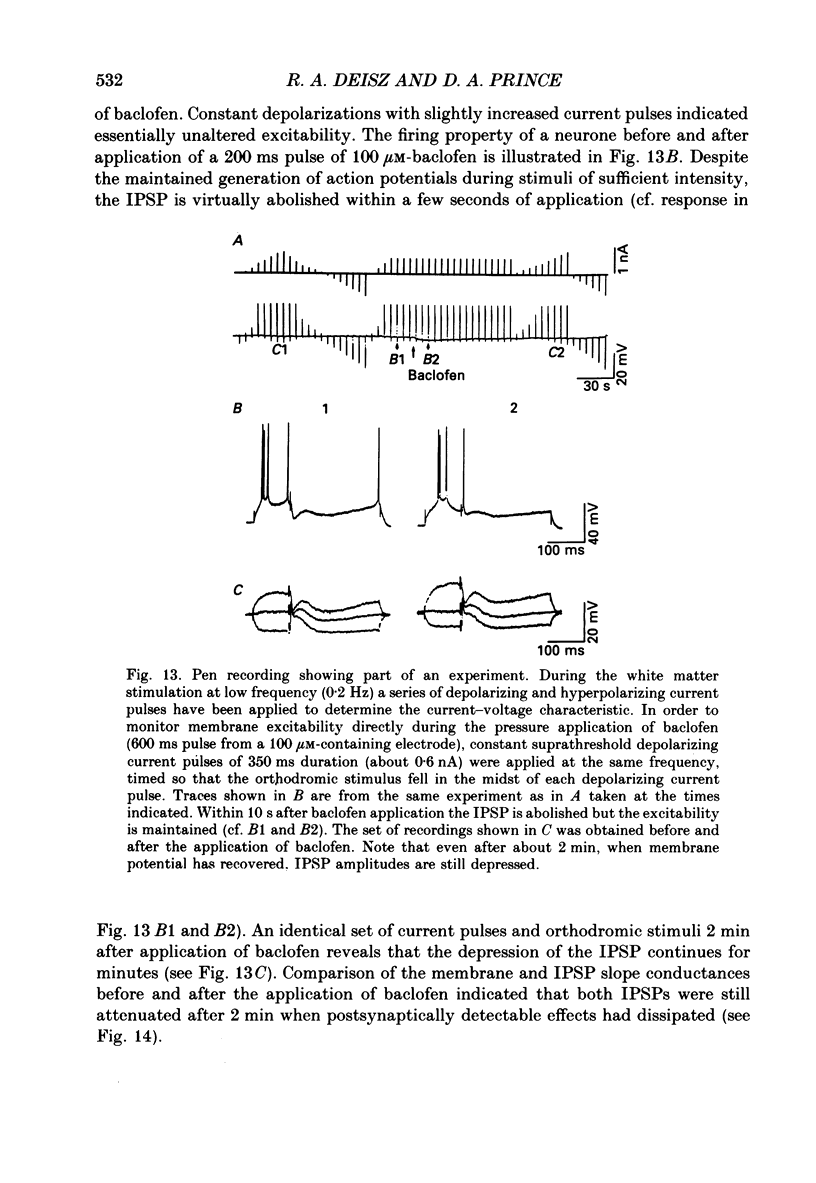
1. The mechanisms involved in the lability of inhibition at higher frequencies of stimulation were investigated in the guinea-pig in vitro neocortical slice preparation by intracellular recording techniques. We attempted to test the possibility of a feedback depression of GABA on subsequent release. 2. At resting membrane potential (Em, -75.8 +/- 5.2 mV) stimulation of either the pial surface or subcortical white matter evoked a sequence of depolarizing and hyperpolarizing synaptic components in most neurones. An early hyperpolarizing component (IPSPA) was usually only obvious as a pronounced termination of the EPSP, followed by a later hyperpolarizing event (IPSPB). Current-voltage relationships revealed two different conductances of about 200 and 20 nS and reversal potentials of -73.0 +/- 4.4 and -88.6 +/- 6.1 mV for the early and late component, respectively. 3. The conductances of IPSPA and IPSPB were fairly stable at a stimulus frequency of 0.1 Hz. At frequencies between 0.5 and 2 Hz both IPSPs were attenuated with the second stimulus and after about five stimuli a steady state was reached. Concomitantly IPSPs were shortened. The average decrease in synaptic conductance between 0.1 and 1 Hz was 80% for the IPSPA and 60% for the IPSPB. At these frequencies the reversal potentials decreased by 5 and 2 mV, respectively; Em and input resistance (Rin) were not consistently affected. 4. The amplitudes of field potentials, action potentials and EPSPs of pyramidal cells were attenuated less than 10% at stimulus frequencies up to 1 Hz, suggesting that alterations in local circuits between the stimulation site and excitatory input onto inhibitory interneurones may play only a minor role in the frequency-dependent decay of IPSPs. 5. Localized application of GABA produced multiphasic responses. With low concentrations and application near the soma an early hyperpolarization prevailed followed by a depolarizing late component. Brief application of GABA at low frequencies induced constant responses; at higher frequencies, the responses sometimes declined. The current-voltage relationships of the two GABA responses were similar to each other and to the early IPSP. An apparently fivefold higher conductance was estimated at lower Ems, suggesting that the GABA response had a voltage sensitivity. The slope conductance of IPSPs was decreased by up to 50% for tens of seconds after postsynaptically detectable effects of GABA had dissipated. 6. Application of the GABA uptake inhibitor nipecotic acid (50-500 microM) reduced the conductance of both components of orthodromically evoked inhibition and shortened the IPSP at low frequencies, but had no additional effects at higher stimulation rates.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







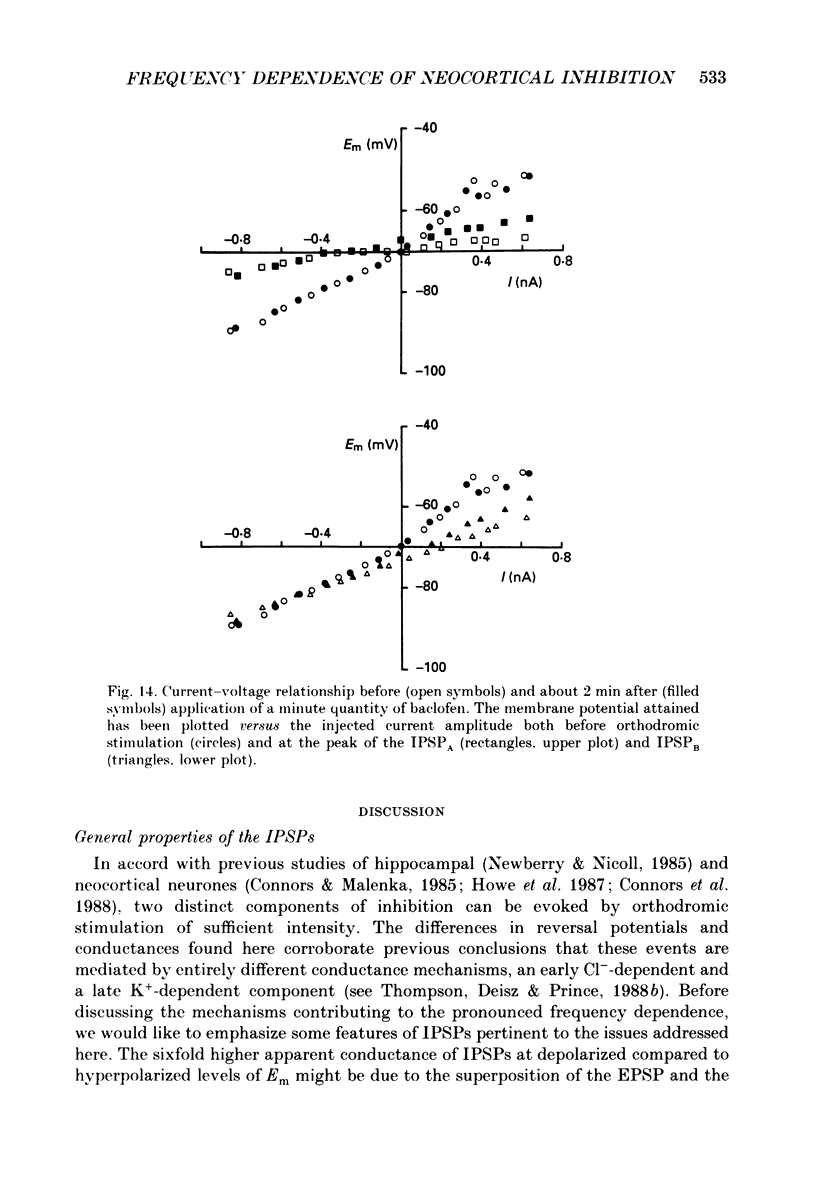








