Abstract
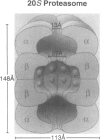
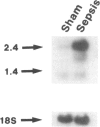
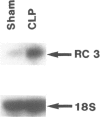
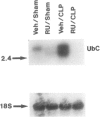
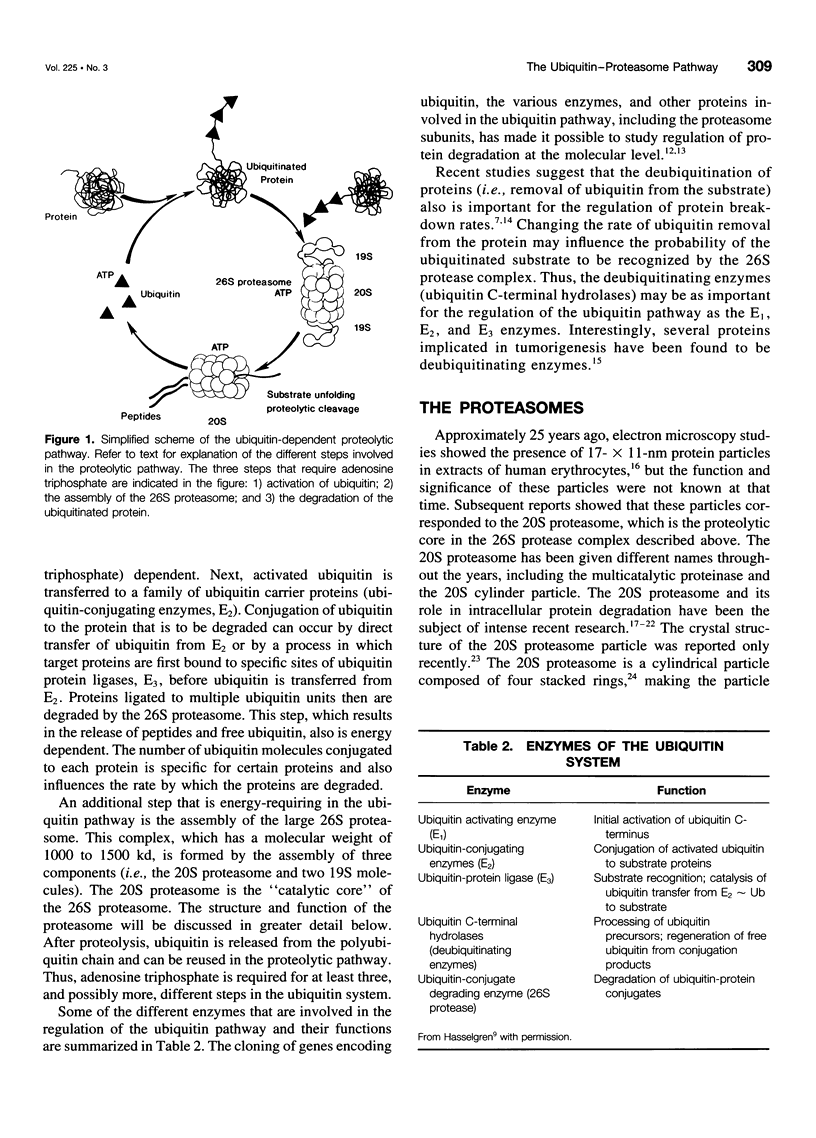
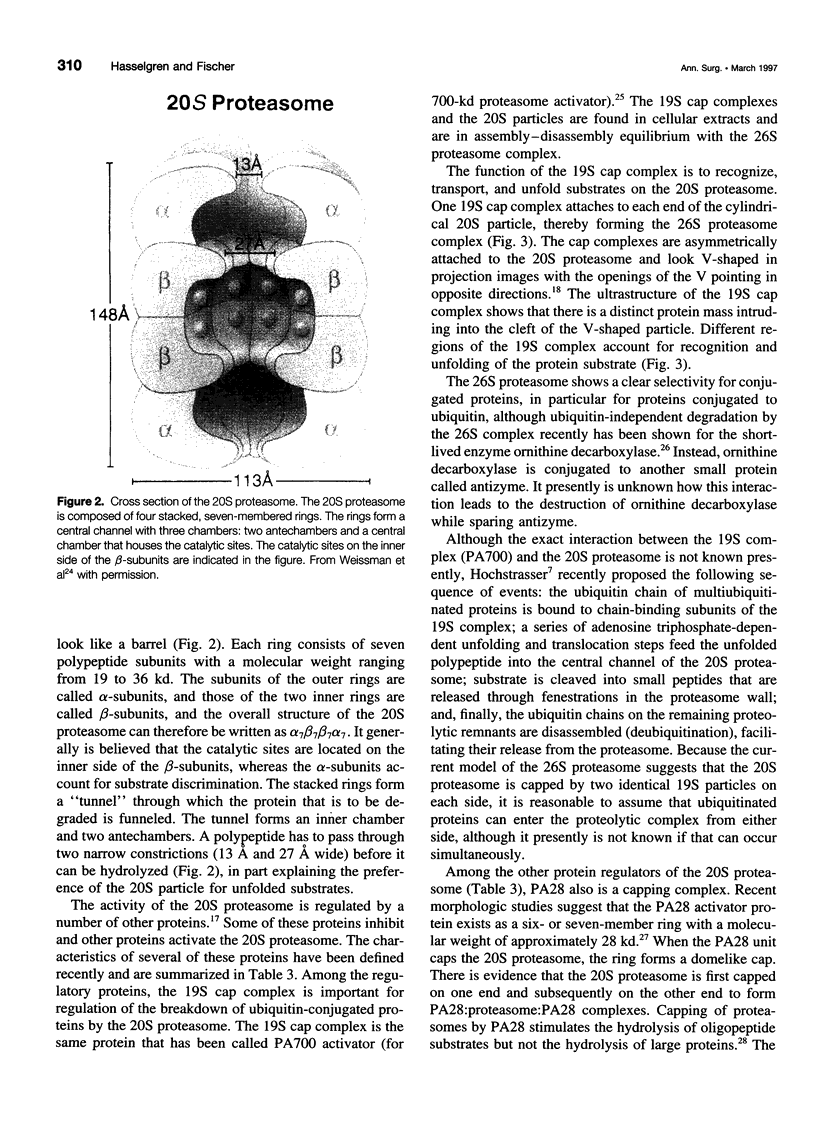
SUMMARY BACKGROUND DATA: Patients with sepsis and other catabolic conditions, such as severe trauma, cancer, and fasting, suffer significant loss of body protein, the majority of which originates from skeletal muscle. Recent evidence suggests that muscle protein breakdown during sepsis is caused by upregulated activity in the ubiquitin-proteasome pathway and is associated with increased expression of the ubiquitin gene. PURPOSE: The purpose of the study was to review the role of the ubiquitin-proteasome pathway in the regulation of muscle proteolysis during sepsis and other catabolic conditions. REVIEW: Proteins that are degraded by the ubiquitin-proteasome mechanism are first conjugated to ubiquitin, a 76-amino-acid, highly conserved residue. Ubiquitinated proteins are recognized by the 26S proteasome, which is a large proteolytic complex consisting of the 19S cap complex and the 20S proteasome. The 20S proteasome is a cylindrical particle composed of four stacked rings, making it look like a barrel. The rings form a "tunnel" in which the target proteins are hydrolyzed, after which ubiquitin is released to be reused in the proteolytic pathway. A unique feature of the ubiquitin-proteasome proteolytic pathway is its energy dependency. CONCLUSIONS: An understanding of the molecular regulation of protein metabolism in patients with sepsis and other catabolic conditions is important because it may form the basis for improved treatment in the future.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Baracos V. E., DeVivo C., Hoyle D. H., Goldberg A. L. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol. 1995 May;268(5 Pt 1):E996–1006. doi: 10.1152/ajpendo.1995.268.5.E996. [DOI] [PubMed] [Google Scholar]
- Barinaga M. A new twist to the cell cycle. Science. 1995 Aug 4;269(5224):631–632. doi: 10.1126/science.7624789. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994 Oct 7;79(1):13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Ciehanover A., Hod Y., Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1100–1105. doi: 10.1016/0006-291x(78)91249-4. [DOI] [PubMed] [Google Scholar]
- Dahlmann B., Kuehn L. The 20S/26S proteasomal pathway of protein degradation in muscle tissue. Mol Biol Rep. 1995;21(1):57–62. doi: 10.1007/BF00990972. [DOI] [PubMed] [Google Scholar]
