Abstract
We studied 61 patients with schizophrenia spectrum disorders who discontinued medication after achieving symptomatic remission. Over 3 years, relapse rates were significantly higher in those not meeting full recovery (p = 0.006) or remission (p < 0.001) criteria, with rates up to twice as high. Significant differences between relapsed and maintained groups included age at onset (p = 0.004), age at discontinuation (p = 0.009), and proportions meeting full recovery (p = 0.001) or remission (p = 0.003). Univariate Cox regression identified older age of onset (p = 0.038), lack of full recovery (p = 0.008) or remission (p = 0.001), and higher positive symptom score (p = 0.018) as predictors of relapse. In multivariate analysis, only full remission remained significant (p = 0.002). Our findings suggest that in making decision about discontinuation, applying more strict approaches, i.e., full recovery or remission criteria and detailed assessment of positive symptoms are critical and essential.
Subject terms: Psychosis, Schizophrenia
Introduction
Current treatment guidelines for discontinuing antipsychotics in first-episode psychosis (FEP) are vague and somewhat heterogenous1–3. To guide clinicians, patients, and their caregivers regarding continuation or discontinuation of medication after remission following first episode psychosis (FEP), it is imperative to identify strong and reliable predictors of relapse in patients discontinuing antipsychotics. Randomized controlled trials (RCTs) comparing discontinuation and maintenance groups have found several predictors of relapse, including a diagnosis of schizophrenia, lower semantic fluency, higher spontaneous eye blinking rate4, lower Positive and Negative Syndrome Scale (PANSS) positive symptom score, enduring remission, and deterioration with prior intermittent treatment5. Predictors from observational studies include premorbid social adjustment, living in Western Europe6, not having been hospitalized in the previous 5 years7, not living with family, and no family history of psychosis8.
However, all these predictors were measured at baseline or upon study entry before randomization, i.e., before discontinuation. Only two studies have reported predictors measured after discontinuation: visual working memory deterioration9 and lower robustness in graph theoretical analysis10. Another critical flaw of the previous discontinuation trials is that most used Andreasen’s remission criteria11 to determine eligibility, which is contrary to a recommendation in the discontinuation guidelines12. To our knowledge, only one study followed the strict recovery criteria of the discontinuation guidelines13. Since the PANSS positive symptom score at study entry was 7.7 in Gaebel’s study5, they probably used a similarly conservative approach. The final controversy is the last antipsychotic dose before complete discontinuation. In discontinuation RCTs, the lowest doses before complete cessation were 1 ~ 3 mg or 5 ~ 20 mg/day of haloperidol equivalent (37.5 ~ 112.5 mg/day or 187.5 ~ 750 mg/day of chlorpromazine equivalent)14, or were not available15. Horowitz and colleagues suggested that the final dose before complete cessation may need to be as low as 1/40th of a therapeutic dose to prevent a large decrease in D2 blockade when stopped15. The discontinuation guidelines recommend a dose equivalent to ≤ 1 mg/day risperidone13. Therefore, we hypothesized that the relapse rate of patients with FEP who met the recovery criteria and discontinued medication would be lower than that of patients who met Andreasen’s remission criteria and discontinued their treatment. Moreover, if we measure some variables of interest after discontinuation and specify the medication status before discontinuation in detail, such as the dose of the last antipsychotic and its duration or whether aripiprazole was used as a last antipsychotic to prevent supersensitivity psychosis, more valid and reliable predictors could be identified.
Therefore, this study analyzed the relapse rates of patients with schizophrenia spectrum disorders (SSDs) who met recovery or remission criteria and discontinued medication. Furthermore, we compared demographic characteristics and clinical variables measured after discontinuation between individuals who relapsed and those who maintained recovery and explored predictors of relapse using Cox regression.
Methods
Participants
This study enrolled 61 individuals who had recovered from SSDs and discontinued their medication. These individuals were participating in one of two prospective cohort studies at Jeonbuk National University Hospital (JBUH): the Korea Early Psychosis Study (KEPS; grant no. HL19C0015)16 and Recovery and Relapse in Early Psychosis (grant no. HI13C1459). Diagnoses were established at the time of enrollment using the criteria of the DSM-517 and the Korean version of the Mini-International Neuropsychiatric Interview18. We considered schizophrenia, schizoaffective disorder, schizophreniform disorder, and other specified schizophrenia spectrum and psychotic disorders (OSSOs) as SSDs. All study participants were between 18 and 60 years old, joined the study voluntarily, and provided informed consent. Healthy controls (HC) were recruited through advertisements in order to obtain norm data of cognitive functioning which was used to calculate Z-score of the patients. They were interviewed using the screening module of the Structured Clinical Interview for DSM Non-Patient Edition for Axis I diagnoses19. Exclusion criteria were as follows: (a) history of previous or current psychiatric disorder, neurological disorder, or significant medical condition and (b) first-degree relatives with psychiatric disorders. All procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation, and with the 1975 Declaration of Helsinki (revised in 2008). This study was approved by the Ethics Committee of JBUH (approval numbers: CUH 2014-06-022 and 2014-11-002).
