Abstract
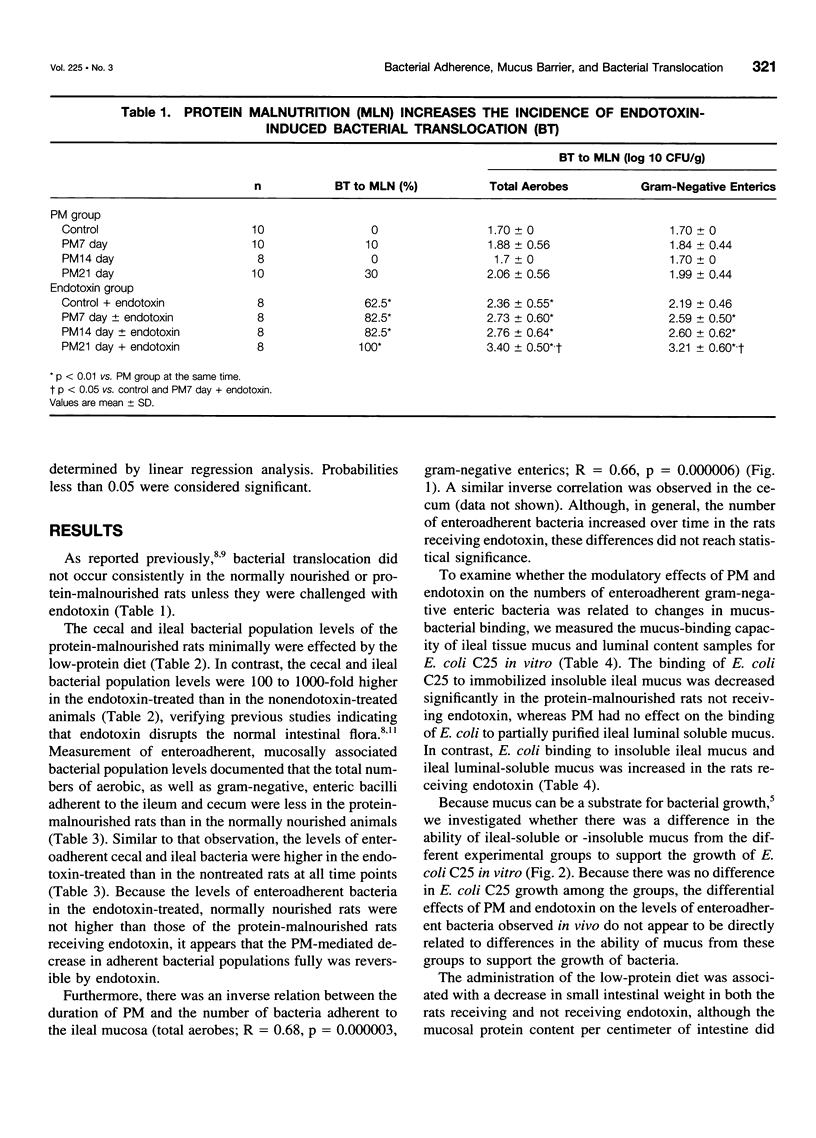
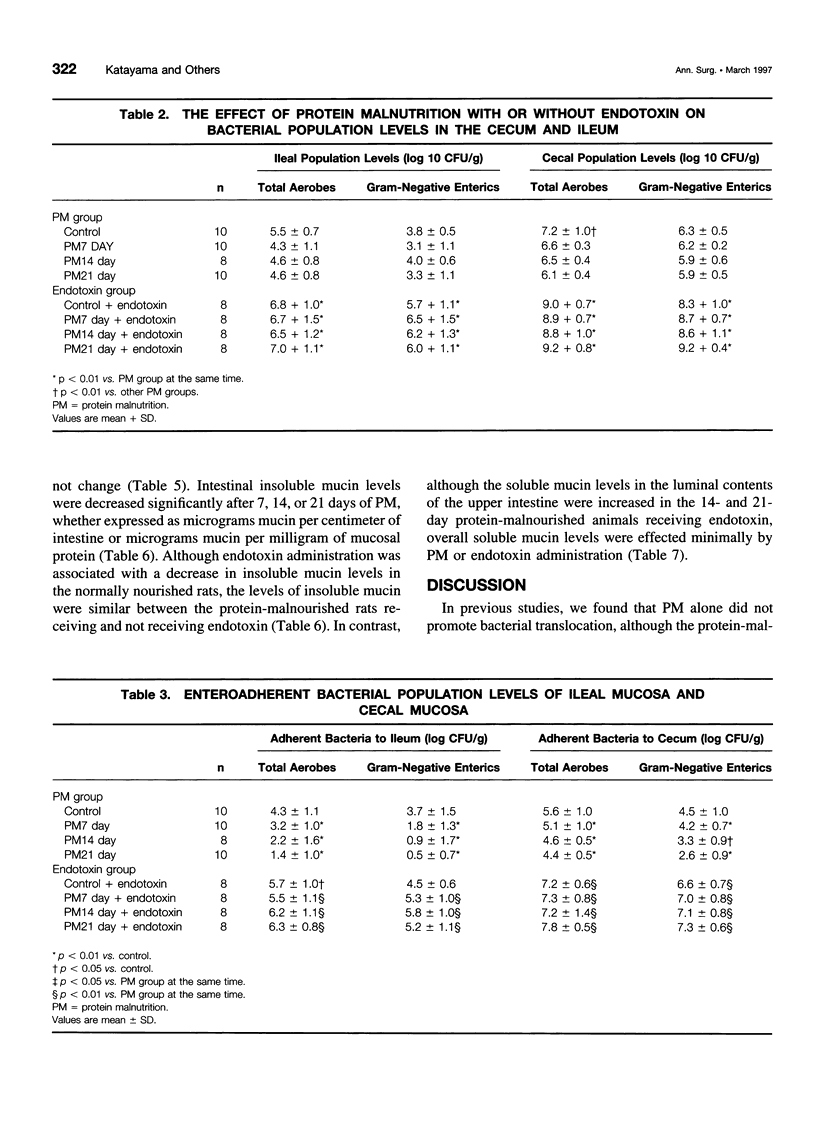
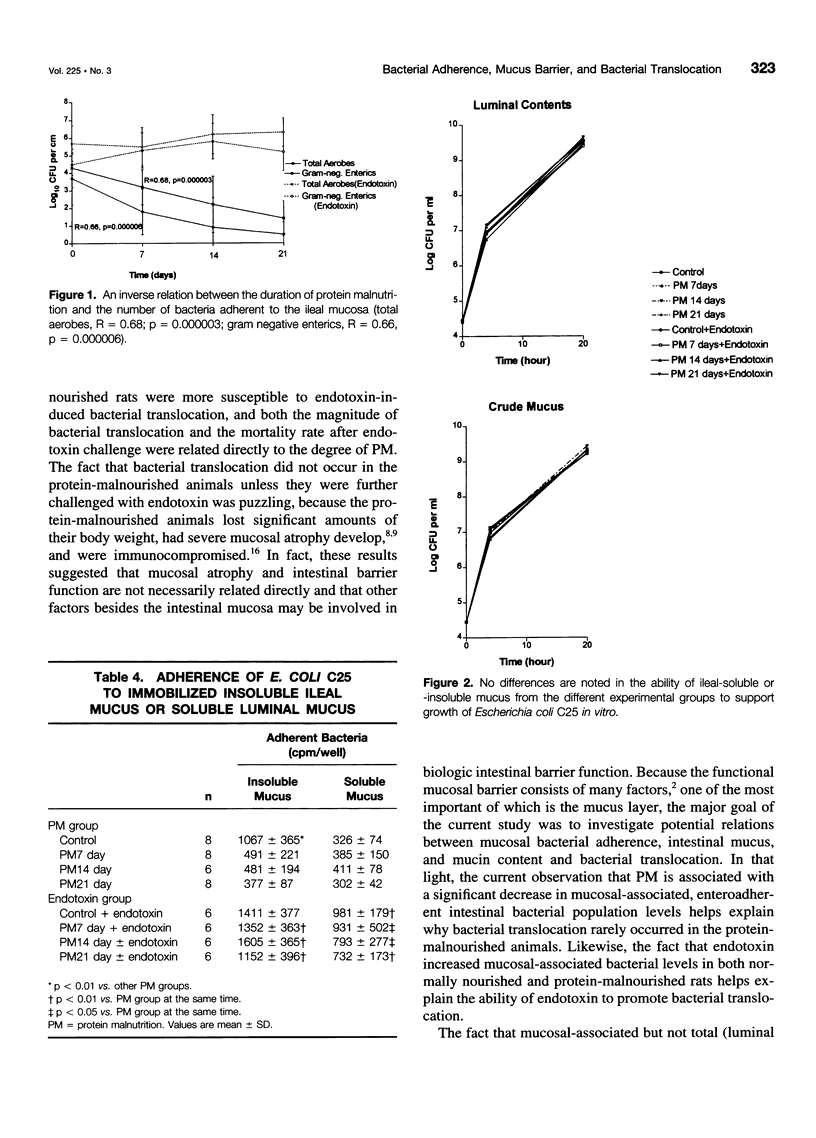
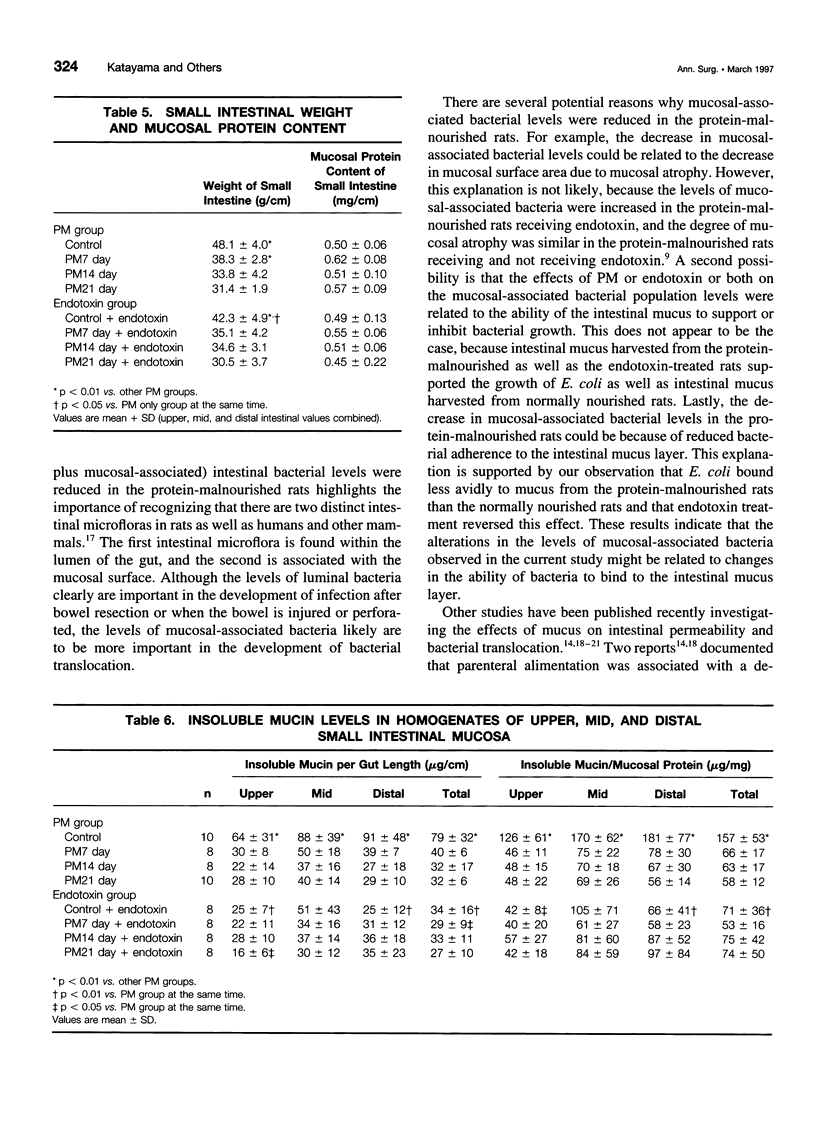
OBJECTIVE: The purpose of the study was to investigate the potential relations between mucosal bacterial adherence, intestinal mucus and mucin content, and bacterial translocation. SUMMARY BACKGROUND DATA: The attachment of bacteria to mucosal surfaces is the initial event in the pathogenesis of most bacterial infections that originate at mucosal surfaces, such as the gut. The intestinal mucus layer appears to function as a defensive barrier limiting micro-organisms present in the intestinal lumen from colonizing enterocytes. Consequently, studies focusing on the biology of bacterial adherence to the intestinal mucosa likely are to be important in clarifying the pathogenesis of gut origin sepsis. METHODS: To explore the relations between intestinal bacterial adherence, mucus bacterial binding, and bacterial translocation, two models were used. One (protein malnutrition) in which profound alterations in intestinal morphology occurs in the absence of significant translocation and one (endotoxin challenge) in which bacterial translocation occurs and intestinal morphology is relatively normal. RESULTS: Protein malnutrition was not associated with bacterial translocation and measurement of enteroadherent, mucosally associated bacterial population levels documented that the total number of gram-negative enteric bacilli adherent to the ileum and cecum was less in