- DeMartino G. N., Moomaw C. R., Zagnitko O. P., Proske R. J., Chu-Ping M., Afendis S. J., Swaffield J. C., Slaughter C. A. PA700, an ATP-dependent activator of the 20 S proteasome, is an ATPase containing multiple members of a nucleotide-binding protein family. J Biol Chem. 1994 Aug 19;269(33):20878–20884. [PubMed] [Google Scholar]
- Falquet L., Paquet N., Frutiger S., Hughes G. J., Hoang-Van K., Jaton J. C. A human de-ubiquitinating enzyme with both isopeptidase and peptidase activities in vitro. FEBS Lett. 1995 Feb 6;359(1):73–77. doi: 10.1016/0014-5793(94)01451-6. [DOI] [PubMed] [Google Scholar]
- Fang C. H., James H. J., Ogle C., Fischer J. E., Hasselgren P. O. Influence of burn injury on protein metabolism in different types of skeletal muscle and the role of glucocorticoids. J Am Coll Surg. 1995 Jan;180(1):33–42. [PubMed] [Google Scholar]
- Fang C. H., Tiao G., James H., Ogle C., Fischer J. E., Hasselgren P. O. Burn injury stimulates multiple proteolytic pathways in skeletal muscle, including the ubiquitin-energy-dependent pathway. J Am Coll Surg. 1995 Feb;180(2):161–170. [PubMed] [Google Scholar]
- Finco T. S., Baldwin A. S. Mechanistic aspects of NF-kappa B regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995 Sep;3(3):263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- Furuno K., Goodman M. N., Goldberg A. L. Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J Biol Chem. 1990 May 25;265(15):8550–8557. [PubMed] [Google Scholar]
- García-Martínez C., Agell N., Llovera M., López-Soriano F. J., Argilés J. M. Tumour necrosis factor-alpha increases the ubiquitinization of rat skeletal muscle proteins. FEBS Lett. 1993 Jun 1;323(3):211–214. doi: 10.1016/0014-5793(93)81341-v. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Margulies D. H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Functions of the proteasome: the lysis at the end of the tunnel. Science. 1995 Apr 28;268(5210):522–523. doi: 10.1126/science.7725095. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Rock K. L. Proteolysis, proteasomes and antigen presentation. Nature. 1992 Jun 4;357(6377):375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. The mechanism and functions of ATP-dependent proteases in bacterial and animal cells. Eur J Biochem. 1992 Jan 15;203(1-2):9–23. doi: 10.1111/j.1432-1033.1992.tb19822.x. [DOI] [PubMed] [Google Scholar]