Procedures
During follow-up, if the individuals met symptomatic remission criteria11 and were willing to discontinue their medication through a shared decision-making process, they were gradually tapered off the medication over 3–6 months. If their symptoms worsened during tapering, they reverted to the previous dosage and were excluded from the study (n = 10) (Fig. 1). Following discontinuation guidelines13, we tried to adjust the dosage of the last antipsychotic before discontinuation to the equivalent of ≤ 1 mg/day risperidone. Patients who made their own decision to discontinue medication were included if they met the symptomatic remission criteria. It is of note that two patients were on paliperidone extended-release injection, 117 mg and 156 mg and discontinued abruptly on their own. After complete discontinuation of their medication (n = 61), they were evaluated for symptomatology, cognitive functioning and self-rated variables within 1 ~ 2 months. They were followed regularly, usually at 2-month intervals, for up to 2 years. On every visit, they were educated and evaluated on the early signs and symptoms of relapse. Thereafter, they were seen and evaluated at spontaneous appointments. Data were collected from Mar 2014 to August 2023.
Fig. 1. Study flow diagram.
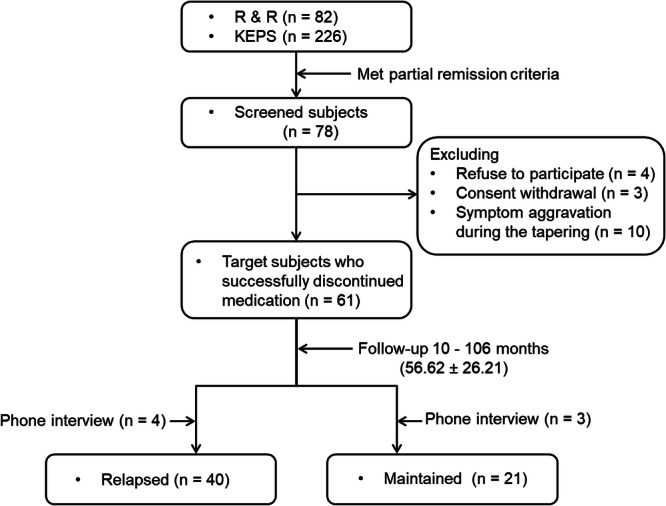
KEPS Korea Early Psychosis Study, R & R Recovery and Relapse in early psychosis.
Assessments
Demographic and clinical data
Data on sex, education (academic years completed), and duration of untreated psychosis (DUP) were obtained at enrollment in both studies. Age and duration of illness (DI) were calculated, and diagnoses and number of episodes were reviewed at the time of discontinuation. The PANSS20 was completed just before discontinuation in cases with a shared decision and after discontinuation for patients who made their own decisions. The Calgary Depression Scale for Schizophrenia (CDSS)21,22, Korea Polyenvironmental Risk Score-II (K-PERS-II)23, and cognitive function test were evaluated within 2 months after discontinuation. The K-PERS-II is an interview-based tool that measures multiple environmental factors associated with schizophrenia. However, some individuals who discontinued their medication on their own were evaluated long after discontinuation (15 individuals were assessed 6 months after discontinuation). The number of individuals evaluated differed depending on the scale (for details, see the sample sizes in the tables). The inter-rater reliability for the PANSS and K-PERS-II scores was ≥ 0.8 (Cohen’s kappa).
The following information regarding medication status before discontinuation was obtained: last antipsychotic dosage and duration, number of individuals who had taken minimum dose (risperidone 1 mg/day) as the last drug, along with the dosage and duration of the medication. number of individuals who used an ultra-minimal dose (aripiprazole ≤ 2 mg) and aripiprazole as the last drug, along with the dosage and duration of the medication. and greatest dose used to treat FEP or relapse/aggravation. The definitions of minimum and ultra-minimal doses were from the discontinuation guidelines12. The chlorpromazine (CPZ) dose equivalents were calculated using the defined daily doses method14.
Self-rated variables
The scales completed after discontinuation were the Brief Core Schema Scales (BCSS)24, Brooding Scale (BS)25, and Early Trauma Inventory Self Report-Short Form (ETISR-SF)26.
Definitions of recovery, remission, and relapse
The criteria for full or partial recovery were as follows: all the scores on P1, P2, P3, N1, N4, N6, G5, and G9 of the PANSS should be ≤ 2 or ≤ 3 and a total score of the Social and Occupational Functioning Assessment Scale (SOFAS) ≥ 71 or ≥ 61 and these conditions should last at least 1-year17. We subdivided symptomatic remission into full (score on eight PANSS items ≤ 2) and partial (score on eight PANSS items ≤ 3)20 remission lasting ≥ 6 months. Relapse was defined as an exacerbation of symptoms, indicated by psychiatric hospitalization, a CGI-S score ≥ 4 with an increase ≥ 2, a CGI-I score ≥ 6 (much worse), a score ≥ 4 for a psychosis item (P1, P2, P3, or P6) with an increase of ≥ 2, a ≥ 25% increase in the total PANSS score (or a ≥ 10-point increase if the baseline score was ≤ 40), or deliberate self-injury/clinically serious suicide ideation/suicide attempt or violent behavior resulting in significant injury to another person or property27. Patients were considered to have had a relapse if the re-emerging symptoms lasted for at least 1 week. Aggravation of symptoms was considered present when symptoms recurred but did not meet the criteria for relapse. As all patients experiencing aggravation wanted to take medication again, we considered them relapsed. In calculating relapse rate, different criteria (all 8 items of the PANSS = 3) for partial recovery and partial remission was used to avoid overlapping individuals.