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Deisz R. A., Lux H. D. Ammonium action on post-synaptic inhibition in crayfish neurones: implications for the mechanism of chloride extrusion. J Physiol. 1982 Aug;329:319–339. doi: 10.1113/jphysiol.1982.sp014305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Deisz R. A. Pentobarbitone interference with inhibitory synaptic transmission in crayfish stretch receptor neurones. J Physiol. 1981 Jun;315:175–187. doi: 10.1113/jphysiol.1981.sp013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Ammonia does not selectively block IPSPs in rat hippocampal pyramidal cells. J Neurophysiol. 1983 Jun;49(6):1381–1391. doi: 10.1152/jn.1983.49.6.1381. [DOI] [PubMed] [Google Scholar]
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980 Aug;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood T. J., Collingridge G. L., Herron C. E., Wheal H. V. Voltage-clamp analysis of somatic gamma-aminobutyric acid responses in adult rat hippocampal CA1 neurones in vitro. J Physiol. 1987 Mar;384:27–37. doi: 10.1113/jphysiol.1987.sp016441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Galvan M. Influence of neuroglial transport on the action of gamma-aminobutyric acid on mammalian ganglion cells. Br J Pharmacol. 1977 Feb;59(2):373–378. doi: 10.1111/j.1476-5381.1977.tb07502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Gration K. A., Usherwood P. N. Influence of glutamate and aspartate on time course of decay of excitatory synaptic currents at locust neuromuscular junctions. Brain Res. 1980 Jun 16;192(1):205–216. doi: 10.1016/0006-8993(80)91020-3. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Malenka R. C., Silva L. R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988 Dec;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. The termination of transmitter action at the crustacean excitatory neuromuscular junction. J Physiol. 1977 Jul;268(3):711–729. doi: 10.1113/jphysiol.1977.sp011878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Game C. J., Lodge D. The in vivo inactivation of GABA and other inhibitory amino acids in the cat nervous system. Exp Brain Res. 1976 Jun 30;25(4):413–428. doi: 10.1007/BF00241731. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Dose M. Comparison of GABA analogues at the crayfish stretch receptor neurone. Brain Res Bull. 1983 Sep;11(3):283–288. doi: 10.1016/0361-9230(83)90161-2. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Dose M., Lux H. D. The time course of GABA action on the crayfish stretch receptor: evidence for a saturable GABA uptake. Neurosci Lett. 1984 Jun 29;47(3):245–250. doi: 10.1016/0304-3940(84)90521-4. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Lux H. D. The role of intracellular chloride in hyperpolarizing post-synaptic inhibition of crayfish stretch receptor neurones. J Physiol. 