- Gray C. W., Slaughter C. A., DeMartino G. N. PA28 activator protein forms regulatory caps on proteasome stacked rings. J Mol Biol. 1994 Feb 11;236(1):7–15. doi: 10.1006/jmbi.1994.1113. [DOI] [PubMed] [Google Scholar]
- Hall-Angerås M., Angerås U., Zamir O., Hasselgren P. O., Fischer J. E. Effect of the glucocorticoid receptor antagonist RU 38486 on muscle protein breakdown in sepsis. Surgery. 1991 Apr;109(4):468–473. [PubMed] [Google Scholar]
- Harris J. R. Release of a macromolecular protein component from human erythrocyte ghosts. Biochim Biophys Acta. 1968 Apr 29;150(3):534–537. doi: 10.1016/0005-2736(68)90157-0. [DOI] [PubMed] [Google Scholar]
- Hasselgren P. O., James J. H., Benson D. W., Hall-Angerås M., Angerås U., Hiyama D. T., Li S., Fischer J. E. Total and myofibrillar protein breakdown in different types of rat skeletal muscle: effects of sepsis and regulation by insulin. Metabolism. 1989 Jul;38(7):634–640. doi: 10.1016/0026-0495(89)90100-5. [DOI] [PubMed] [Google Scholar]
- Hasselgren P. O., James J. H., Fischer J. E. Inhibited muscle amino acid uptake in sepsis. Ann Surg. 1986 Apr;203(4):360–365. doi: 10.1097/00000658-198604000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Murakami Y. Rapid and regulated degradation of ornithine decarboxylase. Biochem J. 1995 Feb 15;306(Pt 1):1–10. doi: 10.1042/bj3060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol. 1995 Apr;7(2):215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- Jentsch S., Seufert W., Hauser H. P. Genetic analysis of the ubiquitin system. Biochim Biophys Acta. 1991 Jun 13;1089(2):127–139. doi: 10.1016/0167-4781(91)90001-3. [DOI] [PubMed] [Google Scholar]
- Koster A. J., Walz J., Lupas A., Baumeister W. Structural features of archaebacterial and eukaryotic proteasomes. Mol Biol Rep. 1995;21(1):11–20. doi: 10.1007/BF00990965. [DOI] [PubMed] [Google Scholar]
- Llovera M., García-Martínez C., Agell N., Marzábal M., López-Soriano F. J., Argilés J. M. Ubiquitin gene expression is increased in skeletal muscle of tumour-bearing rats. FEBS Lett. 1994 Feb 7;338(3):311–318. doi: 10.1016/0014-5793(94)80290-4. [DOI] [PubMed] [Google Scholar]
- Long C. L., Birkhahn R. H., Geiger J. W., Betts J. E., Schiller W. R., Blakemore W. S. Urinary excretion of 3-methylhistidine: an assessment of muscle protein catabolism in adult normal subjects and during malnutrition, sepsis, and skeletal trauma. Metabolism. 1981 Aug;30(8):765–776. doi: 10.1016/0026-0495(81)90022-6. [DOI] [PubMed] [Google Scholar]
- Löwe J., Stock D., Jap B., Zwickl P., Baumeister W., Huber R. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution. Science. 1995 Apr 28;268(5210):533–539. doi: 10.1126/science.7725097. [DOI] [PubMed] [Google Scholar]