The corresponding author (YCC) performed all assessments around the time of discontinuation and during follow-up, which ended in August 2023. During the 8-year follow-up, several patients dropped out with or without relapse (Suppl. Figure 1). The patients who relapsed and dropped out were added to both the numerator and denominator when calculating the relapse rate. The individuals who dropped out without relapse were excluded from the calculation. To accommodate for the worst-case analysis, we treated drop-out without relapse as having relapsed28. Drop-out was defined as not visiting an outpatient clinic for > 3 months before the end of each follow-up year. Seven participants were evaluated in phone interviews and classified as relapsed (n = 4) or maintained (n = 3) (Fig. 1). The follow-up time (including phone interviews) of all individuals ranged from 10 to 106 (average, 56.62 ± 26.21) months (Table 3). Depending on their clinical status during follow-up, participants were categorized as either “relapsed” or “maintained”.
Table 3.
Cumulative relapse rates of subjects after discontinuation.
| Total number at baseline | 1-year | 2-year | 3-year | |
|---|---|---|---|---|
| Full recovery for 1 year | 29 | 10/27 (37.04) | 11/26 (42.31) | 12/22 (54.55) |
| Partial recoverya for 1 year | 20 | 13/20 (65.00) | 16/20 (80.00) | 17/20 (85.00) |
| Full remission for 6 months | 48 | 19/46 (41.30) | 23/45 (51.11) | 24/40 (60.00) |
| Partial remissiona for 6 months | 13 | 10/13 (76.92) | 11/13 (84.62) | 12/13 (92.31) |
| All subjects | 61 | 29/59 (49.15) | 34/58 (58.62) | 36/53 (67.92) |
All values are n/N (%). aScores for all 8 items of the PANSS were “3” to avoid overlapping individuals.
Statistical analysis
We estimated statistical power according to Macdonald (1995)29, by first determining the required number of outcome events (relapses). One systematic review reported a mean relapse rate of 77% and 90% by 1 and 2 years, respectively, following antipsychotic discontinuation30. We aimed to be able to detect an effect that would result in relapse rate of 80% in the discontinuation group. To detect this effect with expected proportion of 0.8 (p) and margin of error of 0.1 (E), we needed a total of at least 62. The formula used was “n = \frac{(Z^2 \cdot p \cdot (1 - p))}{E^2}”.
Demographic and clinical data were compared between the relapsed and maintained recovery groups using independent t-tests or the Chi-square test. In addition, the same data were compared between the drop-out without relapse vs. the drop-out with relapse, relapsed or the rest (individuals remained after excluding the drop-out without relapse). Cognitive function was compared between total patients and HC using t-test or among the relapsed, maintained and HC groups using ANCOVA, with age at discontinuation, education, and gender as covariates. To address multiple testing, a false discovery rate (FDR) was applied to 12 individual tests and four cognitive function domains (attention, memory, executive function and language).
Using demographic and clinical data, self-rated scores, and recovery status as independent variables, relapse after discontinuation was predicted through univariate Cox regression with age at discontinuation as a covariate. Individuals who dropped out without relapse or reached the end of the study without relapse were censored. Variables with a p-value ≤ 0.10 were entered in stepwise multivariate Cox regression models. To mitigate overfitting issue by stepwise regression, we performed further leave-one-out cross-validation and examined the Akaike Information Criterion (AIC). A hazard ratio (HR) > 1 indicates an increased likelihood of experiencing the target event (relapse). Kaplan–Meier survival curves were constructed to visualize the significant predictors. Models were evaluated using the log-rank test. All analyses were performed in R (ver. 4.3.2; R Core Team). Significance was set to an alpha level of 0.05, and all p-values were two-sided.
Results
Ages at onset (p = 0.004) (Table 1) and discontinuation (p = 0.009) (Table 2) were significantly higher in the relapsed group compared to the maintained group. Moreover, the proportions of individuals who met the full recovery (p = 0.001) or remission (p = 0.003) criteria were significantly smaller in the relapsed group (Table 2). Other demographic and clinical variables did not differ significantly between the two groups (Tables 1 and 2, and Suppl. Table 1). When the drop-out without relapse was compared with the drop-out with relapse, relapsed or the rest, no differences were found except the follow-up time and negative symptom score of the PANSS (Suppl. Table 2).
Table 1.
Comparison of demographic and clinical characteristics of patients who relapsed or maintained recovery after discontinuation.
| Total (n = 61) | Relapsed (n = 40) | Maintained (n = 21) | p-value | |
|---|---|---|---|---|
| Male/Female, n (%/%) | 23/38 (37.7/62.3) | 16/24 (40/60) | 7/14 (33.3/6.7) | 0.610 |
| Age at onset | 29.28 ± 10.12 | 32.08 ± 9.68 | 24.33 ± 8.99 | 0.004 |
| Education | 13.73 ± 2.41 | 13.85 ± 2.28 | 13.61 ± 2.67 | 0.725 |
| Age at baseline | 32.00 ± 11.00 | 34.50 ± 10.52 | 26.62 ± 8.69 | 0.005 |
| DI at baseline | 31.00 ± 61.00 (0–378) | 28.80 ± 46.01 (0–157) | 34.47 ± 83.41 (0–378) | 0.732 |
| DUP, Range (month) | 13.85 ± 50.76 | 6.50 ± 11.62 | 27.18 ± 84.00 | 0.275 |
| (0.03–384) | (0.03–65) | (0.07–384) | ||
| Short DUP ( ≤ 13.85a) | 48 (78.7) | 33 (82.5) | 15 (71.4) | 0.316 |
| Long DUP ( > 13.85) | 13 (21.3) | 7 (17.5) | 6 (28.6) | |
| Short DUP ( ≤ 1b) | 31 (50.8) | 20 (50) | 11 (52.4) | 0.860 |
| Long DUP ( > 1) | 30 (49.2) | 20 (50) | 10 (47.6) | |
| Diagnosis, n (%) | 0.763 | |||
| Schizophrenia | 32 (52.5) | 24 (60) | 8 (38.1) | |
| Schizophreniform disorder | 13 (21.3) | 6 (15) | 7 (33.3) | |
| OSSO | 16 (26.2) | 10 (25) | 6 (28.6) | |
| Number of episode, n (%) | 0.244 | |||
| First episode | 47 (77) | 29 (72.5) | 18 (85.7) | |
| Multiple episodes | 14 (23.0) | 11 (27.5) | 3 (14.3) |
DUP Duration of Untreated Psychosis, OSSO Other specified schizophrenia spectrum disorder and other psychotic disorder.