1982 May;326:123–138. doi: 10.1113/jphysiol.1982.sp014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz R. A., Lux H. D. gamma-Aminobutyric acid-induced depression of calcium currents of chick sensory neurons. Neurosci Lett. 1985 May 14;56(2):205–210. doi: 10.1016/0304-3940(85)90130-2. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Prince D. A. Effect of D890 on membrane properties of neocortical neurons. Brain Res. 1987 Sep 29;422(1):63–73. doi: 10.1016/0006-8993(87)90540-3. [DOI] [PubMed] [Google Scholar]
- Desarmenien M., Feltz P., Headley P. M. Does glial uptake affect GABA responses? AN intracellular study on rat dorsal root ganglion neurones in vitro. J Physiol. 1980 Oct;307:163–182. doi: 10.1113/jphysiol.1980.sp013429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R., Korn S. J. Gamma-aminobutyric acid uptake and the termination of inhibitory synaptic potentials in the rat hippocampal slice. J Physiol. 1985 Sep;366:387–409. doi: 10.1113/jphysiol.1985.sp015804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. Voltage dependence of amplitude and time course of inhibitory synaptic current in crayfish muscle. Pflugers Arch. 1977 Oct 19;371(1-2):167–174. doi: 10.1007/BF00580786. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. P., Nakamura J., Shinnick-Gallagher P. Effects of glial uptake and desensitization on the activity of gamma-aminobutyric acid (GABA) and its analogs at the cat dorsal root ganglion. J Pharmacol Exp Ther. 1983 Sep;226(3):876–884. [PubMed] [Google Scholar]
- Gray R., Johnston D. Rectification of single GABA-gated chloride channels in adult hippocampal neurons. J Neurophysiol. 1985 Jul;54(1):134–142. doi: 10.1152/jn.1985.54.1.134. [DOI] [PubMed] [Google Scholar]
- Gutnick M. J., Connors B. W., Prince D. A. Mechanisms of neocortical epileptogenesis in vitro. J Neurophysiol. 1982 Dec;48(6):1321–1335. doi: 10.1152/jn.1982.48.6.1321. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J., Lebeda F. J. Role of uptake in gamma-aminobutyric acid (GABA)-mediated responses in guinea pig hippocampal neurons. Cell Mol Neurobiol. 1985 Dec;5(4):353–371. doi: 10.1007/BF00755401. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Baclofen reduces post-synaptic potentials of rat cortical neurones by an action other than its hyperpolarizing action. J Physiol. 1987 Mar;384:539–569. doi: 10.1113/jphysiol.1987.sp016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. R., Alger B. E. Whole-cell voltage-clamp study of the fading of GABA-activated currents in acutely dissociated hippocampal neurons. J Neurophysiol. 1986 Jul;56(1):1–18. doi: 10.1152/jn.1986.56.1.1. [DOI] [PubMed] [Google Scholar]