the protein-malnourished rats than in the normally nourished animals (p < 0.01). Furthermore, there was an inverse relation between the duration of protein malnutrition and bacterial adherence to the intestinal mucosa (r = 0.62, p < 0.002). In contrast, after endotoxin challenge, the level of enteroadherent bacteria was increased and bacterial translocation was observed. The binding of Escherichia coli to immobilized ileal mucus in vitro was decreased significantly in protein-malnourished rats, whereas E. coli binding to insoluble ileal mucus was increased in the rats receiving endotoxin. CONCLUSIONS: This study indicates that the adherence of bacteria to the intestinal mucosal surface is an important factor in bacterial translocation, that intestinal mucus modulates bacterial adherence, and that increased levels of mucosally associated bacteria are associated with a loss intestinal barrier function to bacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albanese C. T., Cardona M., Smith S. D., Watkins S., Kurkchubasche A. G., Ulman I., Simmons R. L., Rowe M. I. Role of intestinal mucus in transepithelial passage of bacteria across the intact ileum in vitro. Surgery. 1994 Jul;116(1):76–82. [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Berg R. D., Garlington A. W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979 Feb;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cruz N., Alvarez X., Specian R. D., Berg R. D., Deitch E. A. Role of mucin, mannose, and beta-1 integrin receptors in Escherichia coli translocation across Caco-2 cell monolayers. Shock. 