aMean; bMedian; All values are mean ± Standard Deviation (SD) unless otherwise specified.
Table 2.
Comparison of clinical and medication status of patients who relapsed or maintained recovery after discontinuation.
| Variables | Total (n = 61) | Relapsed (n = 40) | Maintained (n = 21) | p-value |
|---|---|---|---|---|
| Age at the time of discontinuation, year | 35.23 ± 11.09 | 37.91 ± 10.49 | 30.38 ± 10.25 | 0.009 |
| DI at the time of discontinuation, month | 67.52 ± 68.89 | 63.03 ± 51.34 | 74.00 ± 94.19 | 0.557 |
| Type of discontinuation method, n (%) | ||||
| Shared decision/Patient’s decision | 49/12 (80.3/19.7) | 33/7 (82.5/17.5) | 16/5 (76.2/23.8) | 0.691 |
| Clinical status before discontinuationa, n (%) | ||||
| Full recovery for 1 year | 29/32 (47.5/52.5) | 13/27 (32.5/67.5) | 16/5 (76.2/23.8) | 0.001 |
| Partial recovery for 1 year | 49/12 (80.3/19.7) | 30/10 (75/25) | 19/2 (90.5/9.5) | 0.149 |
| Full remission for 6 months | 48/13 (79/21) | 27/13 (67.5/32.5) | 21/0 (100/0) | 0.003 |
| Partial remission for 6 months | 61/0 (100/0) | 40/0 (100/0) | 21/0 (100/0) | - |
| Medication status | ||||
| Dose of last antipsychotics (CPZ equivalent dosage), mg/day | 78.09 ± 82.22 | 66.84 ± 66.76 | 99.52 ± 104.19 | 0.142 |
| Duration of last antipsychotics, month | 16.14 ± 14.88 | 16.10 ± 15.72 | 16.21 ± 13.44 (n = 20) | 0.978 |
| Subjects taken/not taken dose of last antipsychotics ≤ risperidone 1 mg/day, n (%) | 54/7 (88.5/11.5) | 37/3 (92.5/7.5) | 17/4 (81.0/19.0) | 0.179 |
| In the subjects taken dose of last antipsychotics ≤ risperidone 1 mg/day (n = 54) | ||||
| Dose of antipsychotics, mg/day | 55.07 ± 31.51 | 52.80 ± 31.19 (n = 37) | 60.00 ± 32.60 (n = 17) | 0.441 |
| Duration of antipsychotics, month | 16.44 ± 15.76 | 16.24 ± 16.20 (n = 37) | 16.88 ± 15.24 (n = 17) | 0.892 |
| Subjects used/not used ultra-minimal dose (aripiprazole ≤ 2 mg/day) before discontinuation, n (%) | 31/30 (50.8/49.2) | 21/19 (52.5/47.5) | 10/11 (47.6/52.4) | 0.717 |
| Subjects used/not used aripiprazole as a last drug before discontinuation, n (%) | 44/17 (72.1/27.9) | 29/11 (72.5/27.5) | 15/6 (71.4/28.6) | 0.929 |
| Highest dose to treat first episode psychosis (n = 60), mg/dayb | 410.38 ± 257.42 | 391.99 ± 220.09 (n = 39) | 444.52 ± 318.68 (n = 21) | 0.456 |
| Highest dose to treat relapse or aggravation (n = 37), mg/dayc | 333.95 ± 293.87 | |||
| Follow-up time, month (Range) | 56.62 ± 26.21 (10–106) | 26.61 ± 4.19 (13–106) | 24.91 ± 5.43 (10–90) | 0.154 |
CPZ Chlorpromazine, DI Duration of Illlness.
aNumber of the subjects who met the criteria/Not met (%/%); All values are mean ± Standard Deviation (SD) unless otherwise specified.
bData of one subject was unknown; cData of three subjects were unknown.
Regarding cumulative relapse rates over 3 years, those who achieved full recovery had lower relapse rates than those who achieved partial recovery (37.04% vs. 65% at 1-year, 42.31 vs.80% at 2-year and 54.55% vs. 85% at 3-year). This was seen in both the full and partial remission groups (Table 3). These patterns were the same as in the results obtained with the worst-case analysis (Suppl. Table 3).