- Jia M., Nelson P. G. Calcium currents and transmitter output in cultured spinal cord and dorsal root ganglion neurons. J Neurophysiol. 1986 Nov;56(5):1257–1267. doi: 10.1152/jn.1986.56.5.1257. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A. Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol. 1961 May;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H., Schachner M. Pharmacological properties of gamma-aminobutyric acid-, glutamate-, and aspartate-induced depolarizations in cultured astrocytes. J Neurosci. 1985 Dec;5(12):3295–3301. doi: 10.1523/JNEUROSCI.05-12-03295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H., Faber D. S., Burnod Y., Triller A. Regulation of efficacy at central synapses. J Neurosci. 1984 Jan;4(1):125–130. doi: 10.1523/JNEUROSCI.04-01-00125.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Krnjević K. Some functional consequences of GABA uptake by brain cells. Neurosci Lett. 1984 Jun 29;47(3):283–287. doi: 10.1016/0304-3940(84)90527-5. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Johnston G. A. Inhibition of GABA uptake in rat brain slices by nipecotic acid, various isoxazoles and related compounds. J Neurochem. 1975 Dec;25(6):797–802. doi: 10.1111/j.1471-4159.1975.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Lerma J., Herreras O., Herranz A. S., Munoz D., del Rio R. M. In vivo effects of nipecotic acid on levels of extracellular GABA and taurine, and hippocampal excitability. Neuropharmacology. 1984 May;23(5):595–598. doi: 10.1016/0028-3908(84)90036-4. [DOI] [PubMed] [Google Scholar]
- Matthews W. D., McCafferty G. P., Setler P. E. An electrophysiological model of GABA-mediated neurotransmission. Neuropharmacology. 1981 Jun;20(6):561–565. doi: 10.1016/0028-3908(81)90208-2. [DOI] [PubMed] [Google Scholar]
- McCarren M., Alger B. E. Use-dependent depression of IPSPs in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1985 Feb;53(2):557–571. doi: 10.1152/jn.1985.53.2.557. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985 Oct;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Miles R. Frequency dependence of synaptic transmission in nucleus of the solitary tract in vitro. J Neurophysiol. 1986 May;55(5):1076–1090. doi: 10.1152/jn.1986.55.5.1076. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Deisz R. A., Dodt H. U., Lux H. D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986 Jun 13;232(4756):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Klee M. R., Zeise M. L. Differences in baclofen-sensitivity between CA3 neurons and granule cells of the guinea pig hippocampus in vitro. Neurosci Lett. 1984 Jun 29;47(3):307–311. doi: 10.1016/0304-3940(84)90531-7. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Ransom B. R., Henkart M., Bullock P. N. Mouse spinal cord in cell culture. IV. Modulation of inhibitory synaptic function. J Neurophysiol. 1977 Sep;40(5):1178–1187. doi: 10.1152/jn.1977.40.5.1178. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistri A., Constanti A. Pharmacological characterization of different types of GABA and glutamate receptors in vertebrates and invertebrates. Prog Neurobiol. 1979;13(2):117–235. doi: 10.1016/0301-0082(79)90016-9. [DOI] [PubMed] [Google Scholar]