1994 Aug;2(2):121–126. doi: 10.1097/00024382-199408000-00007. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Specian R. D., Berg R. D. Endotoxin-induced bacterial translocation and mucosal permeability: role of xanthine oxidase, complement activation, and macrophage products. Crit Care Med. 1991 Jun;19(6):785–791. doi: 10.1097/00003246-199106000-00010. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Winterton J., Berg R. Effect of starvation, malnutrition, and trauma on the gastrointestinal tract flora and bacterial translocation. Arch Surg. 1987 Sep;122(9):1019–1024. doi: 10.1001/archsurg.1987.01400210057008. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Winterton J., Li M., Berg R. The gut as a portal of entry for bacteremia. Role of protein malnutrition. Ann Surg. 1987 Jun;205(6):681–692. doi: 10.1097/00000658-198706000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch E. A., Xu D. Z., Qi L., Specian R. D., Berg R. D. Protein malnutrition alone and in combination with endotoxin impairs systemic and gut-associated immunity. JPEN J Parenter Enteral Nutr. 1992 Jan-Feb;16(1):25–31. doi: 10.1177/014860719201600125. [DOI] [PubMed] [Google Scholar]
- Iiboshi Y., Nezu R., Kennedy M., Fujii M., Wasa M., Fukuzawa M., Kamata S., Takagi Y., Okada A. Total parenteral nutrition decreases luminal mucous gel and increases permeability of small intestine. JPEN J Parenter Enteral Nutr. 1994 Jul-Aug;18(4):346–350. doi: 10.1177/014860719401800412. [DOI] [PubMed] [Google Scholar]