In the neurocognitive assessment, there were significant differences in verbal memory [“delayed recall” (p = 0.022), “learning slope” (p = 0.006), composite score (p = 0.010)], verbal fluency [“animals” (p < 0.001), “stationery” (p < 0.001), “ㄱ” (p = 0.005), “ㅅ” (p = 0.007), “ㅇ”(p < 0.001), composite score (p < 0.001)], and global cognitive functioning (p = 0.002) among the relapsed, maintained, and HC groups. The post-hoc results showed that both patient groups had significant impairments in most of the subtests compared to HCs. Moreover, in some subtests [“learning slope” (p = 0.026) and composite score of verbal memory (p = 0.05), “animals” (p < 0.001), “stationery” (p < 0.001), “ㄱ” (p = 0.005), “ㅅ” (p = 0.007), “ㅇ”(p < 0.001) and composite score (p < 0.001) in the verbal fluency test], significant differences in impairment were observed only between the relapsed and HC groups (Table 4).
Table 4.
Cognitive functioning of patients who relapsed or maintained recovery after discontinuation.
| Total (n = 56) | Relapseda (n = 36) | Maintainedb (n = 20) | HCc (n = 167) | p-valued | p-valuee | Post-hoc testf | |||
|---|---|---|---|---|---|---|---|---|---|
| a vs. b | a vs. c | b vs. c | |||||||
| Auditory CPT | |||||||||
| Correct response | −0.14 ± 1.16 | 0.01 ± 1.10 | −0.41 ± 1.24 | 0.00 ± 1.00 | 0.427 | 0.236 | 0.261 | 0.962 | 0.227 |
| Commission error | −0.18 ± 1.51 | -0.05 ± 1.46 | −0.41 ± 1.61 | 0.00 ± 1.00 | 0.305 | 0.302 | 0.286 | 0.997 | 0.159 |
| Composite score | −0.16 ± 1.29 | -0.02 ± 1.23 | −0.41 ± 0.00 | 0.00 ± 0.88 | 0.389 | 0.209 | 0.210 | 0.995 | 0.135 |
| Verbal memory test | |||||||||
| Delayed recall | −0.38 ± 1.34 | −0.52 ± 1.32 | −0.12 ± 1.37 | 0.00 ± 1.00 | 0.057 | 0.022* | 0.836 | 0.084 | 0.599 |
| Total recall | −0.13 ± 1.31 | −0.29 ± 1.23 | 0.15 ± 1.41 | 0.00 ± 1.00 | 0.486 | 0.188 | 0.721 | 0.677 | 0.962 |
| Learning slope | −0.48 ± 0.90 | −0.48 ± 0.91 | −0.48 ± 0.91 | 0.00 ± 1.00 | 0.002 | 0.006* | 0.998 | 0.026 | 0.134 |
| Composite score | −0.33 ± 1.02 | −0.43 ± 1.01 | −0.15 ± 1.03 | 0.00 ± 0.77 | 0.029 | 0.010* | 0.815 | 0.050 | 0.507 |
| Verbal fluency test | |||||||||
| Animals | −0.79 ± 1.05 | −0.77 ± 1.03 | −0.83 ± 1.12 | 0.00 ± 1.00 | < 0.001 | < 0.001*** | 0.890 | < 0.001 | 0.002 |
| Stationery | −1.25 ± 0.93 | −1.22 ± 0.90 | −1.29 ± 1.00 | 0.00 ± 1.00 | < 0.001 | < 0.001*** | 1.000 | < 0.001 | < 0.001 |
| “ㄱ” | −0.45 ± 0.90 | −0.51 ± 0.86 | −0.32 ± 0.97 | 0.00 ± 1.00 | 0.003 | 0.005* | 0.976 | 0.045 | 0.100 |
| “ㅅ” | -0.46 ± 0.87 | −0.50 ± 0.81 | −0.38 ± 0.99 | 0.00 ± 1.00 | 0.002 | 0.007* | 0.979 | 0.038 | 0.087 |
| “ㅇ” | −0.55 ± 0.86 | −0.56 ± 0.81 | −0.54 ± 0.97 | 0.00 ± 1.00 | < 0.001 | < 0.001*** | 0.804 | 0.019 | 0.014 |
| Composite score | −0.70 ± 0.67 | −0.71 ± 0.60 | −0.67 ± 0.77 | 0.00 ± 0.69 | < 0.001 | < 0.001*** | 0.900 | < 0.001 | < 0.001 |
| Wisconsin card sorting test | |||||||||
| Categories completed | 0.21 ± 0.61 | 0.15 ± 0.64 | 0.30 ± 0.55 | 0.00 ± 1.00 | 0.067 | 0.224 | 0.902 | 0.180 | 0.673 |
| Perseverative error | 0.11 ± 0.79 | 0.01 ± 0.83 | 0.30 ± 0.69 | 0.00 ± 1.00 | 0.435 | 0.296 | 0.996 | 0.585 | 0.889 |
| Composite score | 0.16 ± 0.64 | 0.08 ± 0.66 | 0.30 ± 0.61 | 0.00 ± 0.85 | 0.141 | 0.158 | 0.908 | 0.213 | 0.703 |
| Global composite score | −0.26 ± 0.64 | −0.27 ± 0.61 | −0.23 ± 0.70 | 0.00 ± 0.49 | 0.002 | 0.002 | 0.615 | 0.078 | 0.016 |
CPT Continuous Performance Test, HC Healthy Control.
All values are z-scores relative to healthy controls; aRelapsed;bMaintained;cHealthy Control.dIndependent t-test between total patients and healthy control;eANCOVA with age at discontinuation, education and gender as covariates; fPairwise comparisons were corrected using the Tukey test; Results of false discovery rate corrections which were applied to the 4 domains and 12 tests are shown as *p < 0.05, ***p < 0.001.