- Numann R. E., Wong R. K. Voltage-clamp study on GABA response desensitization in single pyramidal cells dissociated from the hippocampus of adult guinea pigs. Neurosci Lett. 1984 Jun 29;47(3):289–294. doi: 10.1016/0304-3940(84)90528-7. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. An analysis of the inhibitory post-synaptic current in the voltage-clamped crayfish muscle. J Physiol. 1979 Jan;286:265–282. doi: 10.1113/jphysiol.1979.sp012618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S., Yuzaki M. Patch-clamp studies of chloride channels activated by gamma-aminobutyric acid in cultured hippocampal neurones of the rat. Neurosci Res. 1984 Oct;1(5):275–293. doi: 10.1016/0168-0102(84)90034-8. [DOI] [PubMed] [Google Scholar]
- Peet M. J., McLennan H. Pre-and postsynaptic actions of baclofen: blockade of the late synaptically-evoked hyperpolarization of CA1 hippocampal neurones. Exp Brain Res. 1986;61(3):567–574. doi: 10.1007/BF00237582. [DOI] [PubMed] [Google Scholar]
- Schousboe A., Thorbek P., Hertz L., Krogsgaard-Larsen P. Effects of GABA analogues of restricted conformation on GABA transport in astrocytes and brain cortex slices and on GABA receptor binding. J Neurochem. 1979 Jul;33(1):181–189. doi: 10.1111/j.1471-4159.1979.tb11720.x. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: properties of GABA-activated Cl- ion conductance. J Neurophysiol. 1984 Mar;51(3):500–515. doi: 10.1152/jn.1984.51.3.500. [DOI] [PubMed] [Google Scholar]
- Thalmann R. H., Peck E. J., Ayala G. F. Biphasic response of hippocampal pyramidal neurons to GABA. Neurosci Lett. 1981 Feb 6;21(3):319–324. doi: 10.1016/0304-3940(81)90224-x. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Deisz R. A., Prince D. A. Outward chloride/cation co-transport in mammalian cortical neurons. Neurosci Lett. 1988 Jun 17;89(1):49–54. doi: 10.1016/0304-3940(88)90479-x. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Deisz R. A., Prince D. A. Relative contributions of passive equilibrium and active transport to the distribution of chloride in mammalian cortical neurons. J Neurophysiol. 1988 Jul;60(1):105–124. doi: 10.1152/jn.1988.60.1.105. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Prince D. A. Activation of electrogenic sodium pump in hippocampal CA1 neurons following glutamate-induced depolarization. J Neurophysiol. 1986 Aug;56(2):507–522. doi: 10.1152/jn.1986.56.2.507. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. A magnesium-sensitive post-synaptic potential in rat cerebral cortex resembles neuronal responses to N-methylaspartate. J Physiol. 1986 Jan;370:531–549. doi: 10.1113/jphysiol.1986.sp015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A. Dendritic mechanisms underlying penicillin-induced epileptiform activity. Science. 1979 Jun 15;204(4398):1228–1231. doi: 10.1126/science.451569. [DOI] [PubMed] [Google Scholar]