- Ma C. P., Slaughter C. A., DeMartino G. N. Identification, purification, and characterization of a protein activator (PA28) of the 20 S proteasome (macropain). J Biol Chem. 1992 May 25;267(15):10515–10523. [PubMed] [Google Scholar]
- Mayer R. J., Arnold J., László L., Landon M., Lowe J. Ubiquitin in health and disease. Biochim Biophys Acta. 1991 Jun 13;1089(2):141–157. doi: 10.1016/0167-4781(91)90002-4. [DOI] [PubMed] [Google Scholar]
- Medina R., Wing S. S., Haas A., Goldberg A. L. Activation of the ubiquitin-ATP-dependent proteolytic system in skeletal muscle during fasting and denervation atrophy. Biomed Biochim Acta. 1991;50(4-6):347–356. [PubMed] [Google Scholar]
- Mitch W. E., Medina R., Grieber S., May R. C., England B. K., Price S. R., Bailey J. L., Goldberg A. L. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J Clin Invest. 1994 May;93(5):2127–2133. doi: 10.1172/JCI117208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco J. J. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992 May;13(5):173–179. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Parry-Billings M. Properties of glutamine release from muscle and its importance for the immune system. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):63S–67S. doi: 10.1177/014860719001400406. [DOI] [PubMed] [Google Scholar]
- Pagano M., Tam S. W., Theodoras A. M., Beer-Romero P., Del Sal G., Chau V., Yew P. R., Draetta G. F., Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995 Aug 4;269(5224):682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Palombella V. J., Rando O. J., Goldberg A. L., Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994 Sep 9;78(5):773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Papa F. R., Hochstrasser M. The yeast DOA4 gene encodes a deubiquitinating enzyme related to a product of the human tre-2 oncogene. Nature. 1993 Nov 25;366(6453):313–319. doi: 10.1038/366313a0. [DOI] [PubMed] [Google Scholar]
- Peters J. M. Proteasomes: protein degradation machines of the cell. Trends Biochem Sci. 1994 Sep;19(9):377–382. doi: 10.1016/0968-0004(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Price S. R., England B. K., Bailey J. L., Van Vreede K., Mitch W. E. Acidosis and glucocorticoids concomitantly increase ubiquitin and proteasome subunit mRNAs in rat muscle. Am J Physiol. 1994 Oct;267(4 Pt 1):C955–C960. doi: 10.1152/ajpcell.1994.267.4.C955. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gramm C., Rothstein L., Clark K., Stein R., Dick L., Hwang D., Goldberg A. L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994 Sep 9;78(5):761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Rosenblatt S., Clowes G. H., Jr, George B. C., Hirsch E., Lindberg B. Exchange of amino acids by muscle and liver in sepsis. Arch Surg. 1983 Feb;118(2):167–175. doi: 10.1001/archsurg.1983.01390020023004. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Molecular biology of proteasomes. Mol Biol Rep. 1995;21(1):21–26. doi: 10.1007/BF00990966. [DOI] [PubMed] [Google Scholar]
- Taylor R. G., Tassy C., Briand M., Robert N., Briand Y., Ouali A. Proteolytic activity of proteasome on myofibrillar structures. Mol Biol Rep. 1995;21(1):71–73. doi: 10.1007/BF00990974. [DOI] [PubMed] [Google Scholar]
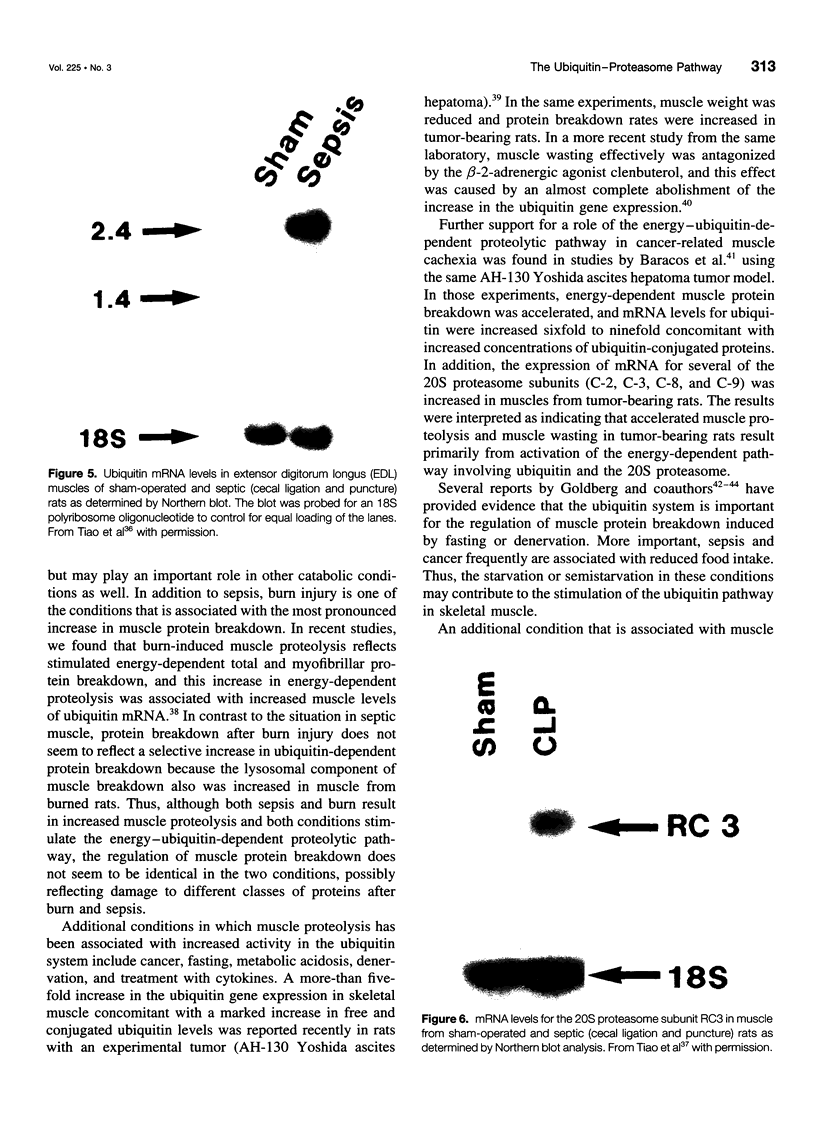
- Tiao G., Fagan J. M., Samuels N., James J. H., Hudson K., Lieberman M., Fischer J. E., Hasselgren P. O. Sepsis stimulates nonlysosomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest. 1994 Dec;94(6):2255–2264. doi: 10.1172/JCI117588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiao G., Fagan J., Roegner V., Lieberman M., Wang J. J., Fischer J. E., Hasselgren P. O. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest. 1996 Jan 15;97(2):339–348. doi: 10.1172/JCI118421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman J. S., Sigler P. B., Horwich A. L. From the cradle to the grave: ring complexes in the life of a protein. Science. 1995 Apr 28;268(5210):523–524. doi: 10.1126/science.7725096. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J Biol Chem. 1980 Jan 10;255(1):107–112. [PubMed] [Google Scholar]
- Wing S. S., Goldberg A. L. Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am J Physiol. 1993 Apr;264(4 Pt 1):E668–E676. doi: 10.1152/ajpendo.1993.264.4.E668. [DOI] [PubMed] [Google Scholar]
- Zamir O., Hasselgren P. O., Kunkel S. L., Frederick J., Higashiguchi T., Fischer J. E. Evidence that tumor necrosis factor participates in the regulation of muscle proteolysis during sepsis. Arch Surg. 1992 Feb;127(2):170–174. doi: 10.1001/archsurg.1992.01420020052008. [DOI] [PubMed] [Google Scholar]
- Zamir O., Hasselgren P. O., O'Brien W., Thompson R. C., Fischer J. E. Muscle protein breakdown during endotoxemia in rats and after treatment with interleukin-1 receptor antagonist (IL-1ra). Ann Surg. 1992 Sep;216(3):381–387. doi: 10.1097/00000658-199209000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]