The predictors in univariate Cox regression were age at onset (HR = 1.030, p = 0.038, 95% confidence interval [CI] = 1.00–1.06), full recovery (HR = 2.485, p = 0.008, 95% CI = 1.272–4.854), full remission (HR = 3.476, p = 0.001, 95% CI = 1.617–4.474), and PANSS positive symptom score (HR = 1.682, p = 0.018, 95% CI = 1.100–2.572) (Suppl. Table 4 and 5). Cognitive variables did not predict relapse (Suppl. Table 6). In stepwise multivariate Cox regression, however, only full remission remained a significant predictor (HR = 3.41, p = 0.002, 95% CI = 1.584–7.344). When analyzed further with leave-one-out cross-validation, AIC value was increased from 217.6 to 219.6 suggesting the original model works better.
According to the Kaplan–Meier curve for all individuals, the relapse rate reached 50% after 369 days (Fig. 2a). Pairwise log-rank tests showed trends toward significant group differences in for the rate of age at onset > 28 years (p = 0.06) and failure to achieve full recovery (p = 0.006), as well as a significant difference in the full remission rate (p < 0.001) (Fig. 2b–d). No significant group difference in the median PANSS positive symptom score was found with the log-rank test (p = 0.735) (Supplementary Fig. 2).
Fig. 2. Kaplan-Meier curve showing time to relapse after antipsychotic discontinuation.
a In the total patients (n = 60). b Age at onset > 28 (median) vs. ≤ 28 (median) (log-rank test P = 0.06). c Met full recovery vs. not met (log-rank test P = 0.006). d Met full remission vs. not met (log-rank test P < 0.001). Note: As relapse date in one patient was unknown, sample size of all curves is 60.
Discussion
In daily practice, clinicians often wonder whether to continue or discontinue antipsychotic medication when patients with FEP show favorable prognostic outcomes, such as being free of psychotic symptoms and demonstrating adequate socio-occupational functioning. To guide the decision, accurate predictors are essential. By measuring variables after discontinuation, applying strict recovery criteria, and taking account of tapering, we found that older age of onset, lack of full recovery or remission, and higher PANSS positive symptom score were significantly associated with an increased risk of relapse.
The relapse rate in the group that did not meet the full recovery or remission criteria was up to twice as high as that of the group that met the criteria. The relapse rate of the group that achieved full recovery or remission was lower than in previous studies applying Andreasen’s criteria8,31,32. However, the relapse rate of the group that achieved partial recovery or remission was similar to those studies8,31,32. Chen and colleagues who applied criteria similar to our full recovery or remission criteria, reported a high relapse rate of 79% 1 year after discontinuation13. However, their duration of symptom maintenance criterion was shorter ( ≥ 8 weeks) and their last antipsychotic dose criterion was higher (does equivalent to 2.5 mg of haloperidol) than ours ( ≥ 1 year or 6 months and does equivalent to 78.09 mg of CPZ). Overall, our findings suggest that applying criteria for recovery or remission stricter than those of Andreasen would reduce the relapse rate after antipsychotic discontinuation.
Regarding the post-hoc results for cognitive function, it was disappointing to see no significant differences between the relapsed and maintained groups. This negative finding was confirmed in the Cox regression and contrasts with Hui4, who identified low semantic fluency as a significant predictor of relapse. However, they evaluated cognitive function before complete discontinuation. Nevertheless, looking at the post-hoc results for the composite score of verbal memory, poorer performance was noted in the relapsed group, but not in the maintained group, compared to HCs. This suggests that verbal memory impairment measured after discontinuation has the potential to be a cognitive marker of relapse. It remains to be determined whether the lack of any difference between our two groups was due to type II error. Overall, even patients who met recovery or remission criteria still had impairments in the verbal memory and verbal fluency domains, although the magnitude was moderate based on z-scores. No group differences in the PANSS, K-PERS-II, or ETI-SR scores were found. Albeit that these are negative findings, it is important to include indices reflecting environmental factors and early traumatic experiences as potential predictors of relapse, as these have often been neglected in previous discontinuation trials.
In our univariate Cox regression, older age at onset predicted relapse after discontinuation. No discontinuation trials have reported this as a predictor of relapse33. Since earlier onset is more often associated with inherent vulnerabilities or severe psychopathologies34,35, and given that several studies reported that an earlier age of onset predicted a poor treatment response in FEP36,37, our result seems counterintuitive. However, Verma’ study reported that younger age predicted recovery at year 2 in patients with FEP38. A possible explanation for this might be that younger persons are more resilient in terms of the psychophysiological response to stress39,40, such that young patients with FEP are more resistant to stress and relapse. Alternatively, it might be due to unmeasured confounding factors related to age at onset. Most importantly, individuals who did not meet the full recovery or remission criteria had a higher risk of relapse. These findings indicate that, at the critical time of considering discontinuation, it is important to check whether patients are symptom-free or have minimal symptoms on the 8 items of the PANSS. Interestingly, individuals with higher positive symptom scores were found to be more likely to relapse, although there was no difference in positive symptom scores between the two groups (7.53 ± 1.03 and 7.26 ± 0.45 in the relapsed and maintained groups, respectively) according to t-tests. Since the mean PANSS positive symptom score for all individuals was very low (7.44) and the difference in these scores between the two groups was very small, this finding suggests that even a small difference in positive symptoms measured around the time of discontinuation may make a significant contribution to relapse over time. This highlights the importance of a careful and detailed evaluation of positive symptoms, including ideas of reference, before making a decision on discontinuation. For this purpose, more systematic and sophisticated tools, such as the Psychotic Symptom Rating Scales (PSYRATS)41, Comprehensive Assessment of At-Risk Mental States (CAARMS)42, and Scale Of Prodromal Symptoms (SOPS)43, work better than the PANSS, which was developed to evaluate more severe psychopathologies in chronic schizophrenia.