- Li M., Specian R. D., Berg R. D., Deitch E. A. Effects of protein malnutrition and endotoxin on the intestinal mucosal barrier to the translocation of indigenous flora in mice. JPEN J Parenter Enteral Nutr. 1989 Nov-Dec;13(6):572–578. doi: 10.1177/0148607189013006572. [DOI] [PubMed] [Google Scholar]
- Mantle M., Thakore E., Atkins E., Mathison R., Davison J. S. Effects of streptozotocin-diabetes on rat intestinal mucin and goblet cells. Gastroenterology. 1989 Jul;97(1):68–75. doi: 10.1016/0016-5085(89)91417-0. [DOI] [PubMed] [Google Scholar]
- Maxson R. T., Dunlap J. P., Tryka F., Jackson R. J., Smith S. D. The role of the mucus gel layer in intestinal bacterial translocation. J Surg Res. 1994 Dec;57(6):682–686. doi: 10.1006/jsre.1994.1201. [DOI] [PubMed] [Google Scholar]
- Rozee K. R., Cooper D., Lam K., Costerton J. W. Microbial flora of the mouse ileum mucous layer and epithelial surface. Appl Environ Microbiol. 1982 Jun;43(6):1451–1463. doi: 10.1128/aem.43.6.1451-1463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth G., Gottwald T., Specian R. D., Mainous M. R., Berg R. D., Deitch E. A. Secretory immunoglobulin A, intestinal mucin, and mucosal permeability in nutritionally induced bacterial translocation in rats. Ann Surg. 1994 Dec;220(6):798–808. doi: 10.1097/00000658-199412000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specian R. D., Oliver M. G. Functional biology of intestinal goblet cells. Am J Physiol. 1991 Feb;260(2 Pt 1):C183–C193. doi: 10.1152/ajpcell.1991.260.2.C183. [DOI] [PubMed] [Google Scholar]