Several limitations of the present study need to be mentioned. First, this was an observational study without an active comparator. Since there are ethical issues regarding assignment to a placebo group, and given that some trials were aborted early because of insufficient recruitment or a high relapse rate31,44, a naturalistic study reflecting real-world practice is the second-best option. Second, as some patients who made the decision to discontinue treatment on their own visited the clinic long after complete cessation of medication, the interval between discontinuation and assessment varied. However, they comprised only about 25% of all participants, satisfied the partial remission criteria at least, and were followed for a sufficiently long time to assess the long-term course. Third, in some individuals, phone interviews were conducted or assessments were incomplete; therefore, there are some cases with missing data, especially for the K-PERS. Fourth, as we published the discontinuation guidelines12 in the middle of the study, the criteria for discontinuation became more stricter in late period of the study. However, this gave us the opportunity to compare relapse rates between the individuals meeting full recovery/remission criteria and those meeting the partial criteria. Despite its shortcomings, the present study evaluated some predictors after discontinuation and incorporated new predictors, such as full recovery criteria, dose of last antipsychotic, and K-PERS-II and ETI-SR scores.
In conclusion, by following patients with SSDs who met at least the partial remission criteria and discontinued their medication, we were able to observe a lower relapse rate in the group that met full the recovery/remission criteria compared to the group that met the partial recovery/remission criteria. In Cox regression, older age of onset, lack of full recovery or remission, and higher PANSS positive symptom score were significant predictors of relapse. These findings suggest that when making a decision about discontinuation, applying stricter criteria, such as those for full recovery or remission, and performing a detailed assessment of positive symptoms is essential.
Supplementary information
Acknowledgements
The corresponding author would like to thank all participants in the study and my family for guidance and support (SDG). Funding This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HR18C0016) and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2024-00335201).
Author contributions
Y.-C.C. conceptualized the study. W.S.K., E.J.J. and Y.-C.C. performed the study and acquired data S.O., T.H.L., F.Z.R. and C.Y.K. conducted statistical analyses. A.S. and Y.-C.C. analysed and interpreted the data. L.L. drafted the manuscript. Y.-C.C. critically revised the manuscript. Y.-C.C. received the grant. All authors approved the final manuscript.
Data availability
The data used in this study can be acquired upon request. Because of restrictions based on privacy regulations and informed consent of participants, data cannot be made freely available in a public repository.
Code availability
All relevant analysis scripts are available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41537-025-00592-3.
References
- 1.Shimomura, Y. et al. Antipsychotic treatment in the maintenance phase of schizophrenia: An updated systematic review of the guidelines and algorithms. Schizophr. Res.215, 8–16 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Sommer, I. E. C., Oomen, P. P. & Hasan, A. Maintenance treatment for patients with a first psychotic episode. Curr. Opin. psychiatry32, 147–156 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Kishi, T. et al. Effect of discontinuation v. maintenance of antipsychotic medication on relapse rates in patients with remitted/stable first-episode psychosis: a meta-analysis. Psychol. Med.49, 772–779 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Hui, C. L. et al. Predicting 1-year risk for relapse in patients who have discontinued or continued quetiapine after remission from first-episode psychosis. Schizophr. Res.150, 297–302 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Gaebel, W. et al. Predictors for symptom re-exacerbation after targeted stepwise drug discontinuation in first-episode schizophrenia: Results of the first-episode study within the German research network on schizophrenia. Schizophr. Res.170, 168–176 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Landolt, K. et al. Predictors of discontinuation of antipsychotic medication and subsequent outcomes in the European First Episode Schizophrenia Trial (EUFEST). Schizophr. Res.172, 145–151 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Moilanen, J. et al. Characteristics of subjects with schizophrenia spectrum disorder with and without antipsychotic medication - a 10-year follow-up of the Northern Finland 1966 Birth Cohort study. Eur. Psychiatry: J. Assoc. Eur. Psychiatrists28, 53–58 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Mayoral-van Son, J. et al. Clinical outcome after antipsychotic treatment discontinuation in functionally recovered first-episode nonaffective psychosis individuals: a 3-year naturalistic follow-up study. J. Clin. psychiatry77, 492–500 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Hui, C. L. et al. Visual working memory deterioration preceding relapse in psychosis. Psychol. Med.46, 2435–2444 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Odkhuu, S. et al. Network biomarkers in recovered psychosis patients who discontinued antipsychotics. Mol. Psychiatry28, 3717–3726 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreasen, N. C. et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am. J. Psychiatry162, 441–449 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Hui, C. L. M. et al. Guidelines for Discontinuation of Antipsychotics in Patients Who Recover From First-Episode Schizophrenia Spectrum Disorders: Derived From the Aggregated Opinions of Asian Network of Early Psychosis Experts and Literature Review. Int. J. Neuropsychopharmacol.25, 737–758 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, E. Y. et al. Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial. BMJ (Clin. Res. ed.)341, c4024 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leucht, S., Samara, M., Heres, S. & Davis, J. M. Dose Equivalents for Antipsychotic Drugs: The DDD Method. Schizophr. Bull.42, S90–S94 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horowitz, M. A. et al. A Method for Tapering Antipsychotic Treatment That May Minimize the Risk of Relapse. Schizophr. Bull.47, 1116–1129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, S. W. et al. Design and Methodology of the Korean Early Psychosis Cohort Study. Psychiatry Investig.14, 93–99 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and statistical manual of mental disorder (5th ed.). Arlington, VA: American Psychiatric Publishing (2013).
- 18.Yoo, S. W. et al. Validity of Korean version of the mini-international neuropsychiatric interview. Anxiety Mood2, 50–55 (2006). [Google Scholar]
- 19.Hahn, O. S. et al. Development of Korean Version of Structured Clinical Interview Schedule for DSM-IV Axis I Disorder: Interrater Reliability. J. Korean Neuropsychiatr. Assoc.39, 362–372 (2000). [Google Scholar]
- 20.Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull.13, 261–276 (1987). [DOI] [PubMed] [Google Scholar]
- 21.Addington, D., Addington, J., & Maticka-Tyndale, E. Assessing depression in schizophrenia: the Calgary Depression Scale. The British journal of psychiatry. Supplement, 39–44 (1993). [PubMed]
- 22.Kim, Y. K. et al. A Study on the Reliability and Validity of the Korean Version of the Calgary Depression Scale for Schizophrenia(K-CDSS). J. Korean Neuropsychiatr. Assoc.44, 446–455 (2005). [Google Scholar]
- 23.Jeon, E. J. et al. Development of the Korea-Polyenvironmental Risk Score for Psychosis. Psychiatry Investig.19, 197–206 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler, D. et al. The Brief Core Schema Scales (BCSS): psychometric properties and associations with paranoia and grandiosity in non-clinical and psychosis samples. Psychological Med.36, 749–759 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Kim, J. H. et al. The Development of the Brooding Scale. Psychiatry Investig.16, 443–449 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bremner, J. D., Bolus, R. & Mayer, E. A. Psychometric properties of the Early Trauma Inventory-Self Report. J. Nerv. Ment. Dis.195, 211–218 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Csernansky, J. G., Mahmoud, R. & Brenner, R. & Risperidone-USA-79 Study Group. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N. Engl. J. Med.346, 16–22 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Clark, T. G., Bradburn, M. J., Love, S. B. & Altman, D. G. Survival analysis part IV: further concepts and methods in survival analysis. Br. J. cancer89, 781–786 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macdonald, A. S. Modelling Survival Data in Medical Research. By D. Collett (Chapman & Hall, 1994). Br. Actuarial J.1, 410–410 (1995). [Google Scholar]
- 30.Zipursky, R. B., Menezes, N. M. & Streiner, D. L. Risk of symptom recurrence with medication discontinuation in first-episode psychosis: a systematic review. Schizophr. Res.152, 408–414 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Boonstra, G., Burger, H., Grobbee, D. E. & Kahn, R. S. Antipsychotic prophylaxis is needed after remission from a first psychotic episode in schizophrenia patients: results from an aborted randomised trial. Int. J. psychiatry Clin. Pract.15, 128–134 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Emsley, R., Nuamah, I., Hough, D. & Gopal, S. Treatment response after relapse in a placebo-controlled maintenance trial in schizophrenia. Schizophr. Res.138, 29–34 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Bowtell, M. et al. Rates and predictors of relapse following discontinuation of antipsychotic medication after a first episode of psychosis. Schizophr. Res.195, 231–236 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Petruzzelli, M. G. et al. Markers of neurodevelopmental impairments in early-onset psychosis. Neuropsychiatr. Dis. Treat.11, 1793–1798 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz, S. K., Ho, B. C. & Andreasen, N. C. Clinical features characterizing young-onset and intermediate-onset schizophrenia. J. neuropsychiatry Clin. Neurosci.12, 502–505 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Crespo-Facorro, B. et al. Predictors of acute treatment response in patients with a first episode of non-affective psychosis: sociodemographics, premorbid and clinical variables. J. Psychiatr. Res.41, 659–666 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Chang, W. C. et al. Prediction of remission and recovery in young people presenting with first-episode psychosis in Hong Kong: a 3-year follow-up study. Aust. N.Z. J. psychiatry46, 100–108 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Verma, S. et al. Symptomatic and functional remission in patients with first-episode psychosis. Acta Psychiatr. Scandinavica126, 282–289 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Uchino, B. N., Holt-Lunstad, J., Bloor, L. E. & Campo, R. A. Aging and cardiovascular reactivity to stress: longitudinal evidence for changes in stress reactivity. Psychol. aging20, 134–143 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Kudielka, B. M., Hellhammer, D. H. & Wüst, S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology34, 2–18 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Haddock, G., McCarron, J., Tarrier, N. & Faragher, E. B. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychological Med.29, 879–889 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Yung, A. R. et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust. N.Z. J. psychiatry39, 964–971 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Miller, T. J. et al. Prodromal Assessment With the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Symptoms: Predictive Validity, Interrater Reliability, and Training to Reliability. Schizophr. Bull.29, 703–715 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Stürup, A. E. et al. Tapered discontinuation vs. maintenance therapy of antipsychotic medication in patients with first-episode schizophrenia: Obstacles, findings, and lessons learned in the terminated randomized clinical trial TAILOR. Front. Psychiatry13, 910703 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study can be acquired upon request. Because of restrictions based on privacy regulations and informed consent of participants, data cannot be made freely available in a public repository.
All relevant analysis scripts are available upon request.



